Abstract
Objectives
Patients with inflammatory bowel disease (IBD) may be at increased risk for pneumocystis jiroveci pneumonia (PCP). Our aims were 1) to determine the incidence and relative risk of PCP in IBD and 2) to describe medication exposures in IBD patients with PCP.
Methods
We performed a retrospective cohort study and a case series using administrative data from IMS Health Inc, LifeLink™ Health Plan Claims Database. In the cohort, IBD patients were matched to 4 individuals with no IBD claims. PCP risk was evaluated by incidence rate ratio (IRR) and adjusted Cox proportional hazards modeling. The demographics and medication histories of the 38 cases of PCP in IBD patients were extracted.
Results
The cohort included 50,932 patients with CD, 56,403 patients with UC, and 1269 with unspecified IBD; matched to 434,416 individuals without IBD. The crude incidence of PCP was higher in the IBD cohort (10.6/100,000) than in the non-IBD cohort (3.0/100,000). In adjusted analyses, PCP risk was higher in the IBD vs. non-IBD cohort (HR 2.96, 95% CI 1.75–4.29), with the greatest risk in Crohn’s disease (CD) as compared to non-IBD (HR 4.01, 95% CI 1.88–8.56). In the IBD case series of PCP cases (n=38), the median age was 49 (IQR 43–57). A total of 20 (53%) were on corticosteroids alone or in combination with other immunosuppression.
Conclusions
Although the overall incidence of PCP is low, patients with IBD are at increased risk. IBD patients with PCP are predominantly on corticosteroids alone or in combination prior to PCP diagnosis.
Keywords: inflammatory bowel disease, complications, pneumocystis, pneumonia, corticosteroids, immunosuppressive drugs
Introduction
Patients with inflammatory bowel disease (IBD), particularly those treated with immunosuppressive agents, are at risk of infectious complications. In fact, infections are among the most common causes of death in patients with IBD, particularly respiratory infections.1 Case reports have described cases of pneumocystis jiroveci pneumonia (formerly known as pneumocystis carinii or PCP) associated with various forms of immunosuppression commonly used in IBD, including azathioprine, corticosteroids, cyclosporine and infliximab.2–7
PCP is an atypical unicellular fungus that produces alveolar infiltrates and local inflammatory reactions which widen the alveolar- arterial oxygen gradient.8, 9. PCP is associated with high rates of morbidity and mortality given its profound effects on gas exchange. PCP is typically associated with human immunodeficiency virus (HIV) and is among the most common acquired immunodeficiency syndrome (AIDS) defining illnesses.10 Its progression in the HIV population is slow and indolent characterized by fever, cough and pulmonary infiltrates whereas a more aggressive course is seen in the non-HIV population likely secondary to a more intense inflammatory response that can culminate in respiratory failure. In the HIV population, patients with a CD-4 lymphocyte count of <200 cells/mm3 are placed on PCP prophylaxis with an agent such as trimethoprim-sulfamethoxazole (TMP-SMX). There is current debate, without consensus guidelines, as to whether prophylaxis against PCP is warranted in patients with IBD on immunosuppression.9 This highlights the need for additional data on the overall incidence and medication-specific risk factors for PCP in the IBD population.
Therefore, we aimed to determine the risk of PCP in patients with IBD as compared to a non-IBD comparison cohort without HIV. We also aimed to describe the medication exposures in patients with IBD and PCP.
Material and Methods
We analyzed the procedural and outpatient pharmaceutical insurance claims contained in the LifeLink™ Health Plan Claims Database (IMS Health Inc, Norwalk, CT) for the period January 1, 1997 through December 31, 2009. This longitudinal, patient-level database has been used in previous epidemiologic studies of IBD.11, 12 The source database contains enrollment information on over 60 million persons from over 98 health plans across the U.S. The included health care plans capture a geographically diverse sample from across the United States. Only health plans that submit data for all members are included in the database, ensuring complete data capture and representative samples. Prior studies have reported the IMS Health database to be representative of the national commercially insured population on a variety of demographic measures.13
Study Design
We performed a retrospective cohort study to determine the overall risk of PCP in IBD patients compared with a non-IBD matched comparison cohort. We then described a case series to determine medication exposures prior to PCP in patients with IBD. A similar retrospective cohort design has previously been used by our group11 and also by Gupta et al14 to evaluate the incidence and risk of infections and malignancies in patients with IBD.
Cohort Study
Patient selection
All patients aged <64 years with at least 12 months of continuous health plan enrollment were eligible for inclusion in this analysis. We chose 64 as the upper age limit to avoid the possibility of missing data resulting from Medicare dual eligibility (which begins at age 65). Individuals were also required to have ongoing pharmacy coverage with their health plan in order to fully capture medication exposures. We identified cases of Crohn’s disease (CD) and ulcerative colitis (UC) by using a previously reported administrative definition expanded to include updated medications recently approved for IBD indications.15 The precise definition included patients with at least 3 health care contacts, on different days, associated with an International Classification of Diseases, 9th Revision, Clinical Modification (ICD-9) diagnosis code for CD (555.xx) or UC (556.xx), or patients with at least 1 claim for CD or UC and at least 1 pharmacy claim for any of the following medications: mesalamine, olsalazine, balsalazide, sulfasalazine, 6-mercaptopurine, azathioprine, methotrexate, infliximab, adalimumab, certolizumab pegol, natalizumab and enteral budesonide. For patients who had claims for both CD and UC, disease assignment was made according to the majority of the last 9 claims or the majority of total claims if there were fewer than 9. We matched each IBD patient to 4 non-IBD subjects by age, gender, U.S. census region and date of health plan coverage. All individuals with any ICD-9 code for human immunodeficiency virus (HIV) (042–044.9) were excluded related to inherent differences in susceptibility to infections.
Cohort lead time and follow-up
For each IBD patient, follow-up began six months after health plan enrollment or on the date of the first claim with a diagnosis code for IBD, whichever came later. For members of the non-IBD comparison cohort, follow up began on the same calendar day as the IBD patient to whom they were matched. A minimum of six months of health plan enrollment was required prior to matching. Thus, each individual had a six month “screening period” immediately preceding cohort entry during which healthcare utilization, comorbidities, and other covariates were assessed. Individuals with a PCP claim in the last month of screening time were excluded, as this was considered to represent a prevalent diagnosis. Each member of the cohort was followed until the first diagnosis of PCP, or, if none, until censoring at the earlier of one of two events: discontinuation of primary or pharmacy insurance coverage, or age > 64.
Assessment of outcome (PCP)
PCP was defined as either 1) an inpatient hospitalization with an ICD-9 code for pneumocystis (136.3), or 2) an outpatient visit with a ICD-9 code for PCP combined with an outpatient prescription for a PCP-specific antibiotic (TMP-SMX, atovaquone, dapsone, pentamidine, and primaquine) filled within 1 week before or after the visit.
Assessment of exposures
We assessed each exposure during the screening period six months prior to the start of follow-up. Health care utilization was estimated in a continuous fashion by the total number of days with at least one health care contact. Utilization has been measured in this fashion in prior pharmacoepidemiology studies.16 Comorbidities were assessed using the Quan update17 of the validated Deyo comorbidity index for administrative data.18 Medication use, including immunosuppressive and biologic medications was assessed in an any/none fashion.
Statistical analysis
We used descriptive statistics to summarize characteristics of patients with and without IBD. Continuous variables are reported as mean +/− standard deviation or median and interquartile range (IQR), and categorical variables are reported as percentages. We then calculated incidence rates of PCP (per 100,000 person-years) and used incidence rate ratios (IRRs) and 95% confidence intervals (CIs) to compare the incidence of PCP in IBD and non-IBD patients. We also performed subsequent analyses, stratifying by age (in decades), and disease type (UC vs CD). We assessed for possible violation of the proportional hazards assumption via log-log plots. As the assumption was not violated, we used Cox proportional hazards models to calculate hazard ratios (HRs) and 95% CIs for the risk of pneumonia in the IBD cohort as compared to the non-IBD cohort, controlling for health care utilization and comorbidities. Analyses were repeated stratified by CD versus UC.
Descriptive Case Series
We next conducted a case-only study of the IBD patients with PCP. We extracted all data, including outpatient medication exposures and hospitalizations, in the 60 days prior to PCP diagnosis. This time frame was chosen in order to capture all immunosuppressive medication therapy that could have an impact on PCP risk. Prior studies of infectious risks in IBD have focused upon a 30 day time frame,14 this was increased to 60 days for the current study in order to completely capture anti-TNF use, which could have been dosed up to 60 days prior to the outcome.
Assessment of exposures
Outpatient Medication Use
The primary medications evaluated included azathioprine and 6-mercaptopurine (thiopurine class), methotrexate, tacrolimus and cyclosporine (calcineurin class), infliximab, adalimumab, certolizumab pegol and natalizumab (biologic class), mycophenolate mofetil, and corticosteroids. Medication exposures were analyzed in the 60 days prior to PCP diagnosis.
Hospitalization
Claims data do not contain information on inpatient medication use, as these charges are packaged into the facility claims. Therefore, we have also evaluated periods of hospitalization in the 60 days prior to PCP diagnosis, as we are unable to assess medication use during these periods.
Statistical analysis
Characteristics of IBD patients with PCP were described using descriptive statistics, including percentages of cases associated with particular classes of medications.
For all analyses, p values were two-sided, and a p value of .05 or less was considered statistically significant. All statistical analyses were performed by using Stata version 10.0 (College Station, TX). The study protocol was granted exemption from review by the Institutional Review Board at University of North Carolina because it involved the use of existing, de-identified data.
Results
The cohort study population included 108,604 patients with IBD. Of these, 50,932 had CD, 56,403 had UC and 1269 had IBD with unknown type. There were a total of 434,416 individuals in the non-IBD comparison cohort. The median length of follow up within this cohort was 24 months (IQR 12–42) with a range from 1–139 months. Length of follow-up was similar for CD (34 months, IQR 19–51) and UC populations (36 months, IQR 21–54), while significantly less in the non-IBD comparison cohort (21 months, IQR 10–38). Characteristics of the patients with IBD as compared to the non-IBD cohort are shown in table 1. Characteristics in general were similar, with increased health care utilization and immunosuppressive medication use among the IBD patients, and among the CD as compared to UC patients.
TABLE 1.
Characteristics of the Population by IBD Overall and CD or UC
| Characteristic | Non-IBD Cohort (n = 434,416)
|
IBD Cohort (n = 108,604)
|
CD (n = 50,932)
|
UC (n = 56,403)
|
||||
|---|---|---|---|---|---|---|---|---|
| n | Median (IQR) or % | N | Median (IQR) or % | n | Median (IQR) or % | n | Median (IQR) or % | |
| Age | 43 (31–52) | 43 (31–52) | 41 (29–51) | 44 (33–53) | ||||
| Gender (% female) | 238,812 | 55.0 | 59,703 | 55.0 | 28,723 | 56.4 | 30,272 | 53.7 |
| Region of the country | ||||||||
| East | 91,923 | 21.2 | 23,069 | 21.2 | 10,686 | 21.0 | 12,061 | 21.4 |
| Midwest | 169,043 | 38.9 | 46,232 | 42.6 | 22,155 | 43.5 | 23,614 | 41.9 |
| South | 97,829 | 22.5 | 20,350 | 18.7 | 9621 | 18.9 | 10,479 | 18.6 |
| West | 75,621 | 17.4 | 18,953 | 17.5 | 8470 | 16.7 | 10,249 | 18.2 |
| Medicationsa | ||||||||
| 5ASAb | 552 | 0.1 | 37,609 | 34.6 | 15,974 | 31.4 | 21,195 | 37.6 |
| Thiopurinec | 257 | 0.1 | 11,155 | 10.3 | 7558 | 18.8 | 3540 | 6.3 |
| Biologicald | 200 | 0.1 | 3013 | 2.8 | 2607 | 5.1 | 396 | 0.7 |
| Calcineurin inhibitore | 136 | 0.0 | 255 | 0.2 | 79 | 0.2 | 173 | 0.3 |
| Health care utilizationf | 1 (0–4) | 4 (2–9) | 5 (2–10) | 4 (1–8) | ||||
| Comorbidities | ||||||||
| Cardiac | 2935 | 0.7 | 1076 | 1.0 | 475 | 0.9 | 584 | 1.0 |
| Diabetes mellitus | 16,888 | 3.9 | 4824 | 4.4 | 2055 | 4.0 | 2705 | 4.8 |
| Liver disease | 2603 | 0.6 | 2112 | 1.9 | 982 | 1.9 | 1104 | 2.0 |
| Renal | 1181 | 0.3 | 845 | 0.8 | 399 | 0.8 | 440 | 0.8 |
| COPD | 17,191 | 4.0 | 6654 | 6.1 | 3233 | 6.4 | 3338 | 5.9 |
| Total comorbiditiesg | 0 (0–0) | 0 (0–0) | 0 (0–0) | 0 (0–0) | ||||
Defined as any use during screening period, any number of days of supply.
Defined as mesalamine, sulfasalazine, balsalazide, and olsalazine.
Defined as mercaptopurine or azathioprine.
Defined as infliximab, adalimumab, or certolizumab pegol.
Defined as cyclosporine or tacrolimus.
Defined as total number of days (continuous) in screening period with at least 1 billed health care contact.
Total number of comorbidities from the Deyo comorbidity index for administrative data.
Source: IMS Health, LifeLink Health Plan Claims Database, from 1997 to 2009.
For patients with IBD, the overall annual incidence of PCP was 10.6/100,000, compared to 3.0/100,000 in the non-IBD cohort (figure 1). We also evaluated the incidence of PCP in the IBD cohort, comparing those with any prescription for an immunosuppressive medication in the screening period to those without an immunosuppressive prescription. The incidence in those with an immunosuppressive medication was higher at 32/100,000 person-years as compared to 5.5/100,000 in those without. IBD patients had an increased risk of PCP (IRR 3.48, 95% CI 2.10–5.81). The risk was somewhat greater for CD as compared to non-IBD (IRR 4.49, 95% CI 2.14–9.75) than for UC as compared to non-IBD (IRR 2.40, 95% CI 1.11–5.10). The incidence of PCP increased with age, with the highest incidence in the 60+ age strata 32.8/100,000. On adjusted Cox analysis, controlling for comorbidities and health care utilization, the relative risk remained elevated in the overall IBD population (HR 2.96, 95% CI 1.75–4.29). Again, for CD the risk was somewhat greater (HR 4.01, 95% CI 1.88–8.56) than for UC (HR 1.97, 95% CI 0.92–4.20).
Figure 1.
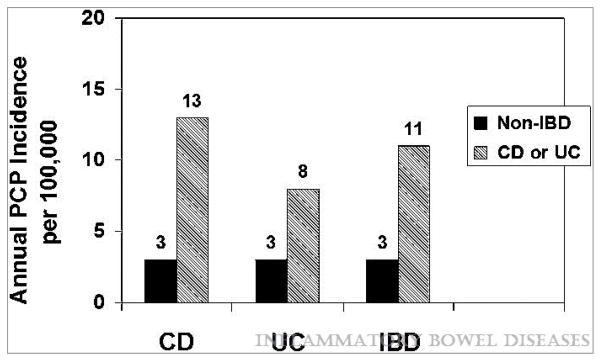
Incidence of pneumocystis jiroveci pneumonia (PCP) in inflammatory bowel disease (IBD) and non-IBD populations
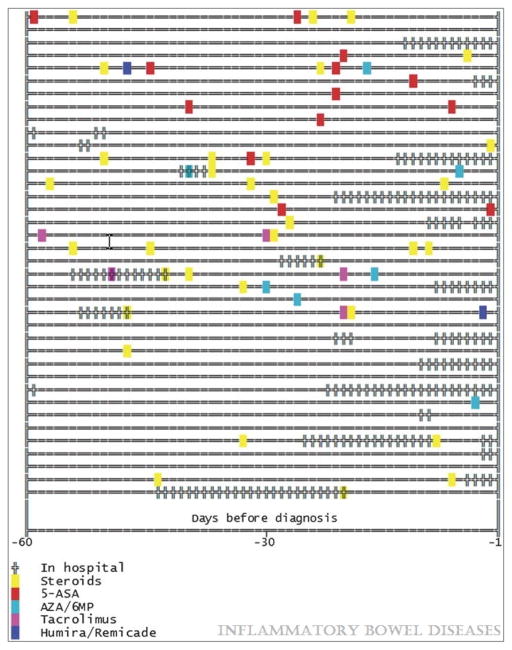
In the case series, a total of 38 individuals with IBD developed PCP. The characteristics of these individuals are shown in table 2. The median age was 49 (IQR 43–57) and gender was roughly equal. Rates of comorbidities among IBD patients with PCP where fairly high, particularly when compared to rates of comorbidities in the IBD population as a whole. Utilization was also high among individuals with PCP. Figure 2 shows medications and hospitalizations in the 60 days prior to PCP diagnosis among these 38 individuals with PCP. A total of 20 (53%) were on corticosteroids alone or in combination with other immunosuppressive medications. A total of 19 (50%) had a hospitalization in the 60 days prior to PCP diagnosis. Only 5 (13%) had no outpatient medication prescriptions or hospitalizations during this time period. In all, 21 (55.3%) were on immunosuppressive drugs, 11 (28.9%) were on corticosteroids alone, 5 (13.2%) on combination thiopurine and corticosteroid, 1 (2.6%) on thiopurine alone and 4 (10.5%) on corticosteroids and 2 other immunosuppressive medications.
TABLE 2.
Characteristics of the IBD Population with PCP
| Characteristics | PCP (n = 38)
|
|
|---|---|---|
| N | % or Median (IQR) | |
| Agea | 49 (43–57) | |
| Gender (% female) | 21 | 55.3 |
| IBD type | ||
| CD | 21 | 55.3 |
| UC | 15 | 39.5 |
| Unknown | 2 | 5.3 |
| Utilizationb | 15 (5–32) | |
| Region | ||
| East | 7 | 18.4 |
| Midwest | 15 | 39.5 |
| South | 8 | 21.1 |
| West | 8 | 21.1 |
| Comorbidities | ||
| Cardiac | 4 | 10.5 |
| Diabetes mellitus | 6 | 15.8 |
| Liver disease | 5 | 13.2 |
| Renal | 5 | 13.2 |
| COPD | 8 | 21.1 |
| Comorbiditiesc | 1 (0–2) | |
Age at time of PCP.
Defined as total number of days (continuous) with any health care contact over 6-month period before PCP diagnosis.
Total number of comorbidities from Deyo comorbidity index.
Source: IMS Health, LifeLink Health Plan Claims Database, from 1997 to 2009.
Figure 2.
Medications and hospitalizations in the 60 days prior to pneumocystis jiroveci pneumonia (PCP) diagnosis in 38 patients with inflammatory bowel disease
In hospital: total duration and timing of hospitalized days prior to PCP diagnosis
Steroids: date where prescriptions filled (any dose or days supply) for corticosteroids
5-ASA: date where prescriptions filled (any dose or days supply) for 5-aminosalicylic acid medications
AZA/6mp: data where prescriptions filled (any dose or days supply) for thiopurine medications (azathioprine, mercaptopurine)
Tacrolimus: date where prescriptions filled (any dose or days supply) for tacrolimus
Humira/remicade: data where prescriptions filled (any dose or days supply) for infliximab (remicade) or adalimumab (humira)
Discussion
In our cohort study, we demonstrated a markedly increased relative risk of PCP in patients with IBD as compared to the general population, with an adjusted effect estimate of HR 2.96, 95% CI 1.75–4.29. However, the absolute risk remained quite low. The risk was higher in the IBD cohort on immunosuppressive medications during screening (32/100,000) than the IBD population on no immunosuppression during this time period (5.5/100,000). Because these calculations incorporated medication use during the screening period, chronic medications were more likely to be captured and corticosteroid use was likely underrepresented. If PCP prophylaxis were 100% effective, and all individuals on any form of immunosuppression were placed on prophylaxis, this would equate to a number needed to treat (NNT) of 3750 to prevent 1 case of PCP in the IBD population. As there was the possibility of underestimation of immunosuppressant exposure status, we recalculated the NNT assuming 25, 50 and 75% underestimation of immunosuppressant exposure among the PCP cases. The NNT remained quite high at 2952, 2432 and 2070 respectively, due to the rarity of the outcome. Therefore, it is evident that other risk factors must also be taken into consideration when targeting a population for prophylaxis. Also, these calculations are for any immunosuppression, and the risk profile may differ in those on multiple immunosuppressive agents. This is particularly important given the increased use of combination immunosuppressive agents to treat certain patients with CD.
Our case series described medication and hospitalization risk factors in the 38 IBD patients who developed PCP. In this group, the majority were on corticosteroids. Other immunosuppressive medications were also common, as was hospitalization in the 60 days preceding the diagnosis. Interestingly, rates of comorbidities were also high in this group of individuals with IBD and PCP. The medication risk factors have been previously described in case series in the IBD population,2–7 and also in the transplant population.
The overall risk of PCP post solid organ transplant (SOT) has been estimated to be between 5 to 15% in the absence of PCP prophylaxis, with attack rates lowest after renal transplant and highest in lung and heart transplant patients.19 The risk factors for PCP have been more extensively studied in this population than the IBD population, likely because of the higher incidence seen with the use of very potent immunosuppressive medications. Risk factors in this population include high-dose corticosteroid therapy, malnutrition and post SOT medications including antilymphocyte agents, calcineurin inhibitors, and biologic agents like alemtuzumab.20, 21 These medications can cause severe neutropenia and lymphopenia, which are believed to increase PCP risk. In addition, previous CMV infection, which has been shown to have immunomodulatory effects; and underlying pulmonary disease, also increase the risk of PCP in SOT patients.22, 23 As a result, post SOT, routine TMP-SMX prophylaxis is recommended in most centers and is continued for at least the first 3 to 12 months, and in some situations for life.24, 25 Similar recommendations do not currently exist in the IBD population. Data on risk of PCP in the IBD population currently consist of descriptive series. Often, the individuals who develop PCP are on multiple immunosuppressive agents.26
There are several strengths to this study of PCP in IBD. We were able to analyze a large sample size. We also had considerable geographic diversity, with patients from all regions of the US. By drawing from a large number of health plans of varying size, type, and location, we believe these results to be broadly generalizable to the commercially insured US population. We attempted to exclude individuals with known HIV, as this population is known to have an increased risk of PCP. We obtained complete data on all billed outpatient prescriptions in order to completely capture medication exposures; rather than relying on patient recall. We were also able to account for health care utilization, as we had access to all health care contacts within the database. Finally, we were able to account for comorbidities through a validated comorbidity index for administrative data.17, 18
There are also several limitations to this study of administrative claims data. There is the possibility of misclassification of exposure and outcome related to the lack of clinical detail. We did use an IBD exposure definition previously used by our group11, 12 that requires multiple health contacts and/or IBD related prescriptions. A similar, but even less specific, administrative case definition has been validated by Herrinton et al.27 The Herrinton definition was found to have a sensitivity exceeding 90% and a PPV exceeding 80% for overall IBD. We used an outcome definition of PCP that required either inpatient claims with an ICD-9 code specific for PCP or outpatient claims with this code in conjunction with appropriate antibiotic dispensing. However, this definition has not been validated. Due to the rarity of the outcome, there were relatively few overall cases of PCP. Another limitation to this study is that the elderly and uninsured were not represented in our population; however, this should not affect the internal validity of the relative risks (IRRs, HRs, ORs) for the under 65 population. Further studies are needed to assess the risk of PCP among the elderly and uninsured. As the risk of PCP increased with age, excluding patients 65 and over may lead to an underestimation of the risk of PCP in this group of patients. While we had access to all billed outpatient prescriptions, we did not have access to those medications given during hospitalizations. As many immunosuppressive medications, such as corticosteroids, are given during hospitalizations for IBD exacerbations, we could not specifically determine medication risk factors in those with a hospitalization preceding their diagnosis of PCP. There were too few cases to evaluate immunosuppression other than as an any/none variable. The type and dose of immunosuppression may play a role in PCP risk, but we were unable to assess this. Additionally, the case series may also have underestimated exposure to medications prescribed more than 60 days prior to PCP diagnosis, for example 90 day prescriptions or prednisone left over from prior prescriptions. Finally, we were unable to account for certain exposures that may be related to the outcome, particularly smoking status. We were able to obtain data on chronic obstructive pulmonary disease (COPD), which has been used as a proxy for smoking status in other studies using administrative data, and controlled for this exposure in our analyses. In our study, COPD was more common among CD patients than UC patients, consistent with prior reports in the literature of increased smoking prevalence among those with CD. However, COPD was also more common in both UC and CD patients as compared to the non-IBD cohort. COPD diagnosis may therefore be a manifestation of both former and current smoking, as UC is often diagnosed after smoking cessation.
In summary, we demonstrated an increased relative risk of PCP in patients with IBD. However, the absolute risk remained quite low, even when considering only those on immunosuppressive medications for IBD, and the NNT to prevent one case of PCP is considerable. Moreover, there can be significant side effects with PCP prophylaxis, including Stevens Johnson’s syndrome, methemoglobinemia, aplastic anemia and an increased risk of pseudomembranous colitis. We therefore would not endorse global PCP prophylaxis in IBD, but rather, consider prophylaxis on an individual basis. We found a particularly increased risk with corticosteroids, prior hospitalization, and comorbid conditions consistent with existing reports in the literature. Any targeted PCP prophylaxis recommendations should consider these potential risk factors.
Acknowledgments
Grant support: This work was supported by a Career Development Award from the Crohn’s and Colitis Foundation of America (MDL), NIH 1K08DK088957-01 (MDK), and NIH P30 DK34987 (RSS).
Footnotes
The statements, findings, conclusions, views, and opinions contained and expressed in this article are based in part on data obtained under license from the following IMS Health Incorporated information service(s): IMS LifeLink™ Health Plan Claims Database (1997–2009), IMS Health Incorporated. All Rights Reserved. The statements, findings, conclusions, views, and opinions contained and expressed herein are not necessarily those of IMS Health Incorporated or any of its affiliated or subsidiary entities.
References
- 1.Hutfless SM, Weng X, Liu L, et al. Mortality by medication use among patients with inflammatory bowel disease, 1996–2003. Gastroenterology. 2007;133:1779–86. doi: 10.1053/j.gastro.2007.09.022. [DOI] [PubMed] [Google Scholar]
- 2.Khatchatourian M, Seaton TL. An unusual complication of immunosuppressive therapy in inflammatory bowel disease. Am J Gastroenterol. 1997;92:1558–60. [PubMed] [Google Scholar]
- 3.Takenaka R, Okada H, Mizuno M, et al. Pneumocystis carinii pneumonia in patients with ulcerative colitis. J Gastroenterol. 2004;39:1114–5. doi: 10.1007/s00535-004-1454-2. [DOI] [PubMed] [Google Scholar]
- 4.Quan VA, Saunders BP, Hicks BH, et al. Cyclosporin treatment for ulcerative colitis complicated by fatal Pneumocystis carinii pneumonia. BMJ. 1997;314:363–4. doi: 10.1136/bmj.314.7077.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Scott AM, Myers GA, Harms BA. Pneumocystis carinii pneumonia postrestorative proctocolectomy for ulcerative colitis: a role for perioperative prophylaxis in the cyclosporine era? Report of a case and review of the literature. Dis Colon Rectum. 1997;40:973–6. doi: 10.1007/BF02051208. [DOI] [PubMed] [Google Scholar]
- 6.Smith MB, Hanauer SB. Pneumocystis carinii pneumonia during cyclosporine therapy for ulcerative colitis. N Engl J Med. 1992;327:497–8. [PubMed] [Google Scholar]
- 7.Kaur N, Mahl TC. Pneumocystis carinii pneumonia with oral candidiasis after infliximab therapy for Crohn’s disease. Dig Dis Sci. 2004;49:1458–60. doi: 10.1023/b:ddas.0000042246.58984.98. [DOI] [PubMed] [Google Scholar]
- 8.Slifkin M, Doron S, Snydman DR. Viral prophylaxis in organ transplant patients. Drugs. 2004;64:2763–92. doi: 10.2165/00003495-200464240-00004. [DOI] [PubMed] [Google Scholar]
- 9.Poppers DM, Scherl EJ. Prophylaxis against Pneumocystis pneumonia in patients with inflammatory bowel disease: toward a standard of care. Inflamm Bowel Dis. 2008;14:106–13. doi: 10.1002/ibd.20261. [DOI] [PubMed] [Google Scholar]
- 10.Jones JL, Hanson DL, Dworkin MS, et al. Surveillance for AIDS-defining opportunistic illnesses, 1992–1997. MMWR CDC Surveill Summ. 1999;48:1–22. [PubMed] [Google Scholar]
- 11.Long MD, Herfarth HH, Pipkin CA, et al. Increased risk for non-melanoma skin cancer in patients with inflammatory bowel disease. Clin Gastroenterol Hepatol. 2010;8:268–74. doi: 10.1016/j.cgh.2009.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Long MD, Porter CQ, Sandler RS, et al. Suboptimal rates of cervical testing among women with inflammatory bowel disease. Clin Gastroenterol Hepatol. 2009;7:549–53. doi: 10.1016/j.cgh.2008.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stempel DA, Mauskopf J, McLaughlin T, et al. Comparison of asthma costs in patients starting fluticasone propionate compared to patients starting montelukast. Respir Med. 2001;95:227–34. doi: 10.1053/rmed.2000.1027. [DOI] [PubMed] [Google Scholar]
- 14.Gupta G, Lautenbach E, Lewis JD. Incidence and risk factors for herpes zoster among patients with inflammatory bowel disease. Clin Gastroenterol Hepatol. 2006;4:1483–90. doi: 10.1016/j.cgh.2006.09.019. [DOI] [PubMed] [Google Scholar]
- 15.Kappelman MD, Rifas-Shiman SL, Kleinman K, et al. The prevalence and geographic distribution of Crohn’s disease and ulcerative colitis in the United States. Clin Gastroenterol Hepatol. 2007;5:1424–9. doi: 10.1016/j.cgh.2007.07.012. [DOI] [PubMed] [Google Scholar]
- 16.Lund JL, Sturmer T, Porter CQ, et al. Thiazolidinedione use and ulcerative colitis-related flares: an exploratory analysis of administrative data. Inflamm Bowel Dis. 2011;17:787–94. doi: 10.1002/ibd.21348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Quan H, Sundararajan V, Halfon P, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care. 2005;43:1130–9. doi: 10.1097/01.mlr.0000182534.19832.83. [DOI] [PubMed] [Google Scholar]
- 18.Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45:613–9. doi: 10.1016/0895-4356(92)90133-8. [DOI] [PubMed] [Google Scholar]
- 19.Radisic M, Lattes R, Chapman JF, et al. Risk factors for Pneumocystis carinii pneumonia in kidney transplant recipients: a case-control study. Transpl Infect Dis. 2003;5:84–93. doi: 10.1034/j.1399-3062.2003.00018.x. [DOI] [PubMed] [Google Scholar]
- 20.Yale SH, Limper AH. Pneumocystis carinii pneumonia in patients without acquired immunodeficiency syndrome: associated illness and prior corticosteroid therapy. Mayo Clin Proc. 1996;71:5–13. doi: 10.4065/71.1.5. [DOI] [PubMed] [Google Scholar]
- 21.Gilroy SA, Bennett NJ. Pneumocystis pneumonia. Semin Respir Crit Care Med. 2011;32:775–82. doi: 10.1055/s-0031-1295725. [DOI] [PubMed] [Google Scholar]
- 22.Phipps LM, Chen SC, Kable K, et al. Nosocomial Pneumocystis jirovecii Pneumonia: Lessons From a Cluster in Kidney Transplant Recipients. Transplantation. 2011;92:1327–34. doi: 10.1097/TP.0b013e3182384b57. [DOI] [PubMed] [Google Scholar]
- 23.Husni RN, Gordon SM, Longworth DL, et al. Cytomegalovirus infection is a risk factor for invasive aspergillosis in lung transplant recipients. Clin Infect Dis. 1998;26:753–5. doi: 10.1086/514599. [DOI] [PubMed] [Google Scholar]
- 24.Fishman JA. Prevention of infection caused by Pneumocystis carinii in transplant recipients. Clin Infect Dis. 2001;33:1397–405. doi: 10.1086/323129. [DOI] [PubMed] [Google Scholar]
- 25.Martin SI, Fishman JA. Pneumocystis pneumonia in solid organ transplant recipients. Am J Transplant. 2009;9 (Suppl 4):S227–33. doi: 10.1111/j.1600-6143.2009.02914.x. [DOI] [PubMed] [Google Scholar]
- 26.Lawrance IC, Radford-Smith GL, Bampton PA, et al. Serious infections in patients with inflammatory bowel disease receiving anti-tumor-necrosis-factor-alpha therapy: an Australian and New Zealand experience. J Gastroenterol Hepatol. 2010;25:1732–8. doi: 10.1111/j.1440-1746.2010.06407.x. [DOI] [PubMed] [Google Scholar]
- 27.Herrinton LJ, Liu L, Lafata JE, et al. Estimation of the period prevalence of inflammatory bowel disease among nine health plans using computerized diagnoses and outpatient pharmacy dispensings. Inflamm Bowel Dis. 2007;13:451–61. doi: 10.1002/ibd.20021. [DOI] [PubMed] [Google Scholar]