Abstract
Platelet transfusions are commonly used treatments that occasionally lead to adverse reactions. Clinical trials, in vitro and animal studies have been performed to try to understand the causes of such reactions. Multiple studies have shown that the supernatant fraction of platelet concentrates contain prothrombotic and proinflammatory mediators. The origin of these mediators was first ascribed to white blood cells contaminating the platelet preparation. However, the accumulation of bioactive mediators after leukoreduction focused attention on platelets themselves during storage. Numerous cytokines, chemokines and prostaglandins are released in stored platelet concentrates. We have focused on a powerful mediator called soluble CD40 ligand (sCD40L, formally known as CD154) as a seminal contributor to adverse reactions. sCD40L can bind and signal the surface receptor, CD40, which is present on various types of human cells including white blood cells, vascular cells and fibroblasts. Downstream results of sCD40L/CD40 signaling include proinflammatory cytokine and chemokine production, prothrombotic mediator release, adherence and transmigration of leukocytes to endothelium and other undesirable vascular inflammatory events. Increased plasma levels of sCD40L can be detected in conditions such as myocardial infarction, stroke, unstable angina, high cholesterol, or other cardiovascular conditions. In retrospective studies, correlations were made between increased sCD40L levels of platelet concentrates and adverse transfusion reactions. We hypothesize that transfusion of partially activated, CD40L-expressing platelets along with sCD40L into a recipient with damaged or dysfunctional vascular tissue results in a “double-hit”, thus inciting inflammation and vascular damage in the recipient.
Keywords: Platelet transfusion, soluble CD40 ligand, CD154, TRALI, platelet concentrate storage
1. Introduction
Platelet transfusions are administered to bleeding thrombocytopenic patients, or prophylactically to patients predicted to have a reduced platelet count after therapies such as anti-neoplastic chemotherapy or hematopoietic stem cell transplantation. Restoring platelet counts through transfusion is performed with one main objective: to prevent bleeding. Unfortunately, complications among platelet transfusion recipients remain as high as 30 percent (1). Despite attempted preventative measures such as premedication and pre-storage leukoreduction, transfusion reactions can occur in forms of fever, rigors, or more severely, transfusion-related acute lung injury (TRALI) and transfusion associated circulatory overload (TACO). TRALI is an inflammatory response attributed to the transfused unit and the responses of recipient inflammatory cells, while TACO is presumed to be primarily caused by the body’s inability to accommodate the infused volume and/or flow rate (2). Over the past 2 decades, clinicians and researchers have been striving to better understand the mechanisms behind adverse transfusion reactions in effort to prevent them from occurring.
2. History of identification of components leading to reactions
Important progress has been made in discovering new methods to lower the incidence of platelet transfusion reactions. Mangano et al were among the first to show that the removal of leukocytes (via centrifugation) from platelet transfusion components decreased, but did not abolish adverse reactions (3). Heddle et al helped to narrow down key mediators contributing to transfusion reactions in a clinical trial that transfused plasma and cellular components separately to patients needing platelet transfusion. They discovered that the plasma components caused more reactions than cellular components, and observed positive correlations between concentrations of plasma interleukin (IL)-1β and IL-6 with adverse reactions (4). This concept supported previous work by Muylle et al who noted IL-6, tumor necrosis factor-α (TNF-α) and IL-1β were abundant in platelet concentrates stored for longer periods of time, and suggested these might be causal of febrile reactions. They concluded these cytokines were most likely synthesized and released during storage of platelet concentrates (5). Time of storage had also been found to play a role in cytokine levels of platelet concentrates, with concentrates stored longer than 3 days causing more reactions than shorter storage concentrates (6).
In a 14 year study of blood component reactions before and after implementation of universal leukoreduction immediately prior to transfusion, strikingly reduced incidences of TRALI, TACO and febrile reactions were observed in the seven years post introduction of universal leukoreduction (7). An immunological-minded alteration of platelet transfusion practice through providing ABO-identical platelets to recipients was implemented to decrease the incidence of reactions. In a study spanning 9 years with 36 months of data collection, febrile and allergic transfusion reactions were significantly reduced after implementation of transfusion with only ABO-identical platelet components (8).
Despite leukoreduction immediately prior to transfusion of platelet concentrates, substances within the supernatant of platelet concentrates still mediate febrile transfusion reactions. In 24 hematology/oncology patients receiving a combined 522 leukoreduced transfusions, it was found that saline washing of the platelet transfusions after leukoreduction failed to prevent, but reduced the frequency of transfusion reactions from 20% to 0.6% (9).
The concept of pre-storage leukocyte removal was investigated as a means to prevent the production of inflammatory agents seen to accumulate during storage. Using a variety of leukocyte removal methods, multiple groups were able to mitigate the increase of cytokines such as TNFα, IL-6 (10), IL-8 and IL-1β (11). In 1996, Muylle et al were able to correlate febrile and allergic reactions (independently) with IL-6 levels, initial white blood cell counts, and storage time. Importantly, their study also included a comparison of adverse reaction events amongst known methods of that time for leukocyte removal (12).
Taken together, these and other studies demonstrate that antigens, cytokines and immune cells in transfused platelet concentrates most likely contribute to adverse transfusion reactions among patients. As demonstrated in the saline-washed leukoreduced platelet transfusion study (9), it appears that platelets themselves contribute to the bioactive mediators released in the concentrates during storage. While many signaling mediators act in concert to contribute to transfusion reactions, much attention and investigation has recently been given to contribution of platelet-derived CD40 ligand (CD40L).
3. CD40 Ligand Background
CD40L, also referred to as CD154, has been classically established as providing co-stimulation from T lymphocytes, binding to its receptor, CD40, on B lymphocytes during an immune response (13). CD40 is part of the TNF receptor family and has a pro-inflammatory stimulation capacity. Besides B cells, other cell types express CD40 on their surface, and therefore have the capacity to be stimulated by CD40L. These cell types include dendritic cells, T cells, fibroblasts, mast cells, smooth muscle cells, neutrophils, endothelial cells, monocytes, macrophages, and platelets. CD40L can also be expressed on the surface of T cells, mast cells, endothelial cells, monocytes, macrophages, and platelets (14). Within the blood, non-cellular bound CD40L is known as soluble CD40L (sCD40L) and exists both as an independent cleaved form and also bound to platelet microparticles. These two populations of sCD40L have not been well differentiated, as the isolation and characterization of microparticles is an emerging process. Therefore, it is important to acknowledge that any measurements or effects of sCD40L could be attributed to one or both forms of the ligand. sCD40L is present in the concentration range of pg to ng/mL in human plasma (15). Platelets contain abundant CD40L in alpha granules, and upon platelet activation, express it on their surface (Fig. 1). Cleavage and microparticle release of sCD40L from activated platelets occurs within seconds to minutes (16), and it is thought that over 95% of sCD40L in plasma originates from platelets (17).
Fig. 1.
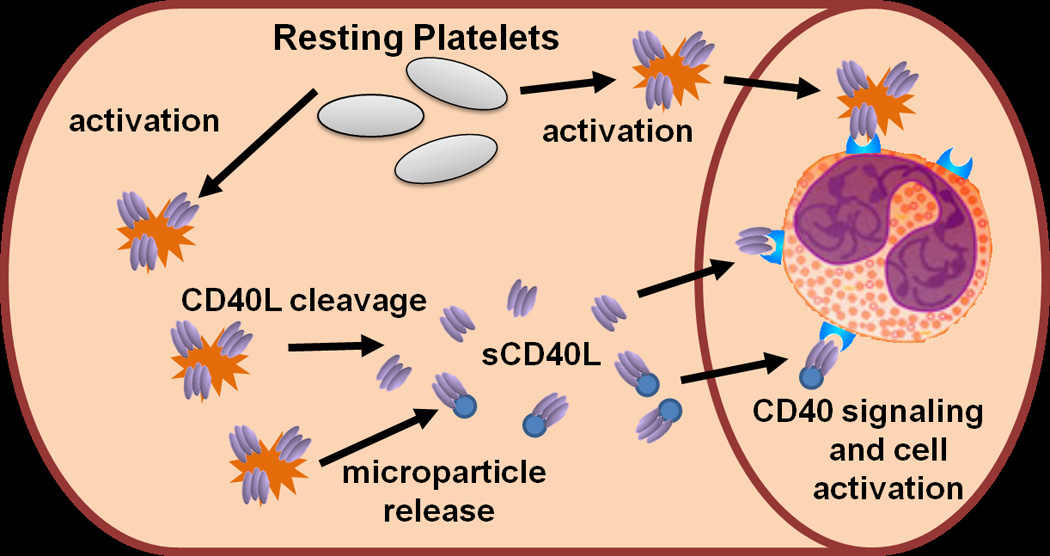
CD40L signaling in the vasculature. Upon activation, platelets (represented by spiked cells) express CD40L (homo-trimeric protein represented by ovals) on their surface within seconds to minutes. Surface CD40L can be shed via plasma proteases and released in a soluble form (sCD40L). Also, during platelet activation, platelets release microparticles (represented by small round circles) that can carry surface CD40L, which is also categorized as sCD40L in most studies. sCD40L or platelet bound CD40L can bind to vascular and blood-borne cells that express CD40 and initiate signaling and cell activation.
4. CD40 Ligand in Vascular Inflammation
Activation of CD40 positive cells by platelet–bound or sCD40L can lead to vascular inflammation. The homo-trimeric form of CD40L binds to CD40 on target cells (Fig. 1), and activates downstream pro-inflammatory transcription factors such as nuclear factor-κB. Examples of resulting inflammatory responses include cytokine and chemokine expression and release, surface expression of adhesion receptors and prostaglandin production (Fig. 2). Van Kooten et al. comprehensively described the in vitro effects of CD40L signaling on CD40-expressing cells. Importantly, the cleaved form of sCD40L retains its ability to bind CD40 to activate target cells, acting in a signaling manner relating to soluble cytokines (18).
Fig. 2.
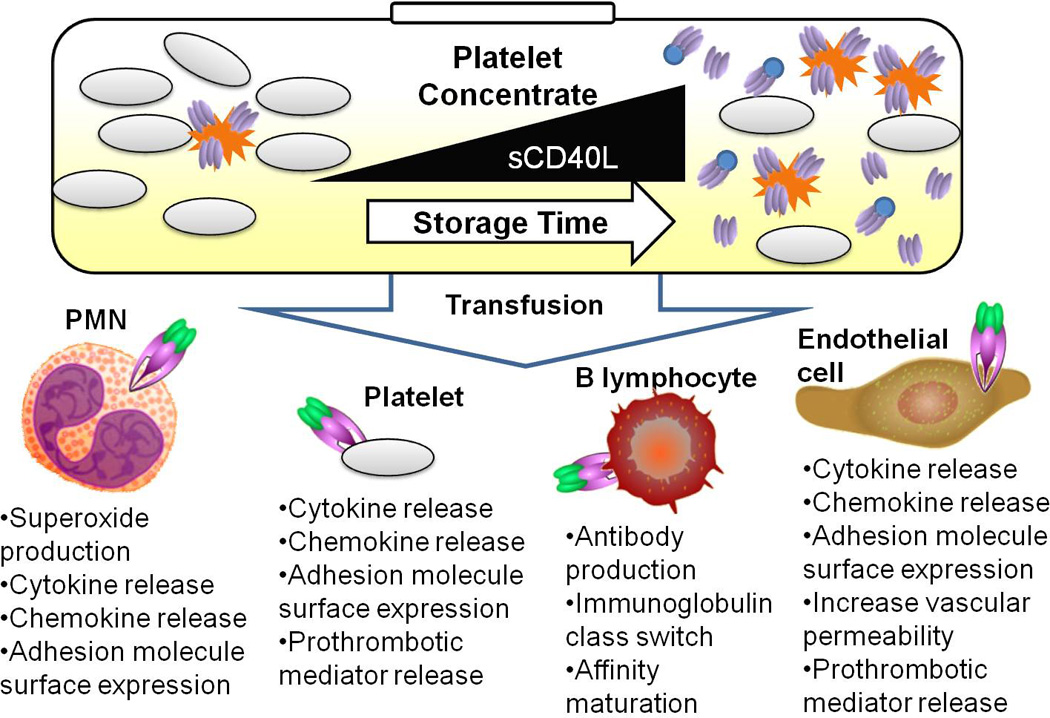
Vascular inflammation induced by sCD40L delivered via transfusion. Platelet concentrates used for transfusion can accumulate activated platelets (represented by spiked cells), as well as cleaved and microparticle-bound sCD40L (homo-trimeric protein represented by ovals and ovals attached to circles) over storage time. Four examples of CD40 receptor expressing cells are depicted: PMN (Polymorphonuclear leukocytes), resting platelets, B lymphocytes, and endothelial cells. Some of the downstream effects of CD40 signaling are listed below each cell type.
Platelet CD40L can activate endothelial cells to produce the chemokines IL-8 and MCP-1, which can subsequently recruit leukocytes to areas of vascular inflammation. Once recruited, the CD40L-induced upregulation of endothelial cell adhesion molecules E-selectin, VCAM and ICAM-1 allows polymorphonuclear leukocytes (PMNs) to bind to and potentially extravasate across the endothelium (16, 19). CD40L signaling has also been shown to enhance the pro-thrombotic vascular environment by causing endothelial cells to express tissue factor (20). When CD40 basal expression is upregulated on dermal endothelial cells, as evidenced in certain skin inflammatory conditions (19), it is probable that the inflamed endothelium could have an increase in sensitivity to platelet-derived CD40L. In the context of a mouse ischemia-reperfusion model, Ishikawa et al showed that CD40-CD40L signaling played an integral role in vascular permeability and compromised blood brain barrier function (21).
Platelet-derived sCD40L was specifically shown to induce IL-6 synthesis and cyclooxygenase-2 expression followed by downstream prostaglandin E2 production in human lung fibroblasts (22). Further, CD40L/CD40 signaling on PMNs was shown to rapidly prime the PMN oxidase which subsequently increased superoxide production. Pre-activation of human microvascular endothelial cells to cause PMN adherence, followed by the addition of sCD40L resulted in significant endothelial cell toxicity, likely through PMN superoxide production (15). Lastly, recombinant CD40L, as well as serum with elevated levels of sCD40L, were both able to cause increased release of MCP-1 from mononuclear cells (23).
Elzey et al demonstrated that wild type platelets transfused into CD40L knockout mice can boost the adaptive immune response to viral infection, through influencing B cells to isotype switch as well as altering T cell function (24). Sprague et al later reported that platelet-derived vesicles were also sufficient to deliver CD40L to B cells to facilitate antibody production (25).
CD40 and CD40L may stimulate a positive feedback loop among activated vascular cells. As platelets, monocytes, macrophages, endothelial cells, mast cells, and T cells express both CD40 and CD40L, the induction of inflammation by a neighboring cell could generate an autocrine or paracrine inflammatory cycle (14). Other functional consequences of CD40 activation in other CD40-expressing cells are elegantly reviewed by van Kooten (18).
5. CD40L and Clinical Correlates
sCD40L levels are elevated in numerous vascular inflammatory disease states. For example, diabetic patients with coronary artery disease display significantly increased plasma levels of sCD40L compared to non-diabetic coronary artery disease patients or healthy controls (26). Middle-aged female patients who developed myocardial infarction, stroke, or cardiovascular death had higher sCD40L plasma levels than healthy control subjects (27). Patients with unstable angina had higher sCD40L levels while their platelets had lower intracellular CD40L levels and release capacity compared to stable angina or control patients (23). sCD40L was also shown to positively correlate with total cholesterol and LDL cholesterol in a study of hypercholesterolemia (28).
Cumulatively, these data suggest the utility of plasma sCD40L measurements as a screening tool for vascular inflammation, endogenous platelet activation, or future risk of development of cardiovascular diseases.
6. sCD40L in Platelet Concentrates
Stored platelet concentrates contain increased levels of sCD40L compared to fresh plasma levels. More specifically, sCD40L was found to steadily increase through day 7 in stored plasma of apheresis-derived platelets (Fig 2.). Additionally, non-leukoreduced packed red blood cells were also found to increase in sCD40L levels at days 28 and 42 compared with day 1 of storage (15).
In a study of 50 male platelet concentrates screened for platelet activation, Bakry et al found a positive correlation between CD40L and Annexin V on the surface of platelets that increased during separation of platelets from whole blood and again when tracked on the third day of storage (29). A more detailed kinetic investigation of the spontaneous release of sCD40L from multiple donor pooled platelet concentrates demonstrated an increase through 72 hours and remained at those levels through 126 hours. Interestingly, aspirin pretreatment failed to inhibit this release, implying sCD40L release may be independent of cyclooxgenase-1 activation (22).
Wenzel et al found that after platelet concentrate transfusion into thrombocytopenic patients, below normal plasma sCD40L levels were returned to a normal range (around 204 pg/mL). Interestingly, per platelet sCD40L release capacity was reduced below healthy ranges after transfusion, possibly indicating that transfused platelets were already activated or aged, losing functional capacity (30). Recently, one group reported no change in recipient plasma sCD40L levels post-transfusion, but noted there were increases in other types of cytokines (31). The contrasting findings of these two studies may stem from differences in initial plasma levels of sCD40L in recipients. Specifically, if patients begin in a normal plasma sCD40L range, through mechanisms of threshold tolerance, it may be the case that above-normal levels are immediately removed from the bloodstream. Additionally, there is no way to account for the quantity of recipient CD40-expressing endothelial and white blood cells in these two studies. Infusion of platelet concentrate-derived sCD40L could immediately be bound by such cells with vacant receptors.
7. sCD40L in Adverse Reactions to Transfusions
Because of its abundance in plasma and platelet concentrates, sCD40L has a strong potential to induce inflammation, especially into an environment that is already perturbed, resulting in increased CD40 expression. With this type of “double-hit” activation, adverse events post-transfusion can plausibly occur.
sCD40L was first reported to be associated with adverse transfusion events in 2006, where cytokines IL-6, IL-8, MCP-1 and sCD40L, but not RANTES were all elevated in the supernatants of leukoreduced transfused platelet concentrates that caused either febrile or allergic reactions (32).
In a retrospective study, levels of sCD40L from platelet concentrates derived from either whole blood or apheresis were higher in transfusion recipients that developed TRALI compared to non-reactive recipients. Measurements of patient plasma showed sCD40L levels in 8 out of 12 TRALI patients were increased after they received transfusions. It is possible that the transfused units containing elevated levels of sCD40L contributed to TRALI in this study (15).
Cognasse et al investigated acute transfusion reactions by contrasting transfused fractionated platelet concentrates which did or did not cause reactions. First, sCD40L was higher in the supernatant fraction, and lower in the platelet lysate fraction within reaction-causing concentrates. Next, they added the supernatant and platelet lysate fractions to B cells to indirectly assay for CD40L activity via B-cell IL-6 production. Again, the highest IL-6 production occurred after B cell treatment with supernatant fractions of the reaction-causing platelet concentrates, as well as the lysates of the control platelet concentrates. To verify specificity, a blocking CD40L antibody was used and IL-6 levels were significantly reduced (33). Together, these findings indicate sCD40L release, probably caused by platelet activation during storage, is associated with transfusion reactions. Importantly, this study also exemplified how the transfusion of sCD40L could have robust activity on various host cells.
In an effort to single out the contribution of sCD40L in transfusion reactions, a depleting antibody was used to remove sCD40L from platelet concentrate supernatants, which were then added to human lung fibroblasts to study inflammatory mediator production. Compared with sCD40L-containing supernatants, the depleted supernatants induced significantly less, and sometimes completely prevented IL-6 release, cyclooxygenase-2 expression and prostaglandin E2 production (22).
Tuinman et al recently failed to find CD40L involvement in an experimental mouse model of TRALI, induced through administration of antibodies to MHC class I (34). Neither the use of ciglitazone to inhibit platelet CD40L expression, nor administration of a blocking antibody against CD40L, had any effect on pulmonary and systemic inflammation in their experiments. However, this artificial mouse model of TRALI may not involve CD40L, or its role may be too minor to detect. While another thiazolidinedione drug, rosiglitazone, has been shown to blunt platelet release of CD40L from human platelets (35), and decrease systemic sCD40L in coronary artery disease and type-2 diabetic patients (26), it has not yet been shown to reduce platelet-derived sCD40L in mice. Even if the correct dosage and timing of ciaglitazone treatment was determined for mice, platelet-derived CD40L would probably not play a role in augmenting a potent anti-MHC inflammatory response, therefore its inhibition may have no effect on the model.
Tuinman et al also investigated plasma levels of sCD40L before and after cardiac surgery, and compared patients who did or did not develop TRALI (34). In this study, there were no differences of sCD40L levels in the plasma of TRALI patients compared with their pre-symptomatic baseline levels, or control patients. One concern is that TRALI in these cases may have been donor antibody mediated, with little or no role for sCD40L. To rule out a role for donor sCD40L, one would need to examine only donor antibody negative cases of TRALI. In addition, the matched control patients of the analysis had an increased incidence of myocardial infarction, hypertension, peripheral artery disease and diabetes. These inflammatory conditions within the control group could conceivably have elevated plasma sCD40L levels to match the inflammatory status seen in the TRALI patients. These data support the fact that vascular inflammation, as measured through elevated plasma sCD40L levels tracked within the same individual over time, can lead to different and sometimes independent cardiovascular injury states.
Conclusions
The vast majority of platelet transfusions with high mediator levels do not lead to adverse reactions. It is possible that when the delicate inflammatory balance of the vasculature is skewed toward a pro-inflammatory status preceding transfusion that platelet derived mediators contribute to an adverse outcome. sCD40L may be one key mediator able to promote a hemostatic imbalance that results in adverse transfusion effects. We hypothesize that transfusion of partially activated, CD40L-expressing platelets along with sCD40L into a recipient with damaged or dysfunctional vascular tissue results in a “double-hit” activation of CD40 expressing cells within the vasculature of the recipient (Fig. 2).
The exact contribution of sCD40L in transfusion-induced adverse reactions has yet to be fully established. Strong correlations of plasma sCD40L concentrations and vascular disease have been demonstrated. The molecular contribution of inflammatory signaling has been modeled in various in vitro and animal settings. The limitations of fully understanding and preventing CD40L-induced inflammatory responses are due to the onset of inflammation being a multifactorial, progressive process that is unlikely to be due to only one mediator. Yet, if inhibiting CD40L has substantial effects on vascular inflammatory disorders, targeting this molecule and blocking its interaction with CD40 provides a potentially attractive avenue of target-based therapy. Simple removal of platelet transfusion supernatant mediators by saline washing or similar techniques may protect selected vulnerable patients from adverse events such as rigors, fever and TRALI.
Acknowledgments
Funding
This work was supported in part by T32-HL066988, HL095467 and ES01247.
Footnotes
No potential conflict of interest relevant to this article was reported
References
- 1.Heddle NM, Klama LN, Griffith L, Roberts R, Shukla G, Kelton JG. A prospective study to identify the risk factors associated with acute reactions to platelet and red cell transfusions. Transfusion. 1993;33(10):794–797. doi: 10.1046/j.1537-2995.1993.331094054613.x. [DOI] [PubMed] [Google Scholar]
- 2.Popovsky MA. Pulmonary consequences of transfusion: TRALI and TACO. Transfus Apher Sci. 2006;34(3):243–244. doi: 10.1016/j.transci.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 3.Mangano MM, Chambers LA, Kruskall MS. Limited efficacy of leukopoor platelets for prevention of febrile transfusion reactions. Am J Clin Pathol. 1991;95(5):733–738. doi: 10.1093/ajcp/95.5.733. [DOI] [PubMed] [Google Scholar]
- 4.Heddle NM, Klama L, Singer J, Richards C, Fedak P, Walker I, et al. The role of the plasma from platelet concentrates in transfusion reactions. N Engl J Med. 1994;331(10):625–628. doi: 10.1056/NEJM199409083311001. [DOI] [PubMed] [Google Scholar]
- 5.Muylle L, Joos M, Wouters E, De Bock R, Peetermans ME. Increased tumor necrosis factor alpha (TNF alpha), interleukin 1, and interleukin 6 (IL-6) levels in the plasma of stored platelet concentrates: relationship between TNF alpha and IL-6 levels and febrile transfusion reactions. Transfusion. 1993;33(3):195–199. doi: 10.1046/j.1537-2995.1993.33393174443.x. [DOI] [PubMed] [Google Scholar]
- 6.Muylle L, Wouters E, De Bock R, Peetermans ME. Reactions to platelet transfusion: the effect of the storage time of the concentrate. Transfus Med. 1992;2(4):289–293. doi: 10.1111/j.1365-3148.1992.tb00172.x. [DOI] [PubMed] [Google Scholar]
- 7.Blumberg N, Heal JM, Gettings KF, Phipps RP, Masel D, Refaai MA, et al. An association between decreased cardiopulmonary complications (transfusion-related acute lung injury and transfusion-associated circulatory overload) and implementation of universal leukoreduction of blood transfusions. Transfusion. 2010;50(12):2738–2744. doi: 10.1111/j.1537-2995.2010.02748.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Henrichs KF, Howk N, Masel DS, Thayer M, Refaai MA, Kirkley SA, et al. Providing ABO-identical platelets and cryoprecipitate to (almost) all patients: approach, logistics, and associated decreases in transfusion reaction and red blood cell alloimmunization incidence. Transfusion. 2011 doi: 10.1111/j.1537-2995.2011.03329.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vo TD, Cowles J, Heal JM, Blumberg N. Platelet washing to prevent recurrent febrile reactions to leucocyte-reduced transfusions. Transfus Med. 2001;11(1):45–47. doi: 10.1046/j.1365-3148.2001.00280.x. [DOI] [PubMed] [Google Scholar]
- 10.Muylle L, Peetermans ME. Effect of prestorage leukocyte removal on the cytokine levels in stored platelet concentrates. Vox Sang. 1994;66(1):14–17. doi: 10.1111/j.1423-0410.1994.tb00270.x. [DOI] [PubMed] [Google Scholar]
- 11.Stack G, Snyder EL. Cytokine generation in stored platelet concentrates. Transfusion. 1994;34(1):20–25. doi: 10.1046/j.1537-2995.1994.34194098597.x. [DOI] [PubMed] [Google Scholar]
- 12.Muylle L, Wouters E, Peetermans ME. Febrile reactions to platelet transfusion: the effect of increased interleukin 6 levels in concentrates prepared by the platelet-rich plasma method. Transfusion. 1996;36(10):886–890. doi: 10.1046/j.1537-2995.1996.361097017174.x. [DOI] [PubMed] [Google Scholar]
- 13.Lederman S, Yellin MJ, Krichevsky A, Belko J, Lee JJ, Chess L. Identification of a novel surface protein on activated CD4+ T cells that induces contact-dependent B cell differentiation (help) J Exp Med. 1992;175(4):1091–1101. doi: 10.1084/jem.175.4.1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rizvi M, Pathak D, Freedman JE, Chakrabarti S. CD40-CD40 ligand interactions in oxidative stress, inflammation and vascular disease. Trends Mol Med. 2008;14(12):530–538. doi: 10.1016/j.molmed.2008.09.006. [DOI] [PubMed] [Google Scholar]
- 15.Khan SY, Kelher MR, Heal JM, Blumberg N, Boshkov LK, Phipps R, et al. Soluble CD40 ligand accumulates in stored blood components, primes neutrophils through CD40, and is a potential cofactor in the development of transfusion-related acute lung injury. Blood. 2006;108(7):2455–2462. doi: 10.1182/blood-2006-04-017251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Henn V, Slupsky JR, Gräfe M, Anagnostopoulos I, Förster R, Müller-Berghaus G, et al. CD40 ligand on activated platelets triggers an inflammatory reaction of endothelial cells. Nature. 1998;391(6667):591–594. doi: 10.1038/35393. [DOI] [PubMed] [Google Scholar]
- 17.André P, Nannizzi-Alaimo L, Prasad SK, Phillips DR. Platelet-derived CD40L: the switch-hitting player of cardiovascular disease. Circulation. 2002;106(8):896–899. doi: 10.1161/01.cir.0000028962.04520.01. [DOI] [PubMed] [Google Scholar]
- 18.van Kooten C, Banchereau J. CD40-CD40 ligand. J Leukoc Biol. 2000;67(1):2–17. doi: 10.1002/jlb.67.1.2. [DOI] [PubMed] [Google Scholar]
- 19.Hollenbaugh D, Mischel-Petty N, Edwards CP, Simon JC, Denfeld RW, Kiener PA, et al. Expression of functional CD40 by vascular endothelial cells. J Exp Med. 1995;182(1):33–40. doi: 10.1084/jem.182.1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Slupsky JR, Kalbas M, Willuweit A, Henn V, Kroczek RA, Müller-Berghaus G. Activated platelets induce tissue factor expression on human umbilical vein endothelial cells by ligation of CD40. Thromb Haemost. 1998;80(6):1008–1014. [PubMed] [Google Scholar]
- 21.Ishikawa M, Vowinkel T, Stokes KY, Arumugam TV, Yilmaz G, Nanda A, et al. CD40/CD40 ligand signaling in mouse cerebral microvasculature after focal ischemia/reperfusion. Circulation. 2005;111(13):1690–1696. doi: 10.1161/01.CIR.0000160349.42665.0C. [DOI] [PubMed] [Google Scholar]
- 22.Kaufman J, Spinelli SL, Schultz E, Blumberg N, Phipps RP. Release of biologically active CD154 during collection and storage of platelet concentrates prepared for transfusion. J Thromb Haemost. 2007;5(4):788–796. doi: 10.1111/j.1538-7836.2007.02412.x. [DOI] [PubMed] [Google Scholar]
- 23.Aukrust P, Müller F, Ueland T, Berget T, Aaser E, Brunsvig A, et al. Enhanced levels of soluble and membrane-bound CD40 ligand in patients with unstable angina. Possible reflection of T lymphocyte and platelet involvement in the pathogenesis of acute coronary syndromes. Circulation. 1999;100(6):614–620. doi: 10.1161/01.cir.100.6.614. [DOI] [PubMed] [Google Scholar]
- 24.Elzey BD, Tian J, Jensen RJ, Swanson AK, Lees JR, Lentz SR, et al. Platelet-mediated modulation of adaptive immunity. A communication link between innate and adaptive immune compartments. Immunity. 2003;19(1):9–19. doi: 10.1016/s1074-7613(03)00177-8. [DOI] [PubMed] [Google Scholar]
- 25.Sprague DL, Elzey BD, Crist SA, Waldschmidt TJ, Jensen RJ, Ratliff TL. Platelet-mediated modulation of adaptive immunity: unique delivery of CD154 signal by platelet-derived membrane vesicles. Blood. 2008;111(10):5028–5036. doi: 10.1182/blood-2007-06-097410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Marx N, Imhof A, Froehlich J, Siam L, Ittner J, Wierse G, et al. Effect of rosiglitazone treatment on soluble CD40L in patients with type 2 diabetes and coronary artery disease. Circulation. 2003;107(15):1954–1957. doi: 10.1161/01.CIR.0000069272.06194.91. [DOI] [PubMed] [Google Scholar]
- 27.Schönbeck U, Varo N, Libby P, Buring J, Ridker PM. Soluble CD40L and cardiovascular risk in women. Circulation. 2001;104(19):2266–2268. doi: 10.1161/hc4401.099447. [DOI] [PubMed] [Google Scholar]
- 28.Cipollone F, Mezzetti A, Porreca E, Di Febbo C, Nutini M, Fazia M, et al. Association between enhanced soluble CD40L and prothrombotic state in hypercholesterolemia: effects of statin therapy. Circulation. 2002;106(4):399–402. doi: 10.1161/01.cir.0000025419.95769.f0. [DOI] [PubMed] [Google Scholar]
- 29.Bakry R, Sayed D, Galal H, Shaker S. Platelet function, activation and apoptosis during and after apheresis. Ther Apher Dial. 2010;14(5):457–464. doi: 10.1111/j.1744-9987.2010.00842.x. [DOI] [PubMed] [Google Scholar]
- 30.Wenzel F, Günther W, Baertl A, Gruber W, Sorg RV, Haas R, et al. Platelet transfusion alters CD40L blood level and release capacity in patients suffering from thrombocytopenia. Transfusion. 2011 doi: 10.1111/j.1537-2995.2011.03438.x. [DOI] [PubMed] [Google Scholar]
- 31.Apelseth TO, Hervig T, Wentzel-Larsen T, Petersen K, Reikvam H, Bruserud Ø. A prospective observational study of the effect of platelet transfusions on levels of platelet-derived cytokines, chemokines and interleukins in acute leukaemia patients with severe chemotherapy-induced cytopenia. Eur Cytokine Netw. 2011;22(1):52–62. doi: 10.1684/ecn.2011.0271. [DOI] [PubMed] [Google Scholar]
- 32.Blumberg N, Gettings KF, Turner C, Heal JM, Phipps RP. An association of soluble CD40 ligand (CD154) with adverse reactions to platelet transfusions. Transfusion. 2006;46(10):1813–1821. doi: 10.1111/j.1537-2995.2006.00979.x. [DOI] [PubMed] [Google Scholar]
- 33.Cognasse F, Payrat JM, Corash L, Osselaer JC, Garraud O. Platelet components associated with acute transfusion reactions: the role of platelet-derived soluble CD40 ligand. Blood. 2008;112(12):4779–4780. doi: 10.1182/blood-2008-05-157578. author reply 80–1. [DOI] [PubMed] [Google Scholar]
- 34.Tuinman PR, Gerards MC, Jongsma G, Vlaar AP, Boon L, Juffermans NP. Lack of evidence of CD40 ligand involvement in transfusion-related acute lung injury. Clin Exp Immunol. 2011;165(2):278–284. doi: 10.1111/j.1365-2249.2011.04422.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Akbiyik F, Ray DM, Gettings KF, Blumberg N, Francis CW, Phipps RP. Human bone marrow megakaryocytes and platelets express PPARgamma, and PPARgamma agonists blunt platelet release of CD40 ligand and thromboxanes. Blood. 2004;104(5):1361–1368. doi: 10.1182/blood-2004-03-0926. [DOI] [PubMed] [Google Scholar]


