Abstract
Clinical management of diabetes must overcome the challenge of in vivo glucose sensors exhibiting lifetimes of only a few days. Limited sensor life originates from compromised enzyme stability of the sensing enzyme. Sensing enzymes degrade in the presence of low molecular weight materials (LMWM) and hydrogen peroxide in vivo. Sensing enzymes could be made to withstand these degradative effects by (1) stabilizing the microenvironment surrounding the sensing enzyme or (2) improving the structural stability of the sensing enzyme genetically. We review the degradative effect of LMWM and hydrogen peroxide on the sensing enzyme glucose oxidase (GOx). In addition, we examine advances in stabilizing GOx against degradation using hybrid silica gels and genetic engineering of GOx. We conclude molecularly engineered GOx combined with silica-based encapsulation provides an avenue for designing long-term in vivo sensor systems.
Keywords: genetic engineering, glucose sensor, hydrogen peroxide, silica, stability
Introduction
Efficient and accurate glucose-sensing devices are a critical component in current diabetes treatment protocols. These devices are often based on the molecular recognition and catalysis of glucose by the enzyme glucose oxidase (GOx). The development of long-term implantable glucose sensors for more efficient diabetes management faces several immediate limitations, most notably sensor lifetime. A long-term implantable sensor based on GOx will require the enzyme to retain a functional level of catalytic activity for months. While GOx maintains this level of activity in vitro, its stability in vivo is of the order of 10–14 days, necessitating novel immobilization and enzyme modification strategies to extend the functional lifetime of these oxygen-sensitive sensors.
Glucose sensor instability depends on many environmental and internal factors. Gough and coauthors1–8 published several papers elaborating on the origins of these environmental and internal factors. Environmental factors occur in vivo due to lack of biocompatibility and include membrane biofouling, electrode passivation, and fibrous encapsulation. Internal factors result from both external penetrants [low molecular weight materials (LMWM)] and internal sensor issues. For an excellent review on the effects of environmental factors on sensor performance, refer to Wisniewski and coauthors.9 The internal factors include lead detachment, electrical short, membrane delamination, membrane degradation, and sensing-enzyme degradation. Gough and coauthors1–8 state that GOx degradation stems from either spontaneous inactivation or peroxide mediated inactivation. They suggest that spontaneous inactivation occurs throughout the immobilized enzyme phase but inactivation occurs by an unknown mechanism. Gough and coauthors3suggest that these mechanisms could include “ a temperature-dependent protein conformational or reversible FAD binding.” Conformational changes due to temperature changes directly relate to GOx stability. We suggest that LMWM degradation and epoxy formation within the immobilized enzyme layer encompasses the spontaneous inactivation observed by Gough and coauthors.1–8 The work of Gough and coauthors1–8 motivated synthesis of this review.
However, sensing-enzyme degradation, a significant internal factor connected to enzyme stability, has not received thorough review. Enzyme degradation severely limits the functional life of GOx in vivo and remains a significant challenge in continuous glucose monitoring. Suspected causes of GOx degradation include hydrogen peroxide (H2O2) generated at the electrode10–15 and intrinsic LMWM from blood and interstitial fluid.16 However, eliminating these factors is not enough to ensure optimum GOx performance; in addition, we must engineer a more stable environment for GOx and make the GOx enzyme itself more intrinsically stable.
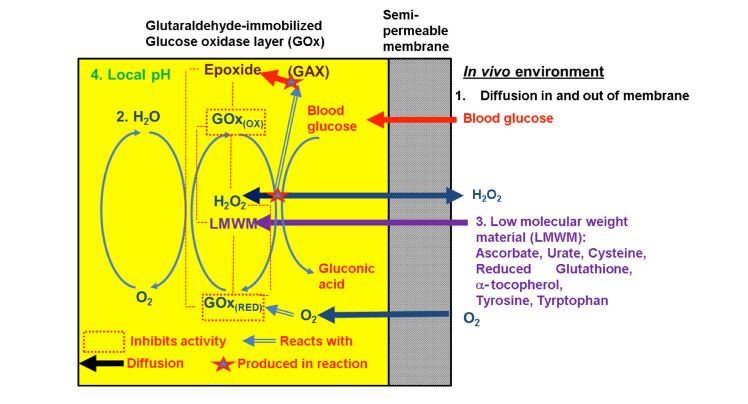
Several groups have attempted to improve stability by engineering various membrane types [silica sol-gel,17–19 glutaraldehyde cross-linking (GAX),20,21 carbon nanotubes22,23] and by manipulating the molecular structure of GOx itself (addition of oligomers and point mutations to improve functionality of GOx). However, each of these strategies fails to create a stable enough environment for GOx to function effectively over the target lifetime of a continuous glucose sensor. A stable environment (Figure 1) requires stoichiometrically controlled diffusion of reactants (oxygen, glucose) and products (H2O2) to and from the electrode and the bulk surface, hydrophilicity within the matrix, a localized pH near the isoelectric point of GOx (isoelectric point 4.2), and a mechanically strong and thin matrix layer on the electrode surface. This review will discuss why these previous stabilization strategies have failed and explore new approaches to achieve a stable GOx environment for the development of a functional, implantable continuous glucose monitor.
Figure 1.
The factors to create an optimum environment for entrapped GOx stability: (1) ample diffusion of key reactants and products in and out of the matrix/GOx/platinum surface, (2) adequate supply of reactants and structural molecules to promote proper folding (potential barriers toward GOx stability), (3) potential degradation from LMWM and H2O2, and (4) loss of localized pH near the isoelectric point of GOx.
Previous Internal Strategy: Glutaraldehyde Cross-Linking of Glucose Oxidase
Enzyme-based glucose sensors provide excellent specificity for a given analyte, yet often suffer from problems with long-term stability and biocompatibility. Glutaraldehyde plays a critical role in the design of biosensors by cross-linking enzymes at amine groups on electrode surfaces. Nonetheless, the effectiveness of GAX-based biosensors is still unclear. In fact, some studies suggest that GAX impairs proper enzyme conformation by cross-linking vital surface residues, resulting in decreased enzymatic activity and limited functionality.24 The following subsection details how GAX alters GOx structure in the absence and presence of H2O2 and LMWM.
Hydrogen Peroxide Degradation of Glucose Oxidase
Glucose oxidase stability decreases over time, in part, due to H2O2 oxidation of active site methionine residues to methionine sulfoxide.10–15 Oxidative damage is a critical issue in maintaining enzyme stability. Methionine sulfoxide formation affects the active site coordination of substrate recognition, catalysis, and specificity. Both soluble and immobilized GOx are susceptible to H2O2-mediated oxidative damage.11,12 Kleppe12 also suggests that H2O2 inactivates the redox states of GOx at different rates. Semiquinone (one electron reduced) and the two-electron-reduced GOx display no activity or severely reduced activity to glucose, respectively. Researchers have shown that H2O2 inactivates the reduced GOx 100 times more than the oxidized GOx.
Studies also demonstrate that H2O2 deactivates oxidized GOx in the absence of glucose slower than reduced GOx in the presence of glucose.14,25 Malikkides and Weiland14 postulated that the predominant mechanism of GOx inactivation by H2O2 involves the attack of peroxide on the glucose–GOx complex. They suggest that glucose changes the oxidized GOx conformational structure by expanding the active site, allowing for attack by peroxide. Bao and coauthors25 determined that immobilized GOx was competitively inhibited by H2O2. The reduced form of GOx competitively binds H2O2 and oxygen with similar specificity. This affinity for H2O2 results in an inactive complex of reduced GOx and glucose, i.e., the semiquinone state discussed previously. Ultimately, glucose sensor stability suffers due to elimination of key active site residues by H2O2 or enhanced susceptibility to oxidative attack of H2O2 in the presence glucose. If H2O2 concentrations reach critical levels, the glucose sensor will experience a decrease in sensor accuracy, substrate sensitivity, and half-life. However, Von Woedtke and coauthors26 report that in vivo glucose sensor operation fails to produce H2O2 levels sufficient to effect GOx. Von Woedtke and coauthors26 concede that local H2O2 concentration depends on the diffusion of glucose, oxygen, and H2O2 on the electrode surface and the configuration of the active electrode surfaces. It is plausible that H2O2 exposure, in addition to potential influence of LMWM, may lead to premature failure of in vivo glucose sensors.
In designing glucose sensors, the central challenge is maintaining consistent GOx conformation, which requires controlling the redox state of GOx during immobilization to the surface of the electrode and during in vivo operation. The differences in the oxidative states of GOx between different glucose sensors also makes it difficult to evaluate glucose sensor performance; lower stability may arise from a combination of design flaws and a higher relative concentration of reduced GOx. However, it is difficult to identify a single cause of GOx instability since, currently, there is no reliable method of measuring the percentage of oxidized/reduced GOx immobilized on the surface of an electrode.
Low Molecular Weight Materials Degradation of Glucose Oxidase
Kerner16 first discovered that LMWM of <10 kDa (e.g., ascorbate, urate, cysteine, reduced glutathione and alpha-tocopherol, tyrosine, and tryptophan) could lead to the rapid degradation of GOx and a dramatically lowered sensitivity. Pinpointing the cause of LMWM degradation of GOx is difficult due to numerous unknown events that occur in the surrounding tissue and within the sensor itself that can lead to gradual failure. For example, enzyme often accumulates at the surface of the sensor, resulting in a substantial concentration of GOx at the membrane–bulk interface.16,26,28–31 This masks the effect of oxidative degradation and prohibits deconvolution of the impact of LMWM from the effect of H2O2.16,26,28–31 As a result, the specific mechanisms of LMWM degradation of GOx continue to elude the scientific community.
Degradation of Glutaraldehyde Cross-Linked Glucose Oxidase
Glutaraldehyde cross-linking severely limits enzyme leaching24,32 in sensor applications; however, it can lead to heavily altered tertiary and secondary structures and compromised stability and activity of the enzyme. Researchers have attempted to improve GOx stability by combining GAX with alternative immobilization strategies. For example, Gouda and coauthors33 showed that the use of lysozyme as a protein-based stabilizing agent (PBSA) resulted in a significant increase in the stability of GOx compared with soluble GOx and immobilized GOx with other PBSAs (PBSA and gelatin). Likewise, Betancor and coauthors34 demonstrated, without the use of PBSAs, that, if GOx was adsorbed onto a cationic support combined with subsequent treatment with glutaraldehyde, it was 100 times more stable than soluble wild-type GOx.
A number of studies suggest that GAX can provide either a destabilizing or stabilizing environment for GOx; however, the parameters controlling GOx stabilization are largely unknown. López-Gallego and coauthors24 suggested that a key factor involves the use of supports that are preactivated35–39 versus nonactivated supports.40,41 Preactivation allows only primary amino groups of the enzyme to react with the aldehyde groups introduced by modification of the amino groups of the support.42,43 With nonactivated supports, primary amino groups as well as other amino groups on the enzyme’s surface react with the support layer, leading to greater conformational changes and lower stability.24
The deleterious effects of GAX on GOx also derive from side reactions of H2O2 with glutaraldehyde to produce epoxides. Peracchia44 demonstrated the production of epoxides from H2O2 and glutaraldehyde using nuclear magnetic resonance spectroscopy. Epoxy groups result from a reaction between H2O2 and double bonds of α-β-unsaturated aldehydes. Unsaturated aldehydes of glutaraldehyde, a product of aldol condensation, are ubiquitous in commercial glutaraldehyde and are very active in protein cross-linking.45–47 These epoxy products interact with GOx as well as with glutaraldehyde during the cross-linking step. For example, the addition of epoxy groups may enhance the activity of glutaraldehyde and result in modification of more than the primary amino group in the presence of preactivated supports. Epoxides may also react with GOx and bind not only the primary amino group, but the imino, hydroxyl, and mercapto groups as well.48 This process is similar to a technique used by Mateo and coauthors49,50 for covalent attachments to epoxy supports. Enzyme attachment to epoxy supports produce intense multipoint covalent attachment, which enhances the stability of the attached enzyme in controlled environments.34 Though the potential of multipoint covalent attachment is possible, baseline glutaraldehyde reactivity with H2O2 may not produce the controlled conditions observed in Mateo’s procedure due to a lack of preactivation of the support. Glutaraldehyde cross-linking shows great promise as a technique to extend GOx stability but requires additional studies to address these side reactions with intermediate species produced in the oxidation of glucose.
Proposed Strategies for Improving Glucose Oxidase Stability
Silica Sol-Gel Encapsulation
Innovations in sol-gel chemistry have led to development of silica hybrids in which organic and inorganic species are mixed at the molecular level.51 These hybrids range from brittle glasses to flexible gel-like materials. Several groups have encapsulated enzymes, antibodies, and other proteins within silica composites to address stability issues in the design of biosensors, biocatalysts, and bioreactors.52–57 To date, most studies on sol-gel entrapped biomolecules use TMOS (tetramethyl orthosilicate) and TEOS (tetraethyl orthosilicate) as silica precursors.58,59 Most TMOS- or TEOS-based silica gels lead to significant changes in enzyme conformation, making them unsuitable for practical applications. However, scientists have developed new sol-gel methods for functional stabilization of biomolecules entrapped in silica gels using different precursors, additives, and aging methods.
For most sensing applications, silica gels in the form of thin films must be uniform. Drying of such films may lead to severe cracking due to differences in drying rates for different pore sizes within the silica gel. Smaller pores remain wet while larger pores dry quickly, creating large internal pressure gradients. These gradients cause fractures during drying or when dry sensors encounter an aqueous environment. Strategies to overcome fracture formation include the use of chemical additives, such as formamide and Triton-X, in the sol-gel precursors.60 Cationic surfactants, such as trimethylalkylammonium chlorides, form electrostatic bonds with deprotonated silanol groups during gelation of the silica gel and prevent fractures after immersion in aqueous solutions. Minimizing these fractures limits leaching of the enzyme from the silica matrix.
Numerous industrial and medical applications use ormosils (e.g., methyltriethoxysilane, propyltrimethoxysilane, dimethyldimethoxysilane) to preserve the native activity of biomolecules. Ormosils are organically modified silanes that incorporate various functional groups (amino, glycidoxy, epoxy, hydroxyl) in alkoxide monomers resulting in modified sol-gels.61,62 The wettability of composite silica gels with ormosils can be controlled by altering the ratio of hydrophilic to hydrophobic monomers,62 thus controlling fracture formation. Wang and coauthors63 reported that the incorporation of copolymers into silica gels enhanced the activity of entrapped GOx for amperometric detection of glucose. Ormosils offer a promising option for enzyme stabilization by preventing surface fractures and stabilizing the enzyme within the silica gel.
In addition, adding polymers such as polydimethylsiloxane, polyamides, polyacrylates, and polyethylene glycol (PEG) may allow control over the inorganic condensation–polymerization process. For example, including polyethers in the sol-gel process allows control over the pore size distribution.64 Adding PEG improves resistance to cracking due to greater hydration of the films during aging, which lowers the hydration stress upon immersion in aqueous solutions.64 The addition of PEG also reduces the surface area the gel without changing the pore size.64 However, PEG may also compromise mobility and alter the conformation of enzymes entrapped in silica gel.66
Several groups have begun to include additives, such as sorbitol and N-methylglycine (osmolytes), in the sol-gel process. Osmolytes can increase the thermal stability and preserve the activity of entrapped enzymes.67 Increased pore size allows also greater diffusion of water to the surface of encapsulated enzymes and allows greater substrate accessibility.68 Enzymes maintain their intrinsic stability largely in an aqueous microenvironment with suitable diffusion to and from the active site of the enzyme.69
While silica gels have potential benefits, sol-gel formation also produces harmful organic solvent byproducts that can destabilize the encapsulated enzymes. For example, methanol and ethanol, common organic solvent byproducts, denature entrapped enzymes. This leads to decreased catalytic activity (kcat), decreased substrate specificity (KM), and increased inhibition (KI) with increased organic solvent concentration. To minimize organic intermediate formation, scientists are using new biocompatible silane precursors (including glycerated silanes68 and sodium silicate70,71) and developing aqueous processing methods to evaporate out the organic solvent or to evaporate in the silica precursor, avoiding organic synthesis all together (chemical vapor deposition).72 Besanger and coauthors58 reported that a new precursor, diglyceryl silane, extends the functional life of entrapped enzymes by liberating glycerol from diglyceryl silane. The glycerol stabilizes the enzyme through molecular crowding and impedes enzyme denaturation by restricting enzyme movement. We reported that glucose sensors fabricated using chemical vapor deposition with GOx dispersed in silica gels condensed at pH 2, 7, and 12. Glucose sensors fabricated at a condensation pH of 12 exhibited the fastest response time, the most extended linear range, greatest specificity, and longest half-life.20 Increased pore size led to increased diffusion of molecular waters and substrates, ensuring extended stability and activity of the entrapped enzymes.
Studies show that macromolecular crowding and increased hydration increase the stability of encapsulated enzymes.73 Zhou and Dill74 report that macromolecular crowding improves stability by introducing folding forces not available for proteins in solution. These forces include ionic and hydrogen bonds as well as Van der Waals and hydrophobic forces. Dipole–dipole forces from molecular waters create a cage structure that stabilizes entrapped enzymes. Zhou and Dill74 predict that these cages increase the stability of an enzyme’s native state by as much as 15 kcal/mol. As reviewed here, polymer dopants, ormosils, proper aging techniques, new silane precursors, and new sol-gel processing techniques are promising approaches for new sensing platforms in long-term in vivo applications.
Glucose Oxidase Molecular Cloning
Despite these promising techniques for improving enzyme stability in vitro, immobilized enzymes demonstrate nominal increases in stability during in vivo applications, resulting in little improvement in sensor lifetimes. However, molecularly engineered enzymes that include protein tags and/or point mutations have shown increased intrinsic molecular stability compared to wild-type homologs. Zhu and coauthors75 adapted directed evolution protocols to improve the catalytic performance, thermal resistance, and pH stability of GOx; this approach resulted in a 4 °C jump in melting temperature, increased functionality at pH range 8–11, and 1.9-fold improvement in kcat. Chen and coauthors76 modified GOx to include a poly-lysine tag on the C-terminus to anchor ferrocenecarboxylic acid mediators to the enzyme, improve enzyme stability, and increase sensitivity of glucose biosensors. This modified GOx maintained a 90% sensor response after 70 days of storage at 4 °C. Finally, Holland and coauthors77 mutated 15 residues in Aspergillus niger GOx to mirror residues found in the more catalytically active Penicillium amag GOx. The mutant GOx displayed a 4.5-fold improvement in kcat and marginal improvement in thermal stability at 50 °C (increase in half-life from 28 to 40 h).77
Another synthetic approach is to engineer recombinant versions of the enzyme at the genetic level to include components or chimeras that lead to stabilization. Engineered protein tags that may improve stability include the chitin binding domain from Pyrococcus furiousus78 and elastin-like polypeptides (ELPs).79 We attached the chitin-binding domain (CBD) to the N-terminus of a xylose isomerase from Thermotoga neapolitana and immobilized the fusion enzyme to chitin beads. Addition of the CBD led to increased isomerization of glucose to fructose, increased melting temperature, and increased half-life of the enzyme (5-fold versus soluble fusion protein and 10-fold versus the soluble wild-type). The xylose isomerase remains folded for a longer period due to the imparted rigidity of the CBD tag anchored to chitin. Similar modifications of GOx may yield enhancements in its stabilities.
The ELP tags can also enhance stability while providing a facile method for purification of enzymes. Shimazu and coauthors79 attached a [78-VPGVG] repeat ELP to the C-terminus of organophosphorus hydrolase (OPH). Incorporation of an ELP fusion sequence markedly improved OPH stability, maintaining 100% activity over 3 weeks.79 The long-term stability of aggregated OPH–ELP in storage far exceeded the stability of soluble OPH–ELP at various temperatures or the stability achieved by the addition of a stabilizing agent (PBSA). Elastin-like polypeptide aggregation drives the fusing enzyme partner out of solution due to dehydration at the surface of the ELP tag while maintaining enzyme structure and activity. In comparison with previously discussed strategies, modification of GOx’s molecular structure is a versatile and perhaps, ultimately, most promising method to improve GOx stability without affecting sensor performance. Engineered GOx may display enhanced in vitro stability that might counterbalance the damaging effects of H2O2 and LMWM in vivo while at the same time incorporate molecular components that allow enhanced protein immobilization to result in improved functional lifetimes for the enzyme and thus implanted sensor devices.
Conclusions
This review discusses GOx degradation in the presence of H2O2, LMWM, and GAX and describes several techniques to overcome these effects. These techniques include silica sol-gel encapsulation (to minimize H2O2 and GAX effects) and molecular cloning (to minimize H2O2 and LMWM effects). Previous studies have demonstrated that (1) silica encapsulation carries less risk of disrupting GOx functionality than techniques utilizing chemical modification of the GOx surface, (2) polymer dopants and ormosils improve stability by increasing the enzyme’s access to molecular waters and substrate, (3) aging of silica prevents enzyme leaching and preserves sensor stability, and (4) molecular engineering of GOx is a versatile and promising method to improve GOx stability without affecting sensor capabilities. Further studies are necessary in combining these approaches to eliminate the effects of H2O2, LMWM, and GAX. In particular, molecularly engineered GOx combined with silica-based encapsulation stands out as a promising approach for designing long-term in vivo sensor systems.
Acknowledgments
We gratefully acknowledge Dr. William (Monty) Reichert of the Duke University for aid in preparing the manuscript.
Glossary
- (CBD)
chitin-binding domain
- (ELP)
elastin-like polypeptide
- (GAX)
glutaraldehyde cross-linking
- (GOx)
glucose oxidase
- (H2 O2)
hydrogen peroxide
- (LMWM)
low molecular weight materials
- (OPH)
organophosphorus hydrolase
- (PBSA)
protein-based stabilizing agent
- (PEG)
polyethylene glycol
Funding
This work was supported by the National Science Foundation’s Research Triangle Materials Research Science and Engineering Centers (DMR-1121107) and by National Science Foundation Grant #CBET-1050176.
References
- 1.Armour JC, Lucisano JY, McKean BD, Gough DA. Application of chronic intravascular blood glucose sensor in dogs. Diabetes. 1990;39(12):1519–1526. doi: 10.2337/diab.39.12.1519. [DOI] [PubMed] [Google Scholar]
- 2.Gough DA. Issues related to in vitro operation of potentially implantable enzyme electrode glucose sensors. Horm Metab Res Suppl. 1988;20:30–33. [PubMed] [Google Scholar]
- 3.Gough DA, Bremer T. Immobilized glucose oxidase in implantable glucose sensor technology. Diabetes Technol Ther. 2000;2(3):377–380. doi: 10.1089/15209150050194242. [DOI] [PubMed] [Google Scholar]
- 4.Gough DA, Kumosa LS, Routh TL, Lin JT, Lucisano JY. Function of an implanted tissue glucose sensor for more than 1 year in animals. Sci Transl Med. 2010;2(42):42ra53. doi: 10.1126/scitranslmed.3001148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jablecki M, Gough DA. Simulations of the frequency response of implantable glucose sensors. Anal Chem. 2000;72(8):1853–1859. doi: 10.1021/ac991018z. [DOI] [PubMed] [Google Scholar]
- 6.Leypoldt JK, Gough DA. Diffusion and the limiting substrate in two-substrate immobilized enzyme systems. Biotechnol Bioeng. 1982;24(12):2705–2719. doi: 10.1002/bit.260241208. [DOI] [PubMed] [Google Scholar]
- 7.Tse PH, Gough DA. Time-dependent inactivation of immobilized glucose oxidase and catalase. Biotechnol Bioeng. 1987;29(6):705–713. doi: 10.1002/bit.260290607. [DOI] [PubMed] [Google Scholar]
- 8.Tse PH, Leypoldt JK, Gough DA. Determination of the intrinsic kinetic constants of immobilized glucose oxidase and catalase. Biotechnol Bioeng. 1987;29(6):696–704. doi: 10.1002/bit.260290606. [DOI] [PubMed] [Google Scholar]
- 9.Wisniewski N, Moussy F, Reichert WM. Characterization of implantable biosensor membrane biofouling. Fresenius J Anal Chem. 2000;366(6-7):611–621. doi: 10.1007/s002160051556. [DOI] [PubMed] [Google Scholar]
- 10.Cho YK, Bailey JE. Enzyme immobilization on activated carbon: alleviation of enzyme deactivation by hydrogen peroxide. Biotechnol Bioeng. 1977;19(5):769–775. doi: 10.1002/bit.260190514. [DOI] [PubMed] [Google Scholar]
- 11.Greenfield PF, Kittrell JR, Laurence RL. Inactivation of immobilized glucose oxidase by hydrogen peroxide. Anal Biochem. 1975;65(1-2):109–124. doi: 10.1016/0003-2697(75)90497-2. [DOI] [PubMed] [Google Scholar]
- 12.Kleppe K. The effect of hydrogen peroxide on glucose oxidase from Aspergillus niger. Biochemistry. 1966;5(1):139–143. doi: 10.1021/bi00865a018. [DOI] [PubMed] [Google Scholar]
- 13.Krishnaswany S, Kittrell JR. Deactivation studies of immobilized glucose oxidase. Biotechnol Bioeng. 1978;20:821–835. [Google Scholar]
- 14.Malikkides CO, Weiland RH. On the mechanism of immobilized glucose oxidase deactivation by hydrogen peroxide. Biotechnol Bioeng. 1982;24(11):2419–2439. doi: 10.1002/bit.260241109. [DOI] [PubMed] [Google Scholar]
- 15.Towe BC, Guilbeau EJ, Coburn JB. In vivo and in vitro deactivation rates of PTFE-coupled glucose oxidase. Biosens Bioelectron. 1996;11(8):791–798. doi: 10.1016/0956-5663(96)85930-6. [DOI] [PubMed] [Google Scholar]
- 16.Kerner W, Kiwit M, Linke B, Keck FS, Zier H, Pfeiffer EF. The function of a hydrogen peroxide-detecting electroenzymatic glucose electrode is markedly impaired in human sub-cutaneous tissue and plasma. Biosens Bioelectron. 1993;8(9–10):473–482. doi: 10.1016/0956-5663(93)80032-k. [DOI] [PubMed] [Google Scholar]
- 17.Chang G, Tatsu Y, Goto T, Imaishi H, Morigaki K. Glucose concentration determination based on silica sol-gel encapsulated glucose oxidase optical biosensor arrays. Talanta. 2010;83(1):61–65. doi: 10.1016/j.talanta.2010.08.039. [DOI] [PubMed] [Google Scholar]
- 18.Jia WZ, Wang K, Zhu ZJ, Song HT, Xia XH. One-step immobilization of glucose oxidase in a silica matrix on a Pt electrode by an electrochemically induced sol-gel process. Langmuir. 2007;23(23):11896–11900. doi: 10.1021/la7020269. [DOI] [PubMed] [Google Scholar]
- 19.Kojuharova A, Popova Y, Kirova N, Klissurski D, Simeonov D, Spasov L. Characterization and application of glucose oxidase immobilized in silica hydrogel. J Chem Technol Biotechnol. 1988;42(2):95–104. [Google Scholar]
- 20.Harris JM, Lopez GP, Reichert WM. Silica-dispersed glucose oxidase for glucose sensing: In vitro testing in serum and blood and the effect of condensation pH. Sens Actuators B Chem. 2012;174:373–379. doi: 10.1016/j.snb.2012.08.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wu B, Zhang G, Shuang S, Choi MM. Biosensors for determination of glucose with glucose oxidase immobilized on an eggshell membrane. Talanta. 2004;64(2):546–553. doi: 10.1016/j.talanta.2004.03.050. [DOI] [PubMed] [Google Scholar]
- 22.Wang Y, Joshi PP, Hobbs KL, Johnson MB, Schmidtke DW. Nanostructured biosensors built by layer-by-layer electrostatic assembly of enzyme-coated single-walled carbon nanotubes and redox polymers. Langmuir. 2006;22(23):9776–9783. doi: 10.1021/la060857v. [DOI] [PubMed] [Google Scholar]
- 23.Wu BY, Hou SH, Yin F, Zhao ZX, Wang YY, Wang XS, Chen Q. Amperometric glucose biosensor based on multilayer films via layer-by-layer self-assembly of multi-wall carbon nanotubes, gold nanoparticles and glucose oxidase on the Pt electrode. Biosens Bioelectron. 2007;22(12):2854–2860. doi: 10.1016/j.bios.2006.11.028. [DOI] [PubMed] [Google Scholar]
- 24.López-Gallego F, Betancor L, Mateo C, Hidalgo A, Alonso-Morales N, Dellamora-Ortiz G, Guisán JM, Fernández-Lafuente R. Enzyme stabilization by glutaraldehyde crosslinking of adsorbed proteins on aminated supports. J Biotechnol. 2005;119(1):70–75. doi: 10.1016/j.jbiotec.2005.05.021. [DOI] [PubMed] [Google Scholar]
- 25.Bao J, Furumoto K, Yoshimoto M, Fukunaga K, Nakao K. Competitive inhibition by hydrogen peroxide produced in glucose oxidation catalyzed by glucose oxidase. Biochem Eng J. 2003;13:69–72. [Google Scholar]
- 26.Von Woedtke T, Fischer U, Abel P. Glucose oxidase electrodes: effect of hydrogen peroxide on enzyme activity? Biosens Bioelectron. 1994;9(1):65–71. doi: 10.1016/0956-5663(94)80016-2. [DOI] [PubMed] [Google Scholar]
- 27.Langemann H, Kabiersch A, Newcombe J. Measurement of low-molecular-weight antioxidants, uric acid, tyrosine and tryptophan in plaques and white matter from patients with multiple sclerosis. Eur Neurol. 1992;32(5):248–252. doi: 10.1159/000116835. [DOI] [PubMed] [Google Scholar]
- 28.Bartlett PN, Whitaker RG. Electrochemical immobilization of enzymes. Part I: theory. J Electroanal Chem. 1987;224:27–35. [Google Scholar]
- 29.Carter RS, Prenosil JE, Bourne JR. Stability studies on the immobilized GOD/catalase enzyme system. Enzyme Eng. 1980;5:321–324. [Google Scholar]
- 30.Reach G. Artificial or bioartificial systems for the totally automatic treatment of diabetes mellitus: the gap between the dream and the reality. Diab Nutr Metab. 1989;2:165–170. [Google Scholar]
- 31.Rebrin K, Fischer U, Hahn von Dorsche H, von Woetke T, Abel P, Brunstein E. Subcutaneous glucose monitoring by means of electrochemical sensors: fiction or reality? J Biomed Eng. 1992;14(1):33–40. doi: 10.1016/0141-5425(92)90033-h. [DOI] [PubMed] [Google Scholar]
- 32.Sun Y, Yan F, Yang W, Sun C. Multilayered construction of glucose oxidase and silica nanoparticles on Au electrodes based on layer-by-layer covalent attachment. Biomaterials. 2006;27(21):4042–4049. doi: 10.1016/j.biomaterials.2006.03.014. [DOI] [PubMed] [Google Scholar]
- 33.Gouda MD, Thakur MS, Karanth NG. Stability studies on immobilized glucose oxidase using an amperometric biosensor – effect of protein based stabilizing agents. Electroanal. 2001;13:849–855. [Google Scholar]
- 34.Betancor L, López-Gallego F, Hidalgo A, Alonso-Morales N, Dellamora-Ortiz G, Guisán JM, Fernández-Lafuente R. Preparation of a very stable immobilized biocatalyst of glucose oxidase from Aspergillus niger. J Biotechnol. 2006;121(2):284–289. doi: 10.1016/j.jbiotec.2005.07.014. [DOI] [PubMed] [Google Scholar]
- 35.Burteau N, Burton S, Crichton RR. Stabilisation and immobilisation of penicillin amidase. FEBS Lett. 1989;258(2):185–189. doi: 10.1016/0014-5793(89)81649-7. [DOI] [PubMed] [Google Scholar]
- 36.Dos Reis-Costa L, Soares AM, França SC, Trevisan HC, Roberts TJ. Immobilization of lipases and assay in continuous fixed bed reactor. Protein Pept Lett. 2003;10(6):619–628. doi: 10.2174/0929866033478573. [DOI] [PubMed] [Google Scholar]
- 37.Magnan E, Catarino I, Paolucci-Jeanjean D, Preziozi-Belloy L, Belleville MP. Immobilization of lipase on a ceramic membrane: activity and stability. J Membr Sci. 2004;241:161–166. [Google Scholar]
- 38.Van Aken B, Ledent P, Naveau H, Agathos SN. Co-immobilization of manganese peroxidase from Phlebia radiata and glucose oxidase from Aspergillus niger on porous silica beads. Biotechnol Lett. 2000;22:641–646. [Google Scholar]
- 39.Quinn ZK, Zhou Xiao DC. Immobilization of β-galactosidase on graphite surface by glutaraldehyde. J Food Eng. 2001;48:69–74. [Google Scholar]
- 40.D’Souza SF, Kubal BS. A cloth strip bioreactor with immobilized glucoamylase. J Biochem Biophys Methods. 2002;51(2):151–159. doi: 10.1016/s0165-022x(02)00008-8. [DOI] [PubMed] [Google Scholar]
- 41.Hwang S, Lee KT, Park JW, Min BR, Haam S, Ahn IS, Jung JK. Stability analysis of Bacillus stearothermophilus L1 lipase immobilized on surface-modified silica gels. Biochem Eng J. 2004;17(85):85–90. [Google Scholar]
- 42.Guisán JM, Penzol G, Armisen P, Bastida A, Blanco RM, Fernandez-Lafuente R, García-Junceda E. Immobilization of enzymes acting on macromolecular substrates: reduction of steric problems. In: Bickerstaff G, editor. Immobilization of enzymes and cells. Totowa: Humana Press; 1997. pp. 261–275. [Google Scholar]
- 43.Monsan P. Optimization of glutaraldehyde activation of a support for enzyme immobilization. J Mol Catal. 1978;3:371–384. [Google Scholar]
- 44.Peracchia C. A system of parallel septa in crayfish nerve fibers. J Cell Biol. 1970;44(1):125–133. doi: 10.1083/jcb.44.1.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bowes JH, Cater CW. The interaction of aldehydes with collagen. Biochim Biophys Acta. 1968;168(2):341–352. doi: 10.1016/0005-2795(68)90156-6. [DOI] [PubMed] [Google Scholar]
- 46.Richards FM, Knowles JR. Glutaraldehyde as a protein cross-linkage reagent. J Mol Biol. 1968;37(1):231–233. doi: 10.1016/0022-2836(68)90086-7. [DOI] [PubMed] [Google Scholar]
- 47.Robertson EA, Schultz RL. The impurities in commercial glutaraldehyde and their effect on the fixation of brain. J Ultrastruct Res. 1970;30(3):275–287. doi: 10.1016/s0022-5320(70)80063-6. [DOI] [PubMed] [Google Scholar]
- 48.March J. Advanced organic chemistry: reactions, mechanisms, and structure. New York: McGraw-Hill; 1968. [Google Scholar]
- 49.Mateo C, Abian O, Fernández-Lorente G, Pedroche J, Fernández-Lafuente R, Guisan JM, Tam A, Daminati M. Epoxy sepabeads: a novel epoxy support for stabilization of industrial enzymes via very intense multipoint covalent attachment. Biotechnol Prog. 2002;18(3):629–634. doi: 10.1021/bp010171n. [DOI] [PubMed] [Google Scholar]
- 50.Mateo C, Fernández-Lorente G, Abian O, Fernández-Lafuente R, Guisán JM. Multifunctional epoxy supports: a new tool to improve the covalent immobilization of proteins. The promotion of physical adsorptions of proteins on the supports before their covalent linkage. Biomacromolecules. 2000;1(4):739–745. doi: 10.1021/bm000071q. [DOI] [PubMed] [Google Scholar]
- 51.Sanchez C, Ribot F. Design of hybrid organic-inorganic materials synthesized via sol-gel chemistry. New J Chem. 1994;18(10):1007–1047. [Google Scholar]
- 52.Avnir D, Braun S, Lev O, Ottolenghi M. Enzymes and other proteins entrapped in sol-gel materials. Chem Mater. 1994;6(10):1605–1614. [Google Scholar]
- 53.Lin J, Brown CW. Sol-gel glass as a matrix for chemical and biochemical sensing. Trends Anal Chem. 1997;16(4):200–211. [Google Scholar]
- 54.Gill I, Ballesteros A. Bioencapsulation within synthetic polymers (part 1): sol-gel encapsulated biologicals. Trends Biotechnol. 2000;18(7):282–296. doi: 10.1016/s0167-7799(00)01457-8. [DOI] [PubMed] [Google Scholar]
- 55.Livage J, Coradin T, Roux C. Encapsulation of biomolecules in silica gels. J Phys Condens Matter. 2001;13:R673–R691. [Google Scholar]
- 56.Jin W, Brennan JD. Properties and applications of proteins encapsulated within sol-gel derived materials. Anal Chim Acta. 2002;461:1–36. [Google Scholar]
- 57.Avnir D, Coradin T, Lev O, Livage J. Recent bio-applications of sol-gel materials. J Mater Chem. 2006;16(11):1013–1030. [Google Scholar]
- 58.Besanger TR, Chen Y, Deisingh AK, Hodgson R, Jin W, Mayer S, Brook MA, Brennan JD. Screening of inhibitors using enzymes entrapped in sol-gel-derived materials. Anal Chem. 2003;75(10):2382–2391. doi: 10.1021/ac026370i. [DOI] [PubMed] [Google Scholar]
- 59.Flora KK, Brennan JD. Effect of matrix aging on the behavior of human serum albumin entrapped in a tetraethyl orthosilicate-derived glass. Chem Mater. 2001;13:4170–4179. [Google Scholar]
- 60.Hench LL, Ulrich DR. Science of ceramic chemical processing. New York: John Wiley and Sons; 1986. p. 52. [Google Scholar]
- 61.Wen J, Wilkes GL. Organic/inorganic hybrid network materials by the sol-gel approach. Chem Mater. 1996;8(8):1667–1681. [Google Scholar]
- 62.Tripathi VS, Kandimalla VB, Ju H. Preparation of ormosil and its applications in the immobilizing biomolecules. Sens Actuators B Chem. 2006;114:1071–1082. [Google Scholar]
- 63.Wang C, Yu B, Knudsen B, Harmon J, Moussy F, Moussy Y. Synthesis and performance of novel hydrogels coatings for implantable glucose sensors. Biomacromolecules. 2008;9(2):561–567. doi: 10.1021/bm701102y. [DOI] [PubMed] [Google Scholar]
- 64.Baker GA, Jordan JD, Bright FV. Effects of poly(ethylene glycol) doping on the behavior of pyrene, rhodamine 6G, and acrylodan-labeled bovine serum albumin sequestered within tetramethylorthosilane-derived sol-gel-processed composites. J Sol-Gel Sci Technol. 1998;11(1):43–54. [Google Scholar]
- 65.Goring GL, Brennan JD. Fluorescence and physical characterization of sol-gel-derived nanocomposite films suitable for the entrapment of biomolecules. J Mater Chem. 2002;12:3400–3406. [Google Scholar]
- 66.Reinhart GD. Influence of polyethylene glycols on the kinetics of rat liver phosphofructokinase. J Biol Chem. 1980;255(22):10576–10578. [PubMed] [Google Scholar]
- 67.Sepasi Tehrani H, Moosavi-Movahedi AA, Ghourchian H, Ahmad F, Kiany A, Atri MS, Ariaeenejad S, Kavousi K, Saboury AA. Effect of compatible and noncompatible osmolytes on the enzymatic activity and thermal stability of bovine liver catalase. J Biomol Struct Dyn. 2012 doi: 10.1080/07391102.2012.742460. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 68.Brennan JD, Benjamin D, DiBattista E, Gulcev MD. Using sugar and amino acid additives to stabilize enzymes within sol-gel derived silica. Chem Mater. 2003;15:737–745. [Google Scholar]
- 69.Iyer PV, Ananthanarayan L. Enzyme stability and stabilization – aqueous and non-aqueous environment. Process Biochem. 2008;43:1019–1032. [Google Scholar]
- 70.Gill I, Ballesteros A. Encapsulation of biologicals within silicate, siloxane, and hybrid sol-gel polymers: an efficient and generic approach. J Am Chem Soc. 1998;120(34):8587–8598. [Google Scholar]
- 71.Li L, Jiang ZY, Wu H, Feng YN, Li J. Protamine-induced biosilica as efficient enzyme immobilization carrier with high loading and improved stability. Mater Sci Eng C Mater Biol Appl. 2009;29:2029–2035. [Google Scholar]
- 72.Gupta G, Rathod SB, Staggs KW, Ista LK, Abbou Oucherif K, Atanassov PB, Tartis MS, Montaño GA, López GP. CVD for the facile synthesis of hybrid nanobiomaterials integrating functional supramolecular assemblies. Langmuir. 2009;25(23):13322–13327. doi: 10.1021/la903475d. [DOI] [PubMed] [Google Scholar]
- 73.Eggers DK, Valentine JS. Molecular confinement influences protein structure and enhances thermal protein stability. Protein Sci. 2001;10(2):250–261. doi: 10.1110/ps.36201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zhou HX, Dill KA. Stabilization of proteins in confined spaces. Biochemistry. 2001;40(38):11289–11293. doi: 10.1021/bi0155504. [DOI] [PubMed] [Google Scholar]
- 75.Zhu Z, Wang M, Gautam A, Nazor J, Momeu C, Prodanovic R, Schwaneberg U. Directed evolution of glucose oxidase from Aspergillus niger for ferrocenemethanol-mediated electron transfer. Biotechnol J. 2007;2(2):241–248. doi: 10.1002/biot.200600185. [DOI] [PubMed] [Google Scholar]
- 76.Chen LQ, Zhang XE, Xie WH, Zhou YF, Zhang ZP, Cass AE. Genetic modification of glucose oxidase for improving performance of an amperometric glucose biosensor. Biosens Bioelectron. 2002;17(10):851–857. doi: 10.1016/s0956-5663(02)00051-9. [DOI] [PubMed] [Google Scholar]
- 77.Holland JT, Harper JC, Dolan PL, Manginell MM, Arango DC, Rawlings JA, Apblett CA, Brozik SM. Rational redesign of glucose oxidase for improved catalytic function and stability. PLoS One. 2012;7(6):e37924. doi: 10.1371/journal.pone.0037924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Harris JM, Epting KL, Kelly RM. N-terminal fusion of a hyperthermophilic chitin-binding domain to xylose isomerase from Thermotoga neapolitana enhances kinetics and thermostability of both free and immobilized enzymes. Biotechnol Prog. 2010;26(4):993–1000. doi: 10.1002/btpr.416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Shimazu M, Mulchandani A, Chen W. Thermally triggered purification and immobilization of elastin-OPH fusions. Biotechnol Bioeng. 2003;81(1):74–79. doi: 10.1002/bit.10446. [DOI] [PubMed] [Google Scholar]