Abstract
Purpose
Racial differences in withdrawal of mechanical ventilation (WMV) have been demonstrated among patients with severe neurologic injuries. We ascertained whether such differences might be accounted for by imbalances in socioeconomic status or disease severity, and whether such racial differences impact hospital mortality or result in greater discharge to long-term care facilities.
Materials and methods
We evaluated WMV among 1885 mechanically ventilated patients with severe neurologic injury (defined as Glasgow Coma Scale <9), excluding those progressing to brain death within the first 48 hours.
Results
Withdrawal of mechanical ventilation was less likely in nonwhite patients (22% vs 31%, P < .001). Nonwhites were younger and were more likely to have Medicaid or no insurance, live in ZIP codes with low median household incomes, be unmarried, and have greater illness severity; but after adjustment for these variables, racial difference in WMV persisted (odds ratio, 0.56; 95% confidence interval, 0.42–0.76). Nonwhite patients were more likely to die instead with full support or progress to brain death, resulting in equivalent overall hospital mortality (40% vs 42%, P = .44). Among survivors, nonwhites were more likely to be discharged to long-term care facilities (27% vs 17%, P < .001).
Conclusions
Surrogates of nonwhite neurologically injured patients chose WMV less often even after correcting for socioeconomic status and other confounders. This difference in end-of-life decision making does not appear to alter hospital mortality but may result in more survivors left in a disabled state.
Keywords: Racial disparity, Medical ethics, Withdrawal of ventilation, Brain injury
1. Introduction
A large part of mortality in the intensive care unit (ICU) occurs after decisions to limit life-sustaining interventions such as mechanical ventilation [1,2]; this is especially true for patients with severe neurologic injuries [3,4]. These difficult decisions typically follow discussions with surrogate decision makers about prognosis and patients’ perspectives on functional recovery and quality of life [5,6]. A number of factors influence these critical end-of-life decisions, including cultural perspectives on life and death, health literacy, and trust in the health care system and reliability of the prognosis [7]. Understanding the determinants of these complex decisions has significant repercussions for the care of critically ill patients as well as overall resource utilization in an era when end-of-life care is increasingly being provided in the ICU [8].
Racial disparities in health care utilization and outcomes after major illness have been noted for some time but have only more recently become a priority in public health research and policy [9]. Although the general pattern of such disparities is that nonwhites receive less resources and have worse outcomes [10–15], it appears that nonwhite patients are actually more likely to receive intensive testing and treatments at the end of life, especially in the ICU [16,17]. Studies have shown that minorities are less likely to implement do-not-resuscitate (DNR) orders and limit or withdraw aggressive treatment, which translates into their dying more often with active interventions in the ICU [18,19].
The source and significance of these racial differences in end-of-life decision making have not been fully elucidated. Although cultural preferences may play a central role, racial differences may also be mediated by gaps in education and health literacy, absence of social support and advance care directives, and lack of trust in the health care system. These influences may be reflected in part by differences of socioeconomic status (SES) among minority patients as well as a greater severity of illness that may impact decisions to withdraw mechanical ventilation. Discovering the primary factors underlying differences in end-of-life decision making is imperative to differentiate whether variance is due to preference or actual bias within the health care system (ie, a true racial disparity). Although the federal government has recently recommitted to eliminating racial disparities over the next decade [20], it remains unclear whether progress has been made in diminishing existing disparities since they were first recognized in the ICU more than a decade ago [3]. In keeping with the Institute of Medicine’s definition of racial disparity, we will use the term racial difference, as it remains unclear whether variation in withdrawal of mechanical ventilation (WMV) is due to a bias or disparity and not due to cultural preference alone [21].
Issues around WMV are particularly salient to critically ill patients with brain injuries, as their pathology often portends a poor prognosis for neurologic recovery rather than the acute systemic multiorgan failure typical in general ICU patients. In this setting, sensitive communication with families about long-term prognosis is even more paramount and subject to biases, mistrust, and varying perspectives on quality of life. Withdrawal of ventilatory support may also seem more incongruous to families in the case of a comatose brain-injured patient than in the case of a patient with refractory shock and/or respiratory insufficiency where death seems an apparent and imminent threat.
A previous study from the 1990s in our Neurology/Neurosurgery Intensive Care Unit (NNICU) found that African Americans were half as likely to discontinue ventilatory support [3]. However, that study did not fully account for SES and severity of illness confounders that may account for some of this association and did not explore whether this large difference in end-of-life decision making led to more minority patients surviving beyond hospital discharge, potentially with persistent neurologic disability. The purpose of the current study is to determine whether racial differences in WMV are still present in a contemporary cohort of patients with severe neurologic insults, accounting for broader spectrum of confounders, including SES and severity of neurologic and physiologic illness. We will then determine whether any racial differences impact overall mortality, specifically whether more nonwhite patients die instead with full ICU support and/or are more likely to be discharged to long-term care (LTC) facilities as a result of less WMV.
2. Materials and methods
We extracted data from a prospectively collected database that includes all NNICU admissions and tracks mortality and hospital disposition. The NNICU is a 20-bed neurologic and neurosurgical unit located in a large urban teaching hospital caring for a significant minority (predominantly African American) population. This analysis was restricted to patients admitted to the NNICU between November 2002 and September 2009 who were mechanically ventilated and whose lowest recorded Glasgow Coma Scale (GCS) score was less than 9 to select severely brain injured patients in whom withdrawal vs continuation of ventilatory support might be a relevant question. We excluded patients who progressed to brain death within 48 hours of ICU admission, in whom withdrawal of ventilation is not commonly discussed, per our usual practice. Patients declared brain dead later than this time were included because they may represent those in whom decisions to not withdraw ventilation permitted their progression to eventual brain death. The Washington University Human Research Protection Office approved the extraction of data for the purpose of this analysis.
Data abstracted included age, race (white vs nonwhite), sex, marital status, and prior functional status. Indicators of SES included type of insurance (private insurance or Medicare vs Medicaid or no insurance) and the patient’s ZIP code. The decision to group Medicare with private insurance was based on the frequent practice of Medicare replacing private employer–based insurance after retirement age. Likewise, Medicaid and no insurance were grouped because they represent those who are dependent on social support for health care. ZIP code was used to determine median household income based on the 2000 US Census (from http://factfinder2.census.gov). We separated ZIP code–based median household incomes into quintiles from lowest to highest and also grouped the lowest 2 quintiles together, roughly corresponding to those living below the poverty level. Severity of illness was derived from admission Acute Physiology and Chronic Health Evaluation (APACHE) II scores as well as the lowest GCS score (recorded daily during the ICU stay). To capture how invested families and the health care team might be in the aggressive/ongoing care of these patients, we identified patients admitted after surgery (either elective or emergent) and noted those who received invasive interventions while in the ICU (surgery/burr holes, intracranial pressure monitoring, lumbar drainage, cerebral angiography, thoracostomy, bronchoscopy, or endoscopy). We created a dichotomous variable including all patients receiving these interventions as a surrogate for aggressiveness of care. Changes in resuscitation status to DNR were also noted.
The primary outcome was WMV, as decided by patients’ surrogate decision makers after discussions with the clinical team. All such cases of terminal extubation were prospectively coded in our ICU database. Secondary end points included in-hospital mortality, separating those dying after WMV from those dying without such limitation of care (either with full support or after determination of brain death). We also measured length of ICU and hospital stay, time to DNR and WMV, and hospital discharge disposition (LTC facilities vs home, rehabilitation, or other hospital).
Data were exported from the ICU database (QuIC; SpaceLabs, Redmond, WA) into SPSS (version 19.0; IBM, Armonk, NY). We compared demographic and severity of illness characteristics between white and nonwhite patients using t tests for normally distributed variables, Mann-Whitney tests for nonnormally distributed continuous or ordinal variables (eg, GCS, APACHE II), and χ2 tests for categorical variables. The univariate effects of race and other variables on WMV were then analyzed using binary logistic regression.
To determine the degree to which other variables accounted for differences in rates of withdrawal between white and nonwhite subjects, we constructed a multivariable regression model for WMV, entering race as the primary independent variable, with additional variables entered in subsequent steps. Measures of SES (ie, income quintile/grouping and insurance status), as established confounders of racial disparity [22], were added to the model first, followed by variables that were significantly different between whites and nonwhites and those that were predictive of WMV in univariate analyses. These variables were added sequentially using a forward stepwise approach (enter P < .2, keep P < .1). The final model produced an adjusted odds ratio (OR) reflecting racial difference in WMV corrected for all these confounding variables. This stepwise approach allowed us to assess the proportion of (crude, unadjusted) racial difference in WMV that was explained by the addition of each subsequent variable by comparing the OR before and after addition of that particular variable [23]. Furthermore, to evaluate whether racial difference had changed over time, we entered year of study as a variable, comparing each subsequent year to 2003 (the first year with sufficient data). Collinearity was assessed by examining tolerance statistics in linear regression models of the same variables as well as ensuring standard errors in the final regression model remained low despite addition of variables.
We compared time to implementation of DNR orders and time to actual WMV between groups using Mann-Whitney U tests. In addition, we evaluated overall mortality to determine whether racial difference resulted in more nonwhites dying by means other than withdrawal of ventilatory support (either by progression to brain death or dying with full ICU support). Finally, we examined the disposition of patients in whom ventilation was not withdrawn to determine whether any difference in WMV resulted in discrepancy in the proportion of patients discharged to LTC.
3. Results
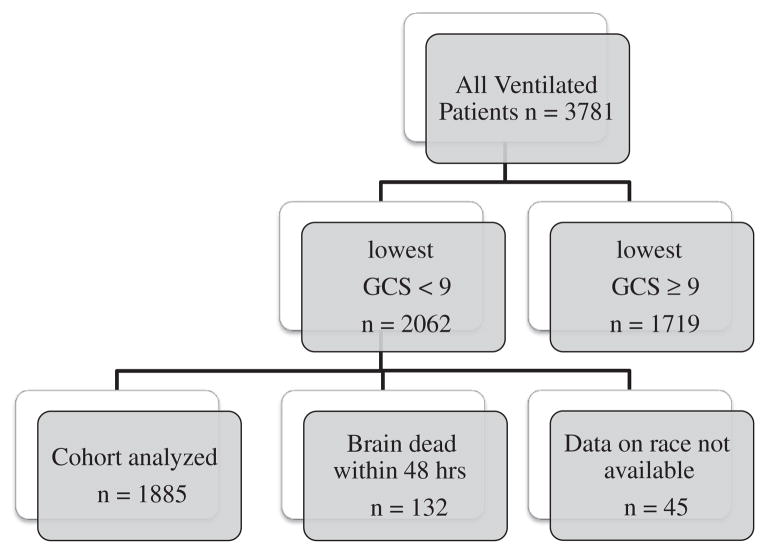
A total of 3781 patients were ventilated in our ICU over this 7-year period. After excluding those whose GCS was never less than 9, 2062 patients with severe neurological injury were eligible for analysis. Of these, 132 were declared brain dead within 48 hours of admission and excluded from analyses of withdrawal of ventilation, leaving 1930 patients for analyses of WMV (Fig. 1). Data on race were only available for 1885 (98%), with 1235 (66%) patients being white and 650 (34%) nonwhite (only 27 of whom were minorities other than African American); and these patients formed the final cohort for analyses of race and WMV. The most common admitting diagnoses were intracranial hemorrhage (parenchymal, subarachnoid, and subdural hematoma) and traumatic brain injury. The overall rate of WMV was 28% (529/1885), but was 40% lower in nonwhites compared to whites (22% vs 31% P < .001). Nonwhites were also significantly younger and were more likely to be unmarried, have Medicaid or no insurance, and reside in ZIP codes with median household incomes in the lowest 2 quintiles (Table 1). Although they were less likely to be admitted after elective surgeries, they were equally likely to receive emergency surgery or aggressive ICU interventions. There was a small difference in admission APACHE II and lowest GCS between groups, with nonwhites being more ill based on these measures.
Fig. 1.
Patient selection.
Table 1.
Comparison of white and nonwhite subjects
| Variable | White (n = 1235) | Nonwhite (n = 650) | P value |
|---|---|---|---|
| Age | 59.4 ± 18.6 | 56.6 ± 17.7 | .001 |
| Sex, male | 675 (55%) | 349 (54%) | .69 |
| Marital status, married | 629 (54%) | 184 (30%) | <.001 |
| Premorbid status, independent | 1070 (91%) | 552 (91%) | .82 |
| Insurance | |||
| Self-pay or Medicaid | 401 (33%) | 296 (46%) | <.001 |
| Private insurance or Medicare | 834 (67%) | 354 (54%) | |
| Median household income (upper limit in year 2000 dollars) | |||
| Quintile 1 (17,920) | 11 (1%) | 31 (5%) | <.001 |
| Quintile 2 (33,000) | 352 (30%) | 464 (74%) | |
| Quintile 3 (52,174) | 620 (52%) | 112 (18%) | |
| Quintile 4 (81,766) | 178 (15%) | 21 (3%) | |
| Quintile 5 | 21 (2%) | 2 (<1%) | |
| Lowest 2 quintiles combined | 363 (31%) | 495 (79%) | <.001 |
| Primary diagnosis | |||
| Intracerebral hemorrhage | 264 (22%) | 172 (28%) | <.001 |
| Subarachnoid hemorrhage | 170 (14%) | 56 (9%) | |
| Traumatic brain injury | 146 (12%) | 73 (12%) | |
| Subdural hemorrhage | 121 (10%) | 45 (7%) | |
| Brain tumor | 69 (6%) | 15 (2%) | |
| Admitted after elective surgery | 133 (11%) | 35 (5%) | <.001 |
| Invasive ICU intervention | 279 (23%) | 134 (21%) | .32 |
| Any surgery and/or ICU intervention | 464 (38%) | 208 (32%) | .02 |
| APACHE II (median, IQR) | 20 (15–24) | 21 (16–25) | <.001 |
| GCS, lowest (median, IQR) | 5 (3–7) | 5 (3–6) | .004 |
| DNR status | 484 (39%) | 230 (35%) | .10 |
| Withdrawal of ventilation | 385 (31%) | 144 (22%) | <.001 |
Apart from race, a number of other variables were associated with WMV (Table 2). Withdrawal of mechanical ventilation was more likely in older patients (34% higher per decade of life). Both markers of severity of illness, APACHE II score and GCS, were strongly associated with higher likelihood of withdrawal. Although no individual income quintile was associated with a higher or lower likelihood of WMV, grouping those in the lowest 2 quintiles together (ie, comparing those living in areas whose median household income was below the poverty level vs those above it), the poorer group had a somewhat lower rate of WMV (OR, 0.82; 95% confidence interval [CI], 0.66–1.00). As hypothesized, patients receiving aggressive ICU or surgical interventions were less likely to undergo WMV.
Table 2.
Variables associated with WMV
| Variable | Frequency of withdrawal | Univariate OR (95% CI) | P value |
|---|---|---|---|
| Race | |||
| Nonwhite | 144 (22%) | 0.63 (0.51–0.78) | <.001 |
| White | 385 (31%) | ||
| Marital status | |||
| Married | 269 (33%) | 1.54 (1.25–1.90) | <.001 |
| Unmarried | 233 (24%) | ||
| Insurance: self-pay or Medicaid | 165 (23%) | 0.66 (0.54–0.82) | <.001 |
| Private insurance or Medicare | 377 (31%) | ||
| Admitted after elective surgery | 14 (8%) | 0.21 (0.12–0.36) | <.001 |
| Admitted other reason | 528 (30%) | ||
| Invasive ICU intervention | 59 (14%) | 0.34 (0.26–0.46) | <.001 |
| No invasive ICU intervention | 483 (32%) | ||
| Surgery or invasive intervention | 94 (14%) | 0.28 (0.22–0.36) | <.001 |
| Neither | 448 (36%) | ||
| Age (per year) | 1.03 (1.02–1.04) | <.001 | |
| APACHE II (per point) | 1.08 (1.06–1.09) | <.001 | |
| GCS, lowest (per point) | 0.68 (0.64–0.73) | <.001 | |
These variables were sequentially entered into the multivariable model for prediction of WMV, with race entered first (Table 3). Addition of income quintile did not modify the OR for WMV by race, meaning that this variable (however it differed between races) did not explain any of the observed differences in WMV between whites and nonwhites. Addition of insurance status reduced racial differences marginally (OR, 0.66–0.69). As disease severity was greater in nonwhites (ie, lower GCS and higher APACHE II) and both these variables were predictors of WMV, their addition to the model resulted in a larger adjusted racial difference (ie, correcting for greater severity, the lower rate of WMV in nonwhites was accentuated). Similar small increments in racial difference were seen with addition of age and aggressive ICU interventions. Adjustment for marital status reduced the racial difference by less than 10%. There was no evidence for collinearity among these variables entered into the model. In the final fully adjusted regression model, the OR for WMV among nonwhites was still significant at 0.56 (95% CI, 0.42–0.76; P < .001), indicating a 44% difference in rate of withdrawal that could not be explained by SES, severity of illness, or other confounding factors. There was no evidence of reduction in racial difference for WMV over time when effects of year of admission were entered into the model.
Table 3.
Multivariate model
| Variable added to model | Adjusted OR for each variable in final model (95% CI) | Stepwise effect on the OR for racial difference (95% CI) | Nagelkerke R2 |
|---|---|---|---|
| Race: nonwhite | 0.56 (0.42–0.76) | 0.66 (0.52–0.82) | 0.11 |
| Income quintile | NS | N/A | N/A |
| Insurance: self-pay or Medicaid | NS | 0.69 (0.53–0.89) | 0.17 |
| GCS, lowest | 0.65 (0.60–0.71) | 0.61 (0.46–0.79) | 0.14 |
| Surgery/ICU intervention | 0.36 (0.27–0.49) | 0.55 (0.42–0.73) | 0.23 |
| Age | 1.03 (1.02–1.04) | 0.56 (0.42–0.75) | 0.27 |
| APACHE II, admit | 1.03 (1.01–1.05) | 0.54 (0.40–0.72) | 0.28 |
| ElectivRe surgery | 0.45 (0.24–0.87) | 0.53 (0.40–0.71) | 0.28 |
| Marital status | 1.31 (1.03–1.68) | 0.56 (0.42–0.76) | 0.29 |
Median time to withdrawal of ventilation was 2 days (interquartile range [IQR], 1–6) in white patients and 3 days (IQR, 1–7) in nonwhites (P = .18). Do-not-resuscitate orders appeared to be used more often among white patients (39% vs 35%, P = .10), although time from admission to DNR was short in both groups (median, 1 vs 2 days; P = .29). Do-not-resuscitate status preceded WMV in almost all cases, usually by a short duration (median, 0–1 day).
Despite significant differences in frequency of death from WMV, overall mortality was similar between whites and nonwhites (42% vs 40%, P = .44). Whereas more white patients died after WMV (75% vs 62%), more nonwhite patients died after progression to brain death (12% vs 3%) or with full support (32% vs 23%, P < .001; Fig. 2). This shift in means of dying translated into a slight increase in the time to death in the nonwhite group (median, 5 days [2–11] vs 4 [1–11]; P = .04). In those surviving hospitalization, nonwhites were more likely to be discharged to an LTC facility (27% vs 17%, P < .001).
Fig. 2.
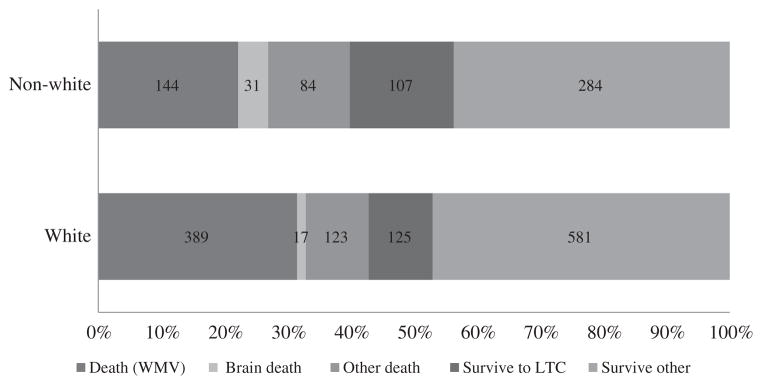
Patient outcomes.
4. Discussion
We confirmed that significant racial differences in limitation of mechanical ventilation persist among a large cohort of ICU patients with severe neurological injuries (defined by GCS < 9). This racial difference is largely unexplained by differences in socioeconomic or marital status, severity of illness, and previous decisions to perform surgery or invasive procedures. After correction for all available confounders, nonwhites were still 44% less likely to withdraw mechanical ventilation. This difference was consistent across all 7 years of the study and remains relatively unchanged from that seen in our previous study a decade earlier, despite adjustment for a greater number of potential confounders. This suggests that the difference reflects some inherent aspect of how surrogates of nonwhite patients make end-of-life decisions in the ICU. This could relate to simply inherent cultural preferences or to systemic barriers in trust, deficiencies in communication by health care providers, health literacy, advanced care planning, or other unknown factors.
Furthermore, despite this large difference in likelihood of WMV, we found no difference of net in-hospital mortality between groups. This suggests that when surrogates chose whether or not to discontinue mechanical ventilation in brain-injured patients, they are largely choosing how a patient will expire and not whether or not they will expire. This novel datum provides important perspectives regarding the implications of the racial differences in WMV on patient outcomes and broader resource utilization. An excess of nonwhite patients surviving only to be discharged to LTC suggests potential for long-term excess costs and disability, an issue that merits further attention.
Our results corroborate the findings of a previous smaller study involving a broad spectrum of patients with severe neurologic injuries [24]. We were able to extend those findings by ensuring that racial difference was not simply confounded by imbalances in SES factors between racial groups that certainly exist. In a more general application, Muni and colleagues [18] evaluated the influence of race on end-of-life care in 15 ICUs and found that, after controlling for SES, nonwhite patients were similarly more likely to die with full ventilatory support (OR, 1.59; 95% CI, 1.3–1.94). Such racial differences in end-of-life decision making may be further amplified in neurologically injured patients, as families’ beliefs and expectations are even more critical in evaluating prognosis and determining whether chances of meaningful neurological recovery match the patient’s wishes and tolerance for potential disability.
Our study does have several limitations. Most importantly, although we adjusted for a number of SES and severity/aggressiveness of care confounders, we did not have specific enough data in such a retrospective study to truly understand all aspects of the decision-making process that may underlie this observed racial difference. We used ZIP code as a surrogate of household income (and SES) but did not have patient-level data on education or religious beliefs. Even in such a single-center study, a lot remains unknown about provider-surrogate communication dynamics. A phenomenon known as statistical discrimination may contribute to persisting racial disparity in the ICU, whereby physician expectations about racial preferences (eg, “African American families are less likely to want limitation of life-sustaining therapies”) influence how health care providers interact with families and may create a self-fulfilling prophecy [25].
The general trend in critical care appears to be toward a limitation of invasive therapy at end of life [1,2,26,27]. This may be due to increased awareness of the importance of addressing goals of care and considering resource utilization. Several studies evaluating the effects of palliative care and ethics consultation have promoted this dimension in improving end-of-life care [28]. However, these studies have not focused specifically on reducing racial disparity and may be directed towards the general population in a way that is less effective for minorities. Although mortality has decreased for leading causes of death overall, racial disparities have persisted [29]. If limiting invasive therapy that has a low likelihood of providing benefit to quality of life is a priority in public health policy, then racial disparity needs to be further researched [30]. Understanding the barriers to various cultural groups making decisions at the end of life will only become more important as globalization in health care continues to expand the burden of acute neurologic illness among groups with varying beliefs and preferences.
Elements worth investigating further include perceptions of bias and lack of trust in the health care provider’s prognosis. Culture preferences likely play a significant role in end-of-life decisions [31]. Previous studies have shown that African Americans are less likely to have advanced care directives and are more likely to choose to die in hospital and continue aggressive interventions even if they only add a week or month of a limited quality of life [32–34]. This may be due to a higher level of confidence in the efficacy of mechanical ventilation and medical interventions in general [34]. Furthermore, studies have found that nonwhite families are more likely to take into account when making such decisions not only the physician’s prognostic information but also their faith and a belief in the power of the patient’s “will to live” [35]. Some may feel that only God has primacy over life and death and that withdrawal is akin to “giving up” [36]. Communication dynamics among family members and with physicians should also be evaluated. For example, Muni et al [18] found that racial groups differ in the amount of discord within families and with physicians, as well as a how prognosis is discussed during family conferences. Health literacy should likewise be investigated, as comatose, brain-injured patients may appear to be resting comfortably on a ventilator. Families may be influenced by the benign physical appearance in underestimating the severity of the underlying illness. Future studies should examine the above subjective elements within a prospective and multicenter framework.
5. Conclusions
Nonwhite patients were less likely to undergo WMV in the face of severe neurologic injury in this single-center retrospective study. This racial difference does not appear to be mediated by imbalances in SES or other available confounding factors. Despite awareness and efforts to reduce differences over the past decade, we found little change in the observed difference over time. Notably, this difference does not lead to lower mortality in nonwhites, suggesting that the effects may mostly be in altering how patients die. We still do not fully understand what lies at the root of these important racial differences, and further studies are needed to determine whether this is a matter of cultural preference or whether it reflects a systemic bias or process/communication issue.
Footnotes
Conflicts of interest and funding: partially supported by National Institutes of Health (National Institute of Neurological Disorders and Stroke) 5P50NS05597704.
References
- 1.Prendergast TJ, Luce JM. Increasing incidence of withholding and withdrawal of life support from the critically ill. Am J Respir Crit Care Med. 1997;155(1):15–20. doi: 10.1164/ajrccm.155.1.9001282. [DOI] [PubMed] [Google Scholar]
- 2.Keenan SP, Busche KD, Chen LM, et al. A retrospective review of a large cohort of patients undergoing the process of withholding or withdrawal of life support. Crit Care Med. 1997;25(8):1324–31. doi: 10.1097/00003246-199708000-00019. [DOI] [PubMed] [Google Scholar]
- 3.Diringer MN, Edwards DF, Aiyagari V, et al. Factors associated with withdrawal of mechanical ventilation in a neurology/neurosurgery intensive care unit. Crit Care Med. 2001;29(9):1792–7. doi: 10.1097/00003246-200109000-00023. [DOI] [PubMed] [Google Scholar]
- 4.Verkade MA, Epker JL, Nieuwenhoff MD, et al. Withdrawal of life-sustaining treatment in a mixed intensive care unit: most common in patients with catastrophic brain injury. Neurocrit Care. 2012;16(1):130–5. doi: 10.1007/s12028-011-9567-y. [DOI] [PubMed] [Google Scholar]
- 5.Cook D, Rocker G, Marshall J, et al. Withdrawal of mechanical ventilation in anticipation of death in the intensive care unit. N Engl J Med. 2003;349(12):1123–32. doi: 10.1056/NEJMoa030083. [DOI] [PubMed] [Google Scholar]
- 6.Smedira NG, Evans BH, Grais LS, et al. Withholding and withdrawal of life support from the critically ill. N Engl J Med. 1990;322(5):309–15. doi: 10.1056/NEJM199002013220506. [DOI] [PubMed] [Google Scholar]
- 7.Curtis JR, Vincent JL. Ethics and end-of-life care for adults in the intensive care unit. Lancet. 2010;376(9749):1347–53. doi: 10.1016/S0140-6736(10)60143-2. [DOI] [PubMed] [Google Scholar]
- 8.Lubitz JD, Riley GF. Trends in medicare payments in the last year of life. N Engl J Med. 1993;328(15):1092–6. doi: 10.1056/NEJM199304153281506. [DOI] [PubMed] [Google Scholar]
- 9.Smedley BD, Stith AY. Unequal treatment: confronting racial and ethnic disparities in health care. National Academy Press; 2003. [PubMed] [Google Scholar]
- 10.McCann J, Artinian V, Duhaime L, et al. Evaluation of the causes for racial disparity in surgical treatment of early stage lung cancer. Chest. 2005;128(5):3440–6. doi: 10.1378/chest.128.5.3440. [DOI] [PubMed] [Google Scholar]
- 11.Liu V, Bhattacharya J, Weill D, et al. Persistent racial disparities in survival after heart transplantation. Circulation. 2011;123(15):1642–9. doi: 10.1161/CIRCULATIONAHA.110.976811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pines JM, Russell Localio A, Hollander JE. Racial disparities in emergency department length of stay for admitted patients in the united states. Acad Emerg Med. 2009;16(5):403–10. doi: 10.1111/j.1553-2712.2009.00381.x. [DOI] [PubMed] [Google Scholar]
- 13.Mathur AK, Osborne NH, Lynch RJ, et al. Racial/ethnic disparities in access to care and survival for patients with early-stage hepatocellular carcinoma. Arch Surg. 145(12):1158–63. doi: 10.1001/archsurg.2010.272. [DOI] [PubMed] [Google Scholar]
- 14.Groeneveld PW, Kruse GB, Chen Z, et al. Variation in cardiac procedure use and racial disparity among Veterans Affairs hospitals. Am Heart J. 2007;153(2):320–7. doi: 10.1016/j.ahj.2006.10.032. [DOI] [PubMed] [Google Scholar]
- 15.Cruz-Flores S, Rabinstein A, Biller J, et al. Racial-ethnic disparities in stroke care: the American experience: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2011;42(7):2091–116. doi: 10.1161/STR.0b013e3182213e24. [DOI] [PubMed] [Google Scholar]
- 16.Degenholtz HB, Thomas SB, Miller MJ. Race and the intensive care unit: disparities and preferences for end-of-life care. Crit Care Med. 2003;31(5 Suppl):S373–8. doi: 10.1097/01.CCM.0000065121.62144.0D. [DOI] [PubMed] [Google Scholar]
- 17.Barnato AE, Chang CCH, Saynina O, et al. Influence of race on inpatient treatment intensity at the end of life. J Gen Intern Med. 2007;22(3):338–45. doi: 10.1007/s11606-006-0088-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Muni S, Engelberg RA, Treece PD, et al. The influence of race/ethnicity and socioeconomic status on end-of-life care in the ICU. Chest. 2011;139(5):1025–33. doi: 10.1378/chest.10-3011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cooper Z, Rivara FP, Wang J, et al. Racial disparities in intensity of care at the end-of-life: are trauma patients the same as the rest? J Health Care Poor Underserved. 2012;23(2):857–74. doi: 10.1353/hpu.2012.0064. [DOI] [PubMed] [Google Scholar]
- 20.Koh HK. A 2020 vision for healthy people. N Engl J Med. 2010;362(18):1653–6. doi: 10.1056/NEJMp1001601. [DOI] [PubMed] [Google Scholar]
- 21.Institute of Medicine. Unequal treatment: confronting racial and ethnic disparities in health care. Washington, DC: National Academy Press; 2002. [PMC free article] [PubMed] [Google Scholar]
- 22.Do DP, Frank R, Finch BK. Does SES explain more of the black/white health gap than we thought? Revisiting our approach toward understanding racial disparities in health. Soc Sci Med. 2012;74(9):1385–93. doi: 10.1016/j.socscimed.2011.12.048. [DOI] [PubMed] [Google Scholar]
- 23.Osborne NH, Upchurch GR, Mathur AK, et al. Explaining racial disparities in mortality after abdominal aortic aneurysm repair. J Vasc Surg. 2009;50(4):709–13. doi: 10.1016/j.jvs.2009.05.020. [DOI] [PubMed] [Google Scholar]
- 24.Mayer SA, Kossoff SB. Withdrawal of life support in the neurological intensive care unit. Neurology. 1999;52(8):1602–9. doi: 10.1212/wnl.52.8.1602. [DOI] [PubMed] [Google Scholar]
- 25.Balsa AI, McGuire TG, Meredith LS. Testing for statistical discrimination in health care. Health Serv Res. 2005;40(1):227–52. doi: 10.1111/j.1475-6773.2005.00351.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Varelas PN, Hacein-Bey L, Schultz L, et al. Withdrawal of life support in critically ill neurosurgical patients and in-hospital death after discharge from the neurosurgical intensive care unit. Clinical article J Neurosurg. 2009;111(2):396–404. doi: 10.3171/2009.3.JNS08493. [DOI] [PubMed] [Google Scholar]
- 27.Koch KA, Rodeffer HD, Wears RL. Changing patterns of terminal care management in an intensive care unit. Crit Care Med. 1994;22(2):233–43. doi: 10.1097/00003246-199402000-00013. [DOI] [PubMed] [Google Scholar]
- 28.Fawole OA, Dy SM, Wilson RF, et al. A systematic review of communication quality improvement interventions for patients with advanced and serious illness. J Gen Intern Med. 2013;28(4):570–7. doi: 10.1007/s11606-012-2204-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Keppel KG, Pearcy JN, Heron MP. Is there progress toward eliminating racial/ethnic disparities in the leading causes of death? Public Health Rep. 2010;5:689–97. doi: 10.1177/003335491012500511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Welch LC, Teno JM, Mor V. End-of-life care in black and white: race matters for medical care of dying patients and their families. J Am Geriatr Soc. 2005;53(7):1145–53. doi: 10.1111/j.1532-5415.2005.53357.x. [DOI] [PubMed] [Google Scholar]
- 31.Sprung CL, Maia P, Bulow HH, et al. The importance of religious affiliation and culture on end-of-life decisions in european intensive care units. Intensive Care Med. 2007;33(10):1732–9. doi: 10.1007/s00134-007-0693-0. [DOI] [PubMed] [Google Scholar]
- 32.Peek ME, Odoms-Young A, Quinn MT, et al. Race and shared decision-making: perspectives of African-Americans with diabetes. Soc Sci Med. 2010;71(1):1–9. doi: 10.1016/j.socscimed.2010.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang AY, Zyzanski SJ, Siminoff LA. Ethnic differences in the caregiver’s attitudes and preferences about the treatment and care of advanced lung cancer patients. Psychooncology. doi: 10.1002/pon.2031. epub ahead of print http://dx.doi.org/10.1002/pon.2031. [DOI] [PMC free article] [PubMed]
- 34.Barnato AE, Anthony DL, Skinner J, et al. Racial and ethnic differences in preferences for end-of-life treatment. J Gen Intern Med. 2009;24(6):695–701. doi: 10.1007/s11606-009-0952-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Boyd EA, Lo B, Evans LR, et al. “It’s not just what the doctor tells me:” factors that influence surrogate decision-makers’ perceptions of prognosis. Crit Care Med. 38(5):1270–5. doi: 10.1097/CCM.0b013e3181d8a217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Torke AM, Garas NS, Sexson W, et al. Medical care at the end of life: views of African American patients in an urban hospital. J Palliat Med. 2005;8(3):593–602. doi: 10.1089/jpm.2005.8.593. [DOI] [PubMed] [Google Scholar]