Abstract
Acrolein, a reactive aldehyde found in cigarette smoke, is thought to induce its biological effects primarily by irreversible adduction to cellular nucleophiles such as cysteine thiols. Here, we demonstrate that acrolein rapidly inactivates the seleno-enzyme thioredoxin reductase (TrxR) in human bronchiolar epithelial HBE1 cells, which recovered over 4-8 hrs by a mechanism depending on the presence of cellular GSH and thioredoxin 1 (Trx1), and corresponding with reversal of protein-acrolein adduction. Our findings indicate that acrolein-induced protein alkylation is not necessarily a feature of irreversible protein damage, but may reflect a reversible signaling mechanism that is regulated by GSH and Trx1.
Keywords: cysteine, selenocysteine, Michael addition, thioredoxin reductase, GSH, thioredoxin
1. Introduction
Acrolein (2-propenal) is a reactive α,β-unsaturated aldehyde and a common environmental pollutant originated from organic combustion, and is also generated as a endogenous metabolite in biological systems resulting from various oxidative processes [1, 2]. The biological actions or toxicity of acrolein are related to its electrophilic character, promoting covalent reactions with cellular nucleophiles such as GSH, protein cysteine, histidine, and lysine residues, and nucleophilic DNA bases. Because acrolein is a major component of tobacco smoke, it is believed to contribute the development of smoking-related diseases such as COPD, asthma, or lung cancer [3, 4]. However, acrolein also shares its electrophilic character with many biological electrophiles with presumed functional properties mediated by electrophile signaling [5, 6].
The toxicity of acrolein is generally thought to be mediated by depletion of cellular GSH or disruption of cellular redox systems, but may also be related to covalent protein modifications leading to loss of protein function [7] or protein miss-folding [8, 9], cross-linking, or aggregation [10]. Among the spectrum of biologically relevant electrophiles, there is a degree of selectivity with respect to their reactivity with nucleophiles, based on the “hard/soft” acid-base principle [11]. Acrolein, as well as other α,β-unsaturated aldehydes or ketones (which include lipid-derived oxidation products such as 4-hydroxy-2-nonenal or cyclopentenone prostaglandins) are known as “soft” electrophiles, and therefore preferentially target “soft” nucleophiles, primarily (protein) cysteine residues. Indeed, kinetic and proteomic data indicate that acrolein preferentially reacts with cysteine residues compared to histidine or lysine residues, which are “harder” nucleophiles [7, 12, 13]. It has been suggested that toxicity associated with acrolein and other α,β-unsaturated aldehydes originates largely from disruption of reversible redox signaling mechanisms, due to more irreversible alkylation of selected sensitive cysteine residues that are also subject to oxidative or nitrosative signaling [14]. Alternatively, the toxic effects of α,β-unsaturated aldehydes such as acrolein, compared to e.g. endogenously generated cyclopentenone prostaglandins, are thought to be related to the fact that the former are slightly “harder” electrophiles and are more reactive to “harder” nucleophiles such as lysine of amino groups in DNA bases in addition to cysteine, especially at higher concentrations [15].
In spite of available evidence for preferred reactivity of acrolein with cysteine thiols or thiolates, which is several orders of magnitude greater compared to reactions with other nucleophiles such as lysine residues [14], the main existing evidence for formation of acrolein-protein adducts in vivo is based on immunological detection of stable Michael adducts with lysine, such as Nε-(3-formyl-3,4-dehydropiperidino)lysine (FDP-lysine) or Nε-(3-methylpyridinium)lysine (MP-lysine). In contrast, relatively little direct evidence exists to date for generation of acrolein-cysteine adducts, and no reports exist of specific acrolein-cysteine adducts in vivo, which is attributed to the relatively limited stability of such adducts even in in vitro systems and secondary reactions of protein-acrolein adducts by Schiff base formation [12].
Several detoxification systems have been shown to metabolize either the unsaturated double bond or the carbonyl moiety of acrolein and other α,β-unsaturated aldehydes, including GSH and glutathione S-transferase (GST) [16], aldehyde dehydrogenase [17], alkenal/one oxidoreductase [18], and aldo/keto reductase [19], and thereby control their biological actions. In contrast, much less is known about the fate of initially formed protein Michael adducts by these electrophiles. Covalent protein adducts of α,β-unsaturated aldehydes may be removed by lysosomal degradation and autophagy [20]. Alternatively, it is feasible that Michael adducts, especially with cysteine, are reversed by cellular reducing systems, which would help explain the relative paucity of demonstrated protein-cysteine adducts in vivo. The reversible nature of acrolein-cysteine adducts is supported by several in vitro studies of Michael addition of electrophiles with GSH, which indicated GST-catalyzed retro-Michael cleavage of GSH conjugates [21-23]. Moreover, such reversibility of cysteine adduction by biologically relevant electrophiles would also support their proposed roles in biological signaling pathways. The objective of the present study was to evaluate the reversible nature of acrolein-dependent inactivation of the seleno-enzyme thioredoxin reductase (TrxR), an important susceptible target for acrolein and other electrophiles [24-26]. Our findings demonstrate that recovery of TrxR activity after its inactivation by acrolein relies importantly on the presence of cellular GSH and thioredoxin-1 (Trx1), and indicate the involvement of these redox systems in reversing acrolein adducts with TrxR and other target proteins.
2. Methods
2.1 Cell culture and treatments
Human bronchial epithelial (HBE1) cells (generously provided by Dr. Reen Wu at the University of California, Davis [27]) were cultured at 37°C in 95% humidified air containing 5% CO2 using Dulbecco’s Modified Eagle’s Medium (DMEM/F-12) supplemented with 50 U/mL penicillin, 50 μg/mL streptomycin, 10 ng/mL choleratoxin (List Biological Laboratories, Inc.), 10 ng/mL epidermal growth factor (Calbiochem), 15 μg/mL bovine pituitary extract, 0.5 mg/mL bovine serum albumin (Invitrogen), 5 μg/mL insulin, 5 μg/mL transferrin, and 0.1 μM dexamethasone (Sigma). For experimentation, cells were plated in 12-well plates and cultured to confluence and placed in 2 mL Hank’s Balanced Salt Solution (HBSS) for treatments with acrolein (to avoid unwanted reactions of acrolein with other constituents present in the culture media), and collected after 30 min for various biochemical analyses, or placed in full culture media for continued incubation. At indicated time points, cells were collected in appropriate lysis buffer for the various analyses described below. Effects on cell viability were assessed by analysis of LDH release (Pierce).
Where indicated, cells were pretreated with 10 μM MG132 (Calbiochem), to inhibit proteasomal degradation, by pretreatment for 30 min prior and during acrolein treatment, and MG132 was again added to the culture media during subsequent incubation. Similarly, cells pretreated for 30 min with 10 μg/mL cycloheximide (CHX; Sigma), to inhibit protein synthesis, which was continued after acrolein treatment for the duration of the experiment.
2.2 Analysis and alteration of cellular GSH levels
GSH was analyzed in cell lysates by derivatization with 2 mM monobromobimane (mBrB), and analyzed by HPLC with fluorescence detection, as previously published [28]. Where indicated, GSH synthesis was inhibited by addition of 100 μM buthionine sulfoximine (BSO) (Sigma) after acrolein treatment.
2.3 SiRNA silencing of Trx1
To suppress endogenous Trx1 levels, cells were seeded at 70% confluence in 24-well plates and transfected with 50 nM TXN1 Smartpool siRNA (Dharmacon) or Non-Targeting siRNA using DharmaFECT transfection reagent according to manufacturere’s instructions. Media was replaced after 24 hrs and cells were used for experimentation 60 hrs after transfection.
2.4 Measurement of TrxR activity using insulin assay
Cells were lysed in 50 mM Tris/Cl (pH 7.4) containing 1 mM EDTA, and the reductase activity of TrxR was measured using a previously described end-point insulin assay [29, 30]. Protein lysates (20 μg) were incubated with 2 mM NADPH, 20 μM E. coli thioredoxin (Trx), and 1.5 mg/mL insulin for 60 min at 37°C after which the reaction was stopped with 8 M guanidine.HCl containing 1 mM 5,5′-dithiobis(2-nitrobenzoic) acid (DTNB). Formation of 2-nitro-5-thiobenzoic acid (TNB) was measured at 412 nm using a BioMate 5 spectrophotometer (Thermo Spectronic), and Trx-dependent reductase activity was determined by calculating the difference in activity with and without Trx.
2.5 Western blotting
Cell lysates were prepared by collecting cells in lysis buffer, containing 1% Triton X-100, 250 mM NaCl, 50 mM HEPES, 10% glycerol, 1.5 mM MgCl2, 1 mM polymethylsulfonyl chloride (PMSF), 1 mM EGTA, 2 mM sodium orthovanadate (Na3VO4), 10 μg/mL aprotinin, and 10 μg/mL leupeptin for 15 min on ice, after which cell suspensions were sonicated for 20 pulses on ice using a sonic dismembrator (Fisher Scientific), and centrifuged (14,000 rpm, 4°C) for 5 min to remove cell debris. Protein was quantified via the bicinchininic acid assay (BCA protein assay kit; Pierce). Aliquots containing 15 μg protein were mixed with 2x reducing sample buffer (containing 4% SDS, 20% glycerol, and 10% β-mercaptoethanol), and separated by SDS-PAGE, transferred to nitrocellulose membranes, and blotted for specific proteins with the following primary antibodies: TrxR1: (1:1000, Abcam), Trx1 (1:2000, Cell Signaling), HO-1 (1:250, Biovision), Cell Signaling), JNK1/2 (1:1000, Cell Signaling), β-actin (1:5000, Sigma). Primary antibodies were detected using HRP-conjugated anti-rabbit or anti-mouse IgG and visualized by SuperSignal West chemiluminescent substrate (Pierce).
2.6 Detection of protein-acrolein adducts using biotin hydrazide labeling
Protein lysates (≥ 300 μg) were mixed with 50 mM biotin hydrazide (Thermo Scientific) solubilized in DMSO at room temperature for 2 hrs with constant rotation, followed by addition of 30 mM NaCNBH4 and incubation on ice for 60 min. Biotin-labeled proteins were purified by avidin chromatography as described previously [31]. Briefly, derivatized lysates were washed 3 times with 300 μL of 20 mM Tris/Cl pH 7.4 using centrifugal filter devices (3,000 MWCO; Millipore) at 10,000 rpm for 10 min, to remove excess biotin hydrazide and NaCNBH4. Labeled proteins were then isolated by affinity chromatography by the addition of 50 μL of a 50% suspension of high capacity neutravidin agarose resin (Thermo Scientific). Beads were washed 6 times with 0.2 M glycine pH 2.8, centrifuged between each wash at ≤ 2500 rpm, then washed once with 20 mM Tris/Cl pH 7.4. Proteins were eluted in 100 μL 2x reducing sample buffer and boiled at 95-100°C for 5 min, for analysis by SDS-PAGE and detection of biotin-labeled proteins by blotting with streptavidin-HRP (1:20,000) or Western blotting for proteins of interest, as described above.
2.7 Statistical analyses
Data for each group were statistically analyzed via t-test or analysis of variance (ANOVA) depending on the amount of groups/experiment and significance was assigned at a maximum cut off of p < 0.05.
3. Results
3.1 Acrolein induces reversible depletion of GSH and reversible inactivation of TrxR
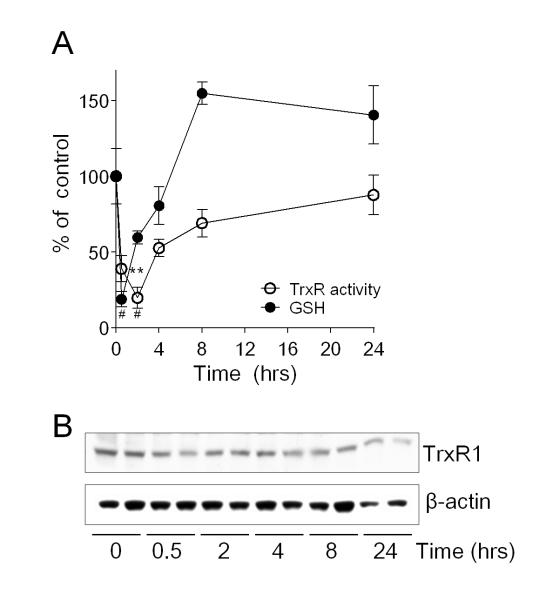
In agreement with earlier findings [32, 33], exposure of HBE1 cells to non-toxic doses of acrolein (30 μM) rapidly and transiently depleted cellular GSH, which was regenerated to initial levels within 2-4 hrs and to levels exceeding initial levels at later time points (8-24 hrs), due to induction of GSH synthesis (Fig. 1A). Similarly, acrolein exposure also rapidly inhibited TrxR activity, consistent with earlier findings [34]. Contrary to a recent report [35], but in accordance with earlier findings [30, 34], TrxR activity was also reversed after acrolein treatment, to near control levels over 4-24 hrs (Fig. 1A). Previous studies suggested that such TrxR recovery is due to induction of TrxR1 mRNA expression [33], however, we did not observe significant increases in TrxR protein levels over the same time period (Fig. 1B).
Figure 1.
Reversible GSH depletion and inactivation of TrxR after acute acrolein exposure. HBE1 cells were exposed to 30 μM acrolein for 30 min, and subsequently incubated for up to 24 hrs. (A) Cellular GSH levels (closed symbols) and TrxR activity (open symbols) were analyzed at indicated times after acrolein treatment, using HPLC and insulin assay, respectively. Data points represent mean ± SEM (n=4). **: p < 0.01, #: p < 0.001. Significance was designated after analysis by one-way ANOVA. (B) Western blot analysis of TrxR1 protein and β-actin at various time points after acrolein treatment (30 μM). A representative blot of 3 separate experiments is shown.
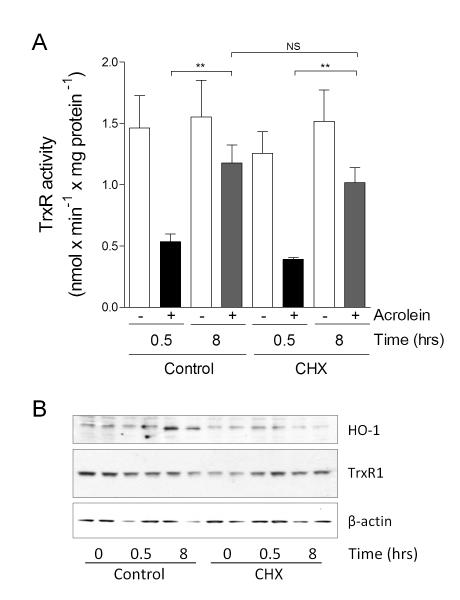
To more directly evaluate the importance of de novo TrxR protein synthesis in recovery of TrxR activity after acrolein exposure, HBE1 cells were pretreated with protein synthesis inhibitor CHX prior to acrolein treatment. As shown in Fig. 2A, recovery of TrxR activity after 8 hrs post-incubation following acrolein exposure was still observed in the presence of CHX, indicating that this recovery of TrxR activity did not depend on protein synthesis. To confirm the efficacy of CHX, we verified its effect on acrolein-mediated induction of heme oxygenase 1 (HO-1), which showed that CHX completely prevented acrolein-induced upregulation of HO-1 (Fig. 2B). Total levels of TrxR were relatively unaltered under the same conditions (Fig. 2B). Thus, these findings indicate that inactivation and recovery of TrxR activity are not related to changes in overall TrxR1 protein levels, but are likely related to reversible post-translational modification of TrxR by e.g. alkylation.
Figure 2.
Recovery of TrxR activity does not require protein synthesis. HBE1 cells were pretreated with CHX followed by a 30 min 30 μM acrolein treatment with or without an 8 hr recovery period. (A) TrxR activity was measured by insulin assay at indicated time-points, and expressed as mean ± SEM (n=4). **: p < 0.01. Significance was designated after analysis by one-way ANOVA. (B) Western blot analysis of HO-1, TrxR1 protein, and β-actin at indicated time points. Representative blots of 2 independent experiments are shown.
3.2 Acrolein induces transient carbonylation of cellular proteins and TrxR1, independent of proteasomal activity
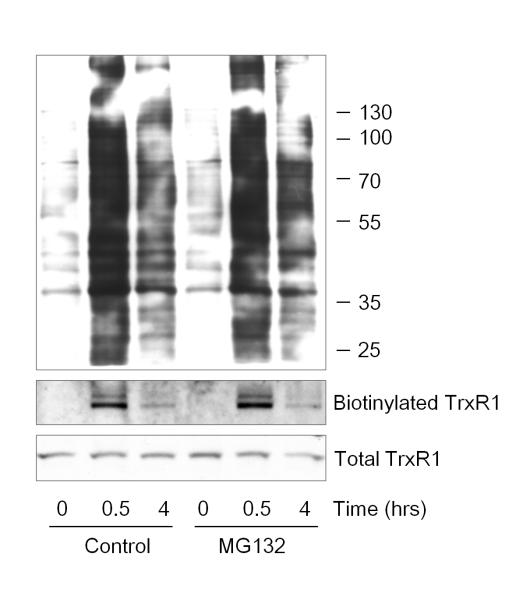
Following up on our previous findings indicating that acrolein-induced inactivation of TrxR is related to alkylation at its selenocysteine residue [7], we explored whether such alkylation may be reversible over time. Using biotin hydrazide tagging to detect protein-acrolein Michael adducts, we detected rapid increases in acrolein-adducted proteins that disappeared with an approximate half-life of 4 hrs, consistent with earlier findings [7, 31]. Importantly, the timing of turnover of protein-acrolein adducts corresponded with the observed kinetics of regeneration of TrxR activity, which also occurred with a half-time of ~4 hrs. We rationalized that the gradual disappearance of carbonylated proteins may be due to proteasomal degradation of alkylated (and thus damaged) proteins or to metabolism of protein-bound carbonyls by e.g. aldehyde dehydrogenases, so that they were no longer be detectable using hydrazide labeling. To address the importance of proteasomal degradation, experiments were performed in the presence of the 26S proteasome inhibitor MG132, which showed that the disappearance of hydrazide-detectable protein carbonyl adducts was not affected (Fig. 3). Similary, acrolein-induced carbonylation of TrxR1 itself, previously demonstrated to result in its inactivation [7], was significantly decreased at 4 h after acrolein exposure, both in the absence or presence of MG132 (Fig. 3). These findings indicate that the reversal of TrxR activity after acrolein exposure is associated with a loss of protein-acrolein adducts within TrxR, independent of protein turnover and potentially by reversal of acrolein-mediated alkylation of TrxR.
Figure 3.
Disappearance of protein-acrolein adducts is not mediated by the 26S proteasome. HBE1 cells were pretreated with MG132, followed by 30 min incubation with 30 μM acrolein with or without a 4 hr recovery period. Carbonylated proteins representing acrolein Michael adducts were analyzed by biotin hydrazide labeling, and biotin labeling of cellular proteins was detected by streptavidin blotting (top panel). Neutravidin-purified biotin hydrazide labeled proteins were analyzed by Western blot using α-TrxR1 (middle panel) in comparison with similar TrxR1 analysis in whole cell lysates (bottom panel). Blots are representative of 2 independent experiments.
3.3 Reversal of TrxR activity and protein carbonylation depend on the presence of GSH and Trx1
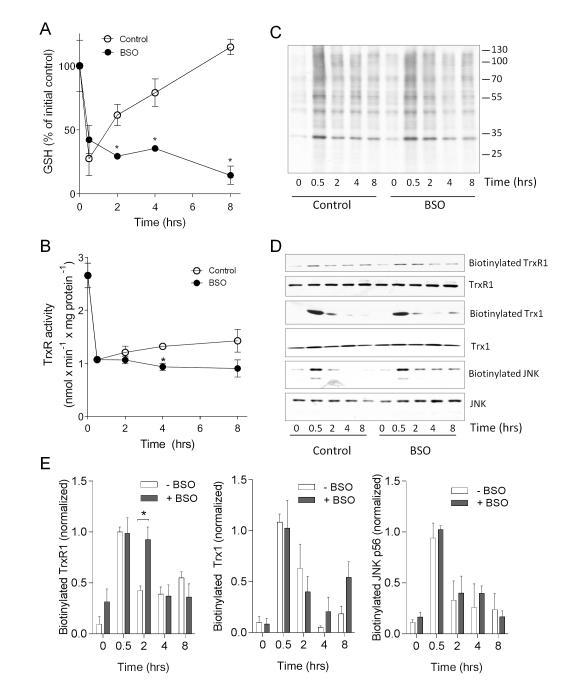
Based on the observed rapid recovery of cellular GSH levels prior to restoration of TrxR activity (Fig. 1), we considered that GSH may be involved in the regeneration of TrxR activity. To test this, HBE1 cells were treated with the GSH synthesis inhibitor BSO, immediately following a 30-min acrolein treatment, to monitor its impact of GSH regeneration on recovery of TrxR activity over time. As expected, BSO treatment completely prevented the rapid repletion of GSH after acrolein treatment (Fig. 4A), although it did not significantly increase cytotoxicity (not shown). Moreover, BSO treatment was also found to significantly attenuate the gradual recovery of TrxR activity over 8 hrs (Fig. 4B), suggesting that this recovery may be related to GSH-dependent reversal of protein alkylation. To test this, we evaluated hydrazide-detectable protein-acrolein adducts in whole cell lysates after acrolein treatment, which indicated that BSO treatment slightly attenuated the disappearance of protein carbonyl adducts in general, especially at 2 hrs (Fig. 4C), suggesting a possible role for GSH in reversal of protein carbonylation by acrolein. To more specifically evaluate carbonylation of TrxR1 or other known target proteins for acrolein, biotin hydrazide-labeled proteins were avidin-purified and analyzed by Western blotting for specific target proteins. Results in Fig. 4D&E indicate that BSO treatment significantly delays loss of carbonylated TrxR1 over time, and also appears to impair loss of carbonylated forms of Trx1 and JNK, two other target proteins for acrolein [26, 31], suggesting a role of GSH-dependent mechanisms in reversal of alkylation of these proteins.
Figure 4.
Recovery of TrxR activity and reversal of protein carbonylation depends on GSH status. Fifteen HBE1 cells were treated with 30 μM acrolein for 15 min, and subsequently incubated for up to 8 hrs in the absence or presence of 100 μM BSO. At indicated time points, cell lysates were evaluated for GSH by HPLC (A) or for TrxR activity using the insulin assay (B). Results are expressed as mean ± SEM, n=4. *: p < 0.05. Significance was designated after analysis by multiple t-tests. Carbonylated proteins were analyzed by biotin hydrazide labeling and streptavidin blot (C) or neutravidin purified for analysis by Western blotting using antibodies against Trx1, JNK, or TrxR1 (D). Comparative Western blot analysis of Trx1, JNK, or TrxR1 was performed on whole cell lysates. Relative band densities of biotinylated TrxR1, Trx1, and JNK were normalized to overall protein levels and expressed relative to levels observed after 30 acrolein treatment (E). Mean ± S.E.M from 4 replicates from 2 separate experiments are shown. *: p < 0.05.
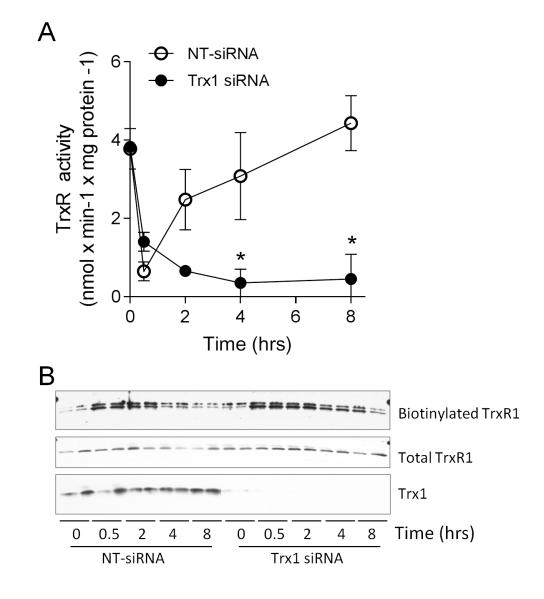
Our recent studies indicated that acrolein exposure induces alkylation of Trx1, which critically depended on the presence of TrxR1 [26]. One potential explanation for this finding is that alkylation of Trx1 occurs by a trans-alkylation mechanism after initial alkylation of TrxR1. Consistent with this suggestion is our observation of sustained and even increasing carbonylation of Trx1 over 2-8 hrs while carbonylation of TrxR1 decreases, particularly in the presence of BSO (Fig. 4E). Consequently, Trx1 (as the substrate for TrxR1) may contribute to regenerating TrxR activity after inactivation by acrolein or other electrophiles. To assess the contribution of Trx1 to regeneration of TrxR activity, Trx1 expression was silenced using siRNA prior to acrolein treatment. As shown in Fig. 5A, Trx1 siRNA did not significantly affect initial inactivation of TrxR by acrolein, but markedly prevented its recovery over a subsequent 8 hr period. Notably, Trx1 siRNA pretreatment slightly enhanced the cytotoxic effects of acrolein (from 15.3 ± 3.2% in NT-siRNA transfected cells to 33.0 ± 6.1% in Trx1-siRNA transfected cells), although this cannot fully explain the complete lack of TrxR recovery in the absence of Trx1. Correspondingly, the reversal of TrxR1 alkylation, as assessed by biotin hydrazide labeling, was also attenuated after Trx1 siRNA knockdown (Fig. 5B).
Figure 5.
Role of Trx1 in reversal of TrxR1 alkylation and recovery of activity. HBE1 cells were pretreated with Trx1-targeted siRNA and exposure to 30 μM acrolein fort 30 min, followed by 8 hr recovery. (A) Analysis of TrxR activity at indicated time points in NT-siRNA or Trx1 siRNA-transfected cells. Data represent mean ± SEM (n=3). *: p < 0.05. Significance was designated after analysis by two-way ANOVA. (B) Purified biotin hydrazide-derivatized proteins were analyzed by Western blot for TrxR1. Total TrxR1 and Trx1 were also analyzed in corresponding whole cell lysates. Representative blots from duplicate experiments are shown.
4. Discussion
Acrolein is a reactive electrophile, and an important environmental pollutant of considerable health concern [1, 36], and is believed to contribute to the adverse health effects of tobacco smoking [7, 26, 31]. However, acrolein is also formed endogenously by various defined oxidative processes, and may therefore share features of other biological electrophiles, such as cyclopentenone prostaglandins [37], nitrated fatty acids [38], or nitrated nucleotides [39], that have been considered to act as intracellular messengers with defined specific cellular functions. Since each of these electrophiles largely act by covalent Michael addition, primarily to susceptible cysteine residues, such electrophile signaling represents another component of thiol-dependent redox signaling, similar to e.g. S-nitrosylation or S-glutathionylation [40, 41]. However, in contrast to these oxidative mechanisms of thiol-based redox signaling, S-alkylation by e.g. α,β-unsaturated aldehydes is typically considered irreversible, although chemical studies indicate that they can reversed in the presence of excess thiols. It is, therefore, plausible that endogenous thiol-based mechanisms exist to reverse S-alkylation in biological systems in vivo, which would further support its importance as a biologically important signaling mechanism. Indeed, our present findings strongly suggest that acrolein-dependent covalent protein modifications are biologically reversible by endogenous reducing mechanisms involving GSH and/or Trx1, and that such reversal mechanisms help restore enzymatic activity of specific target proteins, such as TrxR1. It is feasible that other key target proteins for acrolein or other electrophiles may be restored by similar reducing mechanisms. Although the present studies were performed with a single cell line, our previous studies similarly indicate reversible protein alkylation in other cell types [31], and suggest that these reversal mechanisms may be universal.
It should be noted that the use of biotin hydrazide labeling to detect acrolein-dependent protein Michael adducts has the inherent limitation that it also detects other protein carbonyls that are formed more indirectly by e.g. acrolein-mediated oxidative events. Moreover, disappearance of protein carbonyl moieties over time does not necessarily indicate reversal of Michael adducts, but most likely also represents additional reactions of protein-bound carbonyls (e.g. through Schiff bases [12]) or metabolism of protein-bound carbonyls by e.g. aldehyde dehydrogenases. However, we previously established that acrolein-induced loss of TrxR activity is directly related to Michael addition to its selenocysteine residue [7]. The fact that restoration of TrxR activity corresponds kinetically with disappearance of biotin hydrazide-detectable protein carbonyls within the TrxR1, and is not associated with significant changes in overall TrxR1 protein levels under the same conditions, strongly suggests that such restoration of TrxR is mediated by reversal of acrolein adduction to its selenocysteine residue. Our finding that such reversal depends of the presence of Trx1, a specific substrate for TrxR1, further supports this notion.
Our results indicate the involvement of both GSH and Trx1 in promoting the regeneration of TrxR activity after its inactivation by acrolein. Based on previous findings that indicate a role for GST in catalyzing retro Michael addition by various electrophiles [21-23], we propose that GSH-dependent restoration of TrxR1 activity, or of other proteins such as Trx1 or JNK (Fig. 4), may similarly depend on GST activity. Restoration of TrxR alkylation and activity was also found to critically depend on Trx1, which may be uniquely involved in initial regeneration of TrxR1 by a transalkylation mechanism. Such a mechanism would be consistent with our recent finding that detection of acrolein-adducted Trx1 in acrolein-exposed HBE1 cells was strongly dependent on the presence of TrxR1 [26], which could suggest that alkylation of Trx1 may have largely occurred indirectly after initial alkylation of the more reactive selenocysteine within TrxR1. In turn, reversal of alkylated Trx1 by GSH-dependent mechanisms may contribute to full recovery of TrxR. Future studies using purified or recombinant enzymes in cell-free systems would be required to more definitively confirm the concept of transalkylation in regulation of TrxR.
In summary, our present findings strongly imply that covalent alkylation of protein thiols by biological electrophiles such as acrolein can be reversed by endogenous GSH- and Trx-dependent mechanisms. These findings indicate that, rather than representing irreversible protein damage, protein alkylation by acrolein and other electrophiles may be an important reversible event in adaptive responses to electrophilic stress or function as electrophile-specific signaling mechanism analoguous to e.g. protein S-nitrosylation or S-glutathionylation. A more complete understanding of such reversal mechanisms would not only offer better insight into the biological functions of electrophiles but may also contribute to improved strategies to manage chronic pulmonary diseases associated with environmental acrolein exposure or cigarette smoking.
Highlights.
Acrolein readily and reversibly inactivates thioredoxin reductase (TrxR)
Reversal of TrxR activity occurs independently of protein synthesis.
Reversal of TrxR activity depends on the presence of GSH and Trx1.
We propose that Trx1 and GSH repair alkylated TrxR1 by transalkylation.
Reversible protein alkylation may constitute a biological signaling mechanism.
Acknowledgments
This work was supported by research grants from NIH (R01 ES021476) and the Flight Attendant Medical Research Institute (FAMRI) to AvdV. MJR received a Research Fellowship from the Department of Pathology.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
5. References
- [1].Bein K, Leikauf GD. Acrolein - a pulmonary hazard. Mol Nutr Food Res. 2011;55:1342–1360. doi: 10.1002/mnfr.201100279. [DOI] [PubMed] [Google Scholar]
- [2].Stevens JF, Maier CS. Acrolein: sources, metabolism, and biomolecular interactions relevant to human health and disease. Mol Nutr Food Res. 2008;52:7–25. doi: 10.1002/mnfr.200700412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Feng Z, Hu W, Hu Y, Tang MS. Acrolein is a major cigarette-related lung cancer agent: Preferential binding at p53 mutational hotspots and inhibition of DNA repair. Proc Natl Acad Sci U S A. 2006;103:15404–15409. doi: 10.1073/pnas.0607031103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Moretto N, Volpi G, Pastore F, Facchinetti F. Acrolein effects in pulmonary cells: relevance to chronic obstructive pulmonary disease. Ann N Y Acad Sci. 2012;1259:39–46. doi: 10.1111/j.1749-6632.2012.06531.x. [DOI] [PubMed] [Google Scholar]
- [5].Go YM, Halvey PJ, Hansen JM, Reed M, Pohl J, Jones DP. Reactive aldehyde modification of thioredoxin-1 activates early steps of inflammation and cell adhesion. Am J Pathol. 2007;171:1670–1681. doi: 10.2353/ajpath.2007.070218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Zhang H, Forman HJ. Signaling pathways involved in phase II gene induction by alpha, beta-unsaturated aldehydes. Toxicol Ind Health. 2009;25:269–278. doi: 10.1177/0748233709102209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Spiess PC, Deng B, Hondal RJ, Matthews DE, van der Vliet A. Proteomic profiling of acrolein adducts in human lung epithelial cells. J Proteomics. 2011;74:2380–2394. doi: 10.1016/j.jprot.2011.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Haberzettl P, Vladykovskaya E, Srivastava S, Bhatnagar A. Role of endoplasmic reticulum stress in acrolein-induced endothelial activation. Toxicol Appl Pharmacol. 2009;234:14–24. doi: 10.1016/j.taap.2008.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Vladykovskaya E, Sithu SD, Haberzettl P, Wickramasinghe NS, Merchant ML, Hill BG, McCracken J, Agarwal A, Dougherty S, Gordon SA, et al. Lipid peroxidation product 4-hydroxy-trans-2-nonenal causes endothelial activation by inducing endoplasmic reticulum stress. J Biol Chem. 2012;287:11398–11409. doi: 10.1074/jbc.M111.320416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Burcham PC, Pyke SM. Hydralazine inhibits rapid acrolein-induced protein oligomerization: role of aldehyde scavenging and adduct trapping in cross-link blocking and cytoprotection. Mol Pharmacol. 2006;69:1056–1065. doi: 10.1124/mol.105.018168. [DOI] [PubMed] [Google Scholar]
- [11].LoPachin RM, Gavin T, Decaprio A, Barber DS. Application of the Hard and Soft, Acids and Bases (HSAB) theory to toxicant--target interactions. Chem Res Toxicol. 2012;25:239–251. doi: 10.1021/tx2003257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Cai J, Bhatnagar A, Pierce WM., Jr. Protein modification by acrolein: formation and stability of cysteine adducts. Chem Res Toxicol. 2009;22:708–716. doi: 10.1021/tx800465m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].LoPachin RM, Gavin T, Petersen DR, Barber DS. Molecular mechanisms of 4-hydroxy-2-nonenal and acrolein toxicity: nucleophilic targets and adduct formation. Chem Res Toxicol. 2009;22:1499–1508. doi: 10.1021/tx900147g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].LoPachin RM, Gavin T. Molecular mechanism of acrylamide neurotoxicity: lessons learned from organic chemistry. Environmental health perspectives. 2012;120:1650–1657. doi: 10.1289/ehp.1205432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Higdon A, Diers AR, Oh JY, Landar A, Darley-Usmar VM. Cell signalling by reactive lipid species: new concepts and molecular mechanisms. Biochem J. 2012;442:453–464. doi: 10.1042/BJ20111752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Conklin DJ, Haberzettl P, Prough RA, Bhatnagar A. Glutathione-S-transferase P protects against endothelial dysfunction induced by exposure to tobacco smoke. Am J Physiol Heart Circ Physiol. 2009;296:H1586–1597. doi: 10.1152/ajpheart.00867.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Makia NL, Bojang P, Falkner KC, Conklin DJ, Prough RA. Murine hepatic aldehyde dehydrogenase 1a1 is a major contributor to oxidation of aldehydes formed by lipid peroxidation. Chem Biol Interact. 2011;191:278–287. doi: 10.1016/j.cbi.2011.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Dick RA, Kwak MK, Sutter TR, Kensler TW. Antioxidative function and substrate specificity of NAD(P)H-dependent alkenal/one oxidoreductase. A new role for leukotriene B4 12-hydroxydehydrogenase/15-oxoprostaglandin 13-reductase. J Biol Chem. 2001;276:40803–40810. doi: 10.1074/jbc.M105487200. [DOI] [PubMed] [Google Scholar]
- [19].Srivastava S, Watowich SJ, Petrash JM, Srivastava SK, Bhatnagar A. Structural and kinetic determinants of aldehyde reduction by aldose reductase. Biochemistry. 1999;38:42–54. doi: 10.1021/bi981794l. [DOI] [PubMed] [Google Scholar]
- [20].Hill BG, Haberzettl P, Ahmed Y, Srivastava S, Bhatnagar A. Unsaturated lipid peroxidation-derived aldehydes activate autophagy in vascular smooth-muscle cells. Biochem J. 2008;410:525–534. doi: 10.1042/BJ20071063. [DOI] [PubMed] [Google Scholar]
- [21].Chen J, Armstrong RN. Stereoselective catalysis of a retro-Michael reaction by class mu glutathione transferases. Consequences for the internal distribution of products in the active site. Chem Res Toxicol. 1995;8:580–585. doi: 10.1021/tx00046a012. [DOI] [PubMed] [Google Scholar]
- [22].Meyer DJ, Crease DJ, Ketterer B. Forward and reverse catalysis and product sequestration by human glutathione S-transferases in the reaction of GSH with dietary aralkyl isothiocyanates. Biochem J. 1995;306(Pt 2):565–569. doi: 10.1042/bj3060565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Zhang Y, Kolm RH, Mannervik B, Talalay P. Reversible conjugation of isothiocyanates with glutathione catalyzed by human glutathione transferases. Biochem Biophys Res Commun. 1995;206:748–755. doi: 10.1006/bbrc.1995.1106. [DOI] [PubMed] [Google Scholar]
- [24].Anestal K, Prast-Nielsen S, Cenas N, Arner ES. Cell death by SecTRAPs: thioredoxin reductase as a prooxidant killer of cells. PLoS One. 2008;3:e1846. doi: 10.1371/journal.pone.0001846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Cheng Q, Antholine WE, Myers JM, Kalyanaraman B, Arner ES, Myers CR. The selenium-independent inherent pro-oxidant NADPH oxidase activity of mammalian thioredoxin reductase and its selenium-dependent direct peroxidase activities. J Biol Chem. 2010;285:21708–21723. doi: 10.1074/jbc.M110.117259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Randall MJ, Spiess PC, Hristova M, Hondal RJ, van der Vliet A. Acrolein-induced activation of mitogen-activated protein kinase signaling is mediated by alkylation of thioredoxin reductase and thioredoxin 1. Redox Biology. 2013;1:265–275. doi: 10.1016/j.redox.2013.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Yankaskas JR, Haizlip JE, Conrad M, Koval D, Lazarowski E, Paradiso AM, Rinehart CA, Jr., Sarkadi B, Schlegel R, Boucher RC. Papilloma virus immortalized tracheal epithelial cells retain a well-differentiated phenotype. Am J Physiol. 1993;264:C1219–1230. doi: 10.1152/ajpcell.1993.264.5.C1219. [DOI] [PubMed] [Google Scholar]
- [28].van der Vliet A, O’Neill CA, Cross CE, Koostra JM, Volz WG, Halliwell B, Louie S. Determination of low-molecular-mass antioxidant concentrations in human respiratory tract lining fluids. Am J Physiol. 1999;276:L289–296. doi: 10.1152/ajplung.1999.276.2.L289. [DOI] [PubMed] [Google Scholar]
- [29].Arner ES, Holmgren A. Measurement of thioredoxin and thioredoxin reductase. Curr Protoc Toxicol. 2001 doi: 10.1002/0471140856.tx0704s05. Chapter 7, Unit 7 4. [DOI] [PubMed] [Google Scholar]
- [30].Holmgren A, Bjornstedt M. Thioredoxin and thioredoxin reductase. Methods Enzymol. 1995;252:199–208. doi: 10.1016/0076-6879(95)52023-6. [DOI] [PubMed] [Google Scholar]
- [31].Hristova M, Spiess PC, Kasahara DI, Randall MJ, Deng B, van der Vliet A. The tobacco smoke component, acrolein, suppresses innate macrophage responses by direct alkylation of c-Jun N-terminal kinase. Am J Respir Cell Mol Biol. 2012;46:23–33. doi: 10.1165/rcmb.2011-0134OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Myers CR, Myers JM, Kufahl TD, Forbes R, Szadkowski A. The effects of acrolein on the thioredoxin system: implications for redox-sensitive signaling. Mol Nutr Food Res. 2011;55:1361–1374. doi: 10.1002/mnfr.201100224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Park YS, Misonou Y, Fujiwara N, Takahashi M, Miyamoto Y, Koh YH, Suzuki K, Taniguchi N. Induction of thioredoxin reductase as an adaptive response to acrolein in human umbilical vein endothelial cells. Biochem Biophys Res Commun. 2005;327:1058–1065. doi: 10.1016/j.bbrc.2004.12.104. [DOI] [PubMed] [Google Scholar]
- [34].Yang X, Wu X, Choi YE, Kern JC, Kehrer JP. Effect of acrolein and glutathione depleting agents on thioredoxin. Toxicology. 2004;204:209–218. doi: 10.1016/j.tox.2004.06.056. [DOI] [PubMed] [Google Scholar]
- [35].Myers CR, Myers JM. The effects of acrolein on peroxiredoxins, thioredoxins, and thioredoxin reductase in human bronchial epithelial cells. Toxicology. 2009;257:95–104. doi: 10.1016/j.tox.2008.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Li L, Holian A. Acrolein: a respiratory toxin that suppresses pulmonary host defense. Rev Environ Health. 1998;13:99–108. [PubMed] [Google Scholar]
- [37].Uchida K, Shibata T. 15-Deoxy-Delta(12,14)-prostaglandin J2: an electrophilic trigger of cellular responses. Chem Res Toxicol. 2008;21:138–144. doi: 10.1021/tx700177j. [DOI] [PubMed] [Google Scholar]
- [38].Schopfer FJ, Cipollina C, Freeman BA. Formation and signaling actions of electrophilic lipids. Chem Rev. 2011;111:5997–6021. doi: 10.1021/cr200131e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Nishida M, Sawa T, Kitajima N, Ono K, Inoue H, Ihara H, Motohashi H, Yamamoto M, Suematsu M, Kurose H, et al. Hydrogen sulfide anion regulates redox signaling via electrophile sulfhydration. Nat Chem Biol. 2012;8:714–724. doi: 10.1038/nchembio.1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Benhar M, Forrester MT, Hess DT, Stamler JS. Regulated protein denitrosylation by cytosolic and mitochondrial thioredoxins. Science. 2008;320:1050–1054. doi: 10.1126/science.1158265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Lillig CH, Berndt C, Holmgren A. Glutaredoxin systems. Biochimica et Biophysica Acta (BBA) - General Subjects. 2008;1780:1304–1317. doi: 10.1016/j.bbagen.2008.06.003. [DOI] [PubMed] [Google Scholar]