Abstract
Objectives
An efavirenz-based antiretroviral therapy (ART) regimen is preferred for children more than 3 years of age with tuberculosis. However, rifampin, a key component of antituberculosis therapy, induces CYP2B6. An increased dose of efavirenz is recommended in adults weighing more than 50 kg who require rifampin, but there is scant information in children being treated for tuberculosis.
Design
Plasma efavirenz concentrations were compared in 40 children during concomitant treatment for tuberculosis and HIV-1, after stopping rifampicin, and in a control group of children without tuberculosis. Associations with antituberculosis treatment, metabolizer genotype (based on CYP2B6 516G→T, 983T→C, and 15582C→T), weight, and time after dose were evaluated.
Results
Compared to children with extensive metabolizer genotypes, efavirenz concentrations were increased 1.42-fold (95% confidence interval, CI 0.94–2.15) and 2.85-fold (95% CI 1.80–4.52) in children with intermediate and slow metabolizer genotypes, respectively. Concomitant antituberculosis treatment increased efavirenz concentrations 1.49-fold (95% CI 1.10–2.01) in children with slow metabolizer genotypes, but did not affect efavirenz concentrations in extensive or intermediatemetabolizer genotypes. After adjustment for dose/kg, each kilogram of weight was associated with a 2.8% (95% CI 0.9–4.7) decrease in efavirenz concentrations. Despite higher milligram per kilogram doses, a higher proportion of children in the lowest weight band (10–13.9 kg) had efavirenz concentrations less than 1.0 mg/l than larger children.
Conclusion
Antituberculosis treatment was not associated with reduced efavirenz concentrations in children, which does not support increased efavirenz doses. Children with slow metabolizer genotype have increased efavirenz concentrations during antituberculosis treatment, likely due to isoniazid inhibiting enzymes involved in accessory metabolic pathways for efavirenz.
Keywords: child, CYP2B6, efavirenz, HIV, isoniazid, rifampin, tuberculosis
Introduction
In high burden settings, tuberculosis is common among children with HIV-1 infection [1,2]. Efavirenz with two nucleoside reverse transcriptase inhibitors (NRTIs) is the preferred antiretroviral therapy (ART) regimen for children over 3 years of age if they have tuberculosis [3].
Efavirenz is metabolized predominantly by cytochrome P450(CYP)2B6, a process subject to autoinduction. Rifampin and isoniazid are core components of first-line antituberculosis treatment. Like efavirenz, rifampin induces the expression of CYP2B6, and there is concern that antituberculosis treatment may further reduce plasma efavirenz concentrations due to the inducing effect of rifampin. CYP2A6 and UDP-glucuronosyltransferase 2B7 (UGT2B7) catalyze accessory metabolic pathways of efavirenz [4,5]. Whereas rifampin also induces these enzymes, isoniazid, conversely, inhibits CYP2A6 [6]. The functional genetic polymorphisms CYP2B6 516G→T, 983T→C, and 15582C→T predict increased efavirenz concentrations, and composite CYP2B6 genotypes describe the association of these genetic polymorphisms with efavirenz concentrations [7,8].
There is scant information about the pharmacokinetics of efavirenz in HIV-1-infected children being treated for tuberculosis [9,10]. We previously described similar efavirenz concentrations during and after antituberculosis treatment in 15 children [9]. In this article, we describe the effects of antituberculosis treatment and CYP2B6 polymorphisms on efavirenz concentrations in an independent cohort of children dosed according to the WHO 2006 treatment guidelines [11].
Methods
HIV-1-infected children with tuberculosis underwent pharmacokinetic evaluation after at least 2 weeks of combined treatment with an efavirenz-based ART regimen and antituberculosis treatment containing rifampin. As controls we studied HIV-1-infected children without tuberculosis who were established on efavirenzbased ART. Up to four blood samples were drawn per visit at one or two visits 11–24 h after the evening dose of efavirenz. In children with tuberculosis, pharmacokinetic evaluation was repeated at least 1 month after they completed their antituberculosis treatment. Efavirenz was dosed according to 2006 WHO-recommended weight bands [11]. The daily dose of rifampin (approximately 10 mg/kg) was administered in fixed dose combination (FDC) with isoniazid (approximately 5 mg/kg). During the initial 2-month intensive phase of treatment, the FDC also included pyrazinamide.
Plasma efavirenz concentrations were measured using liquid chromatograph tandem mass spectroscopy as previously described [9]. Intraday and interday precision ranged from 1.2 to 4.1% and 2.5 to 5.3%, respectively. The calibration range was linear over 0.1–15 mg/l and accuracy ranged from 95.2 to 104.6%.
DNA was extracted from whole blood using Wizard Genomic DNA purification kit according to the manufacturer’s protocol (Promega, Madison, Wisconsin, USA). Genetic polymorphisms that predict higher plasma efavirenz concentrations, CYP2B6 516G→T (rs3745274), 983T→C (rs28399499), and 15582C→T (rs4803419) were assayed in the Vanderbilt DNA Resources Core using MassARRAY iPLEX Gold (Sequenom Inc., San Diego, California, USA). Laboratory personnel with no knowledge of the clinical data performed the genotyping. Composite CYP2B6 genotype was determined based on the three CYP2B6 polymorphisms (516G→T, 982T→C, and 15582C→T) [8] as follows: ‘extensive metabolizer’ if genotype showed 516G/G, 983T/T, with either 15582C/C or C/T; ‘intermediate metabolizers’ if genotype showed 516G/T or 983T/C but not both, or if genotype showed homozygosity for 15582T/T; and ‘slow metabolizer’ if genotype showed 516T/T, 983C/C, or the combination of 516G/T with 983T/C.
Seven samples representing one visit for each of three children had plasma efavirenz concentrations below the limit of quantification. As no efavirenz was detectable in any sample at the three visits, yet all three children had efavirenz concentrations more than 1.0 mg/l in all of the samples taken at other visits, the undetectable concentrations (<0.1 mg/l) were excluded from this analysis for presumed nonadherence. Average mid-dose interval (MDI) concentration was defined as the mean of concentrations taken between 12 and 20 h after the dose at the first pharmacokinetic sampling visit. Average MDI concentrations were compared between groups using Wilcoxon rank-sum test (cases vs. controls) and matched-pairs signed-ranks test (within case comparison, during vs. after antituberculosis treatment), respectively. Pearson’s χ2 test was used to evaluate the statistical significance of differences in the proportion of efavirenz concentrations less than 1.0 mg/l between groups.
Multilevel linear mixed-effects (MLME) regression was used to examine the effects of antituberculosis treatment, composite CYP2B6 genotype, time after dose, weight, efavirenz dose per kilogram of body weight, time on ART, age, sex, and BMI on the log-transformed efavirenz concentrations. The model included random effects for individual and visit to account for within individual and visit correlations. Missing genotype values were imputed, using multiple imputation to generate five datasets, by a chained equations approach [12], with estimation results combined by Rubin’s rules [13]. Interaction terms described the additional effects of ‘time after dose’ and ‘tuberculosis treatment’ by metabolizer genotype. The imputed datasets were used in the model to predict the 24-h concentrations of efavirenz.
The Research Ethics Committees of the University of Cape Town and the University of the Witwatersrand approved the study. The Vanderbilt University Institutional Review Board approved this analysis of DNA. Parents or legal guardians gave written informed consent before enrollment of their children, and assent to their participation was obtained from children 7 years of age or older.
Results
A total of 415 efavirenz concentrations from 40 children with tuberculosis and 41 control children without tuberculosis were included in the pharmacokinetic analysis. Composite CYP2B6 genotype data were available for 64 children in whom CYP2B6 extensive, intermediate, and slow metabolizer genotypes were present in 17 (27%), 36 (56%), and 11 (17%), respectively. In the present study, no individuals were homozygous for CYP2B6 15582T/T. Therefore, composite CYP2B6 genotype depended solely on CYP2B6 516G→T and 983T→C polymorphisms. Characteristics of the study participants are summarized in Table 1.
Table 1.
| Children with TB | No TB Controls without tuberculosis |
||
|---|---|---|---|
| During TB treatment | After TB treatment | ||
| N | 40 | 32c | 41d |
| Age (years) | 7.5 (4.6–10.9) | 8.5 (5.0–11.3) | 8.1 (6.4–9.6) |
| Weight (kg) | 19.6 (15.1–25.4) | 23.4 (16.6–28.9) | 22.2 (17.6–26.8) |
| BMI (kg/m2) | 15.8 (15.1–17.1) | 16.1 (15.3–17.6) | 16.1 (15.3–16.8) |
| EFV dose in mg/kg of body weight | 13.9 (13.1–15.0) | 13.7 (12.42–15.14) | 13.4 (12.5–14.2) |
| Time on TB treatment (months) | 3.7 (2.1–5.8) | – | – |
| Time on ART (months) | 2.6 (1.6–4.6) | 7.2 (6.6–9.6) | 24.1 (9.1–49.3) |
| Sex, male/females (% males) | 17/23 (43%) | 12/20 (38%) | 23/18 (56%) |
| Genotyped (n) | 33 | 25 | 31 |
| Extensive metabolizere [n (%)] | 9 (27%) | 7 (28%) | 8 (26%) |
| Intermediate metabolizere [n (%)] | 15 (45%) | 13 (52%) | 21 (68%) |
| Slow metabolizere [n (%)] | 9 (27%) | 5 (20%) | 2 (6.5) |
ART, antiretroviral therapy; EFV, efavirenz; TB, tuberculosis.
Median (IQR) if not otherwise stated.
Characteristics reported are those for the first sampling occasion.
Eight children did not undergo repeated sampling after antituberculosis treatment: one died; ART regimen was changed in four; two were withdrawn for nonmedical reasons; one child was relocated out of the region.
Does not include one child with no detectable efavirenz concentrations, presumably due to poor adherence.
Composite CYP2B6 genotype based on 516G→T, 983T→C, and 15582C→T.
Median CD4+ lymphocyte percentage (CD4%) and plasma HIV-1 RNA were 9.7% [interquartile range (IQR) 4.0, 16.7; n = 30] and 42 000 copies/ml (IQR: 7300–120 000; n = 27) among those children with test results available on starting antituberculosis treatment. After 6 months of antituberculosis treatment, the median (IQR) CD4% had increased to 19.4% (IQR: 12.5–25.3; n = 24) and plasma HIV-1 RNA was less than 400 copies/ml in 20 of 23 children with available test results (efavirenz average MDI concentrations were not significantly different in the children with or without available test results; P = 0.305). Only serious adverse events and AIDS Clinical Trials Group grade 3 or 4 adverse events were recorded. Of these, one adverse event possibly related to treatment was reported in the month after antituberculosis treatment completion. The child had asymptomatic grade 3 elevations of alanine aminotransferase, which returned to normal without treatment adjustment. She had a high average MDI efavirenz concentration (17.17 mg/l) during antituberculosis treatment, which dropped to 4.14 mg/l a month after stopping rifampicin and isoniazid.
Eight children with tuberculosis did not have efavirenz concentration measurement repeated after antituberculosis treatment: one died; four were switched to an alternative antiretroviral regimen due to the development of HIV-1 resistance or other complications; one was withdrawn due to difficulties with phlebotomy; one due to poor adherence; and one relocated to another city.
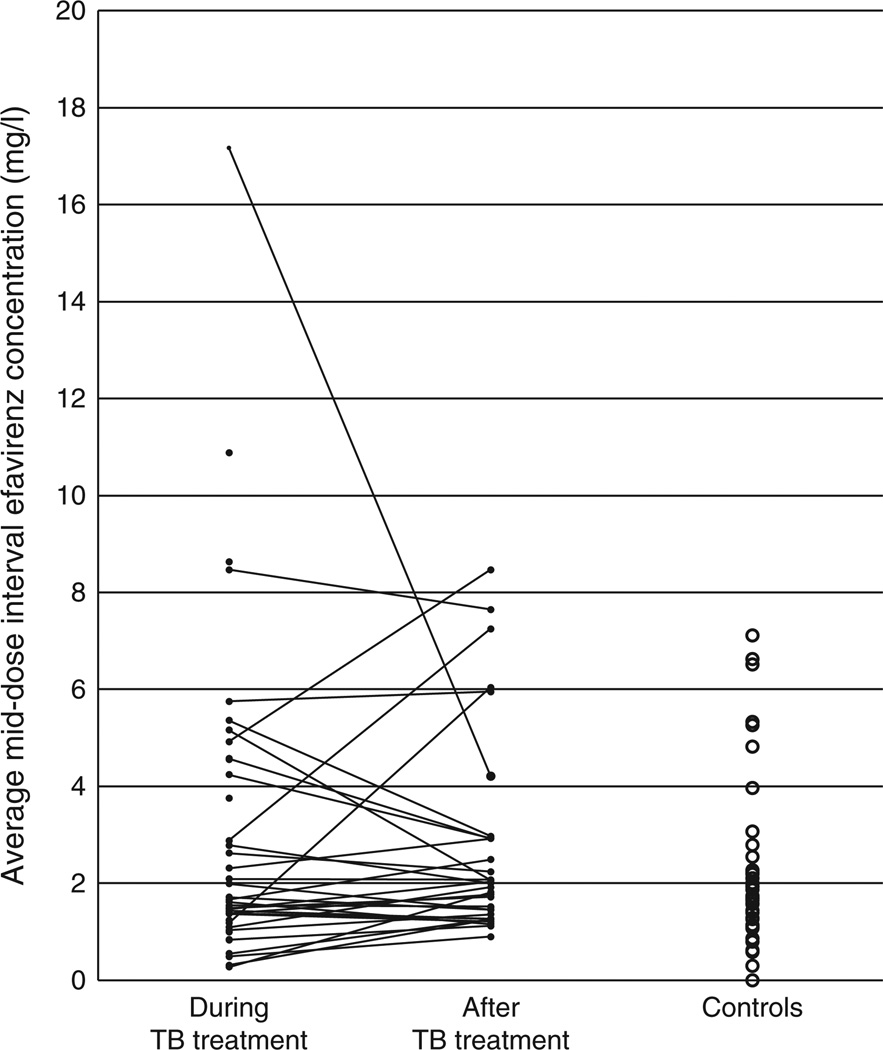
The average MDI efavirenz concentrations during and after antituberculosis treatment, and in controls are represented in Fig. 1. The median (IQR) average MDI efavirenz concentration during antituberculosis treatment was 1.64 (1.21–4.40)mg/l, compared with 1.96 (1.32–2.93) mg/l after antituberculosis treatment (P = 0.64, for 32 children with paired data), and 1.7 (1.14–2.27)mg/l in controls (P = 0.63, compared with ‘on rifampin’).
Fig. 1. Average mid-dose interval concentrations of efavirenz.
Average mid-dose interval concentrations of efavirenz during and after antituberculosis treatment (solid lines join the paired observations), and in controls without tuberculosis (TB).
Using the average MDI concentrations at first pharmacokinetic evaluation, children in the lowest weight band (10–13.9 kg) were more likely to have efavirenz concentrations less than 1.0 mg/l than children weighing 14 kg or more [3/8 (38%) vs. 8/73 (11%), P = 0.037]. Among the 23 children with plasma HIV-1 RNA measurements after 6 months of antituberculosis treatment, the proportion with plasma HIV-1 RNA more than 400 copies/ml did not correspond with plasma efavirenz concentrations less than 1.0 mg/l (1/4 children with efavirenz <1.0 mg/l had plasma HIV-1 RNA >400 copies/ml, as did 2/19 with efavirenz >1.0 mg/l; P = 0.44).
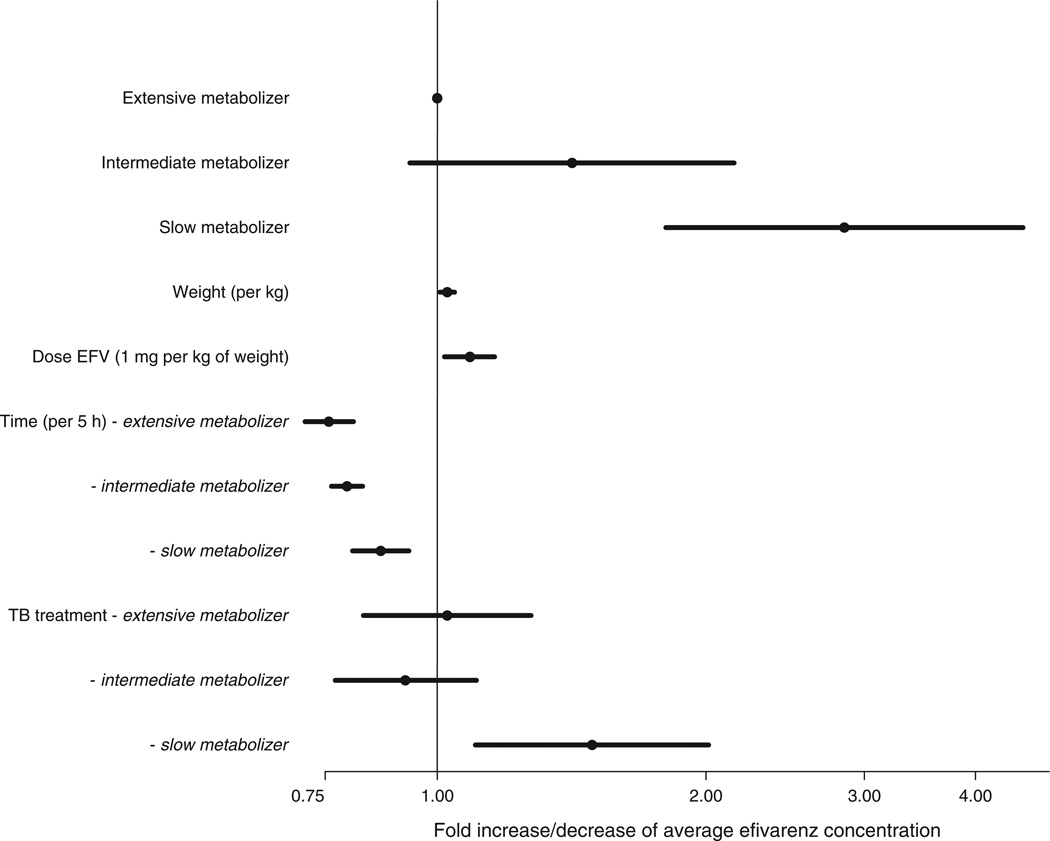
The MLME model described respective 2.85 [95% confidence interval (CI) 1.80–4.52]-fold and 1.42 (95% CI 0.94–2.15)-fold increases in efavirenz concentrations for children with CYP2B6 slow and intermediate metabolizer genotypes, compared with extensive metabolizers. There was no evidence that concomitant antituberculosis treatment was associated with altered efavirenz concentrations in intermediate or extensive metabolizers. However, for slow metabolizers, tuberculosis treatment was associated with a 1.49-fold (95% CI 1.10–2.01) increase in efavirenz concentrations. Among slow metabolizers, efavirenz concentrations declined by 2.9% (95% CI 1.4–4.3) for each hour during the observed portion of the dosing interval, whereas more rapid declines of 4.5% (3.7–5.3) and 5.4% (4.2–6.6), respectively, for each hour, were described in intermediate and extensive metabolizers. After adjustment for dose/kg [8.9% (95% CI 2.0–16.1) increase in efavirenz concentrations for each 1 mg/kg increase in the dose], each kilogram of weight was associated with a 2.8% (95% CI 0.9–4.7) decrease in efavirenz concentrations. Age, sex, BMI, and time on ART were not significantly associated with efavirenz concentrations, so were not included in the final model presented in Fig. 2.
Fig. 2. Factors effecting efavirenz concentrations.
Forest plot representing the results of the multilevel linear mixed-effects regression model describing the effects of antituberculosis (TB) treatment, composite CYP2B6 genotype, time after dose, weight, and efavirenz dose per kilogram of body weight on the log-transformed efavirenz concentrations.
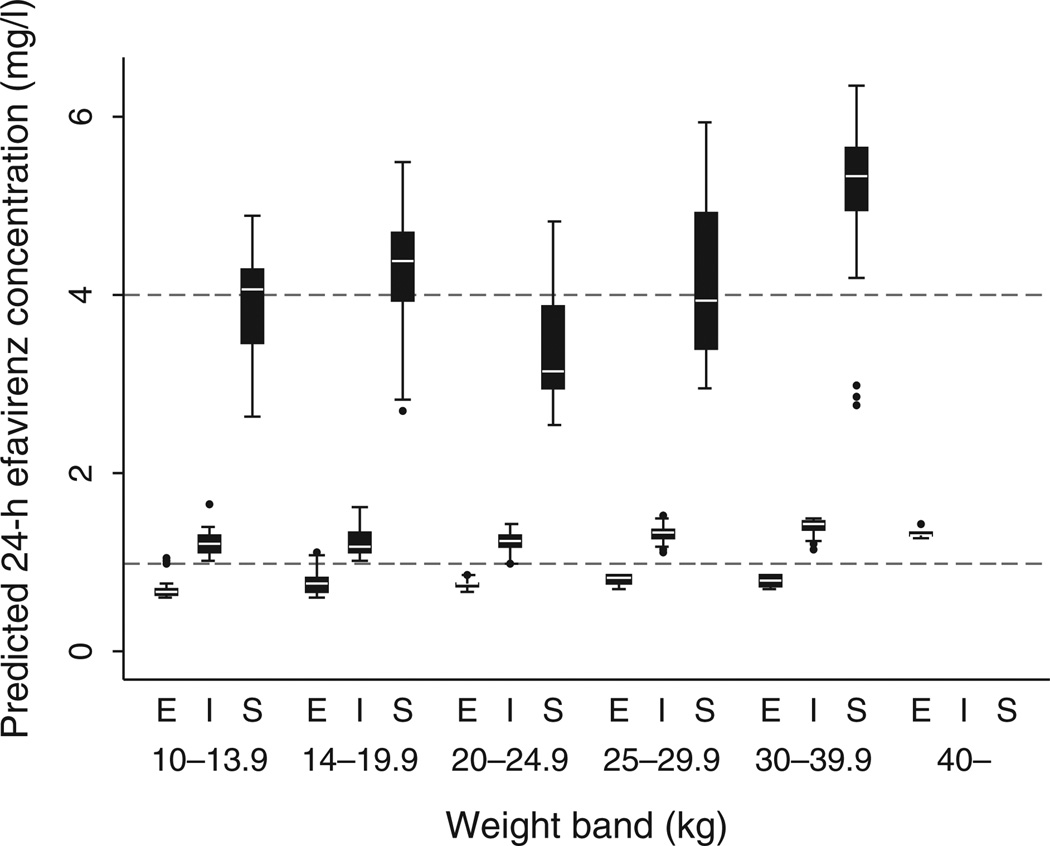
The 24-h trough concentrations of efavirenz predicted after imputation are summarized by genotype and weight band in Fig. 3. Most children with CYP2B6 intermediate metabolizer genotypes had predicted efavirenz trough concentrations within the target range of 1–4 mg/l. Few children with extensive metabolizer genotypes achieved trough concentrations above 1.0 mg/l, whereas many children with slow metabolizer genotypes had concentrations above the target range.
Fig. 3. Predicted 24-h efavirenz concentrations by weight band (in kilograms of body weight), and composite CYP2B6 genotype (E = extensive, I = intermediate, S = slow).
The boxes represent the 25th to 75th percentiles divided by the median and the whiskers represent the 5th–95th percentiles.
Discussion
Our findings do not support the use of increased efavirenz doses in children being treated for tuberculosis with rifampicin-containing regimens, as concomitant antituberculosis treatment was not associated with reduced efavirenz concentrations. This finding is supported by the results of several studies in adults on antituberculosis regimens containing rifampin and isoniazid [14–17].
Unexpectedly, children with CYP2B6 slow metabolizer genotypes had increased efavirenz concentrations during antituberculosis treatment. Although this finding is consistent with several studies in adults [15,16,19], few studies have analyzed the genotype-dependent effect [18]. We hypothesize that the increased efavirenz concentrations during antituberculosis treatment are due to inhibition by isoniazid of accessory metabolizing pathways, which are more important in slow metabolizers. In human liver microsome samples, Ogburn et al. [5] demonstrated that 8-hydroxyefavirenz, which is formed largely by the action of CYP2B6 but also by CYP2A6, accounted for 77.5% of efavirenz metabolized. Efavirenz 7-hydroxylation, which is catalyzed principally by CYP2A6, accounted for 22.5% of efavirenz metabolized. The importance of CYP2A6 for efavirenz elimination among slow metabolizers was suggested in a study of CYP2A6 genetic variants [20], although this association was not replicated in a subsequent study [8]. The CYP2B6 516G→T polymorphism is relatively common in Africa, south-east Asia, and the Caribbean [14–16,21,22], where there is a high incidence of tuberculosis in people with HIV-1 infection. Our results raise the possibility that efavirenz-related central nervous system side-effects, which are associated with higher efavirenz concentrations and early treatment discontinuation, might be more frequent among tuberculosis patients who start efavirenz-based ART [23,24]. Using increased doses of efavirenz during antituberculosis therapy, as has recently been recommended for adults weighing more than 50 kg [25], may further increase this risk among slow metabolizers. Furthermore, the potential drug interactions of isoniazid preventive therapy in HIVinfected patients on efavirenz-based ART should be considered.
The respective contribution of efavirenz, rifampin, and isoniazid to efavirenz metabolism in patients established on combined antituberculosis and ART is not clear. Efavirenz is also metabolized by UGT2B7 directly to efavirenz-N-glucuronide. Although it is usually a minor pathway, it may be more prominent in patients with reduced CYP2B6 function and is inhibited by zidovudine, a commonly coprescribed drug in patients on ART [4]. The magnitude and impact of drug–drug interactions in HIV-1-infected patients with tuberculosis should, therefore, be evaluated in patient populations as studies in healthy volunteers typically fail to account for concomitant drugs other than rifampin, disease factors, or the time course of the combined effects in patients.
The majority of children in our study (83% of those genotyped) had intermediate or extensive metabolizer genotypes, groups that did not have altered efavirenz concentrations during antituberculosis treatment. Ngaimisi et al. [16] found that efavirenz concentrations were reduced to a greater extent in patients on concomitant antituberculosis treatment after just 4 weeks of combined treatment compared to patients without tuberculosis. However, after 16 weeks of ART, the concentrations in patients without tuberculosis had declined to concentrations similar to those at 4 and 16 weeks in the tuberculosis patients. This suggests that antituberculosis treatment did not increase metabolism of the drug more than autoinduction due to efavirenz itself, once that process was complete. Our study was not designed to provide a robust evaluation of the time course of efavirenz concentrations; however, we did not detect an effect of time on ART in the MLME model. It is not known whether children differ from adults in this respect.
The present study had several limitations. Our study had insufficient power to evaluate the impact of efavirenz concentrations on virological outcomes among the children with tuberculosis, and it was not designed to evaluate central nervous system side-effects. No grade 3 or 4 neurological adverse events were reported, but more subtle effects were not recorded. As efavirenz was taken in the evening, the study team did not observe the dose and the absorption phase of concentration–time curve was not characterized. Our data should be extrapolated to other populations with caution. The children in this study were aged between 3 and 15 years; moreover, there is considerable variation in efavirenz concentrations reported in patients from different regions [14–16,21,22]. Lastly, recent guidelines advocate increased doses of rifampicin and isoniazid for children with tuberculosis. The recommended dose of rifampicin has increased from the 10 mg/kg, which was used in our study, to 15 mg/kg and the recommended dose of isoniazid has doubled [26]. It is not known what impact these changes will have on the magnitude of the interactions with efavirenz.
There was wide variability in the effect of antituberculosis treatment on efavirenz concentrations. Although the median change in MDI concentrations (based on average values for each child) was −1%, among the 32 children for whom paired pharmacokinetic data were available, the change ranged from −85 to +308%. Efavirenz concentrations were markedly increased during antituberculosis treatment in one child with a slow metabolizer genotype (Fig. 1). There was no apparent explanation for this outlier, but the individual’s data did influence the MLME model. In a sensitivity analysis that censored this child’s pharmacokinetic data, the model predicted a 28% (95% CI −9 to 81) increase in efavirenz concentrations during antituberculosis treatment for children with a slow metabolizer genotype, whereas the other covariate effects were not substantially altered. This suggests a more modest increase in efavirenz concentrations due to antituberculosis treatment in most slow metabolizers. Clearly, composite CYP2B6 genotype is an important determinant of the effect of antituberculosis treatment on the pharmacokinetics of efavirenz. Additional pharmacogenetic effects warrant investigation, such as effects of more specific CYP2B6 haplotypes [8], and polymorphisms that affect isoniazid and rifampin concentrations, respectively [27,28].
Several studies have described reduced concentrations of efavirenz in children without tuberculosis despite relatively high efavirenz doses per kilogram of bodyweight [29–33]. The weight band-based doses of efavirenz used in this study were recommended by the WHO in 2006. Notwithstanding the higher milligram per kilogram doses prescribed for the lowest weight band (14.4–20 mg/kg), we found that children weighing 10–13.9 kg had reduced efavirenz concentrations when compared to the other weight bands. The WHO increased efavirenz doses in the revised weight band-based guidelines of 2010 based on the pharmacokinetic studies in children. However, children in the lowest weight band were insufficiently represented in those studies and the 200 mg dose recommended for 10–13.9 kg children has not been revised. Our findings suggest that children in this weight band should receive higher doses. However, the most powerful predictor of efavirenz concentrations is CYP2B6 genotype, and few children with the extensive metabolizer genotype achieved predicted 24-h concentrations more than 1.0 mg/l (Fig. 3), which is the lower limit of the recommended target range for trough concentrations of efavirenz [34].
We found highly variable efavirenz concentrations in children, and this variability was increased in a genotypedependent manner among children on antituberculosis therapy. Children with a slow metabolizer genotype who had elevated efavirenz concentrations, experienced an additional increase in efavirenz due to antituberculosis treatment. Adequately powered studies are needed to evaluate the safety and efficacy of efavirenz-based ART in children with tuberculosis. Genotyping for CYP2B6 at treatment initiation with targeted efavirenz dosing could result in a greater proportion of patients achieving efavirenz concentrations within the recommended range, possibly reducing the risk of treatment failure and toxicity. Although our findings support the use of higher efavirenz doses in children who weigh less than 14 kg with and without tuberculosis, in the absence of individualized therapy based on pharmacogenetic testing, our findings do not support dose adjustment in children during rifampin-based antituberculosis treatment.
Acknowledgements
The authors wish to thank the participating children and their parents/caregivers.
The authors also like to thank Mackie Prins, Cynthia Dalamo, Marilyn Solomons, Rae Taylor, Lesley Workman, Angela Oosthuizen, Riki Badenhorst, and others at Red CrossWar Memorial Children’s Hospital HIV clinic in Cape Town, Harriet Shezi Children’s Clinic at Chris Hani Baragwanath Hospital in Soweto, and the University of Cape Town’s Clinical Pharmacology laboratory. They would also like to thank Danielle Richardson of the Vanderbilt Resources Core who did the Vanderbilt portion of genotyping.
H.M.M. designed research, performed research, analyzed data, and wrote the article. M.S. analyzed data and contributed to article. P.Sx., PSm., and D.H. performed research and contributed to article. Y.R., J.N., H.G., H.M., and B.E. performed research. C.M. designed research. G.M. designed research and wrote article.
The European and Developing Countries Clinical Trials Partnership (EDCTP) funded the pharmacokinetic study. Genetic analyses were supported in part by National Institutes of Health (USA) grant AI-077505 (D.W.H.). M.S. is funded via IeDEA-SA by the National Institutes of Allergy and Infectious Diseases (USA) grant 5U01AI069924-05. G.M. is supported in part by the South African National Research Foundation.
H.M. has been principal investigator on research grants to Wits Health Consortium from Gilead Sciences and Tibotec. D.H. has been principal investigator on research grants to Vanderbilt University from Boehringer Ingelheim, Merck, and Gilead Sciences.
Footnotes
Conflicts of interest
For the remaining authors, no conflicts of interest were declared.
References
- 1.Madhi SA, Nachman S, Violari A, Kim S, Cotton MF, Bobat R, et al. Primary isoniazid prophylaxis against tuberculosis in HIV-exposed children. N Engl J Med. 2011;365:21–31. doi: 10.1056/NEJMoa1011214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bakeera-Kitaka S, Conesa-Botella A, Dhabangi A, Maganda A, Kekitiinwa A, Colebunders R, et al. Tuberculosis in human immunodeficiency virus infected Ugandan children starting on antiretroviral therapy. Int J Tuberc Lung Dis. 2011;15:1082–1086. doi: 10.5588/ijtld.10.0538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.World Health Organization. Antiretroviral therapy of HIV infection in infants and children: towards universal access – recommendations for a public health approach: 2010 revision. Geneva, Switzerland: World Health Organization; 2010. [PubMed] [Google Scholar]
- 4.Bélanger AS, Caron P, Harvey M, Zimmerman PA, Mehlotra RK, Guillemette C. Glucuronidation of the antiretroviral drug efavirenz by UGT2B7 and an in vitro investigation of drug-drug interaction with zidovudine. Drug Metab Dispos. 2009;37:1793–1796. doi: 10.1124/dmd.109.027706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ogburn ET, Jones DR, Masters AR, Xu C, Guo Y, Desta Z. Efavirenz primary and secondary metabolism in vitro and in vivo: identification of novel metabolic pathways and cytochrome P450 2A6 as the principal catalyst of efavirenz 7-hydroxylation. Drug Metab Dispos. 2010;38:1218–1229. doi: 10.1124/dmd.109.031393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wen X, Wang JS, Neuvonen PJ, Backman JT. Isoniazid is a mechanism-based inhibitor of cytochrome P450 1A2, 2A6, 2C19 and 3A4 isoforms in human liver microsomes. Eur J Clin Pharmacol. 2002;57:799–804. doi: 10.1007/s00228-001-0396-3. [DOI] [PubMed] [Google Scholar]
- 7.Ribaudo HJ, Liu H, Schwab M, Schaeffeler E, Eichelbaum M, Motsinger-Reif AA, et al. Effect of CYP2B6, ABCB1, and CYP3A5 polymorphisms on efavirenz pharmacokinetics and treatment response: an AIDS Clinical Trials Group study. J Infect Dis. 2010;202:717–722. doi: 10.1086/655470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Holzinger ER, Grady B, Ritchie MD, Ribaudo HJ, Acosta EP, Morse GD, et al. Genome-wide association study of plasma efavirenz pharmacokinetics in AIDS Clinical Trials Group protocols implicates several CYP2B6 variants. Pharmacogenet Genomics. 2012;22:858–867. doi: 10.1097/FPC.0b013e32835a450b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ren Y, Nuttall JJ, Eley BS, Meyers TM, Smith PJ, Maartens G, et al. Effect of rifampicin on efavirenz pharmacokinetics in HIV-infected children with tuberculosis. J Acquir Immune Defic Syndr. 2009;50:439–443. doi: 10.1097/QAI.0b013e31819c33a3. [DOI] [PubMed] [Google Scholar]
- 10.Shah I, Swaminathan S, Ramachandran G, Kumar AK, Goray A, Chaddha U, et al. Serum nevirapine and efavirenz concentrations and effect of concomitant rifampicin in HIV infected children on antiretroviral therapy. Indian Pediatr. 2011;48:943–947. doi: 10.1007/s13312-011-0153-3. [DOI] [PubMed] [Google Scholar]
- 11.World Health Organization. Antiretroviral therapy of HIV infection in infants and children in resource-limited settings: towards universal access. Recommendations for a public health approach. Geneva, Switzerland: World Health Organization; 2006. [Google Scholar]
- 12.White I, Royston P, Wood A. Multiple imputation using chained equations. Stat Med. 2011;30:377–399. doi: 10.1002/sim.4067. [DOI] [PubMed] [Google Scholar]
- 13.Rubin DB. Multiple imputation after 18+ years. J AmStat Assoc. 1996;91:473–489. [Google Scholar]
- 14.Cohen K, Grant A, Dandara C, McIlleron H, Pemba L, Fielding K, et al. Effect of rifampicin-based antitubercular therapy and the cytochrome P450 2B6 516G>T polymorphism on efavirenz concentrations in adults in South Africa. Antivir Ther. 2009;14:687–695. [PMC free article] [PubMed] [Google Scholar]
- 15.Uttayamakul S, Likanonsakul S, Manosuthi W, Wichukchinda N, Kalambaheti T, Nakayama EE, et al. Effects of CYP2B6 G516T polymorphisms on plasma efavirenz and nevirapine levels when co-administered with rifampicin in HIV/TB co-infected Thai adults. AIDS Res Ther. 2010;7:8. doi: 10.1186/1742-6405-7-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ngaimisi E, Mugusi S, Minzi O, Sasi P, Riedel KD, Suda A, et al. Effect of rifampicin and CYP2B6 genotype on long-term efavirenz autoinduction and plasma exposure in HIV patients with or without tuberculosis. Clin Pharmacol Ther. 2011;90:406–413. doi: 10.1038/clpt.2011.129. [DOI] [PubMed] [Google Scholar]
- 17.Luetkemeyer AF, Rosenkranz SL, Lu D, Lizak PS, Ive P, Swindells S, et al. Relationship between weight, efavirenz (EFV) concentrations and virologic suppression in HIV+ patients on rifampin (RIF)-based TB treatment in the ACTG 5221 STRIDE study [abstract]. XIX International AIDS Conference; 22–27 July 2012; Washington DC, USA. [Accessed 25 November 2012]. MOAB0301. http://pag.aids2012.org/abstracts.aspx?aid=4360. [Google Scholar]
- 18.Kwara A, Lartey M, Sagoe KW, Court MH. Paradoxically elevated efavirenz concentrations in HIV/tuberculosis-coinfected patients with CYP2B6 516TT genotype on rifampin-containing antituberculous therapy. AIDS. 2011;25:388–390. doi: 10.1097/QAD.0b013e3283427e05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gengiah TN, Holford NH, Botha JH, Gray AL, Naidoo K, Abdool Karim SS. The influence of tuberculosis treatment on efavirenz clearance in patients co-infected with HIV and tuberculosis. Eur J Clin Pharmacol. 2012;68:689–695. doi: 10.1007/s00228-011-1166-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Di Iulio J, Fayet A, Arab-Alameddine M, Rotger M, Lubomirov R, Cavassini M, et al. In vivo analysis of efavirenz metabolism in individuals with impaired CYP2A6 function. Pharmacogenet Genomics. 2009;19:300–309. doi: 10.1097/FPC.0b013e328328d577. [DOI] [PubMed] [Google Scholar]
- 21.Ramachandran G, Ramesh K, Hemanth Kumar AK, Jagan I, Vasantha M, Padmapriyadarsini C, et al. Association of high T allele frequency of CYP2B6 G516T polymorphism among ethnic south Indian HIV-infected patients with elevated plasma efavirenz and nevirapine. J Antimicrob Chemother. 2009;63:841–843. doi: 10.1093/jac/dkp033. [DOI] [PubMed] [Google Scholar]
- 22.Leger P, Dillingham R, Beauharnais CA, Kashuba AD, Rezk NL, Fitzgerald DW, et al. CYP2B6 variants and plasma efavirenz concentrations during antiretroviral therapy in Port-au-Prince, Haiti. J Infect Dis. 2009;200:955–964. doi: 10.1086/605126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Marzolini C, Telenti A, Decosterd LA, Greub G, Biollaz J, Buclin T. Efavirenz plasma levels can predict treatment failure and central nervous system side effects in HIV-1-infected patients. AIDS. 2001;15:71–75. doi: 10.1097/00002030-200101050-00011. [DOI] [PubMed] [Google Scholar]
- 24.Wyen C, Hendra H, Siccardi M, Platten M, Jaeger H, Harrer T, et al. Cytochrome P450 2B6 (CYP2B6) and constitutive androstane receptor (CAR) polymorphisms are associated with early discontinuation of efavirenz-containing regimens. J Antimicrob Chemother. 2011;66:2092–2098. doi: 10.1093/jac/dkr272. [DOI] [PubMed] [Google Scholar]
- 25.FDA. Sustiva labeling update /dosing adjustment with rifampin. [Accessed 29 October 2012];2012 http://www.fda.gov/ForConsumers/ByAudience/ForPatientAdvocates/HIVandAIDSActivities/ucm294476.htm.
- 26.World Health Organization; Geneva: 2010. [Accessed 21 February 2013]. Rapid advice: treatment of tuberculosis in children. WHO/HTM/TB/2010.13. http://whqlibdoc.who.int/publications/2010/9789241500449_eng.pdf. [PubMed] [Google Scholar]
- 27.Parkin DP, Vandenplas S, Botha FJ, Vandenplas ML, Seifart HI, van Helden PD, et al. Trimodality of isoniazid elimination: phenotype and genotype in patients with tuberculosis. Am J Respir Crit Care Med. 1997;155:1717–1722. doi: 10.1164/ajrccm.155.5.9154882. [DOI] [PubMed] [Google Scholar]
- 28.Chigutsa E, Visser ME, Swart EC, Denti P, Pushpakom S, Egan D, et al. The SLCO1B1 rs4149032 polymorphism is highly prevalent in South Africans and is associated with reduced rifampin concentrations: dosing implications. Antimicrob Agents Chemother. 2011;55:4122–4127. doi: 10.1128/AAC.01833-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ren Y, Nuttall JJ, Egbers C, Eley BS, Meyers TM, Smith PJ, et al. High prevalence of subtherapeutic plasma concentrations of efavirenz in children. J Acquir Immune Defic Syndr. 2007;45:133–136. doi: 10.1097/QAI.0b013e31805c9d52. [DOI] [PubMed] [Google Scholar]
- 30.Hirt D, Urien S, Olivier M, Peyrière H, Nacro B, Diagbouga S, et al. Is the recommended dose of efavirenz optimal in young west African human immunodeficiency virus-infected children? Antimicrob Agents Chemother. 2009;53:4407–4413. doi: 10.1128/AAC.01594-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fillekes Q, Natukunda E, Balungi J, Kendall L, Bwakura-Dangarembizi M, Keishanyu R, et al. Pediatric underdosing of efavirenz: a pharmacokinetic study in Uganda. J Acquir Immune Defic Syndr. 2011;58:392–398. doi: 10.1097/QAI.0b013e318235e560. [DOI] [PubMed] [Google Scholar]
- 32.Saitoh A, Fletcher CV, Brundage R, Alvero C, Fenton T, Hsia K, et al. Efavirenz pharmacokinetics in HIV-1-infected children are associated with CYP2B6-G516T polymorphism. J Acquir Immune Defic Syndr. 2007;45:280–285. doi: 10.1097/QAI.0b013e318040b29e. [DOI] [PubMed] [Google Scholar]
- 33.Starr SE, Fletcher CV, Spector SA, Yong FH, Fenton T, Brundage RC, et al. Combination therapy with efavirenz, nelfinavir, and nucleoside reverse-transcriptase inhibitors in children infected with human immunodeficiency virus type 1. Pediatric AIDS Clinical Trials Group 382 Team. N Engl J Med. 1999;341:1874–1881. doi: 10.1056/NEJM199912163412502. [DOI] [PubMed] [Google Scholar]
- 34.La Porte CJL, Back DJ, Blaschke T, Boucher CAB, Fletcher CV, Flexner C, et al. Updated guideline to perform therapeutic drug monitoring for antiretroviral agents. Rev Antiviral Ther. 2006;3:4–14. [Google Scholar]