Abstract
Background:
Accuracy standards of blood glucose (BG) meters are currently under review. Revised standards are expected to tighten accuracy requirements. Regarding clinical and financial impact of BG meter accuracy, very little data are available. The aim of this study was to analyze potential cost savings related to higher accuracy of glucose meters in Germany.
Methods:
As a model for calculation, a reduction of meter error from 20% to 5% was applied. The health economic analysis was based on four main pillars: (1) number of insulin-treated patients; (2) costs for glucose monitoring in Germany; (3) data of a modeling analysis on the impact on hypoglycemic episodes, glycosylated hemoglobin (HbA1c), and, subsequently, myocardial infarctions; and (4) costs of diabetes-related complications in Germany. A reduction of meter error from 20% to 5% was identified to be associated with a 10% reduction in severe hypoglycemic episodes and a 0.39% reduction in HbA1c, which translates into a 0.5% reduction of myocardial infarctions.
Results:
According to the health economic analysis, the reduction in severe hypoglycemic episodes and myocardial infarctions led to cost savings of €24.14 per patient per year. Considering 390,000 type 1 diabetes patients or 2.3 million insulin-treated patients in Germany, these savings could be equal to a reduction in health care expenditures of more than €9.4 million and €55.5 million, respectively.
Conclusions:
Potential cost savings and clinical effects due to higher accuracy of BG meters should provide an impetus to implementation of tighter accuracy standards and development of glucose meters that provide highest possible accuracy.
Keywords: accuracy, cost analysis, diabetes, hypoglycemia, self-monitoring of blood glucose
Introduction
Self-monitoring of blood glucose (SMBG) enables optimization of diabetes management.1 It also supports preventive strategies of acute and chronic complications of diabetes.2 Self-monitoring of blood glucose (BG) increases the patient’s awareness of hypoglycemic symptoms3,4 and facilitates self-regulatory prevention of significant hypoglycemic episodes.4,5 The need for prevention of hypoglycemic episodes has also been underlined in clinical trials, e.g., Action to Control Cardiovascular Risk in Diabetes; Action in Diabetes and Vascular Disease: Preterax and Diamicron MR Controlled Evaluation; and Veterans Affairs Diabetes Trial.6–8
Studies, e.g., Structured Testing Program and Role of Self-Monitoring of Blood Glucose and Intensive Education, San Carlos, highlight the value of structured SMBG in type 2 diabetes.9–11 Despite the growing evidence for the benefit of SMBG, the potential value of SMBG in type 2 diabetes is still being debated.12–14
The reliability of self-monitored glucose values is a prerequisite for an efficient and safe approach to treat patients to their target. Accuracy of SMBG, therefore, is a key aspect in this regard.
In Europe, BG meters with a Conformité Européenne label need to meet the current standard DIN EN ISO 15197:2003: ≥95% of the BG results shall fall within ±15 mg/dl of the reference method at BG concentrations <75 mg/dl and within ±20% at BG concentrations ≥75 mg/dl.15 The International Organization for Standardization (ISO) standard is currently under review, and the revised standard is expected to tighten accuracy requirements.16 According to the draft of the updated version of the ISO standard, 95% of the BG results shall fall within ±15 mg/dl of the reference method at BG concentrations <100 mg/dl and within ±15% at BG concentrations ≥100 mg/dl.16
Data on the clinical impact of accuracy of BG values are scarce. A modeling analysis reports a significant reduction of hypoglycemic episodes with increasing accuracy of glucose meters.17 The aim of our study was to assess potential cost savings associated with higher accuracy of glucose meters in Germany. A reduction of SMBG meter error from 20% to 5% was used as a model for calculation.
Methods
To analyze potential cost savings due to a higher accuracy of glucose meters in Germany, four domains were identified and included in the analysis:
Number of insulin-treated diabetes patients in Germany;
Costs for glucose monitoring in Germany;
Analysis of impact of higher accuracy on hypoglycemia, glycosylated hemoglobin (HbA1c), and, subsequently, cardiovascular complications; and
Costs of diabetes-related complications in Germany.
Number of Patients with Insulin-Treated Diabetes in Germany
The Robert Koch Institute (central institution for health protection in Germany, serving the Federal Ministry of Health) estimates the number of patients with diabetes in Germany to be 6 million.18 It is assumed that 5–10% of diabetes patients are type 1 patients.19 For the analysis, 390,000 type 1 diabetes patients in Germany, equaling 6.5% of the entire diabetes population, were included. In Germany, the total number of diabetes patients treated with insulin is estimated to be 2.3 million.20 Both numbers were included in the analysis.
Costs for Glucose Monitoring in Germany
Calculation of costs of SMBG in Germany was based on current market prices (December 2012) of SMBG devices and consumable supplies. A glucose meter was calculated at €40 cost. An amount of €0.66 per test strip was applied. The price of lancets was calculated as €0.11 per lancet. Annual costs of SMBG for Germany were based on the assumption of four glucose tests per day.
Analysis of Impact Of Higher Accuracy on Hypoglycemia
To assess the current knowledge on clinical effects of higher accuracy, a literature research was conducted. The target orientated literature research included PubMed/Medline, Cochrane library, and Excerpta Medica Database. Keyword sets combined primarily the word “diabetes (mellitus)” and one or more of the following terms: “complications,” glycemic variability,” “blood glucose,” “self-measurement,” “insulin,” “glycosylated haemoglobin A,” “HbA1c,” “accuracy,” “(self-)monitoring,” “computer simulation,” and “hypoglyc[a]emia.” The main clinical effects are summarized.
Literature research identified 817 references. Several studies provided information on the accuracy of various handheld SMBG meters21–29 but did not give information on the effect of (higher) accuracy on clinical outcomes. After screening of titles and abstracts, two publications were considered potentially relevant and were viewed in full text.17,30 One publication did not provide information on clinical outcomes.30 We identified the publication by Breton and Kovatchev to be applicable for the analysis.17 In the study, the relationship between accuracy of SMBG and risk for hypoglycemia, glucose variability, and long-term glycemic control is assessed.17 This study is based on computer simulation, which includes a validated model of the human metabolic system in patients with type 1 diabetes.31–34
In the study, 16,000 computer simulation trials were performed based on 100 simulated adult patients with type 1 diabetes.17
Analysis of the Impact of Higher Accuracy on Glycosylated Hemoglobin
In order to estimate the deterioration of overall glucose control (HbA1c) due to decreased SMBG inaccuracy, Cryer35,36 simulated the increased risk for hypoglycemia and the related detrimental effect on diabetes control observed in in vivo studies. Based on the formula recommended by the American Diabetes Association,37 the association between SMBG errors and change in HbA1c was incorporated based on the data of the in silico analysis.17
Analysis of the Impact of Higher Accuracy on Cardiovascular Complications
The United Kingdom Prospective Diabetes Study (UKPDS) risk engine provides an equation for estimating the risk of new coronary heart disease (CHD) in people with T2DM based on data from 4540 UKPDS male and female patients.38 The risk engine38 was applied to compute the effects of a reduction in HbA1c on cardiovascular outcome. In the risk engine, CHD is defined as the occurrence of fatal or nonfatal myocardial infarction or sudden death.38 The decrease in HbA1c, which corresponds “in silico” with the improved accuracy (5% versus 20% error), was assessed.17
It was applied because an engine for assessing the risk of cardiovascular outcome in type 1 diabetes is currently not available.
Costs of Severe Hypoglycemia and Myocardial Infarction in Germany
The Diabetes Control and Complications Trial reported a frequency of “severe hypoglycemia (treatment assistance from outside is needed)” of 0.64 per patient and year and a frequency of “very severe hypoglycemia (need for medical assistance or hospitalization due to impaired consciousness or unconsciousness)” of 0.19 per patient and year.39 These results are confirmed by other studies, some of them of German origin.40–43 The fact that 35% of patients with severe hypoglycemia require hospitalization and 65% can be treated by (para)medical personnel (i.e., required ambulance use only)44,45 was also included into the analysis.
Costs for emergency treatment in Germany were calculated based on the Rescue Services Act of Bavaria46 and German Disease-Related Group system.47,48
The incidence of myocardial infarction in Germany is estimated to be 280,000 cases per year,49 whereof 57,000 (20.4%) are fatal.50 The observation that 27% of all patients with myocardial infarction are diabetes patients was included in the analyses of frequency of myocardial infarctions in the diabetes population.51,52 Costs related to diabetic cardiovascular complications were calculated based on the results of the German epidemiological CoDiM-Study published by the London School of Economics.20,53,54
Results
Average annual costs for SMBG in Germany were calculated based on the local prices for test strips, lancets, and glucose meters: 4 × (€0.66 + €0.11) × 365 days + €40 = €1164.20. This amount was incorporated into the analysis.
The impact of SMBG errors at a 5% and 20 % level on detection of hypoglycemia and HbA1c was incorporated as reported in the in silico analysis.17
In the in silico analysis, the probability of missing a significant hypoglycemic event (defined as true glucose level of 60 mg/dl or lower) had been published as depicted in Figure 1.17
Figure 1. (A).
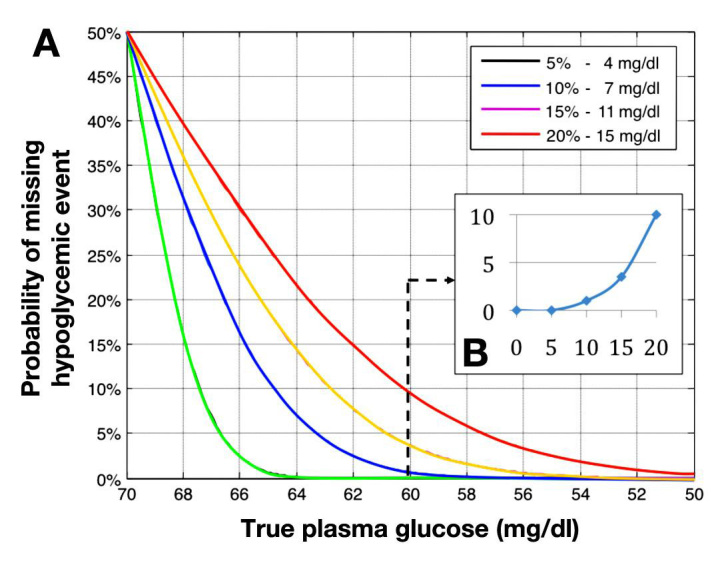
Probabilities of hypoglycemic events in function of measurement errors. (B) Probability for missing a hypoglycemic level of 60 mg/dl as a function of the SMBG error.17
As outlined in Figure 1, the probability of missing a significant hypoglycemic event rises with the increase of SMBG error.17 In the presence of an SMBG error of 5%, a hypoglycemic event will always be detected (Figure 1, green curve). At the 20% error level, as permitted by the current ISO standard,15 1 in 10 hypoglycemic episodes (10%) of 60 mg/dl will remain undetected.17 Based on the in silico analysis, a 10% reduction in severe hypoglycemic events was applied.
As published in the in silico analysis, the average BG is increased by 0.5 mg/dl at 5% error level and by 0.5 mg/dl at a 20% error level. This translates into an increase in HbA1c by 0.01 at 5% error level and by 0.40 at a 20% error level.17 Thus, the reduction of the error range from 20% to 5% will lead to an HbA1c reduction of 0.39 %.17
Applying the UKPDS risk engine, a reduction in HbA1c by 0.39% was found to be associated with a 0.5% reduction in CHD.38
As an interim summary, a 10% reduction in severe hypoglycemic events and a 0.5% reduction in CHD have been identified as clinical effects related to higher accuracy of SMBG meters (5% versus 20%).
As a next step, the costs of hypoglycemic events and the costs for CHD were included in the analysis based on current data from Germany.46–48 Table 1 provides the estimated unit costs for emergency treatment of hypo-glycemia in Germany.
Table 1.
Costs of Severe Hypoglycemia
| Unit | Costs (€) per event | Calculation basis |
|---|---|---|
| Ambulance | 520 | Rescue Services Act of Bavaria46 |
| Hospitalization (very severe hypoglycemia) | 2021 | German DRG K60 E:47 Diabetes mellitus without complicating diagnosis, age >10 years, without serious comorbidities, without ketoacidosis, without complex multimodal treatment. Factor 0.676; base rate (mean of 16 states, year 2012):48 €2990 |
Costs for hospitalization due to severe hypoglycemia were calculated as follows: €520 + €2012 = €2541. Thirty-five percent of all cases of very severe hypoglycemia are hospitalized, 65% treated by ambulance only.44,45 To calculate the average cost for a severe hypoglycemic event, the aspect was incorporated into the analysis: €2541 × 0.35) + (€520 × 0.65) = €1227.
According to the German data of myocardial infarctions,50–52 the occurrence of 75,600 myocardial infarctions annually was calculated for diabetes patients, equaling 1.26% of 6 million diabetes patients. In German type 1 diabetes patients (390,000 patients), this equals 4914 patients with a myocardial infarction annually, of whom 1002 have a fatal event and 3912 have a nonfatal event. In 2.3 million German patients with insulin-treated diabetes, 28,980 patients with a myocardial infarction were calculated per year (equaling 1.26%), of whom 5912 have a fatal event and 23,068 have a nonfatal event.
Costs for myocardial infarction in Germany, as assessed by the German epidemiological CoDiM Study,20,53,54 were calculated to be €9767 per myocardial infarction and €4032 for costs related to the first year of follow-up after an acute myocardial infarction. Adding the two parameters of costs for myocardial infarction, €13,799 per successfully treated myocardial infarction were incorporated into the analysis.
Table 2 summarizes the results of the data incorporated into the analysis:
Table 2.
Summary of Parameters Incorporated into the Analysis
| Type 1 diabetes patients in Germany | 390,000 |
| Insulin-treated patients in Germany | 2.3 million |
| Annual costs for SMBG in Germany (average) | €1164.20 |
| Reduction in severe hypoglycemic events caused by reduction of SMBG meter error from 20% to 5% | 10% |
| HbA1c reduction caused by reduction of SMBG meter error from 20% to 5% | 0.39% |
| Costs of severe hypoglycemia | |
| Ambulance | €520 |
| Hospitalization | €2021 |
| Average cost | €1227 |
| Costs of myocardial infarction | |
| Acute | €9767 |
| Follow-up (first year) | €4032 |
| Successfully treated myocardial infarction | €13,799 |
Cost Analysis of Improvement of Accuracy from 20% to 5%
Severe hypoglycemic episodes have been reported to occur at a rate of 0.19 times per patient and year. Based on the 10% reduction in severe hypoglycemic episodes, savings per patient were calculated as follows: €1227 × 10% × 0.19 = €23.32.
Costs for myocardial infarction in type 1 diabetes patients were calculated as follows: 1002 cases × €9767 + 3912 cases × €13,799 = €9,786,543 + €53,981,688 = €63,768,222.
Total costs of myocardial infarction in the entire group of insulin-treated patients were also analyzed: 5912 cases × €9767 + 23,068 cases × €13,799 = €57,742,504 + €318,315,332 = €376,057,836.
A 0.5 % reduction in fatal and nonfatal myocardial infarction in type 1 diabetes patients translates into savings as follows: €63,768,222 × 0.5% = €318,841 per year or €0.82 per patient with type 1 diabetes and year. Modeling 2.3 million patients with insulin-treated diabetes, savings can be calculated as follows: €376,057,836 × 0.5% = €1,880,289 per year or €0.82 per insulin-treated patient per year.
Adding annual savings due to prevented hypoglycemia (€23.32 per patient per year) and due to myocardial infarction (€0.82 per patient per year), total savings of €24.14 per patient per year were calculated.
Considering the number of 390,000 type 1 diabetes patients in Germany, this will add up to potential annual savings of €9.41 million. Analyzing the savings for 2.3 million patients with insulin-treated diabetes, the sum will add up to €55.52 million.
Table 3 summarizes the results of the cost analysis.
Table 3.
Cost Savings per Patient Related to an Improvement of Accuracy from 20% to 5%
| Annual cost savings per patient | |
| 10% reduction in severe hypoglycemic episodes | €23.32 |
| 0.5 % reduction in fatal and nonfatal myocardial infarction | €0.82 |
| In total | €24.14 |
| Annual savings for the German health care system | |
| 390,000 type 1 diabetes patients | €9.41 million |
| 2.3 million insulin-treated patients | €55.52 million |
Discussion
In our analysis, potential cost savings due to higher accuracy of SMBG devices in Germany were analyzed on the basis of a reduction in SMBG error range from 20% to 5%. In Germany, the clinical effects translate to potential cost savings of €24.14 per patient per year. Based on the German health care system, this may add up to annual savings of €9.4 million in type 1 diabetes patients and €55.5 million in the entire group of insulin-treated patients. In light of annual diabetes-related costs in Germany of €19.1 billion,20 the savings are considered to be a substantial contribution to reducing costs in the health care system of Germany.
Data of an in silico analysis were included in our analysis,17 which demonstrated that a higher accuracy may translate into a 10% reduction of severe hypoglycemic events and a mean HbA1c reduction of 0.39%. Applying the UKPDS risk engine, this reduction in HbA1c will lead to a 0.5 % reduction in CHD.
The reduction in HbA1c, hypoglycemic events, and the occurrence of CHD are key indicators of an improved clinical outcome in the patients. A reduction in HbA1c in response to structured SMBG has also been shown to be related to reductions of the cardiovascular risk biomarker high-sensitivity C-reactive protein.55
The evidence for the clinical benefit of SMBG in diabetes is increasing.9,11,56–58 The current study supports the view that SMBG is cost-effective and contrasts with some previous observations.12–14,59,60 These studies, however, were criticized for not having included a structured educational and therapeutic component in response to BG values.56 Small sample sizes or, in case of meta-analyses, exclusion of relevant data have also been reported to be limitations.61
Current results of SMBG studies are based on data of BG meters, which adhere to less stringent accuracy standards. In the 2003 ISO standard, deviations up to ±15 mg/dl at glucose concentrations <75 mg/dl and up to 20% at glucose concentrations ≥75 mg/dl are allowed.15 According to our analysis, a reduction of SMBG error range from 20% to 5% will be associated with an HbA1c reduction of 0.39%.17 With the technical realization of a 5% error range provided, this reduction in HbA1c may hypothetically be added to decreases in HbA1c related to SMBG in other trials. This may further tone down potential doubts about clinical efficacy of SMBG. Equally, aspects of cost-effectiveness might be redefined.
It is also to be considered that an increase in costs for SMBG may also adversely influence potential savings.
The results of this study also underline the need to develop more accurate BG meters and to implement tighter ISO standards.
Conclusion
The analysis demonstrates that a higher accuracy of BG meters is not only associated with reductions of hypoglycemic events and cardiovascular complications, but also opens up a significant potential for cost savings. The findings should provide an impetus to development of BG meters that provide the highest possible accuracy. Tightening of accuracy standards by health care authorities will further enhance the process.
Glossary
- (BG)
blood glucose
- (CHD)
coronary heart disease
- (HbA1c)
glycosylated hemoglobin
- (ISO)
International Organization for Standardization
- (SMBG)
self-monitoring of blood glucose
- (UKPDS)
United Kingdom Prospective Diabetes Study
Funding:
The generation of the manuscript was supported by an unrestricted educational grant of Bayer HealthCare.
Disclosures:
Oliver Schnell is a member of an expert panel of Bayer HealthCare.
References:
- 1.American Diabetes Association Standards of medical care in diabetes--2012. Diabetes Care. 2012;35(Suppl 1):S11–S63. doi: 10.2337/dc12-s011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kovatchev BP, Otto E, Cox D, Gonder-Frederick L, Clarke W. Evaluation of a new measure of blood glucose variability in diabetes. Diabetes Care. 2006;29(11):2433–2438. doi: 10.2337/dc06-1085. [DOI] [PubMed] [Google Scholar]
- 3.Schnell O, Alawi H, Battelino T, Ceriello A, Diem P, Felton A, Grzeszczak W, Harno K, Kempler P, Satman I, Vergès B. Consensus statement on self-monitoring of blood glucose in diabetes: a European perspective. Diabetes Stoffwechsel Herz. 2009;18(4):285–289. [Google Scholar]
- 4.Peel E, Parry O, Douglas M, Lawton J. Blood glucose self-monitoring in non-insulin-treated type 2 diabetes: a qualitative study of patients’ perspectives. Br J Gen Pract. 2004;54(500):183–188. [PMC free article] [PubMed] [Google Scholar]
- 5.Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). UK Prospective Diabetes Study (UKPDS) Group. Lancet. 1998;352(9131):837–853. [PubMed] [Google Scholar]
- 6.ACCORD Study Group. Cushman WC, Evans GW, Byington RP, Goff DC Jr, Grimm RH Jr, Cutler JA, Simons-Morton DG, Basile JN, Corson MA, Probstfield JL, Katz L, Peterson KA, Friedewald WT, Buse JB, Bigger JT, Gerstein HC, Ismail-Beigi F. Effects of intensive blood-pressure control in type 2 diabetes mellitus. N Engl J Med. 2010;362(17):1575–1585. doi: 10.1056/NEJMoa1001286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.ADVANCE Collaborative Group. Patel A, MacMahon S, Chalmers J, Neal B, Billot L, Woodward M, Marre M, Cooper M, Glasziou P, Grobbee D, Hamet P, Harrap S, Heller S, Liu L, Mancia G, Mogensen CE, Pan C, Poulter N, Rodgers A, Williams B, Bompoint S, de Galan BE, Joshi R, Travert F. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med. 2008;358(24):2560–2572. doi: 10.1056/NEJMoa0802987. [DOI] [PubMed] [Google Scholar]
- 8.Duckworth W, Abraira C, Moritz T, Reda D, Emanuele N, Reaven PD, Zieve FJ, Marks J, Davis SN, Hayward R, Warren SR, Goldman S, McCarren M, Vitek ME, Henderson WG, Huang GD. VADT Investigators. Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med. 2009;360(2):129–139. doi: 10.1056/NEJMoa0808431. [DOI] [PubMed] [Google Scholar]
- 9.Polonsky WH, Fisher L, Schikman CH, Hinnen DA, Parkin CG, Jelsovsky Z, Petersen B, Schweitzer M, Wagner RS. Structured self-monitoring of blood glucose significantly reduces A1C levels in poorly controlled, noninsulin-treated type 2 diabetes: results from the Structured Testing Program study. Diabetes Care. 2011;34(2):262–267. doi: 10.2337/dc10-1732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Franciosi M, Lucisano G, Pellegrini F, Cantarello A, Consoli A, Cucco L, Ghidelli R, Sartore G, Sciangula L, Nicolucci A. ROSES Study Group. ROSES: role of self-monitoring of blood glucose and intensive education in patients with type 2 diabetes not receiving insulin. A pilot randomized clinical trial. Diabet Med. 2011;28(7):789–796. doi: 10.1111/j.1464-5491.2011.03268.x. [DOI] [PubMed] [Google Scholar]
- 11.Durán A, Martín P, Runkle I, Pérez N, Abad R, Fernández M, Del Valle L, Sanz MF, Calle-Pascual AL. Benefits of self-monitoring blood glucose in the management of new-onset type 2 diabetes mellitus: the St Carlos Study, a prospective randomized clinic-based interventional study with parallel groups. J Diabetes. 2010;2(3):203–211. doi: 10.1111/j.1753-0407.2010.00081.x. [DOI] [PubMed] [Google Scholar]
- 12.Tengblad A, Grodzinsky E, Lindstrom K, Molstad S, Borgquist L, Ostgren CJ. Self-monitoring of blood glucose and glycaemic control in type 2 diabetes. Scand J Prim Health Care. 2007;25(3):140–146. doi: 10.1080/02813430701267413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Farmer A, Wade A, Goyder E, Yudkin P, French D, Craven A, Holman R, Kinmonth AL, Neil A. Impact of self monitoring of blood glucose in the management of patients with non-insulin treated diabetes: open parallel group randomised trial. BMJ. 2007;335(7611):132. doi: 10.1136/bmj.39247.447431.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Simon J, Gray A, Clarke P, Wade A, Neil A, Farmer A. Diabetes Glycaemic Education and Monitoring Trial Group. Cost effectiveness of self monitoring of blood glucose in patients with non-insulin treated type 2 diabetes: economic evaluation of data from the DiGEM trial. BMJ. 2008;336(7654):1177–1180. doi: 10.1136/bmj.39526.674873.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.International Organization for Standardization In vitro diagnostic test systems -- requirements for blood-glucose monitoring systems for self- testing in managing diabetes mellitus. ISO 15197:2003. http://www.iso.org/iso/home/store/catalogue_tc/catalogue_detail.htm?csnumber=26309 . [Google Scholar]
- 16.International Organization for Standardization In vitro diagnostic test systems -- requirements for blood-glucose monitoring systems for self-testing in managing diabetes mellitus. ISO 15197. http://www.iso.org/iso/home/store/catalogue_tc/catalogue_detail.htm?csnumber=54976 . [Google Scholar]
- 17.Breton MD, Kovatchev BP. Impact of blood glucose self-monitoring errors on glucose variability, risk for hypoglycemia, and average glucose control in type 1 diabetes: an in silico study. J Diabetes Sci Technol. 2010;4(3):562–570. doi: 10.1177/193229681000400309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Heidemann C, Du Y, Scheidt-Nave C. Diabetes mellitus in Germany. GBE kompakt. Berlin: Robert Koch Institute; 2011. [Google Scholar]
- 19.DiabetesIDE . Deutscher Gesundheitsbericht Diabetes. Mainz: Kirchheim + Co. GmbH; 2012. [Google Scholar]
- 20.Koster I, Huppertz E, Hauner H, Schubert I. Direct costs of diabetes mellitus in Germany – CoDiM 2000–2007. Exp Clin Endocrinol Diabetes. 2011;119(6):377–385. doi: 10.1055/s-0030-1269847. [DOI] [PubMed] [Google Scholar]
- 21.Diabetes Research in Children Network (DirecNet) Study Group. Relative accuracy of the BD Logic and FreeStyle blood glucose meters. Diabetes Technol Ther. 2007;9(2):165–168. doi: 10.1089/dia.2006.0005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fendler W, Hogendorf A, Szadkowska A, Mlynarski W. Non-coding glucometers among pediatric patients with diabetes: looking for the target population and an accuracy evaluation of no-coding personal glucometer. Pediatr Endocrinol Diabetes Metab. 2011;17(2):57–63. [PubMed] [Google Scholar]
- 23.Frank J, Wallace JF, Pardo S, Parkes JL. Performance of the CONTOUR® TS Blood Glucose Monitoring System. J Diabetes Sci Technol. 2011;5(1):198–205. doi: 10.1177/193229681100500128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Freckmann G, Baumstark A, Jendrike N, Zschornack E, Kocher S, Tshiananga J, Heister F, Haug C. System accuracy evaluation of 27 blood glucose monitoring systems according to DIN EN ISO 15197. Diabetes Technol Ther. 2010;12(3):221–231. doi: 10.1089/dia.2009.0128. [DOI] [PubMed] [Google Scholar]
- 25.Hsu CT, Hsiao HC, Lee MS, Chang SF, Lee TC, Tsai YS, Zen JM. Assessing the quality of Bionime self-monitoring blood glucose system Rightest GM110: a critical evaluation of interference and ambient circumstances. Clin Chim Acta. 2009;402(1–2):119–123. doi: 10.1016/j.cca.2008.12.029. [DOI] [PubMed] [Google Scholar]
- 26.Kipnes MS, Joseph H, Morris H, Manko J, Bell DE. Clinical performance of the TRUE2go blood glucose system--a novel integrated system for meter and strips. Diabetes Technol Ther. 2009;11(10):649–655. doi: 10.1089/dia.2009.0020. [DOI] [PubMed] [Google Scholar]
- 27.Kuo CY, Hsu CT, Ho CS, Su TE, Wu MH, Wang CJ. Accuracy and precision evaluation of seven self-monitoring blood glucose systems. Diabetes Technol Ther. 2011;13(5):596–600. doi: 10.1089/dia.2010.0223. [DOI] [PubMed] [Google Scholar]
- 28.Lippi G, Salvagno GL, Guidi GC, Negri M, Rizzotti P. Evaluation of four portable self-monitoring blood glucose meters. Ann Clin Biochem. 2006;43(Pt 5):408–413. doi: 10.1258/000456306778520007. [DOI] [PubMed] [Google Scholar]
- 29.Solnica B, Kusnierz-Cabala B, Slowinska-Solnica K, Witek P, Cempa A, Malecki MT. Evaluation of the analytical performance of the coulometry- based optium omega blood glucose meter. J Diabetes Sci Technol. 2011;5(6):1612–1617. doi: 10.1177/193229681100500640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Boren SA, Clarke WL. Analytical and clinical performance of blood glucose monitors. J Diabetes Sci Technol. 2010;4(1):84–97. doi: 10.1177/193229681000400111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dalla Man C, Raimondo DM, Rizza RA, Cobelli C. GIM, simulation software of meal glucose-insulin model. J Diabetes Sci Technol. 2007;1(3):323–330. doi: 10.1177/193229680700100303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dalla Man C, Rizza RA, Cobelli C. Meal simulation model of the glucose-insulin system. IEEE Trans Biomed Eng. 2007;54(10):1740–1749. doi: 10.1109/TBME.2007.893506. [DOI] [PubMed] [Google Scholar]
- 33.Kovatchev BP, Breton M, Man CD, Cobelli C. In silico preclinical trials: a proof of concept in closed-loop control of type 1 diabetes. J Diabetes Sci Technol. 2009;3(1):44–55. doi: 10.1177/193229680900300106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Magni L, Raimondo DM, Bossi L, Man CD, De Nicolao G, Kovatchev B, Cobelli C. Model predictive control of type 1 diabetes: an in silico trial. J Diabetes Sci Technol. 2007;1(6):804–812. doi: 10.1177/193229680700100603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cryer PE. Hypoglycaemia: the limiting factor in the glycaemic management of type I and type II diabetes. Diabetologia. 2002;45(7):937–948. doi: 10.1007/s00125-002-0822-9. [DOI] [PubMed] [Google Scholar]
- 36.Cryer PE. The pathophysiology of hypoglycaemia in diabetes. Diabetes Nutr Metab. 2002;15(5):330–333. [PubMed] [Google Scholar]
- 37.Nathan DM, Kuenen J, Borg R, Zheng H, Schoenfeld D, Heine RJ. A1c-Derived Average Glucose Study Group. Translating the A1C assay into estimated average glucose values. Diabetes Care. 2008;31(8):1473–1478. doi: 10.2337/dc08-0545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.UKPDS Risk Engine http://www.dtu.ox.ac.uk/riskengine/ [Google Scholar]
- 39.The Diabetes Control and Complications Trial Research Group Hypoglycemia in the Diabetes Control and Complications Trial. Diabetes. 1997;46(2):271–286. [PubMed] [Google Scholar]
- 40.Bragd J, Adamson U, Lins PE, Wredling R, Oskarsson P. A repeated cross-sectional survey of severe hypoglycaemia in 178 type 1 diabetes mellitus patients performed in 1984 and 1998. Diabet Med. 2003;20(3):216–219. doi: 10.1046/j.1464-5491.2003.00902.x. [DOI] [PubMed] [Google Scholar]
- 41.Bott S, Bott U, Berger M, Muhlhauser I. Intensified insulin therapy and the risk of severe hypoglycaemia. Diabetologia. 1997;40(8):926–932. doi: 10.1007/s001250050769. [DOI] [PubMed] [Google Scholar]
- 42.Müller UA, Femerling M, Reinauer KM, Risse A, Voss M, Jörgens V, Berger M, Mühlhauser I. Intensified treatment and education of type 1 diabetes as clinical routine. A nationwide quality-circle experience in Germany. ASD (the Working Group on Structured Diabetes Therapy of the German Diabetes Association) Diabetes Care. 1999;22(Suppl 2):B29–B34. [PubMed] [Google Scholar]
- 43.Schiel R, Muller UA. Structured treatment and teaching programs and an improvement in private health care lead to a better quality of diabetes care. JEVIN, a population-based trial 1989/90 up to 1999/2000. Med Klin (Munich). 2003;98(6):303–312. doi: 10.1007/s00063-003-1264-y. [DOI] [PubMed] [Google Scholar]
- 44.Leese GP, Wang J, Broomhall J, Kelly P, Marsden A, Morrison W, Frier BM, Morris AD. DARTS/MEMO Collaboration. Frequency of severe hypoglycemia requiring emergency treatment in type 1 and type 2 diabetes: a population-based study of health service resource use. Diabetes Care. 2003;26(4):1176–1180. doi: 10.2337/diacare.26.4.1176. [DOI] [PubMed] [Google Scholar]
- 45.Farmer AJ, Brockbank KJ, Keech ML, England EJ, Deakin CD. Incidence and costs of severe hypoglycaemia requiring attendance by the emergency medical services in South Central England. Diabet Med. 2012;29(11):1447–1450. doi: 10.1111/j.1464-5491.2012.03657.x. [DOI] [PubMed] [Google Scholar]
- 46.Bayerische Staatskanzlei. Bayerisches Rettungsdienstgesetz (BayRDG) http://www.gesetze-bayern.de/jportal/portal/page/bsbayprod.psml?showdoccase=1&doc.id=jlr-RettDGBY2008rahmen&doc.part=X&doc.origin=bs . [Google Scholar]
- 47.G-DRG Fallpauschalen-Katalog 2012. http://www.g-drg.de/cms/G-DRG-System_2012/Fallpauschalen-Katalog/Fallpauschalen-Katalog_2012 . [Google Scholar]
- 48.vdek. Landesweite Basisfallwerte. http://www.vdek.com/vertragspartner/Krankenhaeuser/DRG/landesbasisfallwerte/1_lbfw_2012_mit_Tarifrate.pdf . [Google Scholar]
- 49.Hamm CW. Guidelines: acute coronary syndrome (ACS). II: acute coronary syndrome with ST-elevation. Z Kardiol. 2004;93(4):324–341. doi: 10.1007/s00392-004-0109-x. [DOI] [PubMed] [Google Scholar]
- 50.Bruckenberger E. 2009 Multidisciplinary health report on cardiology and cardiac surgery. ISBN 978-3-00-032101-6. [Google Scholar]
- 51.Liebl A, Neiss A, Spannheimer A, Reitberger U, Wieseler B, Stammer H, Goertz A. Complications, co-morbidity, and blood glucose control in type 2 diabetes mellitus patients in Germany--results from the CODE-2 study. Exp Clin Endocrinol Diabetes. 2002;110(1):10–16. doi: 10.1055/s-2002-19988. [DOI] [PubMed] [Google Scholar]
- 52.Roehnisch JU, Behrens S, Maier B. Influence of guideline-based treatment in myocardial infarction patiens with and without diabetes mellitus. Dtsch Aerztebl. 2007;104(40):A2724–A2729. [Google Scholar]
- 53.Koster I, von Ferber L, Ihle P, Schubert I, Hauner H. The cost burden of diabetes mellitus: the evidence from Germany--the CoDiM study. Diabetologia. 2006;49(7):1498–1504. doi: 10.1007/s00125-006-0277-5. [DOI] [PubMed] [Google Scholar]
- 54.Kanavos P, van den Aardweg S, Schurer W. Diabetes expenditure, burden of disease and management in 5 EU countries. London: London School of Economics; 2012. http://www2.lse.ac.uk/LSEHealthAndSocialCare/research/LSEHealth/MTRG/LSEDiabetesReport26Jan2012.pdf . [Google Scholar]
- 55.Schnell O, Amann-Zalan I, Jelsovsky Z, Moritz A, Bermejo JL, Parkin CG, Schweitzer MA, Fisher L, Polonsky WH. Changes in A1C levels are significantly associated with changes in levels of the cardiovascular risk biomarker hs-CRP: results from SteP Study. Diabetes Care. 2013 doi: 10.2337/dc12-1711. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Klonoff DC, Blonde L, Cembrowski G, Chacra AR, Charpentier G, Colagiuri S, Dailey G, Gabbay RA, Heinemann L, Kerr D, Nicolucci A, Polonsky W, Schnell O, Vigersky R, Yale JF. Coalition for Clinical Research-Self-Monitoring of Blood Glucose Scientific Board. Consensus report: the current role of self-monitoring of blood glucose in non-insulin-treated type 2 diabetes. J Diabetes Sci Technol. 2011;5(6):1529–1548. doi: 10.1177/193229681100500630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Schnell O, Alawi H, Battelino T, Ceriello A, Diem P, Felton A, Grzeszczak W, Harno K, Kempler P, Satman I, Vergès B. Addressing schemes of self-monitoring of blood glucose in type 2 diabetes: a European perspective and expert recommendation. Diabetes Technol Ther. 2011;13(9):959–965. doi: 10.1089/dia.2011.0028. [DOI] [PubMed] [Google Scholar]
- 58.International Diabetes Federation. Global guideline for type 2 diabetes (2005) http://www.idf.org/global-guideline-type-2-diabetes-2005 . [Google Scholar]
- 59.Welschen LM, Bloemendal E, Nijpels G, Dekker JM, Heine RJ, Stalman WA, Bouter LM. Self-monitoring of blood glucose in patients with type 2 diabetes who are not using insulin: a systematic review. Diabetes Care. 2005;28(6):1510–1517. doi: 10.2337/diacare.28.6.1510. [DOI] [PubMed] [Google Scholar]
- 60.Malanda UL, Welschen LM, Riphagen II, Dekker JM, Nijpels G, Bot SD. Self-monitoring of blood glucose in patients with type 2 diabetes mellitus who are not using insulin. Cochrane Database Syst Rev. 2012;1:CD005060. doi: 10.1002/14651858.CD005060.pub3. [DOI] [PubMed] [Google Scholar]
- 61.Schnell O, Alawi H, Battelino T, Ceriello A, Diem P, Felton A, Grzeszczak W, Harno K, Hummel M, Kempler P, Satman I, Vergès B. Self-monitoring of blood glucose in patients with type 2 diabetes mellitus who are not using insulin: letter to the editor. Cochrane Database Syst Rev. 2012;1:CD005060. doi: 10.1002/14651858.CD005060.pub3. [DOI] [PubMed] [Google Scholar]


