Abstract
Background:
Glucose measurement is the cornerstone of diabetes control. In the hospital setting, the same device and package of test strips (50 or 100 strips) can be used to monitor glucose in several patients, which can increase cross contamination. The objective of our study is to measure bacterial contamination in glucose test strips, comparing results in individual single-use packets (one hospital) versus multi-use vials (two hospitals) in Spain.
Methods:
Test strips were collected from five different wards. Each hospital also collected two unopened vials from a single ward as controls. They were sent to a reference laboratory for microbiologic study. A number equal or higher than two colony forming units per strip was considered as a positive result.
Results:
Out of 423 glucose test strips collected and cultured, 146 were contaminated (34%); only 7% of individually packed strips were contaminated versus 45% of strips packed in multi-use vials, with a high statistical significance (p < .001).
Conclusions:
In the strips from multi-use vials, a high contamination rate was found and highly pathogenic organisms were identified, such as methicillin-resistant Staphylococcus epidermidis or Staphylococcus hemolyticus. In contrast, in strips packed individually, there was a much lower contamination rate and no such pathogen organisms were found. Therefore, in the hospital setting, the use of blood glucose test strips in individual packages would be more advantageous (mainly from a clinical point of view, but also from a financial one) than those packed in multiuse vials.
Keywords: bacterial contamination, cross contamination, glucose test strips, nosocomial infection
Introduction
Once diabetes is diagnosed, glucose measurement is the cornerstone for its control1. Glucose monitoring is used for checking the efficacy of the treatment, adjusting the dose of the drug, preventing acute and chronic complications, as well as diet intervention or exercise advice.
Glucose levels are measured preferably using portable glucose meters, becoming the standard for the follow up of diabetes patients.2,3 These devices are used at hospitals, health care centers, as well as by patients, which provides them with an essential tool for self-control of their diabetes.
Unlike the home setting, where each patient has his/her individual device, at hospitals and health care centers, the same device and the same package of test strips (50 or 100 strips) can be used to monitor glucose in several patients, which can increase cross contamination due to handling each time a strip is taken from the package.
Nosocomial infections pose a major problem in hospitals, recording prevalence rates in hospitalized patients of 7.11% in Spain, according to the Estudio de Prevalencia de Infecciones Nosocomiales en España (EPINE study),4 and 8.7% worldwide.5 The cost of these infections takes up a significant amount of the health budget: £1.06 billion in the United Kingdom6 and $6.7 billion in the United States7 according to data for the year 2000. In Spain, according to the study of the Ministry of Health and Consumer Affairs, the direct annual costs for nosocomial infections have been estimated at €1 billion.8
Nosocomial infection is one of the most significant adverse events in health institutions. It is estimated that 2 million patients suffer a nosocomial infection every year in the United States.9,10 In Spain, nosocomial infections are the second most common adverse event in hospitals, after effects related to drug administration, with a prevalence of 6.68%.4
According to the World Health Organization,11 there are several factors that stand out and can affect the appearance of nosocomial infections: the microbiological agent, patient susceptibility, environmental factors, and bacterial resistance.
Nosocomial infection is a preventable problem in a high percentage of the cases. In this regard, the EPINE study4 shows that 56% of nosocomial infections occurring at Spanish hospitals can be prevented. The main factor contributing to the increased cost related to nosocomial infections is the prolongation of the patient’s hospital stay.11 The costs also increase due to the greater use of drugs, isolation, and higher use of laboratory and other diagnostic tests.5,11
Therefore, the devices and reagents used for measuring glucose at the patient’s bedside could be a vehicle for trans-mission of these diseases, particularly in patients that can have some degree of immunodepression, such as diabetes patients, neonates, burn patients, or intensive care unit patients.
In the hospital setting, the role played by glucose meters in the transmission of viral diseases, such as hepatitis B12 and hepatitis C,13 due to blood contamination (e.g., splashing, scratching) in the measuring device has been investigated, as the same device is used for measurements in several patients, and bacterial contamination has been poorly studied.14,15 Furthermore, international guidelines have been published that attempt to minimize the risk of hospital infection associated with the use of glucose meters.9,16
The role of glucose measurement test strips as infection transmission agents has not been evaluated in depth to date. These strips can play a major role as they are usually packed in vials of 50 or 100 strips, and once opened, they are exposed to the potential contaminants.
The objective of our study is to measure bacterial contamination in glucose test strips from two different manufacturers, one packed in vials of 50 strips and one packed individually, in several facilities of three Spanish teaching hospitals with over 900 beds.
Material and Methods
This prospective observational study was performed in three hospitals: hospital 1 (900 beds), hospital 2 (1300 beds), and hospital 3 (1400 beds). They used two different types of glucose test strips: hospital 1 (individually packaged strips), hospital 2 (50-strip vial), and hospital 3 (50-strip vial).
During one day, each hospital collected two opened vials from five different wards in specific containers for micro-biologic culture. The vials had to contain less than half of the original amount of strips. Each hospital also collected two unopened vials from a single ward as controls.
The vials and strips were handled exclusively by nurses. Before opening a vial, their hands and the vial were cleaned and disinfected with alcohol.
The data about each vial were as follows:
- Vial data:
- Collection date
- Hospital ward
- For single or multipatient use
- Opened or unopened
- Number of remaining amount of glucose test strips
- Date of vial opening
- Ward data:
- Total number of beds/number of beds available at the time of sampling/number of occupied beds at the time of sampling
- Number of nurses working at the time of sampling
- Number of opened vials at the time of sampling
- If vials were used for single or multiple patients
- If hands and vial disinfection procedures were followed by staff
- Vial storage place
- Hospital data:
- If there was a protocol for handling glucose test strips
- If there was a protocol related to vials for single/multipatient use.
All vials collected were stored at room temperature. The next day, they were sent to a reference laboratory for microbiologic study, independent from the hospitals participating in the study (Laboratorio Echevarne, Barcelona, Spain; http://www.echevarne.com).
Each strip was placed in 10 ml of peptone water and 1% Tween-80 and vortexed. Five mililiters of the suspension was filtered with a sterilized 0.45 μm pore-size membrane. After washing with 100 ml of peptone water twice, the membrane was placed in a tryptic soy agar plate and incubated aerobically at 30–35 °C. The remaining 5 ml of the suspension was also filtered. This membrane was placed in Schaedler agar and incubated at 30–35 °C anaerobically. Viable bacteria were counted after 3 days of culture. This procedure was validated with a bioburden recovery efficiency study. Microorganisms recovered were identified by standard microbiologic methods. If staphylococci were identified, a geno-typing assay was performed by DNA (deoxyribonucleic acid) and polymerase chain reaction extraction followed by screening with DNA strip technology of Staphylococcus aureus and Staphylococcus epidermidis strains, as well as methicillin-resistant genes [methicillin-resistant S. aureus (MRSA) and methicillin-resistant S. epidermidis (MRSE)] and virulence factors (Panton–Valentine leukocidin).
The contamination level of strips was evaluated for each hospital, type of glucose test strip, and hospital ward. A number equal or higher than two colony forming units (CFUs) per strip was considered as a positive result.
The statistical analysis of the results was performed with the software SPSS v13.0.
Results
During the study period, a total of 423 glucose test strips were collected and cultured: 119 from hospital 1; 90 from hospital 2; and 214 from hospital 3. Overall, a total of 146 contaminated strips were detected, which means a rate of 34%. Breaking down this result by hospital, the number of contaminated strips was 8 (7%), 51 (57%), and 87 (41%), respectively (Figure 1).
Figure 1.
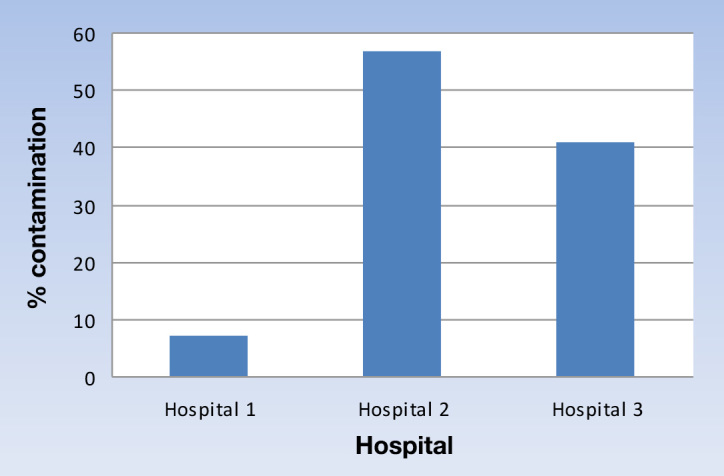
Percentage of contamination of glucose test strips in different hospitals.
The results detailing the contaminated strips related to each hospital are shown in Table 1.
Table 1.
Bacterial Contamination of Glucose Test Strip
| Identified microorganism | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hospital | Ward | Number of test strips | Number of contaminated test strips | Number of test strips with aerobic bacteria | Number of test strips with anaerobic bacteria | Number of test strips with both aerobic and anaerobic bacteria | MRSE | S. epidermidis | S. hemolyticus | Staphylococcus sp | S. lentus | S. capitis | S. caprae | S. warneri | S. cohni | |
| 1 | Intensive care unit | 20 | 0 | |||||||||||||
| Recovery room | 19 | 0 | ||||||||||||||
| Thoracic surgery | 20 | 0 | ||||||||||||||
| Cardiology | 20 | 4 | 3 | 1 | 1 | |||||||||||
| Short stay unit | 20 | 2 | 1 | 1 | ||||||||||||
| Control | 20 | 2 | 2 | |||||||||||||
| TOTAL | 119 | 8 | ||||||||||||||
| 2 | Intensive care unit | 20 | 7 | 2 | 2 | 3 | 4 | |||||||||
| Surgery recovery room | 20 | 15 | 5 | 3 | 7 | 2 | 1 | 2 | 3 | 1 | ||||||
| Neonate | 22 | 14 | 6 | 4 | 4 | 1 | 3 | 1 | 2 | 1 | ||||||
| Blood sampling | 11 | 5 | 2 | 3 | 2 | 1 | ||||||||||
| Control | 17 | 10 | 4 | 6 | 3 | |||||||||||
| TOTAL | 90 | 51 | ||||||||||||||
| 3 | Blood sampling | 28 | 20 | 14 | 6 | 1 | 1 | |||||||||
| Burn unit | 40 | 18 | 5 | 3 | 10 | 2 | 1 | 1 | ||||||||
| Surgery recovery room 1 | 40 | 13 | 7 | 3 | 3 | |||||||||||
| Surgery recovery room 2 | 50 | 21 | 4 | 9 | 8 | 2 | 2 | 1 | 1 | |||||||
| Intensive care unit | 37 | 13 | 1 | 9 | 3 | 1 | 1 | |||||||||
| Control | 19 | 2 | 2 | 1 | ||||||||||||
| TOTAL | 214 | 87 | ||||||||||||||
At hospital 1, only 8 contaminated strips were detected from a total of 119 analyzed. In these positive cases, the low CFU levels (2–6 CFU/strip) are also to be noted. No MRSE was found on the strips from hospital 1.
Hospital 2 evaluated the strips from four wards and one control batch. All strips were in vials containing 50 strips. Fifty-seven percent of the strips evaluated show contamination, mainly contamination by S. epidermidis (43%), 50% of which was MRSE. The highest percentages of contamination were seen in neonate and recovery wards, where there is a high flow of staff due to shifts.
At hospital 3, the strips from five wards of patients and one control batch were evaluated. Eighty-seven contaminated strips were detected from a total of 214 (41%), observing a higher contamination in the sampling and burn patient wards. As in hospital 2, the most common pathogen of all identified was S. epidermidis, 60% of which was MRSE.
The presence of S. aureus and Clostridium difficile was ruled out in the contaminated strips of the three hospitals.
In hospitals 2 and 3, except for one case where a vial of strips was aimed only at use by a single patient in the burn unit, the vials containing strips were shared by several patients.
No differences were found in the contamination of test strips based on the handling of the strips by the nursing staff, existence of a protocol for the management of a glucose meter, need for hand washing, or storage of test strips.
Discussion and Conclusions
The use of portable systems for measuring blood glucose in hospitalized patients, as well as the number of daily controls performed, has increased markedly. In many health institutions, there is no control of the clinical measurements performed out of the laboratory whether it is for acquiring resources or for performing tests or quality assurance of the results obtained.
The transmission of infectious diseases using portable glucose meters has been implicated since 1997, such as transmission of hepatitis C17,18 and hepatitis B,19 constituting a health problem, particularly when glucose meters are shared by different patients.13,20
In the past 2 years, some studies have been published on bacterial contamination in test strips to measure glucose in the hospital setting, such as those performed by Vanhaeren and coauthors21 on 148 strips packaged in multi-use vials obtained from several hospital wards (digestive and geriatric), with a contamination rate of 25.7%.
Our study evaluated the potential bacterial contamination of the test strips used to measure glucose and compare it according to their type of packaging (individual or multi-use vial).
This study was performed on 423 strips, obtained from different hospitalization wards from three teaching hospitals with over 900 beds each, which grants more statistical robustness to the outcomes obtained.
The strip-contamination rate found in our study was 34%, analyzing all data overall. These outcomes are greater than those found by Vanhaeren and coauthors,21 who found 25.7% contamination (enteric and skin flora), but comparable to those obtained by Ng and coauthors.22 The first study was performed in a single hospital, with a single type of strip, while the second was performed jointly at five hospitals using three types of test strip.
The novelty of this study is that the strips evaluated were supplied in different types of packaging (individual and multi-use vials), and their significance in the contamination rates is seen. A new data analysis and breakdown by type of test strip package (individual or multi-use vial) reveals the surprising finding of only 7% contaminated strips when packed individually versus 45% in those packed in multi-use vials, with a high statistical significance (p < .001; Figure 2).
Figure 2.
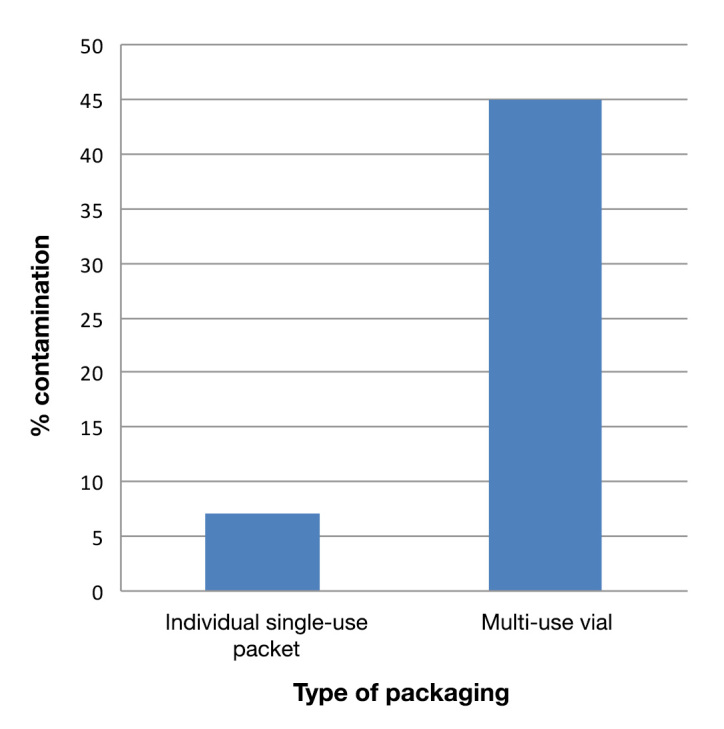
Percentage of contamination of glucose test strips according to packaging.
These data would confirm the recommendations given by several authors and agencies15,16 to prevent transmission of viral infections in diabetes patients monitored with test strips and highlight that each patient should be assigned a single measuring device and, failing that, each patient have their vial of test strips assigned.20,23 Klonoff and Perz24 recommended avoiding the transport of devices and medication in pockets, which is critical, considering that 60% of health staff uniforms can be contaminated by pathogens.25 In this regard, the use of test strips packed individually would significantly reduce the possibility of infection.
Another issue to be highlighted is the type of microorganisms detected in the contaminated glucose test strips (Table 1). In the strips from multi-use vials, some pathogenic organisms were identified: MRSE26 found on strips from surgical rooms and burn units and S. hemolyticus27,28 from a neonate unit and Staphylococcus warneri,29,30 Staphylococcus caprae,31Staphylococcus capitis,32 and Staphylococcus cohnii.33 These findings are consistent with those of Ng and coauthors22 and Carbon.34 Even though the common nosocomial pathogens such as MRSA and vancomycin-resistant Enterococcus were not detected, our results do confirm that bacterial contamination of test strips from open vials is common and suggest that these vials can serve as fomites for patient-to-patient transmission of potentially pathogenic bacteria and organisms important to infection control practices.
The number of CFUs of some of the strips in contaminated multi-use package must also be noted; one case reached 1136 CFU. On the contrary, in strips packed individually, besides finding a much lower contamination rate, with very low CFUs, no such pathogen organisms were identified as in the strips packed in multi-use vials (Table 1). To reduce the risk of bacterial transmission in the clinical environment, the acceptable levels of microbial flora on high-touch surfaces have been defined as <2.5 CFU/cm2 35 or <5 CFU/cm2 36 by various agencies. The total surface area of a blood glucose test strip is approximately 2.7–6.8 cm2, depending on the brand. Many of the clinically relevant bacteria can survive for days to months on dry inanimate surfaces.37 Since a test strip vial is typically accessed multiple times a day, it is logical to predict that transmission of live bacteria can occur.
In this regard, it must be noted that, in the validation of the method applied to measure bioburden on glucose test strips performed by the reference laboratory to perform this study, there was no contaminated strip packed individually, so the appearance of any CFU in these strips could be due to their handling when the sample is obtained (loss of package tightness when the blister is broken).
One of the factors influencing the appearance of nosocomial diseases is the use of contaminated material.11 In this regard, the use of contaminated test strips may be responsible for the occurrence of these diseases, with the increased health costs involved. Therefore, it appears obvious that the use of test strips packed individually can reduce the risk of contracting a nosocomial disease.
Another issue to be considered for the control of nosocomial diseases is the financial aspect. Besides the increased pharmaceutical costs and the prolonged hospital stay due to deterioration in condition, expenditure on glucose meter test strips must be considered. Once their potential infectious effect has been demonstrated, some author guides recommend giving each patient a vial of strips (50–100 strips) for their exclusive use and destroying any unused ones.16 However, we do not think that this practice removes all risks of infection, as it could be transmitted by the nursing staff by cross contamination when performing the test.21 Therefore, it is evident that the use of individually packaged test strips could reduce the costs, as only the necessary strips are used and none would have to be disposed of.
When choosing a system of strips for glucose monitoring in the hospital setting where several operators use the same device, the same device is used for several patients, and the results of the glucose meter are reported in addition to those of the central laboratory, it seems to be obvious that a number of issues must be considered:38
Transferability of the result of the glucose meter with the central laboratory
Traceability of the measure (operator, control, strip)
Patient identification
Automatic transmission of the result to the patient’s history
Compliance with quality specifications
External control system
Reduced risk of transmission of infectious diseases.
Therefore, this decision must not fall solely on the departments of endocrinology, nursing, or supplies, but on professionals in other departments, such as laboratory, preventive medicine, infection diseases, and microbiology.
This study confirms the possibility of bacterial contamination in the test strips used for glucose measuring at the patient’s bedside at the hospitals studied, particularly when these are contained in vials of multiple strips and the same vial is used for different patients, isolating mainly skin colonization pathogens, such as S. epidermidis (with a high percentage of methicillin-resistant pathogens), potentially pathogenic given the characteristics of the patients treated in the wards where they were isolated. This possibility of contamination is less likely when the diagnostic strips are supplied in individual, single-use packets. Most blood glucose monitoring systems are designed primarily for home use by a single patient. When used on multiple patients in the hospital setting, they may pose a higher risk in the transmission of pathogens. It is recommended that health care workers recognize this risk and manufacturers improve the product design to reduce the risk.
Considering that clothing, uniforms, and supplies related to the diagnosis could be contaminated, to ensure the patient’s safety and prevent the transmission of infections, there must be strict aseptic conditions in the diagnostic devices used in the point of care testing setting following the recommendations of international guidelines (e.g., Centers for Disease Control and Prevention and World Health Organization), particularly in the case of patients who may be immunodepressed (such as diabetes patients)39 and there is direct contact with the patient’s blood. In this regard, it would be advisable, from our point of view, to implement local instructions/guidelines for the handling of these devices that prevent bacterial contamination of blood glucose test strips in the hospital setting.
In conclusion, as our study has demonstrated the existence of a high bacterial contamination rate of test strips in multi-use packages, the use of blood glucose test strips in individual packages would be more advantageous (mainly from a clinical point of view, but also from a financial one) than those packed in multi-use vials.
Acknowledgments
We acknowledge the staff from all the participating wards.
Glossary
- (CFU)
colony forming unit
- (EPINE study)
Estudio de Prevalencia de Infecciones Nosocomiales en España
- (MRSA)
methicillinresistant Staphylococcus aureus
- (MRSE)
methicillin-resistant Staphylococcus epidermidis
Funding:
Abbott Diabetes Care provided funding for this study.
References:
- 1.American Diabetes Association. Self-monitoring of blood glucose. Diabetes Care. 1994;17(1):81–86. doi: 10.2337/diacare.17.1.81. [DOI] [PubMed] [Google Scholar]
- 2.The Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329(14):977–986. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- 3.Olveira-Fuster G, Olvera-Marquez P, Carral-Sanlaureano F, Gonzalez-Romero S, Aguilar-Diosdado M, Soriguer-Escofet F. Excess hospitalizations, hospital days, and inpatient costs among people with diabetes in Andalusia, Spain. Diabetes Care. 2004;27(8):1904–1909. doi: 10.2337/diacare.27.8.1904. [DOI] [PubMed] [Google Scholar]
- 4.Sociedad Española de Medicina Preventiva, Salud Pública e Higiene (SEMPSPH) Estudio de Prevalencia de las Infecciones Nosocomiales en España: EPINE 1990--2011: 22 AÑOS. http://www.sempsph.com/images/stories/recursos/pdf/protocolos/2012/378_9-epine_1990-2011.pdf. Accesed November 13, 2012.
- 5.Charvet-Protat S. Medico-economic analysis of nosocomial infections. Presse Med. 2000;29(32):1782–1787. [PubMed] [Google Scholar]
- 6.Plowman R, Graves N, Griffin MA, Roberts JA, Swan AV, Cookson B, Taylor L. The rate and cost of hospital-acquired infections occurring in patients admitted to selected specialties of a district general hospital in England and the national burden imposed. J Hosp Infect. 2001;47(3):198–209. doi: 10.1053/jhin.2000.0881. [DOI] [PubMed] [Google Scholar]
- 7.Haley RW, Fischer RP. Commercial tattooing as a potentially important source of hepatitis C infection Clinical epidemiology of 626 consecutive patients unaware of their hepatitis C serologic status. Medicine (Baltimore) 2001;80(2):134–151. doi: 10.1097/00005792-200103000-00006. [DOI] [PubMed] [Google Scholar]
- 8.Ministerio de Sanidady Consumo. Revisión Bibliográfica sobre Trabajos de Costes de la “No Seguridad del Paciente.” http://www.msc.es/organizacion/sns/planCalidadSNS/docs/CostesNoSeguridadPacientes.pdf. Accessed April 17, 2013.
- 9.Centers for Disease Control (CDC) Public health focus: surveillance, prevention, and control of nosocomial infections. MMWR Morb Mortal Wkly Rep. 1992;41(42):783–787. [PubMed] [Google Scholar]
- 10.Burke JP. Infection control - a problem for patient safety. N Engl J Med. 2003;348(7):651–656. doi: 10.1056/NEJMhpr020557. [DOI] [PubMed] [Google Scholar]
- 11.World Health Organization. Prevention of hospital-acquired infections. A practical guide. 2nd ed. Geneva: World Health Organization; 2003. [Google Scholar]
- 12.Kadi Z, Saint-Laurent P, Cadranel JF, Joly C, Dumouchel P, Jeanne S, Thiers V, Ciurana O, Astagneau P. Retrospective investigation of patients exposed to possible transmission of hepatitis C virus by a capillary blood glucose meter. J Hosp Infect. 2006;63(1):65–69. doi: 10.1016/j.jhin.2005.11.008. [DOI] [PubMed] [Google Scholar]
- 13.Desenclos JC, Bourdiol-Razès M, Rolin B, Garandeau P, Ducos J, Bréchot C, Thiers V. Hepatitis C in a ward for cystic fibrosis and diabetic patients: possible transmission by spring-loaded finger-stick devices for self-monitoring of capillary blood glucose. Infect Control Hosp Epidemiol. 2001;22(11):701–707. doi: 10.1086/501849. [DOI] [PubMed] [Google Scholar]
- 14.Thompson ND, Perz JF. Eliminating the blood: ongoing outbreaks of hepatitis B virus infection and the need for innovative glucose monitoring technologies. J Diabetes Sci Technol. 2009;3(2):283–288. doi: 10.1177/193229680900300208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Louie RF, Lau MJ, Lee JH, Tang Z, Kost GJ. Multicenter study on the prevalence of blood contamination on point-of-care glucose meters and recommendations for controlling contamination. Point Care. 2005;4:158–163. [Google Scholar]
- 16.Centers for Disease Control and Prevention. Diabetes and viral hepatitis: important information on glucose monitoring. http://www.cdc.gov/hepatitis/settings/glucosemonitoring.htm. Accessed November 13, 2012.
- 17.Bronowicki JP, Venard V, Botté C, Monhoven N, Gastin I, Choné L, Hudziak H, Rihn B, Delanoë C, LeFaou A, Bigard MA, Gaucher P. Patient-to-patient transmission of hepatitis C virus during colonoscopy. N Engl J Med. 1997;337(4):237–240. doi: 10.1056/NEJM199707243370404. [DOI] [PubMed] [Google Scholar]
- 18.Izopet J, Pasquier C, Sandres K, Puel J, Rostaing L. Molecular evidence for nosocomial transmission of hepatitis C virus in a French hemodialysis unit. J Med Virol. 1999;58(2):139–144. doi: 10.1002/(sici)1096-9071(199906)58:2<139::aid-jmv7>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 19.Perz JF, Fiore AE. Hepatitis B virus infection risks among diabetic patients residing in long-term care facilities. Clin Infect Dis. 2005;41(5):760–761. doi: 10.1086/432624. [DOI] [PubMed] [Google Scholar]
- 20.Hellinger WC, Grant RL, Hernke DA, Shalev JA, Barber BW, Meek SE, Jones AD, Thompson KM. Glucose meters and opportunities for in-hospital transmission of infection: quantitative assessment and management with and without patient assignment. Am J Infect Control. 2011;39(9):752–756. doi: 10.1016/j.ajic.2010.12.019. [DOI] [PubMed] [Google Scholar]
- 21.Vanhaeren S, Duport C, Magneney M, Dumé L, Dumenil AS, Doucet-Populaire F, Decousser JW. Bacterial contamination of glucose test strips: not to be neglected. Am J Infect Control. 2011;39(7):611–613. doi: 10.1016/j.ajic.2010.11.006. [DOI] [PubMed] [Google Scholar]
- 22.Ng R, Koo S, Johnston R. Multicenter evaluation of bacterial contamination of glucose test strips. Clin Chim Acta. 2012;413:1485–1457. doi: 10.1016/j.cca.2012.06.019. [DOI] [PubMed] [Google Scholar]
- 23.Nichols JH. Estimated strip wastage from glucose meter infection control recommendations. Clin Chim Acta. 2012;413(19–20):1485–1487. doi: 10.1016/j.cca.2012.08.007. [DOI] [PubMed] [Google Scholar]
- 24.Klonoff DC, Perz JF. Assisted monitoring of blood glucose: special safety needs for a new paradigm in testing glucose. J Diabetes Sci Technol. 2010;4(5):1027–1031. doi: 10.1177/193229681000400501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wiener-Well Y, Galuty M, Rudensky B, Schlesinger Y, Attias D, Yinnon AM. Nursing and physician attire as possible source of nosocomial infections. Am J Infect Control. 2011;39(7):555–559. doi: 10.1016/j.ajic.2010.12.016. [DOI] [PubMed] [Google Scholar]
- 26.Otto M. Staphylococcus epidermidis--the ‘accidental’ pathogen. Nat Rev Microbiol. 2009;7(8):555–567. doi: 10.1038/nrmicro2182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rolston KV, Bodey GP. Infections in patients with cancer. In: Kufe DW, Bast RC Jr, Hait WN, Hong WK, Pollock RE, Weichselbaum RR, Holland JF, Frei E, editors. Cancer medicine. 3rd, eds. Hamilton: BC Decker; 2006. pp. 2222–2245. 7th ed. [Google Scholar]
- 28.Froggatt JW, Johnston JL, Galetto DW, Archer GL. Antimicrobial resistance in nosocomial isolates of Staphylococcus haemolyticus. Antimicrob Agents Chemother. 1989;33(4):460–466. doi: 10.1128/aac.33.4.460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Announ N, Mattei JP, Jaoua S, Fenollar F, Sati H, Chagnaud C, Roudier J, Guis S. Multifocal discitis caused by Staphylococcus warneri. Joint Bone Spine. 2004;71(3):240–242. doi: 10.1016/S1297-319X(03)00126-X. [DOI] [PubMed] [Google Scholar]
- 30.Campoccia D, Montanaro L, Visai L, Corazzari T, Poggio C, Pegreffi F, Maso A, Pirini V, Ravaioli S, Cangini I, Speziale P, Arciola CR. Characterization of 26 Staphylococcus warneri isolates from orthopedic infections. Int J Artif Organs. 2010;33(9):575–581. doi: 10.1177/039139881003300903. [DOI] [PubMed] [Google Scholar]
- 31.Carretto E, Barbarini D, Couto I, De Vitis D, Marone P, Verhoef J, De Lencastre H, Brisse S. Identification of coagulase-negative staphylococci other than Staphylococcus epidermidis by automated ribotyping. Clin Microbiol Infect. 2005;11(3):177–184. doi: 10.1111/j.1469-0691.2004.01052.x. [DOI] [PubMed] [Google Scholar]
- 32.Van Der Zwet WC, Debets-Ossenkopp YJ, Reinders E, Kapi M, Savelkoul PH, Van Elburg RM, Hiramatsu K, Vandenbroucke-Grauls CM. Nosocomial spread of a Staphylococcus capitis strain with heteroresistance to vancomycin in a neonatal intensive care unit. J Clin Microbiol. 2002;40(7):2520–2525. doi: 10.1128/JCM.40.7.2520-2525.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Garza-González E, Morfin-Otero R, Martínez-Vázquez MA, Gonzalez-Diaz E, González-Santiago O, Rodríguez-Noriega E. Microbiological and molecular characterization of human clinical isolates of Staphylococcus cohnii, Staphylococcus hominis, and Staphylococcus sciuri. Scand J Infect Dis. 2011;43(11-12):930–936. doi: 10.3109/00365548.2011.598873. [DOI] [PubMed] [Google Scholar]
- 34.Carbon C. MRSA and MRSE: is there an answer? Clin Microbiol Infect. 2000;6(Suppl 2):17–22. doi: 10.1046/j.1469-0691.2000.00005.x. [DOI] [PubMed] [Google Scholar]
- 35.Malik RE, Cooper RA, Griffith CJ. Use of audit tools to evaluate the efficacy of cleaning systems in hospitals. Am J Infect Control. 2003;31(3):181–187. doi: 10.1067/mic.2003.34. [DOI] [PubMed] [Google Scholar]
- 36.Dancer SJ. How do we assess hospital cleaning? A proposal for microbiological standards for surface hygiene in hospitals. J Hosp Infect. 2004;56(1):10–15. doi: 10.1016/j.jhin.2003.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kramer A, Schwebke I, Kampf G. BMC Infectious Diseases. How long do nosocomial pathogens persist on inanimate surfaces? A systematic review. http://www.biomedcentral.com/1471-2334/6/130. Accessed March 7, 2013.
- 38.American Diabetes Association. Bedside blood glucose monitoring in hospitals. Diabetes Care. 2004;27(Suppl 1):S104. doi: 10.2337/diacare.27.2007.s104. [DOI] [PubMed] [Google Scholar]
- 39.Ilyas R, Wallis R, Soilleux EJ, Townsend P, Zehnder D, Tan BK, Sim RB, Lehnert H, Randeva HS, Mitchell DA. High glucose disrupts oligosaccharide recognition function via competitive inhibition: A potential mechanism for immune dysregulation in diabetes mellitus. Immunobiology. 2011;216(>1–2):126–131. doi: 10.1016/j.imbio.2010.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]


