Abstract
Despite advancements in the development of the artificial pancreas, barriers in the form of proprietary data and communication protocols of diabetes devices have made the integration of these components challenging. The Artificial Pancreas Standards and Technical Platform Project is an initiative funded by the JDRF Canadian Clinical Trial Network with the goal of developing device communication standards for the interoperability of diabetes devices. Stakeholders from academia, industry, regulatory agencies, and medical and patient communities have been engaged in advancing this effort. In this article, we describe this initiative along with the process involved in working with the standards organizations and stakeholders that are key to ensuring effective standards are developed and adopted. Discussion from a special session of the 12th Annual Diabetes Technology Meeting is also provided.
Keywords: artificial pancreas, continuous glucose monitoring, diabetes, insulin pump, interoperability, standards
The lack of open standards for communication between insulin pumps, blood glucose meters (BGMs), and continuous glucose monitors (CGMs) has posed considerable challenges to artificial pancreas (AP) research. Though advancements have brought the development of the AP closer to reality, the complexities involved in integrating these varied components into a working closed-loop system presents a significant barrier to research and commercialization. This landscape of closed proprietary systems also limits the potential for innovation from new players in the industry that may be able to speed progress of the AP and new diabetes management tools.
With the aim of addressing these barriers to research, the JDRF has funded the Artificial Pancreas Standards and Technical Platform Project as part of their Canadian Clinical Trial Network initiative. This project will be led by the research and development team at the Centre for Global eHealth Innovation, based at Toronto General Hospital and associated with the University of Toronto, who have a breadth of device integration and diabetes technology experience, specifically relating to their work in developing applications for remote patient monitoring and self-care management of chronic illness.1–3
Given the nature of this work, the success of this project is largely dependent on bringing together key stakeholders whose input is vital in defining the requisite functionality and use cases that need to be developed into device communication standards that will be accepted and adopted by the diabetes device industry. This stakeholder group includes researchers, device manufacturers, regulatory organizations, clinicians, and, of course, people with type 1 diabetes and their families.
In light of this focus on stakeholder engagement, a special session was convened on November 8, 2012, at the 12th Annual Diabetes Technology Meeting in Bethesda, MD. Attendees had the opportunity to learn about the project as well as participate in a discussion that would help provide direction to the work. This article provides background on the project as it was presented as well as highlights the key themes that resulted from the discussion with participants.
Defining the Gap
The need for developing these standards was voiced by many participants at the meeting. Notably, some suggested that it is an endeavor that ought to have been addressed long ago. The need for device communications standards as it relates to AP technology development has been stated previously by members of the research community: “The main issue is that communications with insulin pumps, continuous glucose sensors, and glucometers are not standardized at all and the integration of any new device into the [AP system] will require new developments and the collaboration of manufactures to replicate the communication protocols.”4 Lowering these barriers to technology integration is at the core of this initiative. As diabetes technology has reached a level of maturity where there is a broad base of functionality that is common to devices from diverse manufacturers, the landscape is ripe for establishing interoperability standards.
Medical Device Communication Standards
In the realm of health care technology, the term standard may take on different meanings depending on the context within which it is used. Performance standards would define the allowable ranges of accuracy, whereas safety standards would define the required risk mitigation measures implemented to ensure a medical device poses minimal risk to the patient. Guidance on these types of standards has been discussed previously with their relation to the AP.5
What distinguishes this work from other standard initiatives is its intent to define device communication standards. Fortunately, the issue of device interoperability in AP development is not unique. There are existing initiatives in progress to deal with medical device interoperability in general. One such device communication standard development is IEEE-11073,6,7 which defines its purpose as follows: “[To] enable communication between medical devices and external computer systems.”8 A subset of these standards was developed specifically for personal health devices, identified as IEEE-11073 Personal Health Data (PHD). The IEEE-11073 PHD set of standards addresses “a need for an openly defined, independent standard for converting the collected information into an interoperable transmission format so the information can be exchanged between [personal health devices and manager applications].”8
In order to build effective standards for medical devices, stakeholders must have a suitable forum to discuss, develop, and ratify. The IEEE-11073 PHD working group was established for the purpose of building a standard that would accommodate the resource requirements of personal health devices.9 This group currently consists of 321 members from 201 organizations. Membership is open to all for contribution, with group meetings held weekly by teleconference and face-to-face every 2–3 months. The Working Group has representation from industry, academia, and health care providers. This comprehensive group of contributors provides vital perspectives on requirements and facilitates future adoption of the standard.
Currently, device specializations are under development for insulin pumps and CGMs (see Table 1). A specialization for a glucagon or bihormonal pump would follow the completion of the standards under development and would likely inherit data objects and approaches developed for the insulin pump standard. The device specialization for the BGM has already been approved, though it continues to be reviewed for future revisions.10 It is a primary goal of this initiative to bring the necessary contributors to the table and move these device specializations to completion. If broad participation can be realized, these efforts should result in effective standards that will be adopted by the device manufacturers to facilitate AP research and development.
Table 1.
IEEE-11073 PHD Standards for Personal Health Devices
| AP-related standards | ||
| Device | Standard number | Stage of development |
| Glucose meter | IEEE-11073-10417 | Approved |
| Insulin pump | IEEE-11073-10419 | Under development |
| CGM | IEEE-11073-10425 | Under development |
| Examples of other device standards | ||
| Pulse oximeter | IEEE-11073-10404 | Approved |
| Weighing scale | IEEE-11073-10415 | Approved |
| Basic electrocardiogram | IEEE-11073-10406 | Under development |
The Bluetooth Special Interest Group (SIG) is another important player in the development of device communication standards. The Medical Devices Working Group of the Bluetooth SIG is currently working on wireless communication standards for the insulin pump and the CGM and, like the IEEE-11073 PHD group, has a completed profile defined for the BGM.
Collaboration between the Bluetooth SIG and the IEEE-11073 PHD group is ongoing so as to ensure compatible standards are developed. This compatibility is achieved by modeling data objects so that they can be directly translated from the Bluetooth SIG protocol to the IEEE-11073 PHD standards; this will allow for integration with a broader network of health systems, including electronic health records.11
Compatibility among these standards is at the heart of the efforts of the Continua Health Alliance. Continua is a nonprofit organization of health care and technology companies that has as its goal the establishment of a system of interoperable health solutions. Continua is not a standards organization itself; instead, it works with bodies such as the Institute of Electrical and Electronics Engineers (IEEE) and Bluetooth SIG to develop interoperable standards. Furthermore, they foster collaboration, not only between the member companies, but also with government regulatory agencies, to further the goal of developing safe and effective solutions throughout the health care system.
Given these goals, the project team is working closely with Continua in order to inform and promote this collaborative effort. The IEEE-11073 standard for BGMs has been adopted as part of Continua’s design guidelines, and to date, two devices have been certified by Continua: the Roche Accu-Chek® Mobile in 2011 and the Roche Accu-Chek Smart Pix Device Reader in 2011. In bringing standards development to the forefront in the diabetes community, this initiative endeavors to engage more companies from the diabetes device industry to ensure future adoption of the standards. As part of this initiative, JDRF has signed on as a Promoter Member of the Continua Health Alliance, providing the organization with voting rights on the adoption of guidelines for manufacturers to produce interoperable systems.
Discussion
As presented by Eyal Dassau at the meeting, researchers from the University of California, Santa Barbara, and Sansum Diabetes Research Institute have made significant strides in advancing interoperability by integrating three different insulin pumps and three different CGMs with their Artificial Pancreas System.12 Nevertheless, this work and others like it have been slowed by the need to develop software that will take into account multiple vendor-specific protocols.
Lane Desborough of Medtronic Diabetes (Northridge, CA) presented a review of his experience with standards development in the petrochemical process control and automation industry. In particular, he highlighted how that industry evolved from one of closed, proprietary, single-vendor ecosystems to one of open, standards-based, multivendor ecosystems. These experiences provide insight into both the benefits of a standards-based system of devices and the challenges that are faced in moving toward this goal. Among the key benefits discussed were systems with greater flexibility given greater permutations of device integrations, lowered costs, and a shift toward higher-valued activities.
In the case of AP technology, this idea of a shift toward higher-valued activities is one that should be realized by all parties. Manufacturers would pool their resources to develop effective standards and would thereby be able to shift more of their development efforts to other unmet customer needs. The AP researchers would also be able to focus their efforts on their core competency—development of the critical control algorithms—rather than expend effort on integrating a host of devices using proprietary communication protocols. In the absence of standards, any programming required in developing the AP technology to issue commands, receive data trans-missions, and format received data into data objects that can be processed by the control algorithms must be redone so as to conform to the data communication protocol of each new device introduced to the system (i.e., swapping in a new CGM from a different manufacturer). Standardizing the data objects also lends to more feasible transmission to a health information system, which would facilitate AP telemonitoring and allow for further review and analysis for both clinical and research purposes (see Figure 1).
Figure 1.
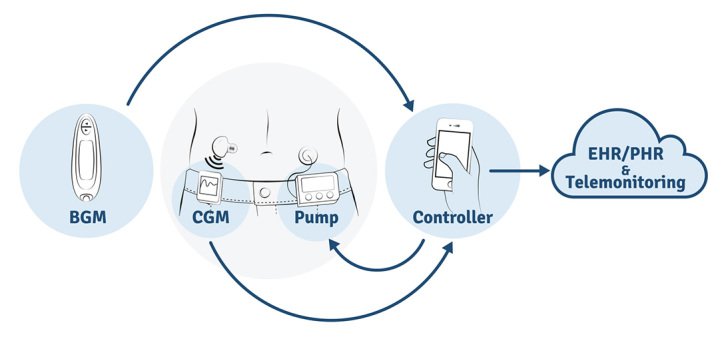
An AP system comprises a BGM, CGM, insulin pump, and controller. The blood glucose measurement from the BGM is used periodically for CGM calibration to provide the most accurate estimates. The AP controller receives continuous glucose measurements from the CGM and performs a series of mathematical calculations. Based on these calculations, the controller communicates delivery instructions to the insulin pump. Communication standards for the CGM and insulin pump will allow consistent device communication with the controller, and device information may be extended to wider interoperability platforms for the purposes of telemonitoring and review on electronic health record/personal health record systems. EHR, electronic health record; PHR, personal health record.
The petrochemical industry experience also brings to light the challenges associated with developing interoperable standards. Notably, the notion of “co-opetion” is raised, where companies simultaneously compete with and complement each other.13 Though competition may make the process difficult, the value achieved through the interaction is greater than that achieved without the interaction. The impact that the Digital Imaging and Communications in Medicine (DICOM) standard and its related technologies have had on medical imaging is one success story that demonstrates this value added. The ability for multiple clinicians to review the same images from different locations speeds decision making and improves patient care.14
The discussion did elicit some concern regarding the complexity of the individual manufacturers’ devices, making it difficult to come to an agreement on a standard that covers the operation of many devices. This legitimate concern can be mitigated with the approach of the standards development. In developing a standard for IEEE-11073, the focus is to develop a framework for the data communication along with a model for the key data objects that are integral to the device in question. Use of the standard is nevertheless made flexible in that it would allow vendor-specific data objects as long as they are implemented using the overarching communication protocol framework (IEEE-11073-20601). The standards are also flexible in that many data objects are made available as an option in an extended configuration. For instance, in BGM specialization, the only mandatory object a vendor must support is that for blood glucose measurement; others—including data objects for carbohydrate counting and medications used—are made available in an extended configuration.
The need for interoperability standards extends beyond the immediate needs of AP research and development. Physician participants see standards-based devices as contributing to the efficiency of their own clinical work. It was noted that the lack of standards has led to a workspace cluttered with a litany of diverse cable connectors, with each device needing its own custom software to enable data download and review. Some industry representatives also noted the benefit of the standards approach given the diversity of communication protocol solutions that exist even within a single manufacturer’s line of devices.
As well, these issues inhibit patients from access to their own data. Amy Tenderich, founder and editor of the DiabetesMine patient advocacy website, expressed her enthusiastic support for the initiative.15 This push for open standards was a driving force behind the DiabetesMine Innovation Summit at Stanford University, and the initiative continues to draw support from the diabetes community.16 Such demand voiced by consumers has long been seen as an important catalyst toward standardization,17 and as such, their vocal support plays a vital role in moving this initiative forward.
Stayce Beck of the Food and Drug Administration noted the organization’s support for the initiative as they see it as an important first step toward device interoperability, which will help to foster innovation. It is notable as well that a key message that came out of the AAMI-FDA Interoperability Summit in October 2012 was that achieving interoperability “protects patients, contributes to clinical decisions and positive patient outcomes, and improves efficiency.”18
Conclusion
This session provided the project team an excellent opportunity to interact with a diverse group of stakeholders and brought a number of new collaborators to the table who will contribute to the initiative. Starting in January 2013, the project team has convened regular meetings with collaborating researchers and industry representatives to discuss the direction of the standards development, focusing on the IEEE-11073 PHD standards for the insulin pump and CGM. Through this ongoing collaboration, the barriers to the interoperability of diabetes devices will eventually be eliminated, fostering greater innovation in the development of the AP and other diabetes management tools.
Glossary
- (AP)
artificial pancreas
- (BGM)
blood glucose meter
- (CGM)
continuous glucose monitor
- (PHD)
personal health data
- (SIG)
special interest group
Funding
This work is funded by the JDRF Canadian Clinical Trial Network.
References
- 1.Cafazzo JA, Casselman M, Hamming N, Katzman DK, Palmert MR. Design of an mHealth app for the self-management of adolescent type 1 diabetes: a pilot study. J Med Internet Res. 2012;14(3):e70. doi: 10.2196/jmir.2058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Logan AG, Irvine MJ, McIsaac WJ, Tisler A, Rossos PG, Easty A, Feig DS, Cafazzo JA. Effect of home blood pressure telemonitoring with self-care support on uncontrolled systolic hypertension in diabetics. Hypertension. 2012;60(1):51–57. doi: 10.1161/HYPERTENSIONAHA.111.188409. [DOI] [PubMed] [Google Scholar]
- 3.Seto E, Leonard KJ, Cafazzo JA, Barnsley J, Masino C, Ross HJ. Mobile phone-based telemonitoring for heart failure management: a randomized controlled trial. J Med Internet Res. 2012;14(1):e31. doi: 10.2196/jmir.1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gómez EJ, Hernando Pérez ME, Vering T, Rigla Cros M, Bott O, García-Sáez G, Pretschner P, Brugués E, Schnell O, Patte C, Bergmann J, Dudde R, de Leiva A. The INCA system: a further step towards a telemedical artificial pancreas. IEEE Trans Inf Technol Biomed. 2008;12(4):470–479. doi: 10.1109/TITB.2007.902162. [DOI] [PubMed] [Google Scholar]
- 5.Klonoff DC. Designing an artificial pancreas system to be compatible with other medical devices. J Diabetes Sci Technol. 2008;2(5):741–745. doi: 10.1177/193229680800200501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.ISO/IEEE 11073-10101-2004 Health informatics. Point-of-care medical device communication. Part 10101: nomenclature.
- 7.ISO/IEEE 11073-10201-2004. Health informatics. Point-of-care medical device communication. Part 10201: domain information model. 2004.
- 8.ISO/IEEE 11073-20601-2010. Health informatics. Personal health device communication. Part 20601: application profile - optimized exchange protocol.
- 9.Clarke M, Bogia D, Hassing K, Steubesand L, Chan T, Ayyagari D. Developing a standard for personal health devices based on 11073. Conf Proc IEEE Eng Med Biol Soc. 2007;2007:6175–6177. doi: 10.1109/IEMBS.2007.4353764. [DOI] [PubMed] [Google Scholar]
- 10.ISO/IEEE 11073-10417-2009. Health informatics. Personal health device communication. Part 10417: device specialization - glucose meter.
- 11.González Gómez R, Hughes RD, Bogia D, Shingala K, Kermes L, Lima M, Herbster R, Pfützenreuter E, Ott L, Barr JR, Schatz G, Strickland R, Bluetooth Special Interest Group Personal health devices transcoding white paper. 2012. https://www.bluetooth.org/docman/handlers/downloaddoc.ashx?doc_id=249499.
- 12.Dassau E, Zisser HC, Palerm CA , Buckingham B, Jovanovic L, J Doyle F., 3rd Modular artificial beta-cell system: a prototype for clinical research. J Diabetes Sci Technol. 2008;2(5):863–872. doi: 10.1177/193229680800200518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brandenburger A, Nalebuff B. Co-opetition. New York: Currency Doubledaay; 1996. [Google Scholar]
- 14.Thrall JH. Reinventing radiology in the digital age: part I. The all-digital department. Radiology. 2005;236(2):382–385. doi: 10.1148/radiol.2362050257. [DOI] [PubMed] [Google Scholar]
- 15.Tenderich A. The Diabetes Technology Society—embracing standards at last? DiabetesMine. http://www.diabetesmine.com/2012/11/the-diabetes-technology-society-embracing-standards-at-last.html.
- 16.Hoskins M. Sailing toward standards for diabetes device interoperability (wind needed) DiabetesMine. http://www.diabetesmine.com/2013/01/sailing-toward-standards-for-diabetes-device-interoperability-wind-needed.html.
- 17.Grove AS. Efficiency in the health care industries: a view from the outside. JAMA. 2005;294(4):490–492. doi: 10.1001/jama.294.4.490. [DOI] [PubMed] [Google Scholar]
- 18.Association for the Advancement of Medical Instrumentation; U.S. Food and Drug Administration. Medical device interoperability: a safer path forward. Priority issues from the 2012 AAMI-FDA Interoperability Summit. Arlington: AAMI; 2012. http://www.aami.org/interoperability/Interoperability_Summit_publication.pdf. [Google Scholar]


