Abstract
The core element of a continuous glucose monitoring (CGM) system is the glucose sensor, which should enable reliable CGM readings in the interstitial fluid in subcutaneous tissue for a period of several days. The aim of this article is to describe the layout and constituents of a novel glucose sensor and the rationale behind the measures that were used to optimize its performance. In order to achieve a stable glucose sensor signal, special attention was paid to the sensor materials and architecture, i.e., biocompatible coating of the sensor, limitation of glucose flux into the working electrode, low oxidation potential by use of manganese dioxide, and a tissue-averaging sensor design. A series of in vitro and in vivo evaluations showed that the sensor enables stable and accurate glucose sensing in the subcutaneous tissue for up to 7 days. Parallel measurements with four sensors in a single patient showed a close agreement between these sensors. In summary, this high-performance needle-type glucose sensor is well suited for CGM in patients with diabetes.
Keywords: continuous glucose monitoring, electrode configuration, glucose oxidase, glucose sensors, sensor design
Introduction
Continuous glucose monitoring (CGM) by means of needle-type glucose sensors that are inserted in subcutaneous tissue has been under development for a number of decades now, and respective systems have been on the market for many years. However, several aspects of currently available CGM systems are still not optimal: duration of usability, measurement performance in all clinically relevant blood glucose (BG) ranges, handling, and reliability.1,2 In order to enable accurate and reliable glucose measurement, CGM system sensors have to be specifically designed for subcutaneous tissue conditions in order to avoid
unpredictable loss of sensitivity over time (sensitivity drift/biofouling),
occasional increase of sensor noise or increase of sensor noise over time,
intermittent loss of sensitivity (“sensor dropouts”),
a different time course of CGM signals of two sensors in the same patient (“in vivo precision”), and
a significant increase in time delays between changes in BG and glucose levels based on CGM measurements.
Roche Diagnostics has developed a prototype of a novel type of glucose sensor that aims to allow a stable and accurate glucose monitoring over a wear time of 7 days. This article describes the layout of this sensor and the different measures undertaken to reduce distortion of the sensor signal.
Sensor Layout
The basic operating principle of the novel glucose sensor is the well-known amperometric detection of glucose, i.e., this is a glucose-oxidase-based enzyme sensor with a three-electrode system and electrochemical detection of hydrogen peroxide. Hydrogen peroxide is oxidized at the working electrode to generate an electrical current that is indicative of the glucose concentration. In amperometric operation, the needle type sensor consists of three electrodes: a working electrode, a reference electrode [silver/silver chloride (Ag/AgC)] and a counter electrode (Figure 1). The geometry of the working electrode consists of several spots distributed over approximately 6 mm of the sensor shaft.
Figure 1.
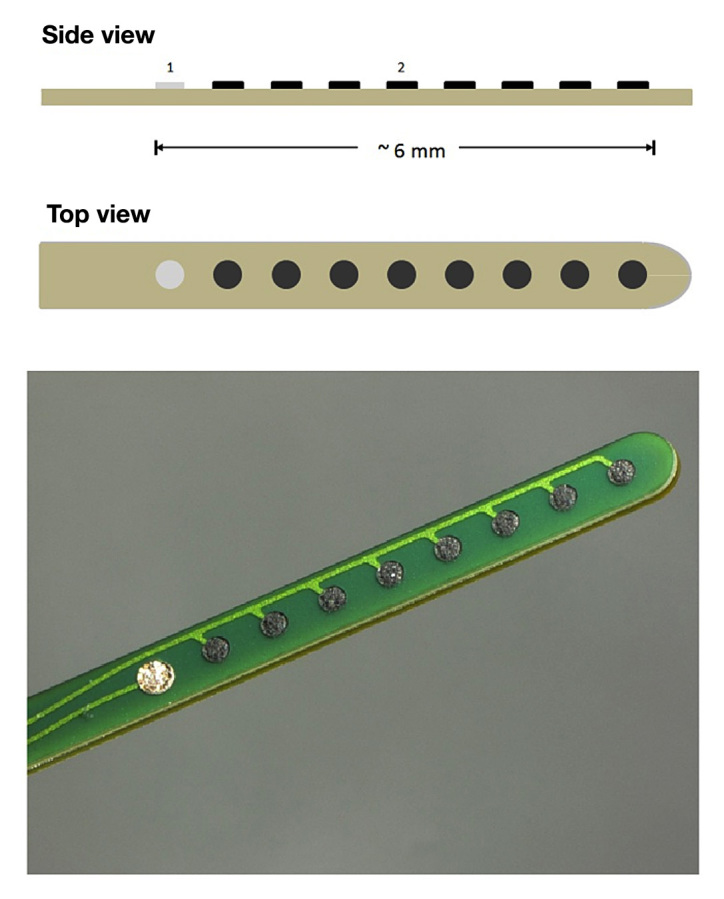
Schematic drawing and photograph of the sensor layout. It is of note that all working electrode spots (2) are on the same electrical lead, i.e., they deliver only one (combined) current; there is only one reference spot (1).
Sources of Signal Distortions
Under in vivo conditions, i.e., after insertion into subcutaneous tissue, glucose sensors often do not provide stable signals in relation to BG levels for a number of reasons (that are not all well understood):
Lag time between changes in glucose levels in interstitial fluid (ISF) and blood (physiological lag time);
Lag time between change in glucose levels near the sensor surface and the measured signal (physical lag time; this is heavily influenced, for example, by the type of membrane selected to protect the sensor and algorithms for signal improvement);
Electrochemically active substances that interfere with the oxidation of hydrogen peroxide (interferences);
Insertion of the sensor induces local traumata that causes wound healing reactions around the sensor;
Movement of the sensor relative to the tissue due to, for example, exercise or pressure during sleep; or
Variations of blood flow in subcutaneous adipose tissue (due to physiological reasons, wound healing processes, or other reasons).
Lag Times
Time delay observed between changes in glycemia (measured in capillary or venous blood samples) and in the ISF signal provided by the CGM system can be partly explained by a physiological phenomenon independent of the CGM sensor: the transport of glucose molecules from the blood capillaries through the interstitial volume to the surface of the CGM sensor.
Another factor in total lag time is induced by the measurement technique itself. This time lag consists partly of the time required by glucose molecules to diffuse through the membranes that are applied on the surface of the sensors. Additionally, there are electrode reaction processes taking place that add a sensor-specific time delay (e.g., diffusion of hydrogen peroxide from glucose oxidase to electrode surface). Another source of time delay is caused by the real-time filtering algorithms used to smooth the noisy sensor raw signal. These kinds of delays are called physical lag time.
The membranes applied to the glucose sensors also limit the amount of glucose that diffuses to the electrodes and the constancy of this process (discussed later). The developers of glucose sensors must find a balance between types of membranes and thickness of the levels applied to best fulfill the different requirements. During the development process of the new glucose sensor, a number of aspects were taken into account, including manufacturing topics, and a membrane type was selected from more than 20 different types of membranes indicated in literature as suitable for glucose sensors. In order to ensure that sensor response correlates only to the glucose concentration and not to other effects (e.g., oxygen concentration, amount of immobilized enzyme, electrode surface), a polyurethane membrane layer is used to control the glucose diffusion to the working electrode. If the glucose diffusion across the membrane is the rate-limiting step, the generation of the electrical current is more independent of the glucose supply to the sensor.
However, if glucose diffusion inside the sensor is too slow, a sensor-induced time lag is generated. The membrane material and its diffusion properties are carefully optimized in order to find a reasonable compromise between the minimization of sensor-induced time lag and the requirement that sensor signal depends solely on the tissue glucose concentration. Another task of the membrane layer is to prevent any leakage of glucose oxidase or other high molecular weight components from the sensor into the surrounding tissue and vice versa to reduce reactions of the body towards the sensor, the so called biofouling.
Algorithm/Smoothing of Data
When an electric signal is generated by the amperometric sensor in relation to the glucose levels around the sensor surface, this signal is subject to extensive data handling to provide the glucose data needed from a clinical point of view. For example, the noise superimposed on the signal has to be reduced by different filtering activities. However, strong filtering itself induces a lag time. Therefore, a sensor signal with a low noise level is advantageous. However, the glucose sensor raw data are not usually included in manufacturer publications, and therefore no quantitative statement can be made about comparing noise levels between different CGM systems.
Interferences
Another relevant source of measurement distortion, i.e., interferences with the glucose measurement, can be electro-chemically active substances that interfere with the oxidation of hydrogen peroxide at the sensor’s working electrode.3 These substances can be exogenous compounds such as paracetamol, ascorbic acid, or salicylic acid as well as endogenous compounds such as uric acid. Exogenous interfering compounds intermittently (e.g., after medication) change the apparent glucose sensitivity by intermittently changing the sensor’s offset current (the current generated if the glucose concentration were zero). The total current measured by a glucose sensor comprises the glucose-related part and the offset current part. Endogenous interfering compounds such as uric acid affect the offset current as well. However, uric acid concentration is usually rather stable over time, causing a constant offset current. The impact of stable concentrations of interfering compounds on the sensor response is minimized because all CGM sensors must be calibrated and recalibrated several times during the usage period.
The working electrode material of the new sensor consists of a mixture of a carbon paste, immobilized glucose oxidase and finely dispersed manganese dioxide, which acts as an electrocatalyst. This allows running the working electrode at an electric potential of 350 mV versus Ag/AgCl. This relatively low potential is intended to keep the effect of electrochemically active compounds (interferents) on the sensor operation at a low level. It is known that the higher the potential, the higher the tendency of the electrode to oxidize electrochemical interferents.3 Thus, the lower the working electrode potential, the lower the influence of electrochemically interfering compounds on sensor operation.
The use of manganese dioxide also offers another advantage; hydrogen peroxide can be degraded to water and oxygen even if no electrical potential is applied to the sensor. Thus, even if the sensor electrodes lost their electrical potential for a short or long period of time, there would be no accumulation of hydrogen peroxide in the sensor–and even more important–in the surrounding tissue.4
Despite the fact that interfering substances can be a serious source of CGM signal distortions, no head-to-head comparisons of the sensitivity of different glucose sensors to such substances have been performed (or published) so far, to our knowledge.
Tissue Reactions/Local Pressure/Blood Flow
Due to the inhomogeneity of adipose tissue, glucose transfer from ISF to the sensor is very much influenced by local phenomena in the tissue/trauma in the subcutaneous tissue close to the surface of the sensor. Variations in tissue pressure and/or in local blood flow also have an impact on diffusion rates of glucose molecules and thereby on sensor signal. It is well-known (but not well studied) that longer-lasting increases in tissue pressure around the sensor can lead to local depletion of glucose levels; this decline in signal intensity is interpreted as a decline in systemic glucose levels. Thus hypoglycemic events at night might not be true low glucose values. Intensive movements of the tissue around the sensor might induce an increase in signal noise. Even under stable physiological conditions there is significant inhomogeneity on a submillimeter scale in adipose tissue with respect to blood flow and glucose/oxygen supply.5–7
The size of the local traumata induced depends on a number of factors: speed of the insertion into the skin (which reflects the properties of the inserter if one is used) and sharpness and other properties of the needle (e.g., diameter, surface). Clearly, experience obtained with lancing devices used for self-monitoring of BG was of help in constructing an inserter/needle geometry that caused minimal tissue damage. The influence between needle properties and sensor performance is not yet well understood. The diameters of the glucose sensors that are on the market differ to a certain extent and there is no clear relationship between sensor performance and its diameter.
The local trauma induced—and thereby the measurement conditions—showed clear changes over time, i.e., over the wear time of several days. There is an immediate wound reaction in the first hours after sensor insertion as a response to the injury induced and a subsequent healing/wound repair reaction. However, due to the local movements of the sensor relative to surrounding tissue, the microenvironment/tissue characteristics around the sensor probably never reach a steady state. Relative movements between the sensor and the surrounding tissue also depend on the rigidity of the sensor, i.e., one would assume that a more flexible needle is advantageous. However, it would be of interest to study which has more relevance: such as more macroscopic or microscopic movements that are more difficult to visualize.
The time course and intensity of all these processes may vary between insertion sites/persons, depending on the actual severity of the injury. The measurement conditions are most probably also heavily influenced by whether larger capillaries or small blood vessels are hit during the insertion process or not. The impact of such local bleedings on sensor performance is not well understood. It is of interest to note that differences reported between insertion sites (e.g., abdomen versus arm) might be of less importance when it comes to measuring quality in comparison with differences on a local level.
Recordings performed with the new sensor thus far (>15,000 h) indicate that the effects discussed here are relatively low, also at night (discussed later). This is most likely the result of the differences in sensor layout in comparison with other glucose sensors.
The layout of the new glucose sensor reduces the effect of the submillimeter inhomogeneity of adipose tissue on the sensor signal; since the total electrical current of the sensor is the sum of the currents registered at each of the working electrode spots, local differences in the generation of current are leveled out (Figure 2A). This realizes a “tissue averaging” effect without additional efforts such as using several independently working electrodes (including the additional electronics necessary for independent monitoring of the electrode spots) and averaging the respective electrical currents. In other words, the electrical currents from each spot are physically combined to one final current that is then measured rather than measuring several currents independently and then computing their sum. As a result, the influence of local variations on the sensor signal is minimized. By increasing the electrode surface, the current density can be reduced by using membrane systems that further decrease the glucose diffusion to the electrode surface without changing the sensor’s sensitivity. Thereby, the local glucose consumption of a single electrode spot is reduced (Figure 2B and 2C). This effect further decreases the impact of local changes in tissue glucose concentration (e.g., due to changes in local blood flow) on the sensor signal.
Figure 2.
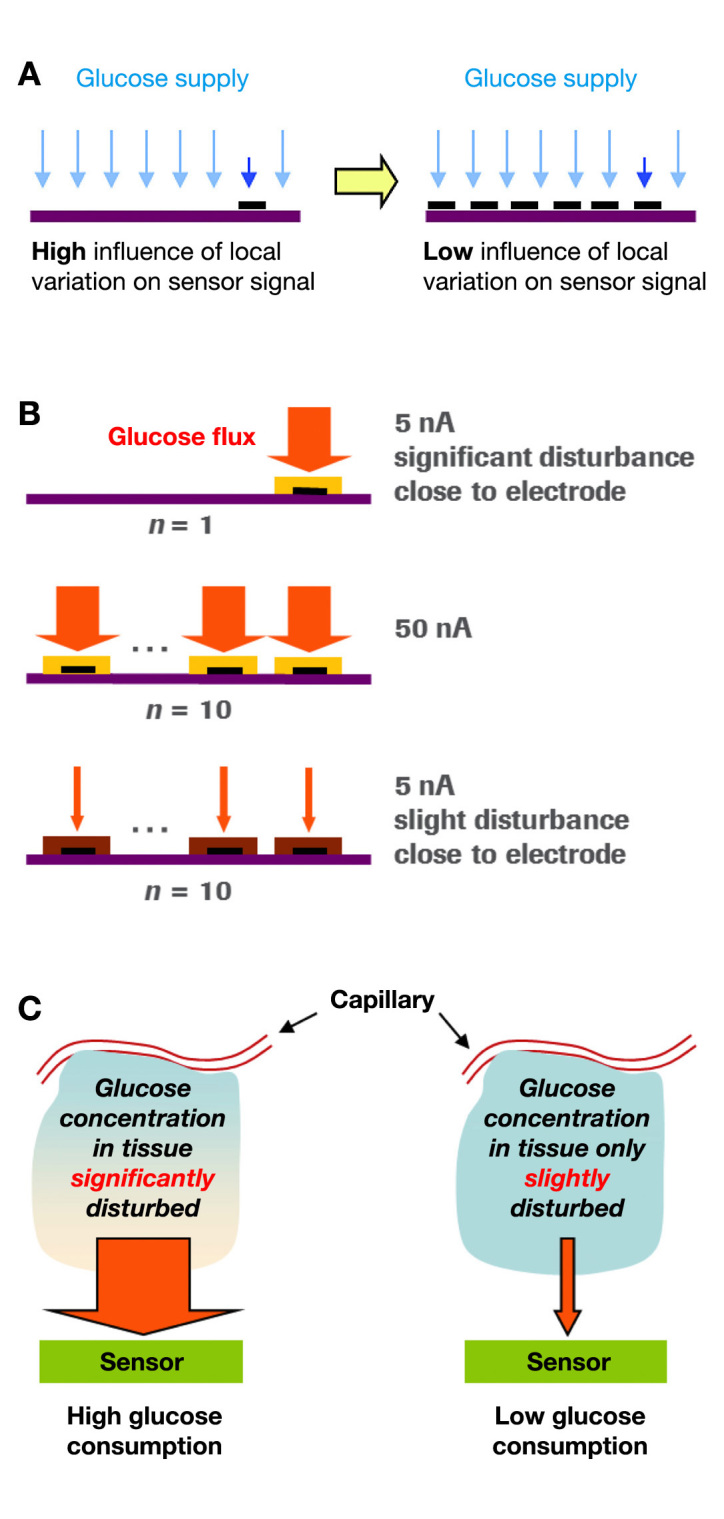
(A) Effect of tissue averaging and (B, C) effect of restriction of glucose flux (glucose consumption) into sensor.
It would also be of interest to perform head-to-head comparisons of different sensors evaluating the impact of such factors in the same subjects in a systematic manner.
One issue with such studies is that the outcome of the measurements (i.e., final glucose data presented by the CGM systems) does not represent the raw data but—as outlined earlier—are subject to considerable data processing. Therefore, differences in the raw signals might be eliminated by these algorithms.8 However, to have a sensor with a low noise level is advantageous, as this lowers the need for the subsequent computation efforts.
Biofouling
Until now, all needle-type sensors show a considerable drift (i.e., decline) in sensor signal over the wear time, i.e., this is limited to several days and not to weeks or even months. The reasons for this decline in sensor signal quality are clearly multifunctional; however, it is of interest to note that huge interindividual differences also exist between patients with diabetes.
By selecting membranes that minimize tissue reactions and the physiologic foreign body response after sensor insertion, the sensor signal can be improved. This also reduces the need for regular recalibrations that are otherwise needed to readjust the sensor signal to the prevailing glycemia. The membranes are to prevent diffusion of proteins toward the sensor surface and also of sensor material escaping into the human body.
In order to optimize the biocompatibility of the sensor, a second membrane material is used to cover the complete sensor shaft that goes into the body. The purpose of this layer is to keep the immunological and inflammation reaction of the tissue in response to the sensor as low as possible.3 This provides stable glucose sensitivity over time and achieves a high degree of sensor reliability over the intended period of use.
In Vivo Evaluation
The in vivo performance of four independent glucose sensors inserted in one subject is illustrated in Figure 3. Data were obtained during a clinical study in a clinical research center (Institute for Diabetes-Technology, Ulm, Germany). The sensors were in use for 7 days, and the subject could move freely in the clinical research center.
Figure 3.
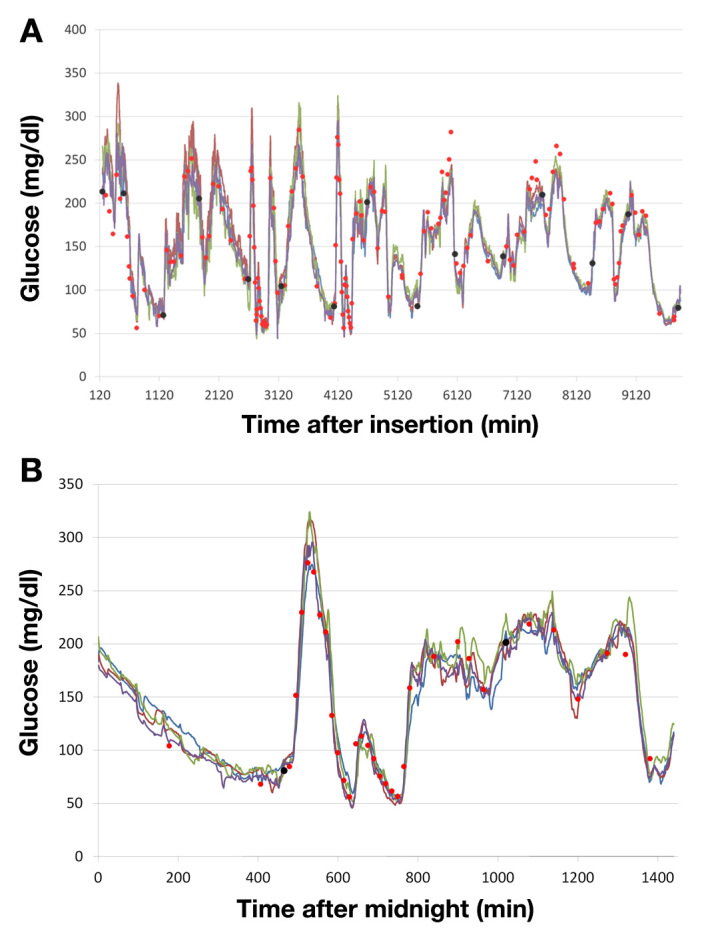
Examplary performance data of four glucose sensors measuring glucose levels at different sites in the same patient (A) over seven days and (B) during one day (cutout of a section of A). Lines of different colors correspond to glucose readings of the sensors. Red dots, BG values; black dots, BG measurements used for calibration.
Sensors was calibrated twice per day. Since a prototype of the CGM system was used, the calibration was done after the experiment as a simulated prospective calibration (sensor raw data were processed as if they were processed in real time). The glucose traces only rarely show dropouts or increased noise and match the BG readings very consistently. In addition, the favorable congruence of the four sensor traces confirms consistent operation of the glucose recording over time.
Summary
By taking four main elements into account in the layout of this novel electrochemical glucose sensor,
Tissue averaging,
Low oxidation potential by use of manganese dioxide,
Limitation of glucose flux into the working electrode, and
Biocompatible coating of the sensor,
stable and precise glucose monitoring is possible, as shown in in vivo studies. The layout of this sensor appears to be responsible for improving the sensor–tissue interaction to such an extent that the impact of physiological effects in the subcutaneous tissue on sensor response is significantly reduced. A CGM system with such a high-performance glucose sensor provides an optimized measurement performance. Further down the road, this would also be well suited for use in future artificial pancreas settings, i.e., closing the loop. As an interim step, precise detection of glucose levels declining into the low glucose range would allow a reliable control of insulin pumps, i.e., stopping insulin infusion to avoid or to reduce duration of hypoglycemic episodes.
Acknowledgments
We acknowledge the constructive support of Prof. Dr. Lutz Heinemann in the writing process of this manuscript.
Glossary
- (BG)
blood glucose
- (CGM)
continuous glucose monitoring
- (ISF)
interstitial fluid
Funding
The research was funded by Roche Diagnostics, Mannheim, Germany.
Disclosures
All authors are employees of Roche Diagnostics, Mannheim, Germany.
References
- 1.Ramchandani N, Arya S, Ten S, Bhandari S. Real-life utilization of real-time continuous glucose monitoring: the complete picture. J Diabetes Sci Technol. 2011;5(4):860–870. doi: 10.1177/193229681100500407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hermanides J, Phillip M, DeVries JH. Current application of continuous glucose monitoring in the treatment of diabetes: pros and cons. Diabetes Care. 2011;34(Suppl 2):S197–S201. doi: 10.2337/dc11-s219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang J. Electrochemical glucose biosensors. Chem Rev. 2008;108(2):814–825. doi: 10.1021/cr068123a. [DOI] [PubMed] [Google Scholar]
- 4.Henninger N, Woderer S, Kloetzer HM, Staib A, Gillen R, Li L, Yu X, Gretz N, Kraenzlin B, Pill J. Tissue response to subcutaneous implantation of glucose-oxidase-based glucose sensors in rats. Biosens Bioelectron. 2007;23(1):26–34. doi: 10.1016/j.bios.2007.03.016. [DOI] [PubMed] [Google Scholar]
- 5.Makale MT, Lin JT, Calou RE, Tsai AG, Chen PC, Gough DA. Tissue window chamber system for validation of implanted oxygen sensors. Am J Physiol Heart Circ Physiol. 2003;284(6):H228–H294. doi: 10.1152/ajpheart.00721.2002. [DOI] [PubMed] [Google Scholar]
- 6.Makale MT, Jablecki MC, Gough DA. Mass transfer and gas-phase calibration of implanted oxygen sensors. Anal Chem. 2004;76(6):1773–1777. doi: 10.1021/ac0352169. [DOI] [PubMed] [Google Scholar]
- 7.Makale MT, Chen PC, Gough DA. Variants of the tissue-sensor array window chamber. Am J Physiol Heart Circ Physiol. 2005;289(1):H57–H65. doi: 10.1152/ajpheart.01001.2004. [DOI] [PubMed] [Google Scholar]
- 8.Facchinetti A, Sparacino G, Guerra S, Luijf YM, DeVries JH, Mader JK, Ellmerer M, Benesch C, Heinemann L, Bruttomesso D, Avogaro A, Cobelli C. AP@home Consortium. Real-time improvement of continuous glucose monitoring accuracy: the smart sensor concept. Diabetes Care. 2013;36(4):793–800. doi: 10.2337/dc12-0736. [DOI] [PMC free article] [PubMed] [Google Scholar]


