Abstract
Introduction
Angiotensin receptor blockers (ARBs) are commonly used antihypertensive medication with several other additional proven benefits. Recent controversy on association of lung cancer and other solid malignancy with the use of ARBs is concerning, although the follow-up studies have shown no such association.
Methods
We used data from the Department of Veterans Affairs electronic medical record system and registries to conduct a retrospective cohort study that compared first-time ARB users with nonusers in 1:15 ratio, after balancing for many baseline differences using inverse probability of treatment weights. We conducted time-to-event survival analyses on the weighted cohort.
Results
Of the 1 229 902 patients in the analytic cohort, 346 (0.44%) of the 78 075 treated individuals had a newly incident lung cancer and 6577 (0.57%) of 1 151 826 nontreated individuals were diagnosed with lung cancer. On double robust regression, the weighted hazard ratio was 0.74 (0.67–0.83, P<0.0001), suggesting a lung cancer reduction effect with ARB use. There was no difference in rates by ARB subtype.
Conclusion
In this large nationwide cohort of United States Veterans, we found no evidence to support any concern of increased risk of lung cancer among new users of ARBs compared with nonusers. Our findings were consistent with a protective effect of ARBs.
Keywords: angiotensin receptor blockers, cancer registry, Department of Veterans Affairs, drug safety, inverse probability of treatment weight, lung cancer, propensity score, survival analysis
INTRODUCTION
Angiotensin receptor blockers (ARBs) are commonly prescribed for patients with hypertension: recently, there was a controversy regarding the utilization of ARBs and its association with an increased risk factor for lung cancer and other solid tumours [1,2]. Lung cancer is a major cause of morbidity with high fatality. The established risk factors for the development of lung cancer include smoking, environmental respiratory carcinogens and genetic factors.
The original report on the increased risk of lung cancer among ARB users originated in the form of a meta-analysis that used aggregate level clinical trial data [1,2]. The finding was subsequently refuted by additional meta-analyses, also using aggregate level data [2–4]. There were two individuallevel research studies [5,6], but they, instead of comparing ARB users vs. non-ARB users, compared ARB users with users of angiotensin-converting enzyme inhibitors (ACEi). In this case, as both the comparators are drugs that work similarly on the renin–angiotensin pathway, it may be speculated that the null association was probably secondary to common biological mechanisms. One study using individual-level data from the United Kingdom General Practice Research Database showed a statistical significant reduction of lung cancer among ARB users, when compared with ACEi users [6]. The Collaborative Transplant Study Report concluded an increased risk of respiratory tract cancer [7]. Hence, there is still some uncertainty on this topic; we used the electronic health record and cancer registry data from the United States Department of Veterans Affairs (VA), to evaluate whether Veterans receiving ARBs were at an increased risk of developing lung cancer compared with non-ARB users.
MATERIALS AND METHODS
Linked individual-level data on all eligible Veteran patients were obtained for fiscal years 1999–2010 from VA data sources including the Central Cancer Registry (CCR), MedSAS, Decision Support System (DSS), Vital status file, health factors file [8] and the Corporate Data Warehouse (CDW) [9]. In this retrospective cohort study, we used the methods developed by Hernan et al. to evaluate whether ARB use was associated with an increased risk for lung cancer [10–12]. We generated a list of Veteran patients who received their first ARB dispensation at any time from 2003 to 2009, by querying two pharmacy data sources (DSS and CDW)from 1999 onwards. The goal was to identify for non-ARBs users and for every ARB user in all the years from 2003 to 2009. To achieve this, we developed a calendar-year based staged random cohort selection method, starting with the year 2003 as follows; first, create a list of patients who were dispensed ARB treatment for the first time in the year 2003. Patients who filled their first ARB prescription without an outpatient clinician encounter within the prior 2 weeks’ time were excluded, as the probability of receiving an ARB prescription without a VA clinical encounter is very low and it might represent patients who were filling a non-VA prescription at a VA pharmacy. These 2003 ARB-dispensed patients were now considered as ‘assigned to receive treatment’ (treated) in the year 2003. To identify their ‘assigned to receive no-treatment’ (not-treated) counterparts for the same year, we randomly identified patients in 1 : 15 ratio from the remaining pool of patients (those who had a VA outpatient clinician encounter in the year 2003 but were not dispensed any ARB from the VA pharmacy until the end of year 2003),with their start date of follow-up being a randomly selected date of the many dates of their respective VA outpatient clinical encounters in the year 2003.
We repeated this patient selection method for the years 2004–2009, with the exception that patients who were already selected into prior year cohorts were not eligible to be selected into subsequent year cohorts; this ensured statistical independence between cohorts. After completing this staged cohort selection process, we pooled all patients together creating the definitive cohort. Also not allowed to be a part of the selection process were patients
who if selected would have at baseline a diagnosis of cancer in the VA CCR (excluding nonmelanoma skin cancer);
who would not have established VA clinical, pharmacy and laboratory care at least 6 months prior to date of treatment assignment;
with missing information of tobacco use in their VA health factor file; or
who would have been either less than 40 or more than 80 years of age (as the age group of 40–80 accounts for >98% of all lung cancer rates).
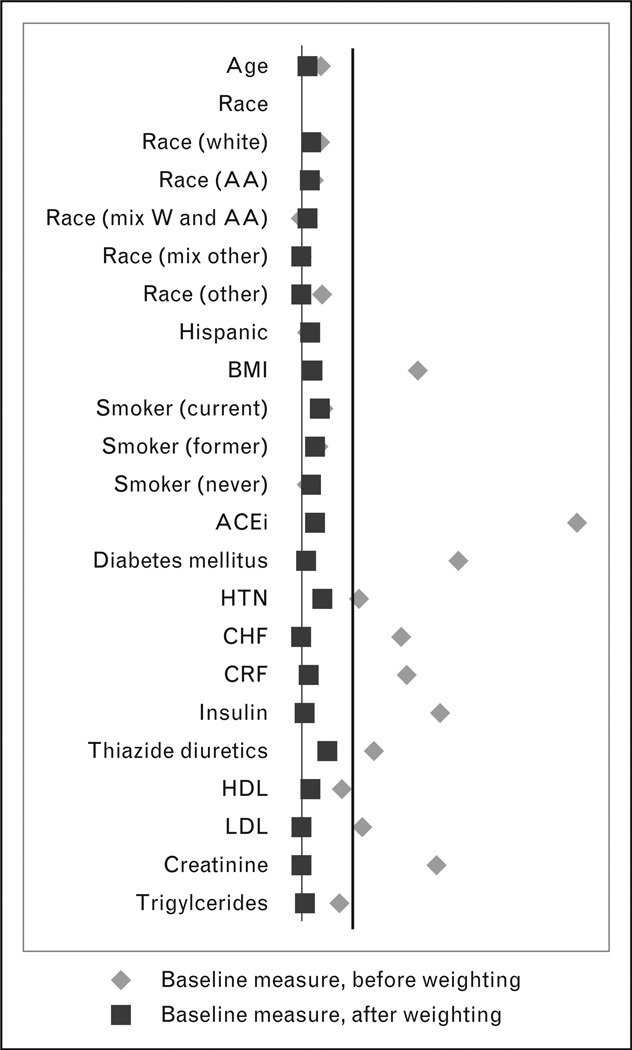
To account for the nonrandom treatment allocation, we computed propensity scores for the intention to start ARB treatment using a comprehensive set of baseline variables (Table 1). These baseline variables were derived from a look-back from 1999, using the most recent data for time-varying variables. To reduce statistical instability, patients in the not-treated group who had propensity scores beyond two standard deviations of the mean treated group propensity score were excluded [13]. We then weighted the cohort with the stabilized inverse probability of treatment weights (IPTWs) and created a weighted cohort. All patients were then followed until one of the following events: their last date of VA healthcare or related benefit; their death; date of diagnosis of lung cancer; or 31 December 2010 (a-priori determined end-point), whichever came first. All analyses were performed using SAS 9.2 (SAS Institute, Cary, North Carolina, USA) using the SAS Grid environment. Data access and analysis was performed by the author G.R. The ability of IPTW to balance baseline covariates was assessed using standardized difference (Fig. 1). Incidence curves were drawn for both types of exposures and for the absolute difference between exposures (Fig. 2). To evaluate effect measures, we conducted double-robust regression [14] by fitting an IPTW-weighted Cox-proportional hazard model with adjustments for selected variables, that is age, race, Hispanic ethnicity, smoking status, BMI [weight (kg)/height (m2)] and diabetes mellitus after checking for proportionality assumption and reported respective hazard ratios with 95% confidence intervals. The advantages of double-robust regression have been previously reported [14].
TABLE 1.
Baseline covariates, before and after weighting with inverse probability of treatment weights
| Treated vs. untreated (not-weighted) 76 797 vs. 1 151 955 |
Treated vs. untreated (weighted) 78 075 vs. 1 151 826 |
|
|---|---|---|
| Demographics | ||
| Age | 63.2 ± (9.3) vs. 62.9 ± (9.7) | 62.9 ± (9.6) vs. 63 ± (9.7) |
| Male | 73 101 (95.2%) vs. 1098,944 (95.4%) | 74 957 (96%) vs. 1 098 716 (95.4%) |
| Race | ||
| White | 60 293 (78.5%) vs. 918 584 (79.7%) | 62 675 (80.3%) vs. 917 671 (79.7%) |
| African-American | 12 829 (16.7%) vs. 182 836 (15.9%) | 12 026 (15.4%) vs. 183 360 (15.9%) |
| Mix of white and African-American race | 1072 (1.4%) vs. 15 744 (1.4%) | 1031 (1.3%) vs. 15 759 (1.4%) |
| Mixed other races | 1399 (1.8%) vs. 20 722 (1.8%) | 1427 (1.8%) vs. 20 730 (1.8%) |
| Other races | 1204 (1.6%) vs. 14 069 (1.2%) | 918 (1.2%) vs. 14 307 (1.2%) |
| Hispanic ethnicity | 4227 (5.5%) vs. 60 932 (5.3%) | 3939 (5%) vs. 61 062 (5.3%) |
| VA and Medicare dual beneficiary | 40 514 (52.8%) vs. 586 712 (50.9%) | 40 166 (51.4%) vs. 588 007 (51%) |
| Religion | ||
| Catholic | 19 440 (25.3%) vs. 287 057 (24.9%) | 19 274 (24.7%) vs. 287 296 (24.9%) |
| Protestant | 47 214 (61.5%) vs. 714 086 (62%) | 48 537 (62.2%) vs. 713 645 (62%) |
| Jewish | 1295 (1.7%) vs. 16 903 (1.5%) | 1141 (1.5%) vs. 17 057 (1.5%) |
| Other | 8848 (11.5%) vs. 133 909 (11.6%) | 9123 (11.7%) vs. 133 828 (11.6%) |
| Smoking status | ||
| Current | 36 362 (47.3%) vs. 565 050 (49.1%) | 39 354 (50.4%) vs. 563 814 (48.9%) |
| Former | 29 604 (38.5%) vs. 428 186 (37.2%) | 28 361 (36.3%) vs. 429 107 (37.3%) |
| Never | 10 831 (14.1%) vs. 158 719 (13.8%) | 10 361 (13.3%) vs. 158 906 (13.8%) |
| Substance abuse | ||
| Alcohol | 8746 (11.4%) vs. 143 521 (12.5%) | 10 694 (13.7%) vs. 142 784 (12.4%) |
| Substance | 6153 (8%) vs. 99 368 (8.6%) | 7467 (9.6%) vs. 98 955 (8.6%) |
| BMI | 31.3 ± (5.9) vs. 30.2 ± (5.6) | 30.2 ± (5.5) vs. 30.3 ± (5.7) |
| Comorbidity | ||
| Diabetes mellitus | 26 552 (34.6%) vs. 264 426 (23%) | 18 708 (24%) vs. 272 706 (23.7%) |
| Essential hypertension | 74 228 (96.7%) vs. 1 131 618 (98.2%) | 76 167 (97.6%) vs. 1 130 276 (98.1%) |
| Myocardial infarction | 1465 (1.9%) vs. 15 480 (1.3%) | 1041 (1.3%) vs. 15 875 (1.4%) |
| Cardiac dysrhythmia | 13 008 (16.9%) vs. 178 070 (15.5%) | 11 778 (15.1%) vs. 179 102 (15.5%) |
| Congestive heart failure | 7008 (9.1%) vs. 56 598 (4.9%) | 4051 (5.2%) vs. 59 582 (5.2%) |
| Acute cerebrovascular disease | 3506 (4.6%) vs. 48 065 (4.2%) | 3507 (4.5%) vs. 48 347 (4.2%) |
| Chronic obstructive pulmonary disease | 15 992 (20.8%) vs. 232 758 (20.2%) | 15 409 (19.7%) vs. 233 146 (20.2%) |
| Asthma | 5130 (6.7%) vs. 69 702 (6.1%) | 4444 (5.7%) vs. 70 119 (6.1%) |
| Chronic renal failure | 4920 (6.4%) vs. 32 282 (2.8%) | 2224 (2.8%) vs. 34 785 (3%) |
| Ulcerative colitis | 646 (0.8%) vs. 10 275 (0.9%) | 692 (0.9%) vs. 10 238 (0.9%) |
| Rheumatoid arthritis | 1620 (2.1%) vs. 25 514 (2.2%) | 1626 (2.1%) vs. 25 430 (2.2%) |
| Osteoarthritis | 24 427 (31.8%) vs. 380 447 (33%) | 25 612 (32.8%) vs. 379 532 (33%) |
| Benign prostatic hyperplasia | 15 197 (19.8%) vs. 239 548 (20.8%) | 15 728 (20.1%) vs. 238 792 (20.7%) |
| HIV | 277 (0.4%) vs. 4530 (0.4%) | 337 (0.4%) vs. 4508 (0.4%) |
| Hepatitis B | 1434 (1.9%) vs. 21 442 (1.9%) | 1478 (1.9%) vs. 21 445 (1.9%) |
| Hepatitis C | 3849 (5%) vs. 59 212 (5.1%) | 4126 (5.3%) vs. 59 112 (5.1%) |
| Mood disorder | 21 642 (28.2%) vs. 324 517 (28.2%) | 22 173 (28.4%) vs. 324 474 (28.2%) |
| Schizophrenia | 2931 (3.8%) vs. 47 188 (4.1%) | 3809 (4.9%) vs. 47 013 (4.1%) |
| Personality disorder | 1848 (2.4%) vs. 29 417 (2.6%) | 2258 (2.9%) vs. 29 320 (2.5%) |
| Epilepsy | 1823 (2.4%) vs. 29 632 (2.6%) | 2189 (2.8%) vs. 29 496 (2.6%) |
| History of coma | 317 (0.4%) vs. 4485 (0.4%) | 313 (0.4%) vs. 4502 (0.4%) |
| History of suicidality | 512 (0.7%) vs. 8314 (0.7%) | 630 (0.8%) vs. 8276 (0.7%) |
| Concomitant medications | ||
| Angiotensin-converting enzyme inhibitors | 54 211 (70.6%) vs. 564 332 (49%) | 38 380 (49.2%) vs. 579 712 (50.3%) |
| Antidepressants | 12 668 (16.5%) vs. 182 006 (15.8%) | 12 115 (15.5%) vs. 182 448 (15.8%) |
| Betablockers | 22 123 (28.8%) vs. 296 580 (25.7%) | 18 713 (24%) vs. 298 609 (25.9%) |
| Calcium channel blocker | 9946 (13%) vs. 122 186 (10.6%) | 7544 (9.7%) vs. 123 722 (10.7%) |
| Glucocorticoids | 2820 (3.7%) vs. 37 399 (3.2%) | 2283 (2.9%) vs. 37 673 (3.3%) |
| Insulin | 8685 (11.3%) vs. 59 044 (5.1%) | 4211 (5.4%) vs. 63 384 (5.5%) |
| Statins | 5405 (7%) vs. 68 124 (5.9%) | 4294 (5.5%) vs. 68 890 (6%) |
| 5-alpha-reductase inhibitor | 1658 (2.2%) vs. 24 412 (2.1%) | 1596 (2%) vs. 24 437 (2.1%) |
| Thiazide diuretics | 25 926 (33.8%) vs. 326 393 (28.3%) | 20 921 (26.8%) vs. 330 064 (28.7%) |
| Baseline laboratory | ||
| Alanine aminotransferase | 33.5 ± (19.6) vs. 33.3 ± (19.9) | 33 3 ± (18.6) vs 33.3 ± (20.1) |
| Asparatate aminotransferase | 28.5 ± (16.9) vs. 28.8 ± (16.2) | 29 ± (18.4) vs. 28.7 ± (16.2) |
| International normalized ratio | 1.4 ± (0.5) vs. 1.4 ± (0.5) | 1.4 ± (0.4) vs. 1.4 ± (0.5) |
| Platelet count | 158.5 ± (33.5) vs. 157.4 ± (31.7) | 157 ± (30) vs. 157.5 ± (31.9) |
| Albumin | 4.1 ± (0.4) vs. 4.1 ± (0.3) | 4.1 ± (0.4) vs. 4.1 ± (0.3) |
| High-density lipoprotein | 43.2 ± (7.6) vs. 43.7 ± (7.5) | 43.8 ± (7.7) vs. 43.7 ± (7.5) |
| Hemoglobin | 14.4 ± (1.4) vs. 14.5 ± (1.2) | 14.5 ± (1.3) vs. 14.5 ± (1.2) |
| Low-density lipoprotein | 105 ± (30.3) vs. 107.9 ± (28.5) | 107.8 ± (30.2) vs. 107.8 ± (28.5) |
| Potassium | 4.3 ± (0.5) vs. 4.3 ± (0.4) | 4.3 ± (0.4) vs. 4.3 ± (0.4) |
| Creatinine | 1.2 ± (0.5) vs. 1.1 ± (0.4) | 1.1 ± (0.3) vs. 1.1 ± (0.4) |
| Total cholesterol | 176.9 ± (39.4) vs. 178.6 ± (37) | 178 ± (38.4) vs. 178.5 ± (37.2) |
| Trigylcerides | 164.1 ± (90.8) vs. 158.6 ± (85.2) | 158.4 ± (86.5) vs. 158.9 ± (85.5) |
Figure 1.
Balance achieved for selected covariates. Only selected variables reported. Black vertical line represents standardized difference of 10. Postweighting, all variables had a standardized difference of less than 10. ACEi, angiotensin-converting enzyme inhibitors; CCB, calcium channel blockers; CHF, congestive heart failure; CRF, chronic renal failure; HDL, high-density lipoprotein; HTN, essential hypertension; LDL, low-density lipoprotein; race (AA), African–American; race (Haw or PI), race Hawaiian or Pacific Islander; race (mixed other), mixed other race; race (others), other race; race (W and AA), both white and AA.
Figure 2.
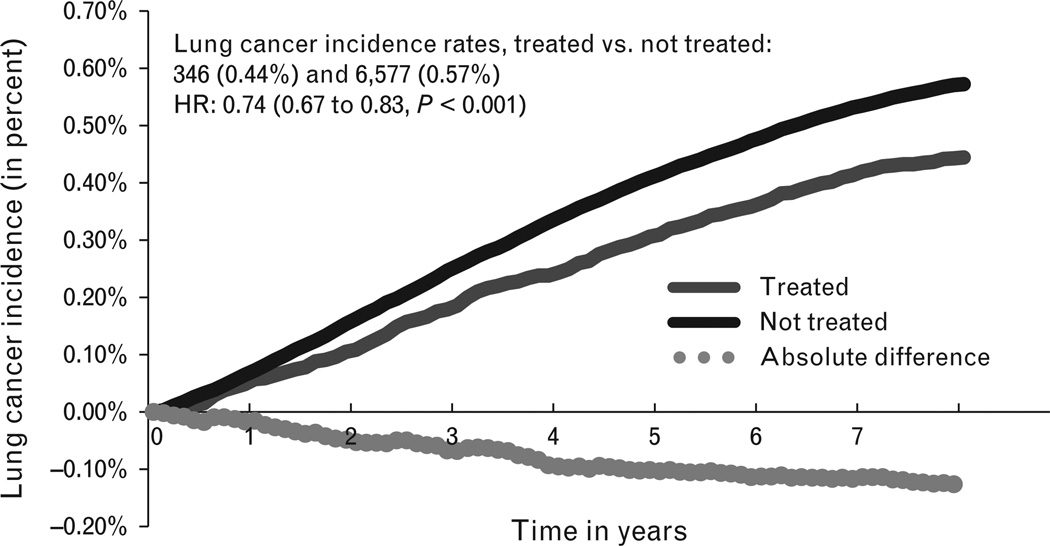
Weighted incidence curve for lung cancer. HR, hazard ratio.
In addition to our main analysis, we described the trends in utilization of ARBs in the VA. We also conducted two subanalyses; we first stratified by ARB subtypes to evaluate any possible individual drug effect on lung cancer risk. Because the rate of dispensation of telmisartan and olmesartan was together less than 0.25%, these two groups, being very small, were dropped: the ARB subtype analysis was restricted to candesartan, irbesartan, losartan and valsartan. Second, we stratified by smoking status, to evaluate the association between ARB use and lung cancer among three different stratums of smoking status.
This study was approved by the Institutional Review Board (IRB) of the Williams JB Dorn VA Medical Center and VA regulatory oversight organizations including the National Data Systems and the Patient Care Services. All data were stored and analysed using the resources of the Veterans Affairs Informatics and Computing Infrastructure (VINCI) by the author G.R.
RESULTS
The final cohort consisted of 1 228 960 unique patients. Baseline covariates with the greatest influence on the propensity to prescribe ARB were diabetes mellitus, serum creatinine level, concurrent or past use of ACEis, insulin use, BMI, congestive heart failure, chronic renal failure, thiazide diuretics, low-density lipoprotein levels and essential hypertension. Baseline comparisons between treated and not-treated groups, both with and without weighting, are reported in Table 1 and standardized differences between the two groups are shown in Fig. 1. Because the untreated group was limited to those with propensity scores that were within two standard deviations of the treated group: the treated and not-treated appeared similar even before weighting with IPTW, improving statistical validity. The characteristics of the two groups represent patients likely to receive ARB and are not representative of the general VA patient population, explaining the high rate of hypertension (more than 96%) in all groups.
A total of 78 075 (6.4%) patients were classified as treated with a total follow-up time of 350 878 person-years (mean 4.5±2.1 years) compared with 1 151 826 (93.7%) classified as not-treated with a total follow-up time of 5 098 085 person-years (mean 4.4±2.0 years). The number of lung cancers diagnosed in the treated and not-treated groups were 346 (0.44%) and 6577 (0.57%), respectively [relative risk (RR) 0.78 (0.70–0.86); P<0.001]. The 10-year number needed to treat (NNT) to reduce lung cancer incidence by one case is 329. The difference in incidence rates for lung cancer in the two groups is shown in Fig. 2. After double-robust regression, the adjusted hazard ratio for ARB was 0.74 (0.67–0.83, P<0.0001) (Table 2).
TABLE 2.
Adjusted hazard ratio by double-robust regression
| Variables | Hazard ratio |
|---|---|
| Angiotensin receptor blocker | 0.74 (0.67–0.83, P < 0.0001) |
| Age group (Reference ’≥70’) | |
| 65–70 years | 1.05 (0.99–1.13, P = 0.1191) |
| 60–65 | 1.00 (0.94–1.07, P = 0.9025) |
| 55–60 | 0.58 (0.54–0.62, P < 0.0001) |
| 50–55 | 0.39 (0.35–0.43, P < 0.0001) |
| 45–50 | 0.20 (0.16–0.24, P < 0.0001) |
| 40–45 | 0.07 (0.04–0.11, P < 0.0001) |
| Race (reference ’white’) | |
| African-American | 1.19 (1.11–1.27, P < 0.0001) |
| Mix of white and | 1.21 (0.99–1.46, P = 0.059) |
| African-American race | |
| Mixed other races | 0.50 (0.36–0.7, P < 0.0001) |
| Other races | 1.74 (1.51–2, P < 0.0001) |
| Hispanic ethnicity | 0.45 (0.38–0.53, P < 0.0001) |
| Smoker (reference ’Never’) | |
| Current | 6.54 (5.65–7.57, P < 0.0001) |
| Former | 2.84 (2.44–3.31, P < 0.0001) |
| BMI | 0.92 (0.91–0.92, P < 0.0001) |
| Diabetes mellitus | 1.02 (0.96–1.08, P = 0.5557) |
Smoking status at the start date of follow-up had the largest impact on the incidence of lung cancer with current smokers accounting for 5123 (0.9%), former smokers accounting for 1614 (0.4%) and never smoker accounting for 187 (0.1%). On subanalysis with recalculated IPTW within the different strata of smokers, the reduction in cancer incidence among current smokers persisted with a hazard ratio of 0.72 (0.64–0.82; P<0.001). Similar subanalysis among never-smokers and former smokers demonstrated an association in the same direction, but it did not reach statistical significance.
The number of patients who were censored, dead or lost-to-follow-up were similar between treated and not-treated groups at 67 615 (86.6%); 3981 (5.1%); 6133 (5.5%) vs. 979 036 (85.0%); 61 365 (5.3%); and 104 848 (9.1%). The number of patients in the treated group who were on ARB treatment for at least 75% of their respective duration of follow-up was 41 338 (53.0%); 27 121 (34.7%) were still on treatment at the end-of-follow-up. The number of patients who were on ARB for 25% or less of their respective duration of follow-up was 19 405 (24.9%). The number of patients in the not-treated group who were subsequently started onARB was small at 70 735 (6.1%), with the mean time to initiation of ARB being 2.9 years (±1.9).
Losartan [37 382 (48.7%)] was the most common ARB subtype prescribed in the VA followed by irbesartan [17 992 (23.4%)], valsartan [17 477 (22.8%)] and candesartan [3757 (4.9%)]. There were temporal differences in the pattern of utilization of ARBs between 2003 and 2009. Irbesartan accounted for more than 58% of ARB dispensations in the calendar year 2003 and 2004, but in the calendar years 2005 to 2009, losartan became the most common ARB accounting for 49.5, 66.4, 69.2, 70.2 and 73.6% of all ARBs across those 5 years. Olmesartan and telmisartan had the lowest dispensation rates of 0.05 and 0.19%, respectively, with no observed lung cancers, and therefore were excluded from further ARB subtype subanalysis. Candesartan was preferentially dispensed to patients with cardiac arrhythmia or those prescribed beta-blockers, whereas valsartan was used preferentially among those with a diagnosis of heart failure. The preferred ARB type among patients with diabetes mellitus was losartan. The crude rates of lung cancer were highest for candesartan and irbesartan, but this was not found in the time-to-event survival analysis model adjusted for smoking status, BMI, diabetes mellitus, race, ethnicity and age, in which no ARB subtype was found to be significantly different than losartan in relation to lung cancer (Table 3).
TABLE 3.
Subanalysis among treated for the relationship between angiotensin receptor blocker subtype and lung cancer incidence
| Stratified by smoking statusa | HR for ARB (95% CI) | P |
| All current smokers | 0.72 (0.64–0.82) | <0.001 |
| All former smokers | 0.86 (0.70–1.07) | 0.175 |
| All never smoked | 0.42 (0.18–1.02) | 0.06 |
| Stratified ARB subtype in a cohort of all ARB usersb | HR for ARB subtype (95% CI) | P |
| Losartan | 1 (reference) | |
| Candesartan | 1.00 (0.67–1.51) | 0.79 |
| Irbesartan | 0.94 (0.73–1.22) | 0.95 |
| Valsartan | 0.94 (0.69–1.27) | 0.99 |
ARB, angiotensin receptor blocker; CI, confidence interval; HR, hazard ratio.
Adjusted for age, race, Hispanic ethnicity, diabetes mellitus and BMI.
Adjusted for age, race, Hispanic ethnicity, smoking status, diabetes mellitus and BMI for all patients.
DISCUSSION
We used retrospective data from the nationwide cohort of United States Veterans, to evaluate the impact of real-world use of ARBs on the long-term risks for the development of lung cancer. Our analysis included a total cohort of more than one million individual patients with over five million combined person-years of follow-up, potentially the largest long-term medication safety research study. We found that intention to dispense ARBs was not associated with an increased risk of lung cancer. On the contrary, it appeared to have a protective effect [hazard ratio 0.74 (0.67–0.83, P<0.0001)], with a small absolute risk reduction of 0.30 lung cancers per 1000 person-years in the ARB-treated group. As shown in weighted incidence curves (Fig. 2), the difference in risk among ARB-prescribed and nonprescribed group starts to emerge after the third year and consistently increases over the consequent years. On stratified analysis, the protective effect was statistically significant only among those who at the start of follow-up were current smokers.
Our study findings are in contrast with those reported by Sipahi et al. [1], who reported a modest increase in risk. The meta-analyses showed that exposure to maximal daily doses of ARBs for at least 3 years increased lung cancer, but the excess risk does not become apparent at lower levels of total exposure. Although we were unable to include dose information in our analysis – due to data unavailability – it is most likely that lower drug doses are prescribed in the real world (especially in an elderly VA cohort). Further, the cancer protective effect appeared to be more prominent around the thirrd year after start of treatment. Our subanalysis did not reveal any ARB subtype effect, but this cannot be ruled out, as the use of telmisartan, the most prevalent ARB subtype in the study from Sipahi et al. [1], was almost negligible in the VA.
The Collaborative Transplant Study concluded that among kidney transplant recipients, ACEi/ARB treatment was associated with a significant increase in the rate of respiratory/ intrathoracic tumours in the subpopulation of patients with a history of smoking [7]. In contrast, we found that smokers on ARB (dominantly losartan) were in fact associated with a lower risk of lung cancer than nonsmokers on ARB.
To our knowledge, this is the first United States Veteran population-based study that evaluated the relationship between ARBs dispensation and lung cancer, the first to use individual-level linked claims, electronic medical records and cancer registry data inside the new VINCI environment. Such large studies that evaluate safety concerns are few. Recently, a Danish nationwide cohort study used predominant administrative claims data to show no significant cancer risk increase among new ARB users compared with new ACEi users [adjusted rate ratio, 0.99; 95% confidence interval (CI) 0.95–1.03] and, specifically, no significant difference in lung cancer risk (adjusted rate ratio, 0.92; 95% CI 0.82–1.02) [5]. Similar to our findings, a study based on Taiwan National Health Insurance Database reported an independent association of ARB use with a decreased risk for cancer occurrence among patients with systemic hypertension (hazard ratio 0.66, 95% CI 0.63–0.68, P<0.001) [15]. Such beneficial results might be worth investigating, especially as any efficient cardiovascular therapy might prolong life and thereby expose the patient to an increased risk of cancer of all forms [16].
We believe that this study is the largest to date to investigate the relationship between ARB exposure and lung cancer risk. More epidemiological and clinical data are accumulating in this area and this investigation is timely, especially considering the recent meta-analysis carried out by Sipahi et al. [1]. With observational data, one can never be sure that a model for the treatment assignment mechanism (IPTW-weighted Cox-proportional hazard model) or a model for the counterfactual data is correct, so to partly overcome this limitation, we conducted a double-robust regression. In such a model, the resulting estimator is double-robust when either a model for the treatment assignment mechanism or a model for the distribution of the counterfactual data is correctly specified, giving the investigators two opportunities for bias control [14], and improving on previous approaches. Another strength aspect of the design is using IPTW on the basis of propensity score to balance the two comparative groups allowed us to control for measured confounders and to approximate randomized trial designs.
Nevertheless, although one can measure certain confounders, many can never be measured. This is especially an important limitation, as some of these confounders may rise due to medical activities that are not captured in the VA medical records: for instance, some patients who were classified as not-receiving ARB might have received ARBs outside the VA. However, this specific effect is probably minimal, as lower copay and governmental benefits administered by the VA pharmacy are strong incentives for Veterans to dispense their ARBs consistently via VA pharmacy. Although it is reasonable to expect that the treated and not-treated groups in this observational study are balanced on the variables used to generate the propensity scores, unlike a true-randomized experiment, the IPTW procedure will not balance unmeasured confounding such as latent or undiagnosed disease or disease severity. Thus, the bias from lack of information on unmeasured confounding variables cannot be excluded. However, most of the established confounders such as diabetes mellitus, age and smoking are measured in our study, and have been used in the computation of propensity scores; thus, the bias from major confounders is less likely. In addition, we have incorporated variables that may approximate instrumental variables (such as serum creatinine) that are expected to be related to ARB dispensation but unrelated (or very weakly related) to outcome; instrumental variables when balanced using propensity scores are thought to balance unmeasured confounders.
Other limitations are that nonadherence to the dispensed drugs might bias results towards the null, especially if ARBs were associated with lung cancer; however, a bias to the null would not explain the observed protective effect, which is away from the null. Also, by comparing treated to not-treated, a surveillance bias for lung cancer might have been created because patients under antihypertensive treatment are more likely to have opportunities to report symptoms of lung cancer to their physicians. However, this surveillance bias is also expected to reduce the effect measure towards one or the null and thus reduce the probability of detecting a reduced risk of lung cancer. Also, as this is an intention-to-treat analysis, cross-contamination between the two groups may bias the results. To investigate the risk of cross-contamination, we examined our data and found that only 6.1% of the not-treated group was prescribed ARBs after enrolment (started ARBs during interval follow-up); this crossover is also likely to move our findings towards the null, if ARBs are indeed protective. The observed rate of lung cancer for each age group may appear to be lower than the expected rate for a comparable US general population. This may be explained by nondifferential bias from incomplete reporting within a hospital-based cancer registry or the use of cancer as a baseline exclusion criterion. Finally, we are aware that our average follow-up time of 4.5 years may not fully account for the possible causal biological effect of ARB intake on cancer occurrence, but in this follow-up period, no increased risk was observed.
In conclusion, in this large nationwide cohort of United States Veterans, we found no evidence to support a concern of increased risk of lung cancer among those dispensed ARB (predominantly losartan) compared with those not dispensed by presenting evidence of a protective relationship. These findings are considered additional reassurance to the Foods and Drug Administration conclusion that ARBs are at least not harmful for lung cancer incidence. Also, such findings are useful to stimulate further research on the subject, if indeed ARBs had any protective association.
ACKNOWLEDGEMENTS
We wish to thank the VINCI program, the VA CCR and staff at the William JB Dorn VA Medical Center. J.R.H. was supported by an Established Investigator Award in Cancer Prevention and Control from the Cancer Training Branch of the National Cancer Institute (K05 CA136975); the South Carolina Cancer Disparities Community Network from the National Cancer Institute’s Center to Reduce Cancer Health Disparities (Community Networks Program Center) (U54 CA153461, J.R.H., principal investigator); and the South Carolina Cancer Prevention and Control Research Network (U48 DP001936, J.R.H., principal investigator) from the Centers for Disease Prevention and Control.
Abbreviations
- ACEi
angiotensin-converting enzyme inhibitors
- ARB
angiotensin receptor blockers
- FDA
Food and Drug Administration
- VA
Department of Veterans Affairs
- VINCI
Veterans Affairs Informatics and Computing Infrastructure
Footnotes
Disclaimer: The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the VA or the United States government.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Sipahi I, Debanne SM, Rowland DY, Simon DI, Fang JC. Angiotensin-receptor blockade and risk of cancer: meta-analysis of randomised controlled trials. Lancet Oncol. 2010;11:627–636. doi: 10.1016/S1470-2045(10)70106-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.FDA Drug Safety Communication: no increase in risk of cancer with certain blood pressure drugs – angiotensin receptor blockers (ARBs) [[Accessed 31 May 2012]]; www.fda.gov.
- 3.Bangalore S, Kumar S, Kjeldsen SE, Makani H, Grossman E, Wetterslev J, et al. Antihypertensive drugs and risk of cancer: network meta-analyses and trial sequential analyses of 324,168 participants from randomised trials. Lancet Oncol. 2011;12:65–82. doi: 10.1016/S1470-2045(10)70260-6. [DOI] [PubMed] [Google Scholar]
- 4.Collaboration TAT. Effects of telmisartan, irbesartan, valsartan, candesartan, and losartan on cancers in 15 trials enrolling 138 769 individuals. J Hypertens. 2011;29:623–635. doi: 10.1097/HJH.0b013e328344a7de. [DOI] [PubMed] [Google Scholar]
- 5.Pasternak B, Svanstrom H, Callreus T, Melbye M, Hviid A. Use of angiotensin receptor blockers and the risk of cancer. Circulation. 2011;123:1729–1736. doi: 10.1161/CIRCULATIONAHA.110.007336. [DOI] [PubMed] [Google Scholar]
- 6.Bhaskaran K, Douglas I, Evans S, van Staa T, Smeeth L. Angiotensin receptor blockers and risk of cancer: cohort study among people receiving antihypertensive drugs in UK General Practice Research Database. BMJ. 2012;344:e2697. doi: 10.1136/bmj.e2697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Opelz G, Döhler B. Treatment of kidney transplant recipients with ACEi/ARB and risk of respiratory tract cancer: a collaborative transplant study report. Am J Trans. 2011;11:2483–2489. doi: 10.1111/j.1600-6143.2011.03681.x. [DOI] [PubMed] [Google Scholar]
- 8.McGinnis KA, Brandt CA, Skanderson M, Justice AC, Shahrir S, Butt AA, et al. Validating smoking data from the Veteran’s Affairs Health Factors dataset, an electronic data source. Nicotine Tobacco Res. 2011;13:1233–1239. doi: 10.1093/ntr/ntr206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Department of Veterans Affiars (VA) VA Information Resource Center (VIReC) web site. [[Accessed 2 March 2012]]; www.virec.research.va.gov.
- 10.Hernan MA. A definition of causal effect for epidemiological research. J Epidemiol Community Health. 2004;58:265–271. doi: 10.1136/jech.2002.006361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hernan MA, Robins JM. Estimating causal effects from epidemiological data. J Epidemiol Community Health. 2006;60:578–586. doi: 10.1136/jech.2004.029496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.D’Agostino RB., Jr Propensity score methods for bias reduction in the comparison of a treatment to a nonrandomized control group. Stat Med. 1998;17:2265–2281. doi: 10.1002/(sici)1097-0258(19981015)17:19<2265::aid-sim918>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 13.Kurth T, Walker AM, Glynn RJ, Chan AK, Gaziano JM, Berger K, Robins JM. Results of multivariable logistic regression, propensity matching, propensity adjustment, and propensity-based weighting under conditions of nonuniform effect. Am J Epidemiol. 2006;163:262–270. doi: 10.1093/aje/kwj047. [DOI] [PubMed] [Google Scholar]
- 14.Bang H, Robins JM. Doubly robust estimation in missing data and causal inference models. Biometrics. 2005;61:962–973. doi: 10.1111/j.1541-0420.2005.00377.x. [DOI] [PubMed] [Google Scholar]
- 15.Huang CC, Chan WL, Chen YC, Chen TJ, Lin SJ, Chen JW, Leu HB. Angiotensin II receptor blockers and risk of cancer in patients with systemic hypertension. Am J Cardiol. 2011;107:1028–1033. doi: 10.1016/j.amjcard.2010.11.026. [DOI] [PubMed] [Google Scholar]
- 16.Messerli FH, Bangalore S, Torp-Pedersen C, Staessen JA, Kostis JB. Cardiovascular drugs and cancer: of competing risk, smallpox, Bernoulli, and d’Alembert. Eur Heart J. 2013;34:1095–1098. doi: 10.1093/eurheartj/ehs158. [DOI] [PubMed] [Google Scholar]