Abstract
Eye gaze is a powerful cue for orienting attention in space. Studies examining whether gaze and symbolic cues recruit the same neural mechanisms have found mixed results. We tested whether there is a specialized attentional mechanism for social cues. We separately measured BOLD activity during orienting and reorienting attention following predictive gaze and symbolic cues. Results showed that gaze and symbolic cues exerted their influence through the same neural networks, but also produced some differential modulations. Dorsal fronto-parietal regions in left IPS and bilateral MT+/lateral occipital cortex only showed orienting effects for symbolic cues while right pIPS showed larger validity effects following gaze cues. Both exceptions may reflect the greater automaticity of gaze cues: symbolic orienting may require more effort, while disengaging attention during reorienting may be more difficult following gaze cues. Face-selective regions, identified with a face localizer, showed selective activations for gaze cues reflecting sensory processing but no attentional modulations. Therefore, no evidence was found linking face-selective regions to a hypothetical, specialized mechanism for orienting attention to gaze cues. However, a functional connectivity analysis showed greater connectivity between face-selective regions and right pIPS, pSTS and IFG during gaze cueing, consistent with proposals that face-selective regions may send gaze signals to parts of the DAN and VAN. Finally, although the default-mode network is thought to be involved in social cognition, this role does not extend to gaze orienting as these regions were more deactivated following gaze cues and showed less functional connectivity with face-selective regions during gaze cues.
INTRODUCTION
Shifts of visual attention can be automatically driven by stimulation (i.e. exogenous cues) or voluntarily deployed (i.e. endogenous cues). Both voluntary attention, as studied with symbolic cues (e.g. an arrow pointing to a location) (Corbetta and Shulman, 2002; Yantis et al., 2002; Corbetta et al., 2008), and reflexive attention, as studied with highly salient but non-informative peripheral cues (Lepsien and Pollmann, 2002; Kincade et al., 2005; Mayer et al., 2006), recruit a dorsal fronto-parietal attention network (DAN) with principal nodes in intra-parietal sulcus/superior parietal lobule (IPS/SPL) and frontal eye field (FEF). Once attention has been focused on an object, the appearance of a novel or behaviorally relevant object may produce a reorienting response. Reorienting recruits the DAN but also a ventral fronto-parietal attention network (VAN) related to detection of unattended but novel or task relevant stimuli, with principal nodes in temporal-parietal junction (TPJ) and ventral frontal cortex/insula (Arrington et al., 2000; Corbetta et al., 2000; Shulman et al., 2009; Corbetta and Shulman, 2002; Corbetta et al., 2008 for a review).
Gaze is a very powerful cue for orienting that seems more automatic than purely symbolic cues, although it does not share all the features of exogenous cues (Frischen et al., 2007). Studies of gaze perception and social cognition raise the possibility that orienting by gaze may be subserved by a special attentional system and thus may involve brain regions not recruited by other orienting cues, particularly in temporal cortex and TPJ. Perret et al. (1985) found gaze-direction responsive cells in the monkey’s superior temporal sulcus (STS), a result confirmed in humans (Allison et al., 2000; Hoffman and Haxby, 2000; Kingstone et al., 2004) while a neighboring region in TPJ, similar to that observed during reorienting (Mitchell, 2008; Scholz et al., 2009), has been linked to social cognition and theory of mind (Saxe and Kanwisher, 2003). Studies that have compared the brain regions recruited by gaze and symbolic orienting, however, have reported mixed results, with some finding common activations (Tipper et al., 2008; Greene et al., 2009; Sato et al., 2009) and others finding gaze-specific activations in occipito-temporal regions (Hietanen et al., 2006; Engell et al., 2010) that appear distinct from those observed during gaze perception or theory of mind.
Additionally, PPI analyses (Nummenmaa and Calder, 2009) have been conducted to understand the dynamics and relative contribution of face processing regions and attentional networks to the processing of the spatial information associated with gaze. Fusiform gyrus and superior temporal sulcus, both part of the face network (Haxby et al., 2000), have been found to be more connected to regions of the DAN and VAN (Corbetta et al., 2008) when paying attention to faces in which there was a horizontal shift in gaze relative to faces in which the eyes opened and closed. One interpretation of these results is that face processing regions send information to attentional networks during gaze shifts.
Overall, it is currently unclear whether social and symbolic orienting involves common or distinct brain systems, and whether face processing regions are part of a specialized social orienting network or whether their role is restricted to feeding information to supramodal attentional systems. Discrepant results across studies may have reflected methodological factors, such as the use of blocked rather than event related designs or the use of highly schematic faces rather than more realistic computer-generated displays or photographs. Most importantly, no prior study comparing social and symbolic cues has separately measured the brain activations for orienting attention in space and reorienting attention for target detection. An experimental design in which the activations following a cue to orient attention are separated from the activations related to the presentation of a subsequent target is critical for clearly identifying the different activations for orienting attention and re-orienting to a target.
Therefore, the current work improved on previous studies in two important ways. First, we used an experimental design that allowed separate measurements of the activity associated with orienting of attention and reorienting of attention following both gaze and symbolic cues (Corbetta et al., 2000; Ollinger et al., 2001a, 2001b). The event-related character of the design also allowed trials with directional and non-directional cues to be intermixed. Second, we used a large set of computer generated dynamic, realistic faces with smooth eye movements that should powerfully recruit gaze-specific mechanisms. In addition, we conducted separate localizer scans to identify face processing regions and separately tested them for effects of attentional orienting and re-orienting. This design feature provided a targeted assessment of the role of face-selective regions in gaze orienting. All these improvements over previous studies allowed us to test the hypothesis that different neural networks are responsible for social and symbolic cueing. Finally, we carried out functional connectivity analyses on task-regressed data to measure interactions between regions during gaze and symbolic orienting and re-orienting. These analyses tested the hypothesis that supramodal attention systems (DAN and VAN) dynamically interact with face specific regions in occipital and temporal cortex when social stimuli are processed.
METHODS
Participants
Twenty six participants were run in the imaging session. All participants were right handed, were screened for neurological or psychiatric conditions, had normal or corrected to normal vision, and were not taking any psychoactive medications. Participants were recruited from the Washington University community and gave informed consent according to the guidelines of the Institutional Review Board of the Washington University School of Medicine. Participants ran a behavioral version of the experiment prior to the imaging session. Participants were invited to return for the imaging session if they showed a cueing effect (i.e. faster reaction times for valid than invalid trials) for both arrow and gaze cues and were able to maintain fixation during the task. Of the 46 participants that completed the behavioral session, 18 did not meet the criteria for inclusion in the scanning experiment (inhibition of return, IOR, instead of facilitation effect in gaze trials, n=7; IOR in arrow trials, n=7: IOR in both tasks, n=1; poor compliance with the task, n=2; unable to maintain fixation, n=1). Two subjects that met all the criteria did not attend their scheduled imaging session. Of the 26 subjects that were scanned, 2 were excluded due to excessive movement inside the scanner.
Apparatus
Stimulus presentation and data collection were carried out with E-Prime 2 (Schneider et al., 2002) running on a Dell Precision T3400 computer (behavioral session) and a Dell Latitude D630 laptop (imaging session). Responses were recorded with an e-prime button box for the behavioral session and an mri-compatible fiber-optic button box connected to an e-prime button box for the scanning experiment. In the imaging session, stimuli were projected to the head of the bore of the scanner via a liquid crystal display projector (Sharp LCD C20X) and viewed with a mirror attached to the head coil. In both settings the button box was placed so both keys were located orthogonal to the location of the target stimuli on the screen (i.e. it was placed parallel to the body axis) in order to prevent stimulus location-response hand congruency effects.
Stimuli
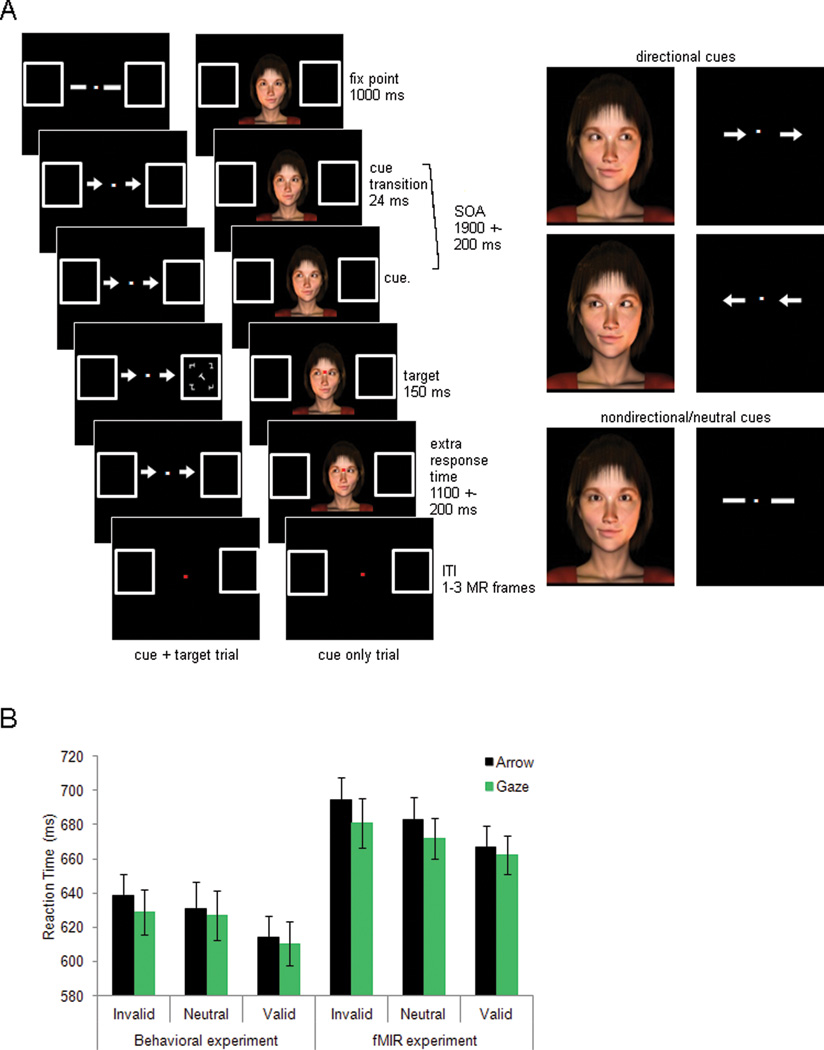
Participants performed a spatial cueing task. Two types of cues were used. Arrow cues consisted of two light grey arrows presented on both sides of a fixation dot. Arrows subtended 0.89°; visual angle horizontally and were located 1.15°; right and left of fixation (distance from fixation to center of the arrow). Gaze cues consisted of computer generated realistic images of male/female faces showing the neck and shoulders (Figure 1A). All gaze cue images were presented with the same t-shirt color to minimize saliency based on features unrelated to face identity. Each gaze display subtended 10.92 × 11.42°; and the actual face was 6–8°; horizontal × 8.9°; vertical. Both cue stimuli were designed so that the size and screen location of the cue (arrows or eyes) were comparable. A total of 152 different stimuli were used (76 female and 76 male faces). In order to minimize previous exposure, four female faces not used in the imaging experiment were used as gaze stimuli in the behavioral experiment. Target stimuli consisted of a white rotated target letter (“L” or “T”) surrounded by four composite T/L white distracters. Letters and distracters could appear in one of four different orientations (45°;, 135°;, 225°; and 315°;). Targets (0.88°; × 0.66°;) were presented 7.6°; to the left or right of the fixation point inside one of two place-holder boxes (4°; × 4°;) that were present throughout the trial. A fixation dot was present during the entire trial. On gaze-cue trials, the dot was superimposed on the face stimulus at the intersection of the eye line with the nose.
Figure 1.
A). Procedure. Example of a valid cue+target arrow trial, a cue-only neutral gaze trial and the different types of cues. B). Behavioral results for all subjects (n=47) during the behavioral experiment (left panel), and for subjects (n=24) during the scanning session (right panel). Error bars show the standard error of the mean.
Stimuli for the localizer experiment consisted of 54 photographs of scenes (inside or outside of buildings, 27 each) and 54 photographs of faces (male or female, 27 each). These images have previously been used by Tosoni et al. (2008). Each black and white image subtended 6°; × 6°;.
Procedure
Behavioral session
Participants received 12 blocks of trials, 36 trials per block. The first 10 blocks involved trials with a cue followed by a target (cue+target trials) while the last two blocks mixed cue+target trials with trials where no target followed the cue (cue only trials). The first 10 blocks only included directional trials (i.e. trials where the cue pointed to the left or right side of the screen) while the last two blocks also included non-directional trials (i.e. trials where the cue did not offer directional information). The first 10 blocks were used to check for a cueing effect and the last two blocks were used to acquaint participants with the task as it would be run in the scanner. Accuracy feedback was given after each block. Gaze cue trials and arrow cue trials were blocked, presented in alternating order and counterbalanced across participants. As shown in Figure 1a, every trial started with the presentation of a face stimulus looking straight ahead (gaze trials) or two thick lines (arrow trials) for 1000ms. Subsequently, on gaze directional trials, the eyes looked 45°; to the left or right for 24 ms and then 85°; left or right where they remained until the end of the trial. This succession of images produced a vivid sensation of the eyes moving to one side. On arrow directional trials, the two thick lines changed into arrows pointing to the left or right. Following a variable SOA (1900±200ms) the target was flashed in one of two placeholders for 150ms, and participants were required to make an identity discrimination (i.e. letter “L” or “T”) by pressing one of two keys with the right index or middle finger (counterbalanced). The target could appear at the location signaled by the cue (valid trials; 75%) or at the opposite location (invalid trials: 25%). The visual cue (face or arrows) remained on the screen until the participant responded or for a maximum duration of 1500ms. An inter-trial interval (ITI) of 1000ms followed. During the ITI period, both the fixation point and place-holders remained on screen, and the fixation point changed color (white to red) to indicate the end of the trial. As previously described, the last two blocks introduced two additional conditions so that participants could become familiar with the experiment as presented in the scanner. First, cue only trials were introduced. On these trials the cue stimulus was not followed by the presentation of a target. Instead, the color of the fixation point changed from white to red to indicate the end of the trial at the same time as a target would have been presented on a cue+target trial. Second, non-directional trials were also included. On non-directional gaze trials, the eyes blinked (for 24ms) and opened again for the remainder of the trial. On non-directional arrow trials, the two thick lines became thin (for 24ms) and then returned to their original thickness for the remainder of the trial. Therefore, non-directional cues underwent stimulus changes over time, as did directional cues but did not provide directional information. In these last two blocks (one arrow block and one gaze block), cue + target trials accounted for 78% of the total and cue only trials accounted for the remaining 22%. In each of these conditions, directional cues were present in 57% of the trials and non-directional cues appeared in the remaining 43%. Of the directional cues, 75% were valid in predicting target location and 25% invalid.
Imaging session
Participants were comfortably placed in the scanner bed, they performed two practice blocks (one per cue type) while the scanner was being calibrated. Participants performed 16 experimental scans (36 trials/scan) and used the same key response finger mapping and the same cue type counterbalancing order as in the behavioral experiment. Trial structure and percentage of trial conditions were the same as those described for the last two blocks of the behavioral task with the following differences. The cue remained on the screen until 1100±200ms after target offset (the end of the second MR frame). The ITI was extended to 2.06, 4.12 or 6.18s (1, 2 or 3 MR frames). Therefore the average duration of a trial was 8.24 s (4 MR frames).
Face localizer
Sixteen of the 24 participants received two scans designed to localize face selective regions. They performed a 1-back task responding whenever the same image was presented twice consecutively (twenty times per scan). Each scan consisted of 8 face blocks, 8 scene blocks and 8 fixation blocks semi-randomly intermixed (no consecutive blocks of the same type were allowed; Figure 3A). Each experimental block lasted 16.5 s (8 MR frames) and contained 16 images shown for 800ms with a 200ms ITI. Fixation blocks had a variable duration of 10.3–14.4 s (5–7 frames). Each image was presented, on average, 4.7 times during the experiment, across different blocks.
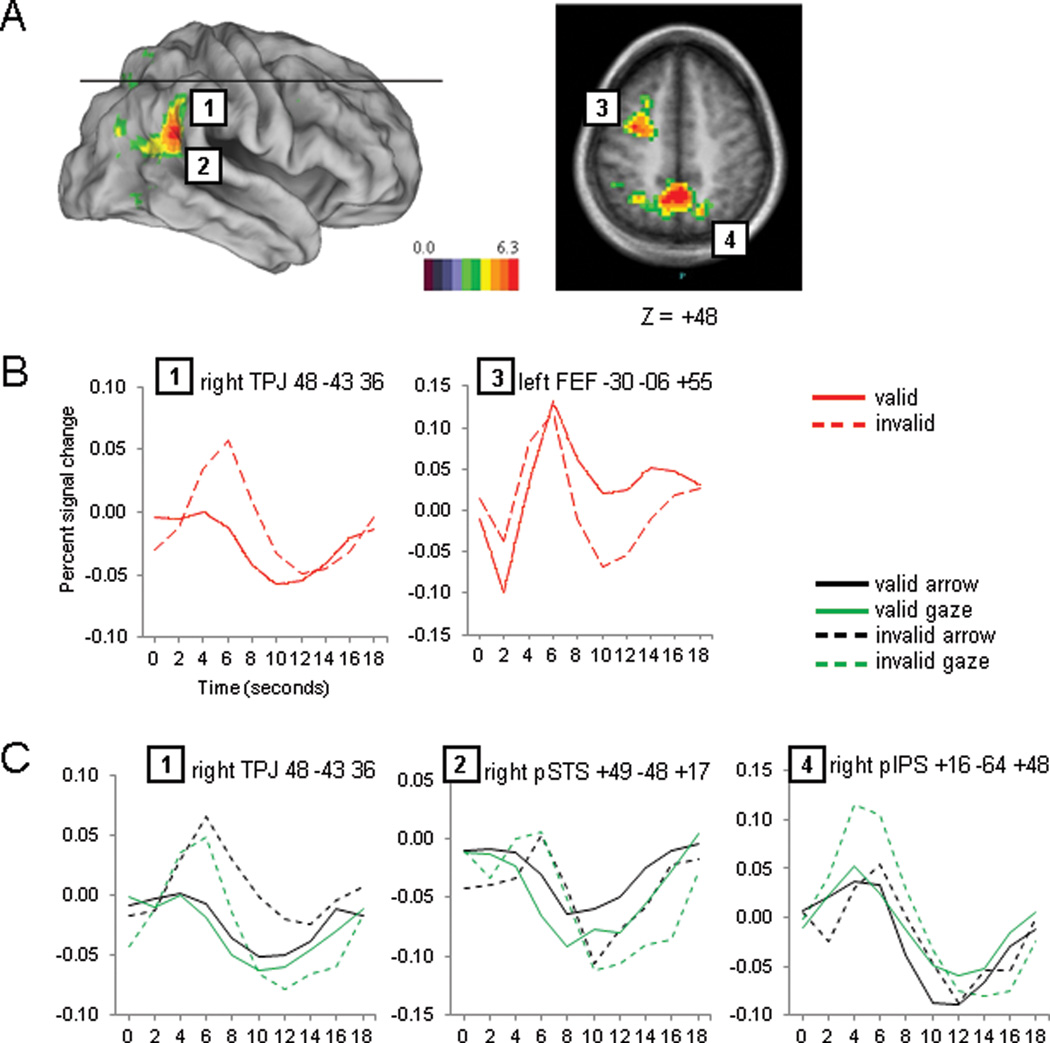
Figure 3.
Target period. A). Voxel-wise map of the validity effect. B). Time courses of the validity effect for two representative regions. C). Time courses for the regional analysis of Cue Type × Cue Validity × Time performed on regions from the validity map shown in panel A.
Eye movement recording
In order to assure that participants maintained fixation throughout the trials, eye movements were recorded during both behavioral and imaging sessions (behavioral session: Eyelink1000, SR Research Ltd. Ottawa, Canada; imaging session: ISCAN ETL-200, ISCAN Inc. MA, USA) fMRI acquisition and data analysis.
Acquisition and preprocessing
Imaging was performed on a Siemens Allegra 3T scanner with a gradient-echo echo planar imaging sequence to measure BOLD contrast. Thirty-two contiguous 4mm slices were acquired, 4×4 mm in-plane resolution, echo time (TE) = 25ms, flip angle = 90°;, repetition time (TR) = 2.06 s. Structural images included a sagittal MP-RAGE T1-weighted sequence (TR = 1810 ms, TE = 3.93ms, flip angle = 12°;, inversion time = 1200ms, voxel size = 1 × 1 × 1.25 mm).
Compensation of acquisition time by sinc interpolation was carried out to align all the slices of each frame to the start of that frame. Whole-brain normalization was performed to correct for changes in signal intensity across scans. Data was realigned within and across scans to correct for head movements. Images were re-sampled into 3mm isotropic voxels and registered to an atlas-space representative target volume (Talairach and Tournoux, 1988) using a 12-parameter affine transformation. Movement correction and atlas transformation was accomplished in one re-sampling step to minimize blur and noise. All preprocessing steps, as well as statistical analyses were carried out using in-house software.
Task evoked BOLD signal analysis
The BOLD signal was analyzed with the general linear model (GLM) to estimate the activations associated with each experimental event type. We estimated the BOLD activity without assuming a hemodynamic response by using a finite-impulse-response (FIR) model that involved 10 timepoint regressors for each event type (Ollinger et al., 2001a). Overall, the regression model included ten timepoint regressors (one for each of 10 consecutive MR frames) for each of six cue event-types (left directional, right directional and non-directional arrow cues and gaze cues) and each of eight target event-types (left valid, right valid, left invalid and right invalid for arrow cue trials and gaze cue trials). A separate set of ten timepoint regressors was estimated for trials involving an incorrect response. Non task-related regressors were included for baseline, linear trend and low frequency (<0.009 Hz) components of the BOLD signal. For each event type, the set of 10 timepoint regressors constitutes its event-related BOLD ‘time course’.
The whole-brain maps of parameter estimates resulting from these GLMs were smoothed with a Gaussian filter with a full-with-at-half-maximum of 6mm and analyzed with voxel-wise as well as ROI ANOVAs in which subject was a random factor. An initial voxel-wise analysis of the cue period using 10 timepoint regressors revealed a large number of significant activations that, when further studied, showed to be due to a large activation increase in the time course of the response for the non directional arrow trials after time point 7 (i.e. 14 seconds after cue presentation). Foci that showed this effect were not seen in a separate voxelwise ANOVA in which only the first 7 time points of the estimated time course were used. Thus the analyses we report are those carried out on the 7 time point data set. Therefore, voxel wise ANOVAs were performed with factors Cue Type (2), Cue Directionality (2) and Time (7), for the cue period, and Cue Type (2), Cue Validity (2) and Time (10), for the target period. Statistical images were corrected for violations of sphericity and corrected over the brain for multiple comparisons using a joint z-score/cluster size criteria of z = 3 and cluster size of at least 13 contiguous voxels (Forman et al., 1995) determined by in-house simulations to correspond to a whole-brain multiple comparison corrected p-value of p<0.05 (McAvoy et al., 2001). An automated algorithm that searches for local maxima and minima was used to identify the peak coordinates for each region in Region of Interest (ROI) analyses. Each ROI included all voxels in the multiple-comparison corrected z-map that fell within a 16mm diameter sphere centered on the peak coordinate. These ROIs were then analyzed using a ‘regional ANOVA’. In a regional ANOVA, a parameter estimate from the voxelwise GLM was first averaged across all the voxels in the ROI to arrive at a parameter estimate for the ROI. After applying this averaging procedure to each of the parameter estimates relevant for a particular analysis, the parameter estimates for the ROI were then entered into an ANOVA. The ROIs that were analyzed in regional ANOVAs were always identified from the voxel wise activation map using a term in the ANOVA that was orthogonal to the term tested in the regional ANOVA. For example, ROIs that were formed from voxels that showed a significant interaction of Cue Directionality by Time were statistically evaluated in a regional ANOVA with respect to the interaction of Cue Type by Cue Directionality by Time. This procedure ensured that the selection of the ROIs did not bias the results of the regional ANOVA.
Face localizer BOLD analysis
Analysis of the face localizer scans involved the steps previously defined plus the following specific steps aimed at establishing the face specific regions in an objective manner. We first identified the coordinates of ‘face-selective’ regions reported in previous studies (i.e. bilateral fusiform face area, FFA, bilateral occipital face area, OFA, and right posterior superior temporal sulcus, pSTS) (Hoffman and Haxby, 2000; Grill-Spector et al., 2004; Andrews et al., 2010). For each face region, we then found the mean and standard deviation (SD) of the reported coordinates and created a sphere that was centered on the average coordinate and had a radius of 2 SD. We used this sphere as a search region within each of the 16 subjects that performed our localizer experiment in order to define ‘face-selective’ ROIs for that subject. First, we thresholded the subject’s voxelwise statistical map for the contrast faces>scenes at a z-threshold corresponding to p=0.001, uncorrected. Then, we found the voxel within the search region that yielded peak activity. Finally, we formed a 16mm diameter ROI centered on the peak activation for that subject. Using this procedure we identified left and right FFA, in 13/16 participants; rOFA, in 14/16 participants; left OFA, in 13/16 participants; and right pSTS, in 9/16 participants. Lowering the threshold to p=0.023 (z=2) allowed the identification of left OFA in 1 more participant, right FFA, left FFA and right OFA in 2 more participants, and right pSTS in 5 more participants. In addition to the individually defined face ROIs, a group level set of face ROIs was also created by averaging the peak coordinates for each individually-defined ROI across our subjects (e.g. averaging the coordinates of all the individually defined lFFA) and forming a 16mm diameter ROI centered on that peak. This group level set of ROIs was used in group (n=24) based analyses.
Task regressed functional connectivity preprocessing and analysis
After the task-evoked preprocessing was finished, the BOLD time series underwent spatial smoothing with a 6mm full-with-at-half-maximum Gaussian blur, temporal filtering to exclude frequencies below 0.009 and above 0.08Hz, removal by regression of six parameters for head motion (3 translation, 3 rotation), a whole brain signal (except the ventricles), a signal from a ventricular region, a signal from a white matter region and temporal derivatives of these regressors to account for the time-shifted versions of spurious variance. Task-evoked activity was also regressed out by removing a simplified version of the design matrix (collapsing left and right trials) used to analyze the task-evoked BOLD activity. This design matrix included two cue types (arrow and gaze) two cue directionality types (directional and non-directional), two target types (valid and invalid), ten time points and one regressor for error trials. All blocks belonging to the symbolic task (i.e. arrow cue) and social task (i.e. gaze cue) were separately concatenated to form a time series for each cue type. For each time-series, voxelwise analyses were carried out by computing the correlation over the time series between a set of seeds and each voxel in the brain. We placed seeds in the five group level defined face network regions. We then compared the connectivity between the face network and the rest of the brain during symbolic and social (task-regressed) blocks for each subject. Specifically, we computed the voxelwise difference map (social minus symbolic) for each of the five face seeds, averaged the five maps to increase the stability of the results, and then performed a voxelwise random effects group analysis using the averaged single-subject difference maps. Subject was treated as a random factor. We also carried out voxelwise t-tests for the individual seeds that compared connectivity in the social vs. symbolic blocks. All results were multiple-comparison corrected with a joint z-score/cluster-size (z=2.5; n=35, p<=0.05) criterion based on monte-carlo simulations (McAvoy et al., 2001).
RESULTS
Behavioral data
Participants performed the task in the scanner with high accuracy (0.94). A repeated measures ANOVA with the factors Cue Type (arrow vs. gaze) and Cue Validity (valid, invalid and neutral) showed no main effects of Cue Type or Cue Validity and no significant interaction (all Fs<1). Reaction times (RTs) for incorrect trials and those above or below 2.5 standard deviations from the mean (regarded as outliers) were excluded from the RT analysis. A repeated-measures ANOVA on the reaction times collected in the scanner (right panel of Figure 1B) showed that gaze trials involved faster responses than arrow trials (F(1,23)=5.42, p=0.0290) and that valid targets were discriminated faster than invalid targets (F(2,46)=13.28, p=0.0001). This validity effect was found on both arrow and gaze trials (arrow trials: invalid vs. valid, 695 ms vs. 667 ms, F(1,23)=25.96, p=0.0001; gaze trials: invalid vs. valid, 681 ms vs. 662 ms, F(1,23)=6.22, p=0.0203), and no interaction of Cue Validity by Cue Type was observed (F(1,46)=1.1, n.s.). RTs to non-directional trials were between those for valid and invalid trials (683 ms for arrows and 672 ms for gaze).
The results from the pre-scan behavioral session for the entire sample of 46 participants (i.e. those participants were selected for the imaging experiment together with those that did not meet the criteria to be scanned) (left panel of Figure 1B) also showed a main effect of Cue Validity (F(2,47)=6.37, p=0.003), with no effect of Cue Type (F<1) and no interaction of Cue Validity and Cue Type (F<1). Therefore, the critical behavioral result found in the imaging sample (i.e. a cueing effect) was not an artifact of subject selection but assured that imaging of the corresponding brain activations would be more robust since our subjects showed consistent effects of cueing. The fact that a large proportion of participants did not meet the criteria to come back (a nominal cueing effect for both arrow and gaze trials) suggests that individual differences in cueing effects are obscured by the fact that group analyses are routinely reported. Overall, these results show that the paradigm yielded significant and equivalent attentional cueing effects in the gaze and arrow conditions. Also, non-directional cues appeared to act as an appropriate ‘neutral-attention’ condition in which attention was not strongly committed to a location, since RTs were intermediate between those for valid and invalid trials. The intermediate performance for non-directional cues indicates that those cues provided similar temporal or alerting information as directional cues.
Neuroimaging data
1. Are there different mechanisms for symbolic and social cueing?
To address this issue we analyzed the task evoked activity during orienting of attention (cue period) and reorienting of attention/target detection (target period). We also specifically analyzed activity in face processing regions to test whether they were the site of a specialized social attentional cueing mechanism.
Task evoked activity: cue period
Cue Type effect
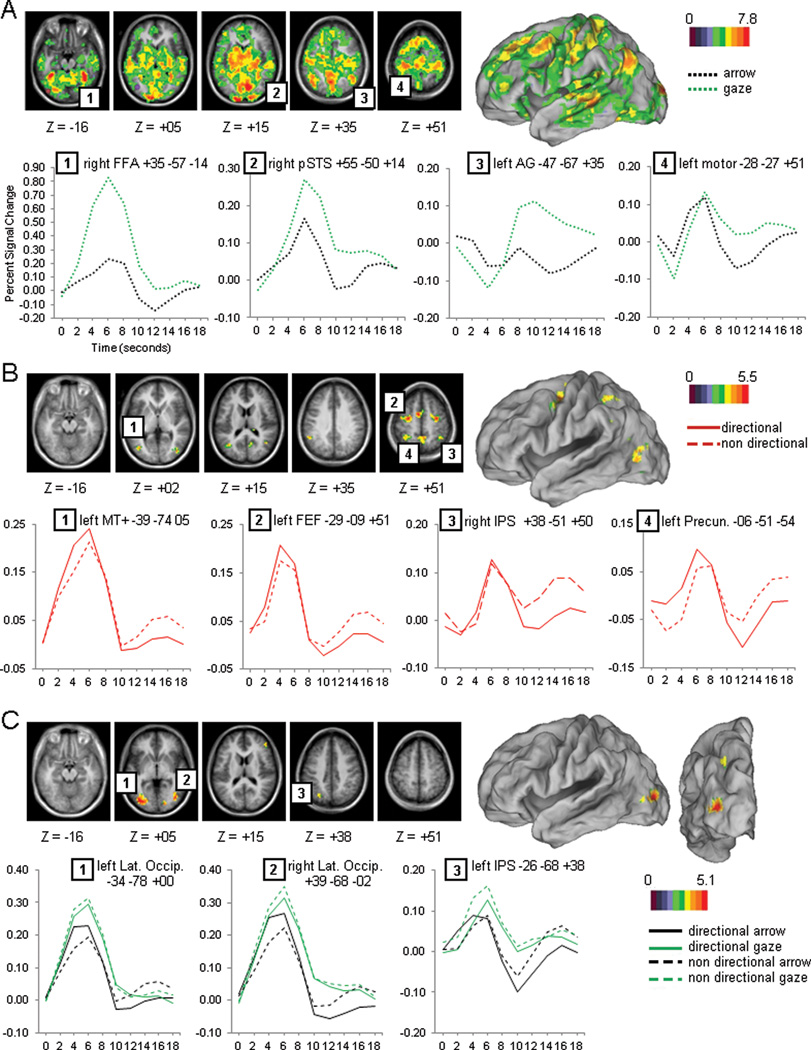
The gaze and arrow cue stimuli had very different sensory properties and produced widespread differences in brain activity involving both cortical and subcortical regions. A voxelwise ANOVA with Cue Type (gaze, arrow), Cue Directionality (directional, non-directional), and Time (7 time points) as factors indicated a large set of regions that showed significantly different BOLD responses for arrows and gaze cues, as indexed by a significant interaction of Cue Type by Time (Figure 2A). Within the visual system, regions that showed larger activations for gaze than arrow cues were primarily observed within striate and extra-striate cortex, including bilateral ventral occipital cortex, fusiform gyrus, medial occipital, superior occipital, and right posterior superior temporal sulcus (pSTS) (see Figure 2A and Table 1 for peak coordinates). A significant effect of cue type was also observed in regions likely belonging to the default mode network (DMN). During the gaze but not arrow condition, DMN regions were initially deactivated followed by a later activation (Figure 2A shows the time course for a representative region of the DMN, the angular gyrus (AG), and a region outside of the DMN, central sulcus).
Figure 2.
Cue period. A). Voxel-wise map and time courses for the Cue Type by Time image. B). Voxel-wise map and time courses for the Cue Directionality by Time image. C). Voxel-wise map and time courses for the Cue Type by Cue Directionality by Time image.
Table 1.
Stereotaxic coordinates (in Talairach & Tournoux space) for activation peaks shown by experimental condition. Peak z-score and activation voxel size are also shown.
| Right | Left | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Region | X | Y | Z | z-score | voxel size |
X | Y | Z | z-score | voxel size |
| CueType × time | ||||||||||
| Fusiform Gyrus | 35 | −57 | −14 | 11.11 | 80 | −34 | −47 | −18 | 10.13 | 79 |
| ventral occipital | 28 | −76 | −8 | 9.22 | 80 | −22 | −81 | −8 | 9.35 | 70 |
| medial occipital | 4 | −67 | 13 | 10.58 | 74 | −12 | −89 | −4 | 9.65 | 71 |
| posterior superior occipital | 17 | −96 | 19 | 10.27 | 65 | −13 | −98 | 10 | 10.09 | 71 |
| p STS | 55 | −50 | 14 | 5.85 | 45 | |||||
| AG | 50 | −58 | 25 | 7.77 | 70 | −47 | −67 | 35 | 8.20 | 27 |
| central sulcus | 29 | −31 | 55 | 8.08 | 78 | −28 | −27 | 51 | 9.13 | 82 |
| CueDirectionality × time | ||||||||||
| FEF | 32 | −10 | 47 | 5.51 | 57 | −29 | −9 | 51 | 5.13 | 64 |
| a IPS | 38 | −51 | 50 | 3.96 | 19 | −28 | −50 | 47 | 3.82 | 43 |
| precuneus | 4 | −48 | 48 | 4.70 | 43 | −6 | −51 | 54 | 3.85 | 30 |
| SMA | −7 | 0 | 52 | 5.52 | 41 | |||||
| TPJ | −51 | −49 | 29 | 3.87 | 21 | |||||
| MT+ | −39 | −74 | 5 | 4.09 | 37 | |||||
| TOJ | 38 | −60 | 19 | 4.43 | 19 | −33 | −66 | 17 | 4.06 | 23 |
| Lateral Occipital | 38 | −76 | −4 | 4.81 | 39 | −34 | −73 | −7 | 4.86 | 34 |
| a ITS | 52 | −15 | −8 | 4.26 | 29 | |||||
| CueType × CueDirectionality × time | ||||||||||
| pIPS | −26 | −68 | 38 | 3.70 | 15 | |||||
| Lateral Occipital | 39 | −68 | −2 | 4.53 | 28 | −34 | −78 | 0 | 5.42 | 71 |
| CueValidity × time | ||||||||||
| SPL/precuneus | 4 | −55 | 50 | 6.47 | 81 | −9 | −57 | 50 | 6.07 | 75 |
| FEF | −30 | −6 | 55 | 5.93 | 74 | |||||
| IPS | 27 | −59 | 56 | 3.59 | 17 | −31 | −53 | 50 | 4.39 | 36 |
| aIPS | −45 | −45 | 38 | 4.71 | 42 | |||||
| vIPS | −27 | −72 | 28 | 3.85 | 17 | |||||
| pIPS | 16 | −64 | 48 | 4.47 | 38 | |||||
| TPJ | 48 | −43 | 36 | 4.32 | 50 | |||||
| pSTS | 49 | −48 | 17 | 6.03 | 81 | |||||
| CueValidity × CueType × time | ||||||||||
| pIPS | 13 | −63 | 43 | 3.84 | 14 | |||||
| Face Localizer (Group Regions) | ||||||||||
| FFA | 39 | −45 | −14 | −39 | −52 | −17 | ||||
| OFA | 41 | −81 | −14 | −41 | −82 | −15 | ||||
| pSTS | 55 | −52 | 16 | |||||||
Cue Directionality effect
Only a small set of regions responded to the directional information conveyed by the visual cue and therefore were related to the deployment of attention to a particular location in the visual field. These regions were identified by a significant interaction of Cue Directionality by Time and are shown in Figure 2B. Significant activations were observed in regions associated with the Dorsal Attention Network, including bilateral FEF, anterior IPS, SPL/precuneus, and left MT+. Other significant regions included left TPJ, bilateral temporo-occipital junction (TOJ), bilateral LO regions just ventral to MT+, right anterior inferior temporal sulcus (aITS), and supplementary motor area (SMA), (see Table 1 for peak coordinates). In all regions, the activity during directional trials was greater than during non-directional trials.
Cue Type × Directionality interaction
A critical test of our primary hypothesis was whether there were regions in which the BOLD response for orienting attention to a location was different in the arrow and gaze conditions. These regions were identified by the interaction of Cue Type by Cue Directionality by Time. Because voxelwise analyses of interaction effects can be insensitive, we conducted separate tests of this interaction using a regional analysis and a voxelwise analysis (see below). For the regional analysis, we formed ROIs based on those voxels that showed an effect of Cue Directionality by Time in the previous analysis, i.e. voxels that showed an effect of attentional orienting. Importantly, the Cue Directionality by Time term used to form the ROIs was independent of the critical Cue Type by Cue Directionality by Time interaction term. We then conducted regional ANOVAs (see methods) on these ROIs. Therefore, while the interaction of Cue Directionality by Time pointed to regions that showed an orienting effect, this analysis would show if any of those had a different pattern of activity for arrow or gaze cueing trials. A significant Cue Type by Cue Directionality by Time interaction was found in four regions: left anterior IPS (F(6,138)=2.70, p=0.0164), left MT+ (F(6,138)=3.30, p=0.0045), and a bilateral region in LO cortex just ventral to MT+ (left: F(6,138)=3.50, p=0.0029; right: F(6,138)=5.71, p=0.0002). For all four regions, the cue directionality effect was larger for arrow than gaze cues. A separate regional ANOVA on the arrow cues with Cue Directionality and Time as factors indicated larger activations for directional than non-directional arrow cues in all four regions (left IPS (F(6,138)=7.06, p=0.0001); left MT+ (F(6,138)=8.79, p=0.0001); left LO: F(6,138)=10.75, p=0.0001; right LO: F(6,138)=10.95, p=0.0001). A similar ANOVA on the gaze cues found no differences between directional and non-directional gaze in three of the four regions (Fs<1), with a larger activation in LO for non-directional than directional cues (F(6,138)=2.53, p=0.0234). That is, LO showed a pattern that was opposite to the pattern found for arrows. Therefore, most regions of the DAN that had been identified in the Cue Directionality × Time interaction did not show significantly different effects for arrow and gaze cues, as indexed by the lack of a higher order interaction. Four regions, however, did show enhanced activity for arrow cues relative to gaze cues.
To examine the robustness of the above results, we also tested voxelwise for the interaction of Cue Type × Cue Directionality × Time. These results largely corroborated those from the regional analysis. A significant interaction was observed in left posterior IPS and a bilateral region corresponding to or very near MT+ (Figure 2C; see Table 1 for peak coordinates). Even though the overall response was larger for gaze than arrow cues, only arrow cues showed a directionality effect (directional cues showed higher activity than non-directional cues). A separate analysis of each cue type in these ROIs confirmed an effect of Cue Directionality by Time for arrow cues (left pIPS (F(6,138)=4.32, p=0.0005; left LO-MT+: F(6,138)=10.46, p=0.0001; right LO-MT+: F(6,138)=9.94, p=0.0001) and no differences, or an inverted effect for gaze cues (left pIPS (F(6,138)=2.22, p=0.0446, all others non significant).
The ROIs that showed differential cueing effects for arrow and gaze conditions in the voxelwise analysis were similar to but not identical to those identified from the regional analysis of the Cue Directionality × Time ROIs. The LO foci identified both in the voxelwise and the regional analyses were partially overlapping (the vector distance separating the peaks of the activations was 8.60 mm for left LO and 8.31 mm for right LO), but the aIPS focus identified in the ROI analysis was more anterior than the pIPS focus observed in the voxelwise interaction map (vector distance = 20.2 mm).
Overall, the results from both voxelwise and regional analyses suggested similar mechanisms for symbolic and social orienting of attention, with some differences in a subset of regions within the DAN (bilateral LO/MT+ and left IPS) that were more strongly recruited by arrow cues.
Task evoked activity: target period
Validity effect
We next determined whether there were differences in reorienting attention to unexpected targets following social and symbolic invalid cues. We followed an approach similar to that used for the cue period. We first identified ROIs involved in reorienting attention based on a voxelwise analysis of the effects of Cue Validity by Time. We then conducted a regional ANOVA to determine whether those ROIs showed different effects of reorienting following arrow and gaze cues, as determined by significant interaction of Cue Type by Cue Validity by Time. Since we were interested in studying reorienting of attention, only those trials with a directional cue were included in this analysis.
The voxelwise image of Cue Validity by Time (Figure 3A) showed a widespread set of significant activations that included VAN regions in right TPJ and pSTS and DAN regions in bilateral SPL/precuneus, left FEF and bilateral IPS. In all regions invalid trials, in which attention had to be disengaged and moved to another location, showed higher activity than valid trials (see the time courses of right TPJ and left FEF in Figure 3B for an example). An regional ANOVA on the ROIs that showed a Cue Validity × Time effect (Figure 3A) found a significant interaction of Cue Type by Cue Validity by Time only for R pIPS (F(9,207)=2.98, p=0.0023) as shown in Figure 3C, graph 4. Pair-wise comparisons showed that the interaction was due to a large validity effect for gaze trials (F(9,207)=5.31, p=0.0001) and a small validity effect for arrow cue trials (F(9,207)=2.45, p=0.0114). Although not reaching significance, a similar pattern was also found in bilateral SPL/precuneus. No interaction was found for either TPJ or pSTS (Figure 3C, graphs 1 and 2). Since these regions have been associated with gaze cueing and social cognition, we were interested in whether they showed reorienting effects. Thus, we conducted separate planned comparisons for the effect of Cue Validity × Time following arrow and gaze cues. These regional ANOVAs showed a significant validity effect for both gaze and arrow trials in pSTS (F(9,207)=5.75, p=0.0001 and F(9,207)=2.22, p=0.0223 respectively) as well as in rTPJ (F(9,207)=3.15, p=0.0014 and F(9,207)=2.80, p=0.004 respectively), confirming the lack of differences in the validity effect following gaze and arrow cues.
Finally, we conducted a voxelwise test for the interaction of Cue Validity by Cue Type by Time in order to corroborate the regional analyses. The interaction image showed a significant activation in right pIPS that overlapped with the ROI previously observed in the above regional analysis (vector distance between the peaks of the two foci = 5.92 mm; see Table 1). Inspection of the BOLD time courses for this region revealed a large validity effect for gaze trials and a small inverted effect for arrow trials. These observations were backed up by pair-wise tests (F(9,207)=6.06, p=0.0001 and F(9,207)=2.30, p=0.0174 for gaze and arrows respectively). The voxelwise analysis of the interaction also yielded other foci, but the time courses for these regions were difficult to interpret as the significant modulation depended on large baseline shifts and changes in the tails of the time courses.
Overall, both the regional and voxelwise analyses of the target period indicated a substantial overlap between regions underlying social and symbolic reorienting with the exception of a region of the DAN, right pIPS, that was more recruited by reorienting following a social than symbolic cue.
Therefore, analyses of both the cue and target periods support the hypothesis that symbolic and social orienting are largely realized by the same neural network, with some interesting exceptions that will be considered in the discussion.
Task evoked activity: Analysis of face regions
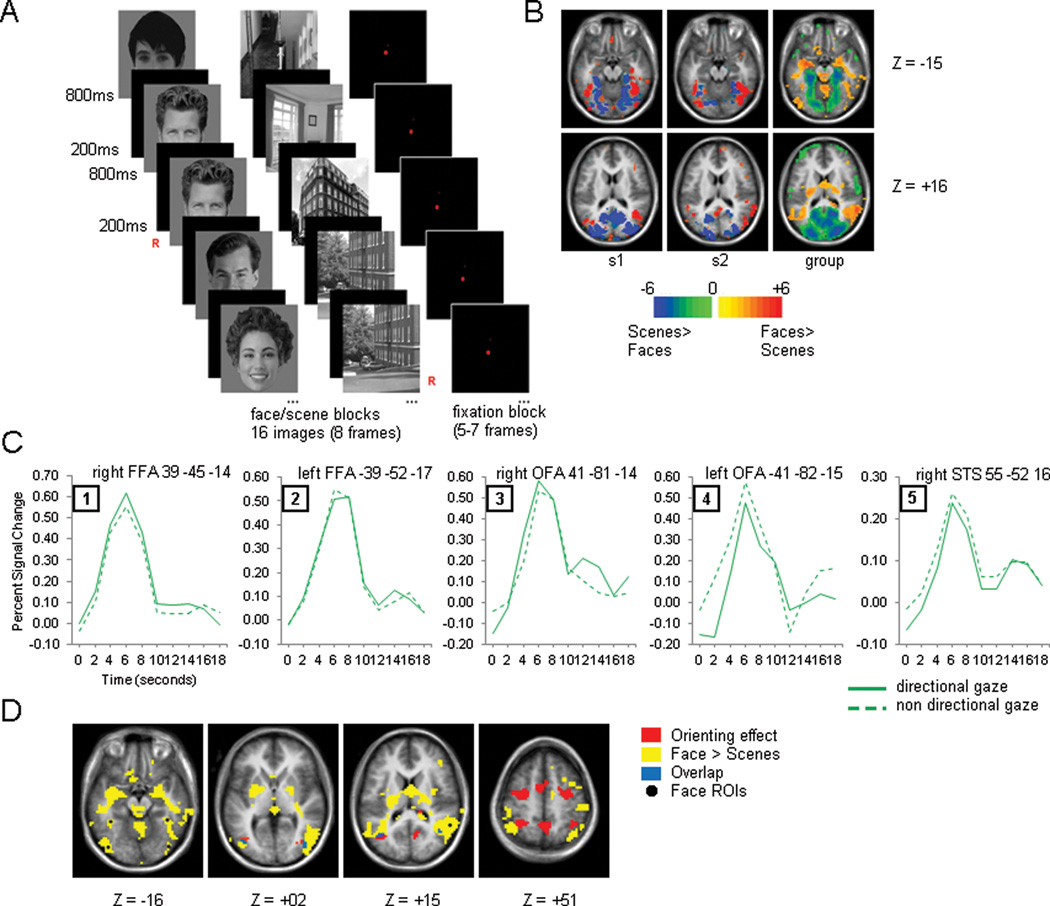
We conducted a targeted analysis on face specific regions identified from a face functional localizer in order to test whether they were involved in orienting attention during social cue conditions. Figure 4B presents voxelwise statistical maps of the faces > scenes contrast for two individuals and for the average of all 16 participants).
Figure 4.
Localizer experiment. A). Experimental procedure showing face, scenes and fixation blocks. B). Faces>scenes (warm colors) and scenes>faces (cold colors) contrasts for two representative participants and the group average (n=16). C). Time courses for the Directionality effect for the five group defined face regions. D). Overlap of the Cue Directionality effect and the face>scenes contrast.
An ROI analysis on five face regions during gaze trials with Cue Directionality and Time as factors yielded no significant effects in any of the five selected ROIs (bilateral FFA, bilateral OFA and right pSTS). Since only 16 participants had localizer data for these regions, we performed a second analysis with the group average face seeds (see Methods, third row in Figure 4B) and applied those ROIs to the 24 participants. Again, none of the five face regions showed a significant interaction (Figure 4C). Therefore, regions specialized in face processing do not orient attention to a location based on gaze information.
To further show the independence of face processing regions from regions directing spatial attention we conducted a conjunction analysis that compared the topography of the orienting response (as given by the Cue Directionality × Time interaction) and the face localizer results (Figure 4D). All of the main regions active during the orienting response were different from the regions activated during the face localizer. Only very small regions of overlap were found in bilateral LO and bilateral posterior temporo-occipital junction. In neither case, however, did the overlap suggest a role for face regions in orienting attention. In the preceding analysis of the cue period, bilateral LO cortex had actually shown stronger activity for directional than non-directional cues for arrow cues but not for gaze cues. The same analysis indicated that gaze cues activated the region more strongly than arrow cues (Cue Type × Time, (F(4,92)=22.65, p=0.0001 and F(4,92)=20.53, p=0.0001 for right and left LO respectively), accounting for the overlap. The second region that showed an overlap (bilateral posterior temporo-occipital junction) did not correspond to any of the regions from the previous analyses that showed an interaction between Cue Directionality and Cue Type. A previous regional analysis, however, had indicated that these bilateral regions showed a Cue Directionality effect (stronger activity for directional than neutral cues, F(4,92)=5.75, p=0.0007 and F(4,92)=3.53, p=0.0140 for right and left respectively) and a Cue Type effect (stronger activity for gaze than arrow cues (F(4,92)=8.38, p=0.0001 and F(4,92)=5.17, p=0.0008 for right and left respectively), consistent with the overlap in the conjunction map.
Therefore, these new analyses failed to find a pattern of activations consistent with a face specific orienting mechanism in any of the five face specific processing regions or in those foci where both the directional image and the localizer image overlapped.
2. Are face selective regions interacting dynamically with supramodal attentional networks?
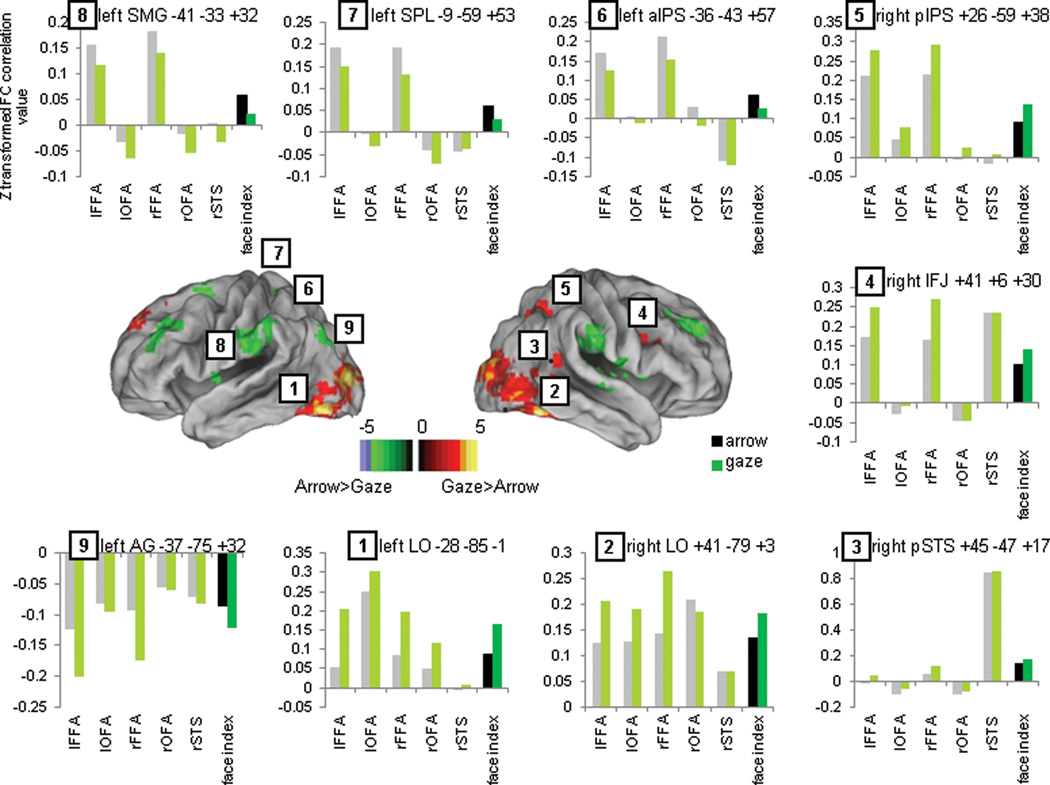
Although face-selective regions did not play a direct role in orienting attention, they may nevertheless selectively interact with regions that orient attention when gaze cues are present. For example, they could send sensory information to those regions. Therefore, we conducted functional connectivity analyses to determine whether face selective regions interacted dynamically with a supramodal attentional network more for gaze than arrow cues. While these analyses cannot inform regarding the directionality of potential interactions, they do provide evidence for the presence of an interaction between regions The functional connectivity analysis was carried out on the residual BOLD fluctuations after regressing out the task (He et al., 2007). The analysis compared the voxelwise functional connectivity (FC) map of the face network (5 group seeds) during social-cueing blocks and symbolic-cueing blocks. Because of the slow fluctuations of the BOLD signal, we could not separately measure functional connectivity during cue and target periods. Figure 5 depicts the voxelwise differences between social-cueing blocks and symbolic-cueing blocks in the functional connectivity of the face network with the rest of the brain. In order to understand the sign of the differences, we extracted peaks from the voxelwise map and obtained average connectivity values for each cueing condition (see dark green-black bar plots in Figure 5 for connectivity values of the “face network” with each specific region –e.g. region 1: left LO-face network connectivity for arrow and gaze conditions).
Figure 5.
Voxelwise map of statistical differences between the face network functional connectivity voxel-wise map during social and symbolic trials (warm colors represent areas where the face network shows stronger positive connectivity or weaker negative connectivity during social trials and cold colors represent areas where the face network shows stronger positive connectivity or weaker negative connectivity during symbolic trials) and bar plots showing the connectivity strength between the face network (dark green and black bars) or each individual face seed (light green and gray bars) and some representative peak regions.
During social-cueing blocks, the face network showed stronger connectivity to large bilateral clusters of ventral, posterior and lateral occipital cortex, bilateral amygdala, right posterior STS, right inferior frontal junction (IFJ) and right posterior IPS (warm colors, regions 1–5). Other regions showed weaker correlations (left SPL, left anterior IPS and bilateral supramarginal gyrus: cold colors, regions 6–8) or stronger anti-correlations (i.e. more negative) during social-cueing blocks. This enhanced negative relationship was mostly found in regions of the DMN (right medial superior temporal gyrus, left angular gyrus, anterior cingulate cortex, posterior cingulate cortex and bilateral superior frontal sulcus; e.g. region 9). Therefore, during social-cueing blocks, face-specific visual processing areas showed stronger coupling with LO, right pIPS, pSTS and IFG, weaker coupling with bilateral SMG and left DAN, and stronger negative coupling with the DMN as compared to symbolic-cueing blocks. The connectivity values for each individual face region revealed that OFA was mostly connected to the LO regions. PSTS was mostly connected to the right IFJ region and a focus slightly anterior to the face-selective pSTS focus that corresponded to the TPJ/pSTS region identified in the voxelwise analysis of the Validity effect. Bilateral FFA was strongly connected to a much larger set of regions that included all visual as well as attentional regions. Finally, all face regions were strongly anticorrelated with the DMN regions (Figure 5, gray and light green bars; e.g. region 1, mean connectivity of each face network node and left LO)
We conclude that the face network is more strongly coupled with a specific region of the right posterior DAN (pIPS), VAN (right TPJ/right pSTS), a pivot region between DAN and VAN (r IFG) and more strongly negatively coupled to the DMN during social-cueing than symbolic-cueing blocks.
DISCUSSION
Our first goal was to test for differences between the spatial attentional mechanisms underlying social and symbolic cueing. We found largely overlapping activity associated with both types of cueing, with some interesting exceptions. Both cues produced significant differences in the preparatory activations for directional and non-directional spatial information within the IPS/SPL and FEF nodes of the DAN, adding to previous findings associating this network with spatial attention (Kastner and Ungerleider, 2001; Corbetta and Shulman, 2002). Some dorsal attentional regions in left IPS and bilateral MT+/LO cortex, however, showed a directionality effect only for arrow cues. Similarly, reorienting to unexpected targets during both gaze and arrow cue trials activated the principal posterior node of the VAN, right TPJ, as well as frontal and parietal regions of the DAN. Yet a difference in the magnitude of activation was also observed in R IPS, a region of the DAN, although now activation was stronger following a gaze than arrow cue.
We found no evidence that social cueing involved face selective regions in ventral occipital and posterior temporal cortex. Face-selective regions showed equivalent activations for directional and non-directional symbolic cues. Also, no region overlapping the face activation map and the cue directionality map showed an interaction between the orienting effect in social and symbolic trials. Finally, the temporo-parietal region, a region near the face specific pSTS that has been related to both attentional and social processes such as mentalizing, did not show any difference in the reorienting response following social and symbolic cues.
Another goal of our study was to test whether face–selective regions influence a supramodal orienting system by dynamically coupling their activity with the DAN and VAN during social cueing blocks. The functional connectivity analysis of the task regressed data showed stronger correlations between the face network and bilateral LO, right pSTS, right pIPS and right IFG during the gaze than arrow blocks as well as stronger anticorrelations with the DMN. Conversely, weaker connectivity during gaze than arrow blocks was found with bilateral SMG and left DAN regions. The increased connectivity with right pIPS may reflect visual input from face regions to part of the DAN during social orienting, with the caveat that functional connectivity measurements do not indicate the direction of an interaction between regions.
1. Evidence for one supramodal attentional mechanism
Orienting of attention
Dorsal fronto-parietal network
While both gaze and symbolic cues produced preparatory activity related to spatial attention in the DAN, activity in some parts of that network such as regions of bilateral MT+/LO and left IPS was significantly greater for arrow cues. Since IPS has been associated with the maintenance of attention (Corbetta and Shulman, 2002; Shulman et al., 2009) one possible explanation is that gaze cues are more effective or automatic in maintaining attention to a peripheral location and do not require as much top down control or effort as arrow cues. The greater attention-related activations in MT+/LO regions following arrow cues may reflect a role in translating information provided by the cue into a particular spatial location.
Although large activation differences were found between social and symbolic cues as shown by the Cue Type × Time interaction, it is striking that none of those regions showed a differential pattern for directional vs. non-directional cues. Instead, this pattern was only observed in canonical DAN regions. Overall, these results are in line with previous fMRI studies that have reported similar activations for gaze and arrow (or peripheral) cues (Tipper et al., 2008; Greene et al., 2009; Sato et al., 2009), but with a design that controlled for several important variables, such as the separation of orienting from target detection and reorienting. Our conclusions are also consistent with those of Brignani et al. (2009), who measured event-related potentials to central, predictive arrow and schematic eye-gaze cues. While these cues induced different strengths of activation, they recruited the same cortical network as shown by similar ERP components and the same number of topographical cortical maps. Conversely, only Hietanen et al. (2006) presented evidence to suggest different mechanisms for symbolic and social orienting. In their study, arrow cues activated the dorsal fronto-parietal network while activations associated with gaze cues were restricted to occipital regions, However, their SOA was relatively short (200ms) and the central cue did not disappear before the target was presented, a design very similar to that used in studies reporting that non-directional gaze cues capture attention (Senju and Hasegawa, 2005). Therefore, it is possible that in Hietanen et al. (2006), both non-directional and directional cues captured attention, resulting in null activation of the DAN by gaze cues.
These considerations raise the issue of whether the non-directional gaze cues in our study might also have captured attention to a greater degree than the non-directional symbolic cues, weakening the attentional effects related to gaze cues. However, our study involved much longer SOAs than those in Hietanen et al. (2006). Senju and Hasegawa (2005) found that when using longer SOAs the non-directional gaze condition did not seem to capture attention but behaved as an averted gaze (i.e. eyes looking down) or a condition where eyes were closed. Therefore, the use of long SOAs in the current study likely led to better differential activation of the DAN by directional and non-directional gaze cues. It is also worth noting that, unlike previous fMRI studies, we used predictive gaze and symbolic cues, which likely recruited the endogenous system more strongly than the non-predictive cues used in other studies. Behavioral studies (Hill et al., 2010) have shown reflexive and voluntary effects of predictive social cues at very short (reflexive) and longer (voluntary) SOAs, respectively.
Face regions
As in previous studies (Kingstone et al., 2004; Grosbras et al., 2005), larger activations were found in pSTS for gaze cues, consistent with its role in gaze processing (Akiyama et al., 2006). However, this region was not differentially active during processing of directional vs. non-directional cues, either in voxelwise or in ROI analysis. Furthermore, the portion of the STS specifically involved in face processing, as identified by the face localizer, did not show a directionality effect. Indeed, none of the face specific regions identified with the face localizer did. Last, the overlap analysis showed that the face network and orienting network mainly had nonoverlapping topographies. The small overlapping clusters showed both a main effect of directionality and cue type but no interaction that would signal a specialized system for directional information conveyed by a social cue. Therefore, based on our results, there is little evidence to support the hypothesis that regions comprising the core face network (Haxby et al., 2000) directly control shifts of attention to a peripheral stimulus that is the object of another person’s gaze.
Reorienting of attention and target detection
Dorsal fronto-parietal network
While the reorienting of attention to an unexpected target modulated the DAN and VAN following both gaze and arrow cues, a region in the right pIPS showed greater activity for gaze-related reorienting. This result fits nicely with the findings from the cue period. Since orienting to gaze is likely more reflexive than symbolic orienting (Ristic et al., 2007), stronger voluntary control may be needed to disengage attention from its current locus. This effect would be particularly powerful in the current paradigm as the gaze cue remained on-screen until the target was presented. Additionally, it has been suggested that the right hemisphere is differentially involved in gaze cueing (Greene and Zaidel, 2011). We found that left IPS showed greater activity during orienting following arrow cues, while the right pIPS showed greater activity during reorienting following gaze cues, providing qualitative support for this hypothesis. As in the case of orienting, the cue-specific effects of reorienting may have reflected our use of predictive cues. Under non-predictive conditions, reorienting attention away from a social cue may be less effortful, reducing the need for increased activation of the DAN.
Ventral fronto-parietal network
We were interested in whether the VAN was differentially modulated by reorienting to unexpected targets following gaze and arrow cues since the posterior node of the VAN, right TPJ/pSTS, includes or is close to regions that have been related to gaze perception, social cognition, and theory of mind (Saxe and Wexler, 2005; Nummenmaa and Calder, 2009). Gaze cueing, as well as joint attention (Tomasello, 1995) are thought to be precursors for the development of social interactions and theory of mind, as they involve assigning intentions to a person based on their gaze (Charman et al., 2000; Emery, 2000; Pruett et al., 2011). We found no differences in the activity of right pSTS and TPJ to unexpected targets following arrow or gaze cues. Only one other study has focused on the differences between social and symbolic reorienting of attention. Engell et al. (2010) found stronger VAN involvement for arrows and no validity effect on gaze trials. They suggested that the VAN is involved in social cueing regardless of cue validity, explaining the lack of difference between valid and invalid targets. Contrary to this interpretation we found that the TPJ component of the VAN was equivalently modulated by gaze and arrow cue validity.
Our results from cue and target period, as well as from the ROI analyses performed on the face specific regions, support the hypothesis of a single attentional mechanism that is activated regardless of the type of cue that is driving the orienting and reorienting of attention. This general conclusion is not inconsistent with the fact that some specific regions of the DAN were differentially recruited during orienting (left IPS and LO/MT+ for arrows) or reorienting (right pIPS for gaze). Further testing is necessary, however, to show that regions in the DAN, that show directional cueing effects for gaze and arrow cues, also process spatial information for the two cue types in a comparable fashion. For example, a multivoxel analysis could determine if the same pattern of voxelwise activity is associated with a rightward shift of attention for gaze cues and symbolic cues. An equivalence would provide stronger support for the claim that a single attentional mechanism is active for social and symbolic cues.
2. Evidence for dynamic coupling of face regions and a supramodal attentional mechanism
The functional connectivity analyses showed that the face processing regions identified by the functional localizer were strongly connected to bilateral LO regions, right IFJ, right pSTS and right pIPS and this coupling was significantly stronger during social-cueing than arrow-cueing blocks. Right pIPS is part of the dorsal network involved in orienting of attention, right pSTS is part of the ventral network involved in reorienting, and right IFJ has been proposed as a region mediating the interactions between DAN and VAN to communicate and coordinate both networks (Corbetta et al., 2008; Asplund et al., 2010). The right pSTS region that showed strong connectivity with the face network was slightly anterior to the one identified with the face functional localizer and overlapped the TPJ/pSTS region found in the Validity effect. The pIPS region showed a partial overlap with the pIPS region that was identified in the analysis of Cue Validity and was more strongly activated following invalid gaze than arrow cues. Therefore, the face network was connected to regions of both DAN and VAN, as well as to a pivot region that interacts with both, and this coupling was stronger during trials with social information.
Nummenmaa (Nummenmaa et al., 2010) used a psychophysiological interaction (PPI) analysis to look at the dynamic connectivity of the face network with attentional networks. They found that right FFA and right pSTS showed stronger connectivity with multiple nodes of the DAN and VAN when participants were paying attention to gaze shifting faces vs. faces opening and closing their eyes. Right pSTS was more strongly connected to right FEF and IPS and bilateral SMG/STG and MT during gaze shifts than open-close trials and right FFA had a positive change in coupling with right SMG and MFG. OFA did not significantly change its connectivity across conditions and no negative couplings between regions were found. They compared two conditions involving face processing that differed in the movements made by the eyes (gaze shift vs. open/close). We compared conditions involving gaze shifts (directional cues) and open/close eyes (non-directional cues) to directional and non-directional conditions in which no social information was processed. Therefore the results of Nummenmaa and Calder showed that the coupling of the face network with attentional regions is stronger for directional vs. non-directional gaze cues, but did not rule out the possibility that a similar directional vs. non-directional difference is present for other types of cues. Our results showed that differences in the coupling of face regions and attentional regions were stronger for gaze than arrow cues. This difference in the specificity of the analysis might explain why we found stronger coupling between face regions and IPS, but not with FEF.
Our analysis does not indicate whether couplings are associated with orienting or reorienting or are sustained throughout the trial. The analysis also does not indicate whether the interactions between regions are directional, i.e. primarily from one region to the other. However, the functional connectivity analysis is consistent with the notion that, after processing the face stimuli, face regions relayed their output to regions that coded and manipulated spatial information (i.e. MT+/LO) and to regions involved in attentional orienting (right pIPS), reorienting (right pSTS) and their interaction (right IFJ). Also, the fact that face regions were more connected to right hemisphere attentional regions but less connected to the left DAN (i.e. left FEF and left pIPS) during social-cueing than symbolic-cueing blocks, qualitatively supports the notion that social orienting is predominantly lateralized to the right hemisphere.
Last, our functional connectivity analysis showed strong anticorrelations between the face network and the DMN that were stronger (i.e. more negative) during social-cueing blocks. Similarly, during the cue period, DMN regions showed initial deactivations that were much stronger for gaze than arrow cues. Therefore, while previous reports have shown an overlap between DMN and the social brain, both in task evoked studies and in functional connectivity studies (Schilbach et al., 2008; Mars et al., 2012), this correspondence does not extend to the use of gaze information to control orienting. It is likely the case that only more abstract social reasoning involves the DMN although further studies will be necessary to answer this question.
Overall, our task-evoked analyses show that dorsal and ventral fronto-parietal mechanisms for orienting and reorienting attention to space are engaged by both symbolic and social cues. The detailed recruitment of these mechanisms, however, depends to some extent on the nature of the cue. Also, these fronto-parietal mechanisms show enhanced interactions with face-selective regions when the direction of attention is based on gaze information. The most likely interpretation of these interactions is that during social cueing, face-selective regions extract gaze information and pass it forward to the attentional networks.
Acknowledgments
This work was supported by a Fulbright Scholar Grant (AC) and NIMH Grant RO1 MH 71920-06 (MC). We would like to thank Avi Snyder and Mark McAvoy for their help with approaches to data processing and statistical analysis.
Footnotes
Conflict of interest: none
REFERENCES
- Akiyama T, Kato M, Muramatsu T, Saito F, Umeda S, Kashima H. Gaze but not arrows: a dissociative impairment after right superior temporal gyrus damage. Neuropsychologia. 2006;44:1804–1810. doi: 10.1016/j.neuropsychologia.2006.03.007. [DOI] [PubMed] [Google Scholar]
- Allison, Puce, McCarthy Social perception from visual cues: role of the STS region. Trends Cogn Sci (Regul Ed) 2000;4:267–278. doi: 10.1016/s1364-6613(00)01501-1. [DOI] [PubMed] [Google Scholar]
- Andrews TJ, Davies-Thompson J, Kingstone A, Young AW. Internal and external features of the face are represented holistically in face-selective regions of visual cortex. J Neurosci. 2010;30:3544–3552. doi: 10.1523/JNEUROSCI.4863-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arrington CM, Carr TH, Mayer AR, Rao SM. Neural mechanisms of visual attention: object-based selection of a region in space. J Cogn Neurosci. 2000;12(Suppl 2):106–117. doi: 10.1162/089892900563975. [DOI] [PubMed] [Google Scholar]
- Asplund CL, Todd JJ, Snyder AP, Marois R. A central role for the lateral prefrontal cortex in goal-directed and stimulus-driven attention. Nat Neurosci. 2010;13:507–512. doi: 10.1038/nn.2509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brignani D, Guzzon D, Marzi CA, Miniussi C. Attentional orienting induced by arrows and eye-gaze compared with an endogenous cue. Neuropsychologia. 2009;47:370–381. doi: 10.1016/j.neuropsychologia.2008.09.011. [DOI] [PubMed] [Google Scholar]
- Charman T, Baron-Cohen S, Swettenham J, Baird G, Cox A, Drew A. Testing joint attention, imitation, and play as infancy precursors to language and theory of mind. Cognitive Development. 2000;15:481–498. [Google Scholar]
- Corbetta M, Kincade JM, Ollinger JM, McAvoy MP, Shulman GL. Voluntary orienting is dissociated from target detection in human posterior parietal cortex. Nat Neurosci. 2000;3:292–297. doi: 10.1038/73009. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Kincade JM, Shulman GL. Neural systems for visual orienting and their relationships to spatial working memory. J Cogn Neurosci. 2002;14:508–523. doi: 10.1162/089892902317362029. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Patel G, Shulman GL. The reorienting system of the human brain: from environment to theory of mind. Neuron. 2008;58:306–324. doi: 10.1016/j.neuron.2008.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbetta M, Shulman GL. Control of goal-directed and stimulus-driven attention in the brain. Nat Rev Neurosci. 2002;3:201–215. doi: 10.1038/nrn755. [DOI] [PubMed] [Google Scholar]
- Emery NJ. The eyes have it: the neuroethology, function and evolution of social gaze. Neuroscience & Biobehavioral Reviews. 2000;24:581–604. doi: 10.1016/s0149-7634(00)00025-7. [DOI] [PubMed] [Google Scholar]
- Engell AD, Nummenmaa L, Oosterhof NN, Henson RN, Haxby JV, Calder AJ. Differential activation of frontoparietal attention networks by social and symbolic spatial cues. Soc Cogn Affect Neurosci. 2010;5:432–440. doi: 10.1093/scan/nsq008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forman SD, Cohen JD, Fitzgerald M, Eddy WF, Mintun MA, Noll DC. Improved assessment of significant activation in functional magnetic resonance imaging (fMRI): use of a cluster-size threshold. Magn Reson Med. 1995;33:636–647. doi: 10.1002/mrm.1910330508. [DOI] [PubMed] [Google Scholar]
- Frischen A, Bayliss AP, Tipper SP. Gaze cueing of attention: visual attention, social cognition, and individual differences. Psychol Bull. 2007;133:694–724. doi: 10.1037/0033-2909.133.4.694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene DJ, Mooshagian E, Kaplan JT, Zaidel E, Iacoboni M. The neural correlates of social attention: automatic orienting to social and nonsocial cues. Psychol Res. 2009;73:499–511. doi: 10.1007/s00426-009-0233-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene DJ, Zaidel E. Hemispheric differences in attentional orienting by social cues. Neuropsychologia. 2011;49:61–68. doi: 10.1016/j.neuropsychologia.2010.11.007. [DOI] [PubMed] [Google Scholar]
- Grill-Spector K, Knouf N, Kanwisher N. The fusiform face area subserves face perception, not generic within-category identification. Nat Neurosci. 2004;7:555–562. doi: 10.1038/nn1224. [DOI] [PubMed] [Google Scholar]
- Grosbras M-H, Laird AR, Paus T. Cortical regions involved in eye movements, shifts of attention, and gaze perception. Hum Brain Mapp. 2005;25:140–154. doi: 10.1002/hbm.20145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Grünau M, Anston C. The detection of gaze direction: A stare-in-the-crowd effect. Perception. 1995;24:1297–1313. doi: 10.1068/p241297. [DOI] [PubMed] [Google Scholar]
- Haxby, Hoffman, Gobbini The distributed human neural system for face perception. Trends Cogn Sci (Regul Ed) 2000;4:223–233. doi: 10.1016/s1364-6613(00)01482-0. [DOI] [PubMed] [Google Scholar]
- He BJ, Snyder AZ, Vincent JL, Epstein A, Shulman GL, Corbetta M. Breakdown of functional connectivity in frontoparietal networks underlies behavioral deficits in spatial neglect. Neuron. 2007;53:905–918. doi: 10.1016/j.neuron.2007.02.013. [DOI] [PubMed] [Google Scholar]
- Hietanen JK, Nummenmaa L, Nyman MJ, Parkkola R, Hämäläinen H. Automatic attention orienting by social and symbolic cues activates different neural networks: an fMRI study. Neuroimage. 2006;33:406–413. doi: 10.1016/j.neuroimage.2006.06.048. [DOI] [PubMed] [Google Scholar]
- Hill JL, Patel S, Gu X, Seyedali NS, Bachevalier J, Sereno AB. Social orienting: reflexive versus voluntary control. Vision Res. 2010;50:2080–2092. doi: 10.1016/j.visres.2010.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman EA, Haxby JV. Distinct representations of eye gaze and identity in the distributed human neural system for face perception. Nat Neurosci. 2000;3:80–84. doi: 10.1038/71152. [DOI] [PubMed] [Google Scholar]
- Kastner S, Ungerleider LG. The neural basis of biased competition in human visual cortex. Neuropsychologia. 2001;39:1263–1276. doi: 10.1016/s0028-3932(01)00116-6. [DOI] [PubMed] [Google Scholar]
- Kincade JM, Abrams RA, Astafiev SV, Shulman GL, Corbetta M. An event-related functional magnetic resonance imaging study of voluntary and stimulus-driven orienting of attention. J Neurosci. 2005;25:4593–4604. doi: 10.1523/JNEUROSCI.0236-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kingstone A, Tipper C, Ristic J, Ngan E. The eyes have it!: an fMRI investigation. Brain Cogn. 2004;55:269–271. doi: 10.1016/j.bandc.2004.02.037. [DOI] [PubMed] [Google Scholar]
- Lepsien J, Pollmann S. Covert reorienting and inhibition of return: an event-related fMRI study. J Cogn Neurosci. 2002;14:127–144. doi: 10.1162/089892902317236795. [DOI] [PubMed] [Google Scholar]
- Mars RB, Neubert F-X, Noonan MP, Sallet J, Toni I, Rushworth MFS. On the relationship between the “default mode network” and the “social brain.”. Front Hum Neurosci. 2012;6:189. doi: 10.3389/fnhum.2012.00189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer AR, Harrington D, Adair JC, Lee R. The neural networks underlying endogenous auditory covert orienting and reorienting. Neuroimage. 2006;30:938–949. doi: 10.1016/j.neuroimage.2005.10.050. [DOI] [PubMed] [Google Scholar]
- McAvoy MP, Ollinger JM, Buckner RL. Cluster size thresholds for assessment of significant activation in fMRI. NeuroImage. 2001;13:S198. [Google Scholar]
- Mitchell JP. Activity in right temporo-parietal junction is not selective for theory-of-mind. Cereb Cortex. 2008;18:262–271. doi: 10.1093/cercor/bhm051. [DOI] [PubMed] [Google Scholar]
- Nummenmaa L, Calder AJ. Neural mechanisms of social attention. Trends Cogn Sci (Regul Ed) 2009;13:135–143. doi: 10.1016/j.tics.2008.12.006. [DOI] [PubMed] [Google Scholar]
- Nummenmaa L, Passamonti L, Rowe J, Engell AD, Calder AJ. Connectivity analysis reveals a cortical network for eye gaze perception. Cereb Cortex. 2010;20:1780–1787. doi: 10.1093/cercor/bhp244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ollinger JM, Corbetta M, Shulman GL. Separating processes within a trial in event-related functional MRI. Neuroimage. 2001a;13:218–229. doi: 10.1006/nimg.2000.0711. [DOI] [PubMed] [Google Scholar]
- Ollinger JM, Shulman GL, Corbetta M. Separating processes within a trial in event-related functional MRI. Neuroimage. 2001b;13:210–217. doi: 10.1006/nimg.2000.0710. [DOI] [PubMed] [Google Scholar]
- Perrett DI, Smith PAJ, Potter DD, Mistlin AJ, Head AS, Milner AD, Jeeves MA. Visual Cells in the Temporal Cortex Sensitive to Face View and Gaze Direction. Proceedings of the Royal Society of London Series B, Biological Sciences. 1985;223:293–317. doi: 10.1098/rspb.1985.0003. [DOI] [PubMed] [Google Scholar]
- Pruett JR, Jr, LaMacchia A, Hoertel S, Squire E, McVey K, Todd RD, Constantino JN, Petersen SE. Social and non-social cueing of visuospatial attention in autism and typical development. J Autism Dev Disord. 2011;41:715–731. doi: 10.1007/s10803-010-1090-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ristic J, Wright A, Kingstone A. Attentional control and reflexive orienting to gaze and arrow cues. Psychon Bull Rev. 2007;14:964–969. doi: 10.3758/bf03194129. [DOI] [PubMed] [Google Scholar]
- Sato W, Kochiyama T, Uono S, Yoshikawa S. Commonalities in the neural mechanisms underlying automatic attentional shifts by gaze, gestures, and symbols. Neuroimage. 2009;45:984–992. doi: 10.1016/j.neuroimage.2008.12.052. [DOI] [PubMed] [Google Scholar]
- Saxe R, Kanwisher N. People thinking about thinking people The role of the temporo-parietal junction in “theory of mind.”. Neuroimage. 2003;19:1835–1842. doi: 10.1016/s1053-8119(03)00230-1. [DOI] [PubMed] [Google Scholar]
- Saxe R, Wexler A. Making sense of another mind: the role of the right temporo-parietal junction. Neuropsychologia. 2005;43:1391–1399. doi: 10.1016/j.neuropsychologia.2005.02.013. [DOI] [PubMed] [Google Scholar]
- Schilbach L, Eickhoff SB, Rotarska-Jagiela A, Fink GR, Vogeley K. Minds at rest? Social cognition as the default mode of cognizing and its putative relationship to the “default system” of the brain. Conscious Cogn. 2008;17:457–467. doi: 10.1016/j.concog.2008.03.013. [DOI] [PubMed] [Google Scholar]
- Schneider, Eschman, Zuccolotto . E-Prime reference guide. Pittsburgh, PA: Psychology Software Tools Inc; 2002. [Google Scholar]
- Scholz J, Triantafyllou C, Whitfield-Gabrieli S, Brown EN, Saxe R. Distinct regions of right temporo-parietal junction are selective for theory of mind and exogenous attention. PLoS ONE. 2009;4:e4869. doi: 10.1371/journal.pone.0004869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senju A, Hasegawa T. Direct gaze captures visuospatial attention. Visual Cognition. 2005;12:127–144. [Google Scholar]
- Shulman GL, Astafiev SV, Franke D, Pope DLW, Snyder AZ, McAvoy MP, Corbetta M. Interaction of stimulus-driven reorienting and expectation in ventral and dorsal frontoparietal and basal ganglia-cortical networks. J Neurosci. 2009;29:4392–4407. doi: 10.1523/JNEUROSCI.5609-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Co-planar stereotaxic atlas of the human brain: 3-dimensional proportional system: an approach to cerebral imaging. Thieme. 1988 [Google Scholar]
- Tipper CM, Handy TC, Giesbrecht B, Kingstone A. Brain responses to biological relevance. J Cogn Neurosci. 2008;20:879–891. doi: 10.1162/jocn.2008.20510. [DOI] [PubMed] [Google Scholar]
- Tomasello M. Joint attention as social cognition. In: Moore C, Dunham PJ, editors. Joint attention: its origins and role in development. Lawrence Erlbaum Associates; 1995. p. 103. [Google Scholar]
- Tosoni A, Galati G, Romani GL, Corbetta M. Sensory-motor mechanisms in human parietal cortex underlie arbitrary visual decisions. Nat Neurosci. 2008;11:1446–1453. doi: 10.1038/nn.2221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yantis S, Schwarzbach J, Serences JT, Carlson RL, Steinmetz MA, Pekar JJ, Courtney SM. Transient neural activity in human parietal cortex during spatial attention shifts. Nat Neurosci. 2002;5:995–1002. doi: 10.1038/nn921. [DOI] [PubMed] [Google Scholar]