Abstract
The rhombic lip gives rise to neuronal populations that contribute to cerebellar, proprioceptive and interoceptive networks. Cell production depends on the expression of the basic helix-loop-helix (bHLH) transcription factor Atoh1. In rhombomere 1, Atoh1-positive cells give rise to both cerebellar neurons and extra-cerebellar nuclei in ventral hindbrain. The origin of this cellular diversity has previously been attributed to temporal signals rather than spatial patterning. Here, we show that in both chick and mouse the cerebellar Atoh1 precursor pool is partitioned into initially cryptic spatial domains that reflect the activity of two different organisers: an isthmic Atoh1 domain, which gives rise to isthmic nuclei, and the rhombic lip, which generates deep cerebellar nuclei and granule cells. We use a combination of in vitro explant culture, genetic fate mapping and gene overexpression and knockdown to explore the role of isthmic signalling in patterning these domains. We show that an FGF-dependent isthmic Atoh1 domain is the origin of distinct populations of Lhx9-positive neurons in the extra-cerebellar isthmic nuclei. In the cerebellum, ectopic FGF induces proliferation while blockade reduces the length of the cerebellar rhombic lip. FGF signalling is not required for the specification of cerebellar cell types from the rhombic lip and its upregulation inhibits their production. This suggests that although the isthmus regulates the size of the cerebellar anlage, the downregulation of isthmic FGF signals is required for induction of rhombic lip-derived cerebellar neurons.
Keywords: Lhx9, Otx2, Gbx2, Isthmo-optic nucleus, Deep cerebellar nuclei, FGF receptor knockout, Cerebellum, Chick, Mouse
INTRODUCTION
Recent years have seen a startling conceptual simplification of the genetic programmes underlying the development of the cerebellum and proprioceptive networks. At the heart of this revision has been a series of increasingly complex fate maps that build on Wilhelm Harkmark’s original ablation studies in the chick embryo in the 1950s (Harkmark, 1954; Wingate, 2001). More modern anatomical (Wingate and Hatten, 1999; Gilthorpe et al., 2002) and, in particular, genetic fate maps (Rodriguez and Dymecki, 2000; Machold and Fishell, 2005; Wang et al., 2005; Rose et al., 2009) have pinpointed the origin of the glutamatergic precerebellar and cerebellar neurons in the rhombic lip, a thin strip of cells that borders the expanded roof plate of the fourth ventricle. The production of this distinct range of cell types is entirely dependent on the expression of the basic helix-loop-helix (bHLH) transcription factor Atonal 1 (Atoh1/Math1) (Ben-Arie et al., 1997; Wang et al., 2005; Rose et al., 2009). These cells form an integrated network that relays proprioceptive information both to the thalamus and cerebellum.
Expression of Atoh1 in rhombic-lip-derived neurons is both induced by dorsally derived TGFβ signals (Alder et al., 1999; Lee et al., 2000) and actively maintained by the roof plate organiser (Broom et al., 2012). Although Atoh1 is transient (in all but specialised granule cell precursors of the cerebellum) this expression is sufficient to conditionally drive reporters that have been used to map all rhombic lip derivatives in transgenic mice (Machold and Fishell, 2005; Wang et al., 2005; Rose et al., 2009) and fish (Kani et al., 2010). Increasing resolution in these fate maps has revealed that, for the cerebellar rhombic lip of rhombomere (r) 1, there is a perhaps surprising diversity of neuronal derivatives from the Atoh1 pool born within a very narrow temporal window, before the generation of cerebellar cell types. In particular, fate maps identify small groups of isthmic nuclei such as the cholinergic parabigeminal nucleus and the dorsal nucleus of the lateral lemniscus that lie outside the cerebellum and at the border of the midbrain (Machold and Fishell, 2005; Rose et al., 2009). These isthmic nuclei play important roles in regulating bilateral correlation of midbrain sensory maps for both vision and audition, respectively (Butler and Hodos, 1996). Isthmic nuclei with divergent anatomical conformations show a fragmented distribution of isthmic nuclear structures across vertebrate phyla. This includes specialised cell groups such as the isthmo-optic nucleus that projects to the retina in birds, some basal fish and a subset of reptiles (Butler and Hodos, 1996). This overall variability highlights different developmental constraints on isthmic rhombic lip derivatives when compared with other, conserved outputs of the Atoh1 pool in the cerebellum. What are the likely candidates for regulating the diversity of these different derivatives?
A primary determinant of cell fate in the rhombic lip is the temporal patterning cues (Gilthorpe et al., 2002; Machold and Fishell, 2005) through extrinsic signals that are yet to be determined (Wilson and Wingate, 2006). However, a highly diverse set of nuclei in ventral r1 is produced in a very short temporal window, and therefore a temporal signal is not likely to be sufficient to produce such fine grain specificity in different cell types. A possible additional source of patterning information is spatial cues, which can be divided into dorsalising signals from the roof plate boundary organiser (Broom et al., 2012) and rostrocaudal cues originating from the midbrain/hindbrain isthmus. As the linear pool of Atoh1-expressing cells comprises the most dorsal cell population in the neural tube, variability in dorsoventral patterning can be ruled out as a source of diversity. However, the isthmus, which comprises the anterior boundary of r1, has a profound role on the establishment of the entire region via the action of the secreted morphogen FGF8 (Joyner, 1996; Reifers et al., 1998). This includes restricting the encroachment of Otx2-positive midbrain into r1 (Foucher et al., 2006; Sato and Joyner, 2009) and preventing the hypoplasia of the medial [embryonically rostral (Sgaier et al., 2005)] cerebellar vermis, which accompanies downregulation of FGF signalling (Meyers et al., 1998; Chi et al., 2003; Basson et al., 2008). Whether the isthmus also confers rostrocaudal pattern within cerebellar territory is less clear, despite evidence for its highly polarised effects on midbrain maturation (Martinez et al., 1999; Shamim et al., 1999; Zervas et al., 2004). Studies in chick suggest that segmental identity overrides isthmic signalling in inducing rhombic lip cell types (Eddison et al., 2004). However, the sequence of specification of rhombic lip derivatives has not been examined in embryos with attenuated FGF signalling at the isthmus.
To investigate the effects of spatial organisation on Atoh1 expression and its derivatives in r1, we have contrasted mouse transgenic manipulation of FGF signalling with experiments in the chick embryo model. The latter is amenable to a range of approaches, including an unbiased assessment of the interactions between different tissues through ablation studies in culture and focal targeting of genetic manipulations by in ovo electroporation. Through these, we show that the Atoh1 domain is cryptically organised into two territories that are separately maintained by FGF and roof plate signals. These respective isthmic and rhombic lip domains gives rise to different derivatives: the former giving rise to isthmic nuclei. We propose that this pool of isthmic, FGF-dependent Atoh1 progenitors may be a conserved feature of r1 patterning, suggesting a re-evaluation of genetic fate maps and a re-definition of ‘rhombic lip’ by its inductive relationships rather than gene expression alone.
RESULTS
Atoh1 is expressed in three distinct domains in r1 in chick and mouse
In examining the expression of Atoh1 in the chick embryo at embryonic day (E) 5, we identified a prominent site of expression in dorsal, isthmic r1 apparently distinct from the rhombic lip, before the formation of an external granule layer (EGL) (Fig. 1A). This isthmic Atoh1 domain extends from the pial to ventricular surface of the tissue (Fig. 1B) and is bordered rostrally by Otx2-positive midbrain (Fig. 1C) and caudally by Ptf1a-expressing progenitors in the cerebellar ventricular zone (Fig. 1D). At later stages (E6/7) when Atoh1-positive granule cell precursors accumulate in an EGL, the isthmic Atoh1 domain becomes segregated from the developing cerebellum into a domain that lies rostral to the cerebellar plates (Fig. 1E). Analysis of mouse embryos at an equivalent stage (E14.5) reveals a similar, although markedly smaller domain of Atoh1 expression abutting the isthmus (Fig. 1F). By E8 in chick, the rostral domain of expression is downregulated and Atoh1 is exclusively expressed in a distinct EGL (data not shown).
Fig. 1.
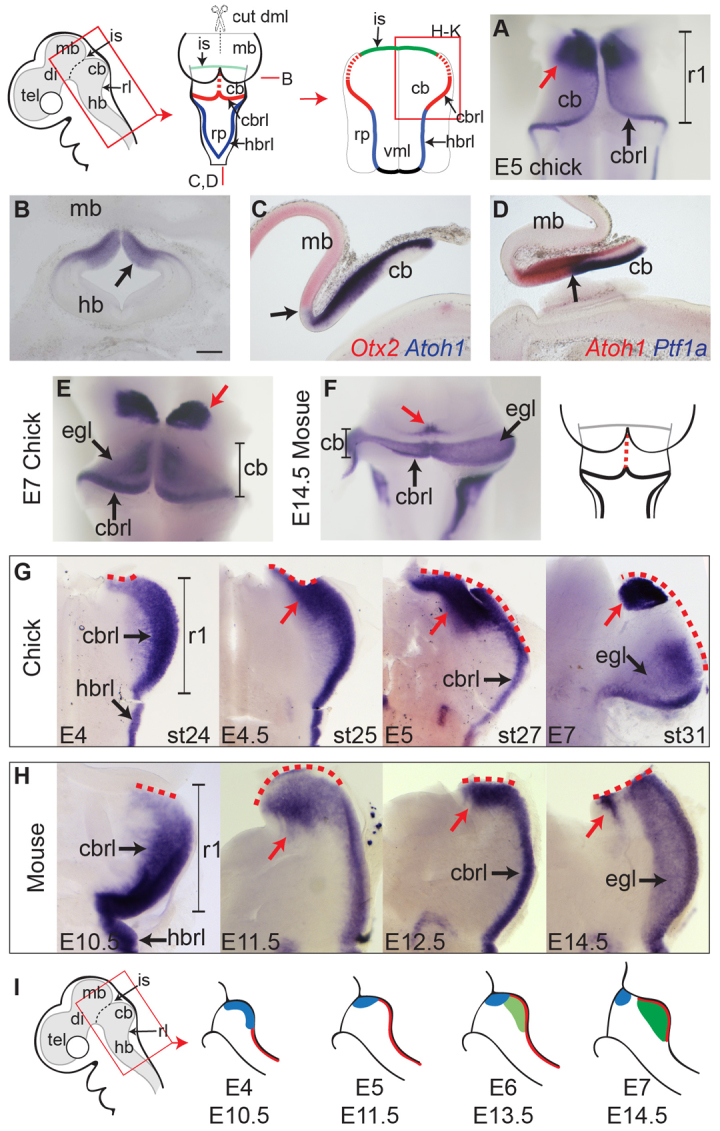
Distinct Atoh1 domains in r1. (A) In a dorsal view of the cerebellum at E5, Atoh1 expression is seen in a distinct rostral/isthmic domain (red arrow) and at the cerebellar rhombic lip. (B-D) Coronal (B) and sagittal (C,D) sections at E5 (see schematic, top left) reveal Atoh1 expression in the isthmic domain extends to the ventricular surface (B, arrow) and abuts the expression of Otx2 rostrally (C, arrow) and Ptf1a (which defines Purkinje cell precursors), caudally (D, arrow). (E,F) Dorsal view of E7 chick (E) and E14.5 mouse (F) cerebellum and rostral hindbrain showing Atoh1 expression in an isthmic domain (red arrow) segregated from the developing external granule layer of the cerebellum. (G,H) Timecourse of Atoh1 expression in chick (G) and mouse (H) shown in flat-mounted hindbrain preparations. Atoh1 expression in the cbrl is initially broader than in the hb rhombic lip (G,H, left) and is subsequently confined to rostral cerebellum (red arrow). A dashed red line indicates the cut edge of the dorsal midline (schematic diagram above). (I) Schematic diagram of hindbrain at different stages indicating the dynamic, broad Atoh1 domain (blue) in comparison to cerebellar rhombic lip (red) and external granule layer (green) domains of Atoh1 expression. Scale bars: 200 μm (B-D). cb, cerebellum; cbrl, cerebellar rhombic lip; di, diencephalon; dml, dorsal midline; egl, external granule layer; hb, hindbrain; hbrl, hindbrain rhombic lip; is, midbrain-hindbrain isthmus; mb, midbrain; rl, rhombic lip; rp, roof plate; tel, telencephalon; vml, ventral midline.
We examined the establishment of the isthmic Atoh1-positive domain by a stage by stage expression analysis in both chick (Fig. 1G) and mouse (Fig. 1H). At E4 [Hamburger and Hamilton stage (st.) 24] in chick (Fig. 1G) and at E10.5 in mouse (Fig. 1H), the Atoh1-positive rhombic lip in the presumptive cerebellum (r1) is broader than in the hindbrain. Between E4 and E5 (st.24-st.27 in chick) (Fig. 1G), this broad domain of Atoh1 expression is refined to the rostral pole of the cerebellar primordium. In mouse, a similar refinement results in a broad Atoh1 domain at the fused midline region of the cerebellum at E11.5 and E12.5 and subsequently a spatially separate domain by E14.5 (Fig. 1H). As in chick (Fig. 1G), this isthmic domain in mouse lies outside the cerebellum. Fig. 1I summarises this dynamic Atoh1 expression in the formation of three distinct domains: a broad rostral domain (blue), which is initially seen throughout r1 and becomes refined to an extra-cerebellar territory; the rhombic lip (red); granule cell precursors of the EGL (green).
The isthmic Atoh1 domain gives rise to a distinct pool of Lhx9-positive non-cerebellar neurons
To fate map the isthmic Atoh1 domain, we employed a mouse Atoh1 enhancer element (Helms et al., 2000) linked to Cre recombinase (Kohl et al., 2012) that conditionally labels cells when co-electroporated with a plasmid containing gfp proceeded by a floxed stop-cassette. Rostral r1 was co-electroporated at E5 and examined 2 days later when fluorescent cells were found rostral to the cerebellum at the isthmus (Fig. 2A, n=9). The absence of cells labelled in the EGL confirms the specificity of targeting to rostral r1 without electroporating the more caudal region of the r1 rhombic lip. In mouse, non-cerebellar derivatives of the Atoh1-positive rhombic lip express Lhx9 (Rose et al., 2009). In chick, this transcription factor is expressed in a continuous domain in r1 extending from dorsal (rostrally) to ventral (caudally) (Fig. 2B), including the isthmic Atoh1-positive domain at E5 (Fig. 2C). Serial coronal sections through this region in electroporated embryos show that neurons labelled by GFP under the Atoh1 conditional reporter (Fig. 2D) express Lhx9 (Fig. 2E) and are displaced laterally relative to the isthmic Atoh1 domain (Fig. 2F). These cells extend axons rostrally over across the optic tectum and a subset of axons turn ventrally/rostrally (Fig. 2G). The position of labelled cells with tectal projections identifies them as neurons of the nucleus isthmi pars parvocellularis (Hunt and Künzle, 1976), which is a part of the isthmic complex involved in integration of visual information. Although we were unable to establish the termination of ventrally/rostrally turning axons, their initial trajectory is characteristic of retinal projections of the isthmo-optic nucleus (McGill et al., 1966; Cowan and Clarke, 1976).
Fig. 2.
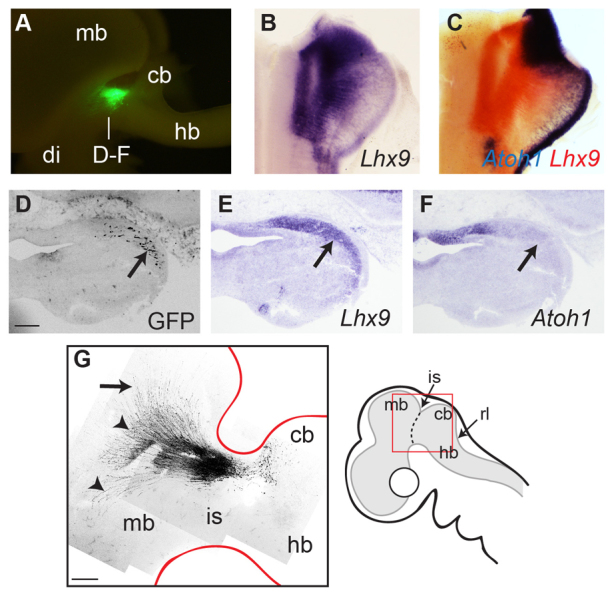
Isthmic Atoh1 gives rise to specific nuclei. (A) Lateral view of an E7 chick brain showing specific green fluorescent protein (GFP) cell labelling at the isthmus following co-electroporation of Atoh1-cre + lox-stop-lox-gfp constructs into isthmic Atoh1 domain at E5. (B,C) Lhx9 expression at E5 (B, blue; C, red) overlaps isthmic Atoh1 (C, blue), extending from dorsal/rostral to ventral/caudal rhombomere 1. (D-F) Following electroporation of Atoh1-cre + lox-stop-lox-gfp at E5, serial coronal sections at E7 show migrated neurons (D, arrow) coincident with Lhx9 expression (E) lateral to isthmic Atoh1 (F). (G) Composite confocal image of GFP-labelled cells in the isthmic region (schematic, right) of a flat-mount of the embryo in A: axons extend over the tectum (arrow) and rostrally/ventrally (arrowheads). Scale bars: 200 μm (D-F); 0.5 mm (G). cb, cerebellum; di, diencephalon; hb, hindbrain; is, midbrain-hindbrain isthmus; mb, midbrain.
Atoh1 expression at the isthmus and rhombic lip is regulated by the midbrain/hindbrain boundary
The striking, dynamic re-positioning of the isthmic Atoh1-positive domain suggests a dependence on proximity to the boundary of the midbrain and hindbrain, which in early development constitutes an important organiser of regional cell fate (Joyner, 1996). To determine whether isthmic Atoh1 expression is dependent on signalling from the isthmic organiser, we designed a series of in vitro explant experiments in which different tissues were selectively and precisely removed (Fig. 3A). Specific removal of roof plate tissue, confirmed by absence of Gdf7 expression (Fig. 3B), from E6 hindbrain tissue preparations encompassing intact territory from the midbrain-hindbrain isthmus shows the full complement of Atoh1 expression domains when fixed immediately after dissection (Fig. 3B). Over the course of 32 hours in vitro, expression of Atoh1 in the rhombic lip is selectively abolished (Broom et al., 2012); however, Atoh1 expression in the isthmic domain is retained (Fig. 3C). By contrast, removing the midbrain at E5 while leaving roof plate intact (Fig. 3D) eliminates isthmic Atoh1 expression but leaves rhombic lip expression intact after 48 hours in culture (n=6). Removal of roof plate and the unilateral midbrain at both E4 (Fig. 3E) and E5 (Fig. 3F) abolishes Atoh1 expression in the rhombic lip and isthmic domain ipsilateral to midbrain ablation (n=35). In a subset of these explants, removal of midbrain territory was confirmed by in situ hybridisation for Otx2 (Fig. 3F). This suggests a model whereby the midbrain/hindbrain boundary maintains Atoh1 expression in rostral r1 independently of the roof plate. This is further supported by the observation that unilateral midbrain ablation at E3 reduces the breadth of Atoh1 expression at the rhombic lip before the establishment of a distinct isthmic domain (n=6) (Fig. 3G).
Fig. 3.
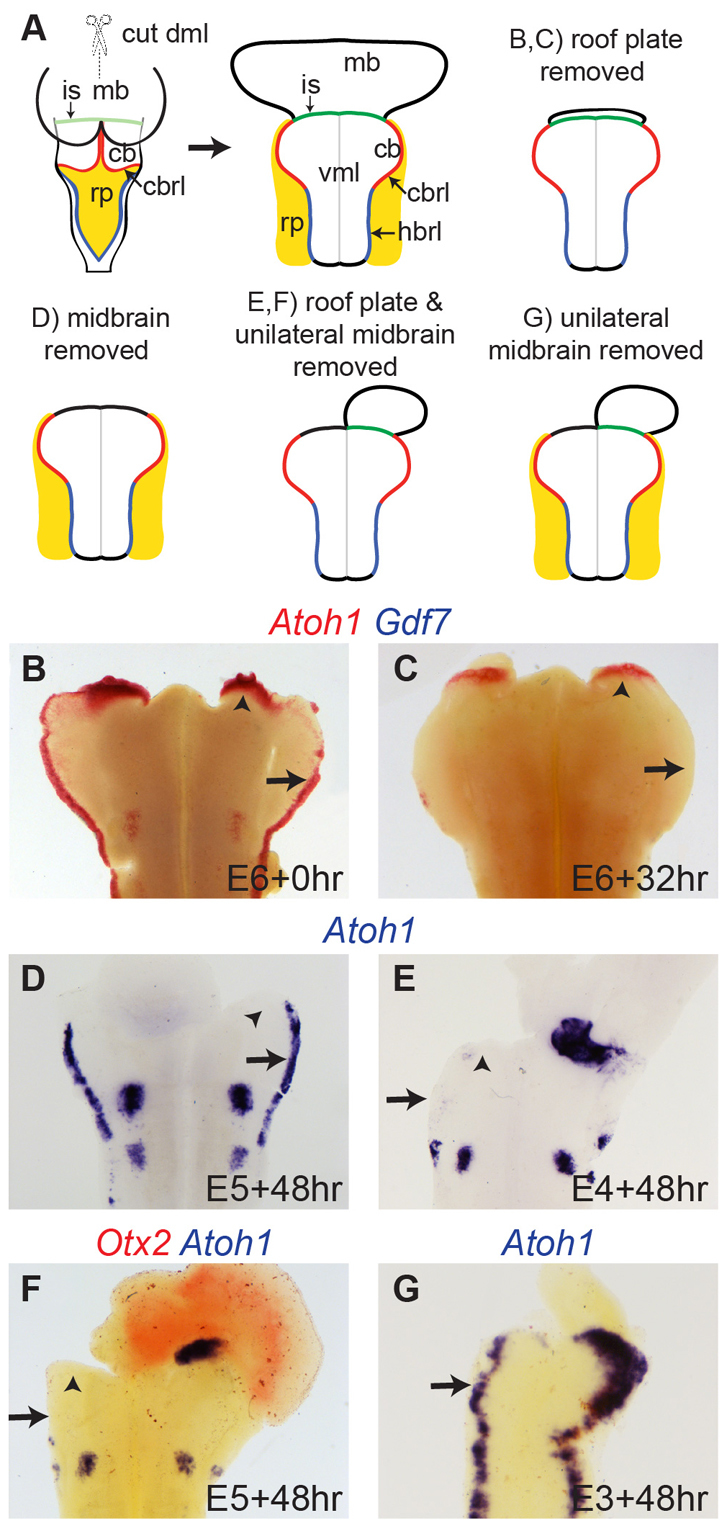
Atoh1 domains are independently regulated by different organisers. (A) Schematic showing midbrain/hindbrain tissues dissected in flat-mount and different combinations of rp and mb tissue removal. (B) Immediately after dissection (0 hours), E6 tissue with rp ablation but intact mb-hb boundary, shows normal Atoh1 (red). (C) After 32 hours in vitro, Atoh1 is lost in rl (arrow) but maintained at the isthmus (arrowhead). (D) With rp intact but mb ablated, Atoh1 is expressed at rl (arrow) but not mb-hb boundary. (E,F) Unilateral midbrain (Otx2, red in F) and bilateral rp ablation results in loss of all Atoh1 ipsilateral to mb ablation. (G) Unilateral ablation of mb alone causes an ipsilateral reduction in width of Atoh1 expression at the cbrl (arrow). In all images, ventral ‘spots’ of Atoh1 are non-rl-derived respiratory paramotor nuclei. cb, cerebellum; cbrl, cerebellar rhombic lip; hbrl, hindbrain rhombic lip; is, midbrain-hindbrain isthmus; mb, midbrain; rp, roof plate; vml, ventral midline.
Isthmic-dependent features of Atoh1 expression and rhombic lip length are dependent on FGF signalling
In early embryonic development, the midbrain-hindbrain boundary signalling is mediated by the secreted morphogen FGF8. Induction of Fgf8 relies on the interaction between Otx2-positive midbrain and Gbx2-positive hindbrain territory. We therefore examined whether the expression of Otx2, Gbx2 and Fgf8 beyond E3 support a continued role for this signalling mechanism in later stages of r1 development. From E5, Otx2 remains uniformly expressed throughout the midbrain, whereas Gbx2 expression is limited to dorsal r1 (Fig. 4A). At high magnification at E5, the isthmus is bordered by a narrow strip of Gbx2-expressing cells in r1 adjacent to the midbrain (Fig. 4B). Correspondingly, Fgf8 is both expressed at the isthmus but upregulated dorsally, where it extends caudally into dorsal r1 (Fig. 4C), mirroring the expression of Gbx2. The caudal extension of Fgf8 in dorsal r1 overlaps Atoh1 expression (Fig. 4D) corresponding to the ventricular layer expression of Atoh1 in the isthmic domain (Fig. 4E). This suggests a functional link between midbrain-hindbrain boundary signalling, Fgf8 expression and Atoh1 maintenance. Correspondingly, response to FGF signals, indicated by expression of the downstream effector of FGF signalling, Sprouty2 (Spry2 - Mouse Genome Informatics), becomes progressively excluded from caudal r1 from E3 to E5 (Fig. 4F,G).
Fig. 4.
FGF signal blockade downregulates Atoh1. (A-G) Expression of Otx2, Gbx2 and Fgf8 at E5 (A-E,G) and E4 (F) in flat-mount (A-D,F,G) and coronal sections (E). (A,B) Otx2 (red) is expressed throughout mb, whereas Gbx2 (blue) is expressed in cb and a thin stripe at the isthmus (arrow in B: a high magnification view of A). (C-E) Dorsal expansion of Fgf8 (arrows) correlates with broader Atoh1 expression (red). (F,G) Sprouty2 is expressed in rostral cb that diminished between E4 (F) and E5 (G). (H-J) Co-electroporation of dn-fgfr3c (dnR) and gfp at E2 causes a caudal shift at E5 (indicated by line) of Otx2 (red) and ipsilateral downregulation of Atoh1 (blue). There is no expanded Atoh1 domain caudal to this shifted boundary (arrows). (K,L) Co-electroporation of dn-fgfr3c + gfp into caudal r1 at E3 downregulates Atoh1 and reduces the size of the ipsilateral cerebellar anlage. (M,N) Cerebellar asymmetry at E4 following dnR or GFP electroporation into r1 at E3, measured as the ratio of cbrl length (M) or dorsoventral dimension (N). Each point represents the ratio of unelectroporated:electroporated for a single embryo (median ratio in red). **P<0.001, *P<0.05 Mann-Whitney U-test. cb, cerebellum; hb, hindbrain; is, midbrain-hindbrain isthmus; mb, midbrain.
To determine whether FGF8 signal transduction is necessary for the Atoh1 expression in r1, we overexpressed a truncated human FGF receptor, dn-fgf3c. This fgfr3 has been shown to bind and sequester FGF8 ligand but as the receptor lacks an intracellular domain it does not cause a downstream activation of the ERK pathway when electroporated in mouse cortex (Toyoda et al., 2010). Although FGFR3 is not the predominant receptor present in r1 (Walshe and Mason, 2000; Blak et al., 2005; Blak et al., 2007; Saarimäki-Vire et al., 2007), this truncated receptor is expected to act in a dominant-negative manner by competitively inhibiting the other FGF receptors in r1. We first confirmed the function of this construct in chick through electroporation at E2 into the midbrain-hindbrain region. Ectopic dn-fgfr3c was sufficient to ablate Sprouty2 expression at the isthmus at E3 (supplementary material Fig. S1A-C) and induce a caudal expansion of Otx2-positive midbrain tissue at the expense of Gbx2-positive hindbrain tissue, recapitulating the early effects of a loss of isthmic FGF signalling (Sato and Nakamura, 2004) (supplementary material Fig. S1D-F). Electroporation of dn-fgfr3c at E2 results at E5 in a loss of isthmic Atoh1 expression coincident with Otx2 upregulation in cerebellum (Fig. 4H). Caudal to the domain of Otx2 upregulation, Atoh1 is expressed at the rhombic lip. However, dn-fgfr3c suppresses the formation of the prominent isthmic expression domain at the shifted Otx2-Gbx2 boundary (Fig. 4I,J). Because electroporations at E2 result in a significant reorganisation of the midbrain-hindbrain boundary, we performed targeted electroporations of dn-fgfr3c into caudal r1 at E3. At this later stage, blockade of FGF signalling can be targeted to caudal r1, leaving the midbrain-hindbrain boundary unaffected. This allowed us to examine the effects of FGF signal blockade independently of changes to isthmic organiser activity. Accordingly, locally disrupted FGF signal transduction results in a reduction, but not complete loss of Sprouty2 expression in caudal r1 at E4 (supplementary material Fig. S1G,H). Downregulation of Sprouty2 correlates with a local narrowing of the Atoh1 expression domain at the rhombic lip at E4 (Fig. 4K,L) and an overall reduction in cerebellar size. To quantify this change we measured the anteroposterior length of the rhombic lip between the isthmus and lateral angle of the rhombic lip and the distance between the ventral midline and most dorsal point of the cerebellum in flat-mounted electroporated embryos. We find that the anteroposterior length of the cerebellar rhombic lip is significantly reduced (Mann-Whitney U-test: z=-4.28: P<0.0001) compared with the control side of the embryo (Fig. 4M) whereas the dorsoventral dimension shows a smaller but still significant reduction (Mann-Whitney U-test: z=-2.03: P=0.0424) (Fig. 4N).
Overexpression of Fgf8b results in upregulation of Atoh1
We next determined whether FGF8 is sufficient to induce Atoh1 expression by using a full-length mouse Fgf8b, the most active isoform of Fgf8 which accounts for the majority of isthmic FGF function (Sato et al., 2001; Guo et al., 2010). Electroporating Fgf8b into the midbrain-hindbrain region at E2/3 is sufficient to upregulate Sprouty2 (a downstream target of FGF signalling) and transform midbrain territory into r1 (Sato et al., 2001) (supplementary material Fig. S2). When examined at E5, embryos electroporated at E2 with full-length Fgf8b displayed high levels of Atoh1 expression extending into the midbrain (Fig. 5A). In flat-mounted half brain preparations, induction is limited to dorsal neural tube (Fig. 5B) despite extensive electroporation (GFP in Fig. 5B, right), suggesting a dorsoventral restriction in competence to express Atoh1. Its expression is highest at the dorsal midline and at the anterior boundary of the ectopic Atoh1 territory. This is consistent with a transformation of midbrain to an r1/cerebellar fate with a corresponding rostral shift of the midbrain/hindbrain boundary and Atoh1-positive isthmic domain.
Fig. 5.

Ectopic Fgf8 induces Atoh1 expression. (A-E) Expression of Atoh1 at E5 (A,B,D,E) and Sprouty2 at E4 (C) following co-electroporation of Fgf8b + gfp at E2 (A,B) and E3 (C-E), in dorsal whole-mount (A) and flat-mount views (B-E). The arrow indicates electroporated right side of the embryo (left of picture in flat-mounts). Note that the fluorescent signal is quenched by in situ hybridisation staining in dorsal regions. Electroporation at E2 shifts the isthmus rostrally (A,B, solid line). Electroporation at E3 into caudal cb induces Sprouty2 (C) and Atoh1 (D). At high magnification (E), ectopic Atoh1 proximal to the rl is not maintained in distal derivatives (arrow). (F,G) Electroporation of Fgf8b + gfp at E4 is sufficient to maintain Atoh1 in vitro 48 hours after ablation of rl and mb. cb, cerebellum; cbrl, cerebellar rhombic lip; hbrl, hindbrain rhombic lip; is, midbrain-hindbrain isthmus; mb, midbrain; rp, roof plate; vml, ventral midline.
By electroporating Fgf8b at later stages we were able to target ectopic gene expression into caudal r1 rhombic lip and induce a local upregulation of Sprouty2 at E4 without disrupting the midbrain-hindbrain boundary (Fig. 5C). When electroporated at E3, Fgf8b induces a sustained Atoh1 expression at E5 in streams of migratory cells from the rhombic lip of caudal r1 (Fig. 5D). At high magnification, Atoh1 expression is absent in cells furthest from the rhombic lip, suggesting that FGF-induced upregulation is transient (Fig. 5E, compare left and right). To test whether FGF is sufficient to regulate Atoh1 in the absence of either roof plate or isthmic signals, we electroporated Fgf8b into r1 at E4 and immediately dissected away the midbrain and roof plate tissue before placing the isolated cerebellum in culture (Fig. 5F). After 48 hours in vitro, Atoh1 expression was uniformly abolished at the rhombic lip and in the isthmic domain; however, Atoh1 was expressed in migrating cells originating from electroporated precursors (n=12/22) (Fig. 5G). This suggests that even when inductive roof plate cues are removed FGF8 signalling is sufficient to induce or maintain Atoh1 in dorsal r1.
Ectopic FGF signalling drives proliferation and inhibits specification of late-born, cerebellar cell types from the rhombic lip
To explore whether FGF signalling alters the fate of Atoh1-positive derivatives, we looked at the gene expression and morphology of rhombic lip derivatives following overexpression of Fgf8b. We first examined Lhx9, which is expressed in both isthmic nuclei and early-born rhombic lip derivatives. Overexpression of Fgf8b at E3 in the rhombic lip migratory stream leads to a disruption in the normally continuous domain of Lhx9 expression at E6 (Fig. 6A-C). In ventral r1, there are small gaps in Lhx9 expression (Fig. 6B,C), whereas ectopic Lhx9 is apparent in streams of migratory cells (Fig. 6A,B, arrowheads). This suggests that Lhx9 expression is not simply downstream of FGF signalling but secondary to more complex changes in cell fate. We therefore looked in detail at the position and morphology of rhombic lip derivatives expressing ectopic Fgf8b.
Fig. 6.
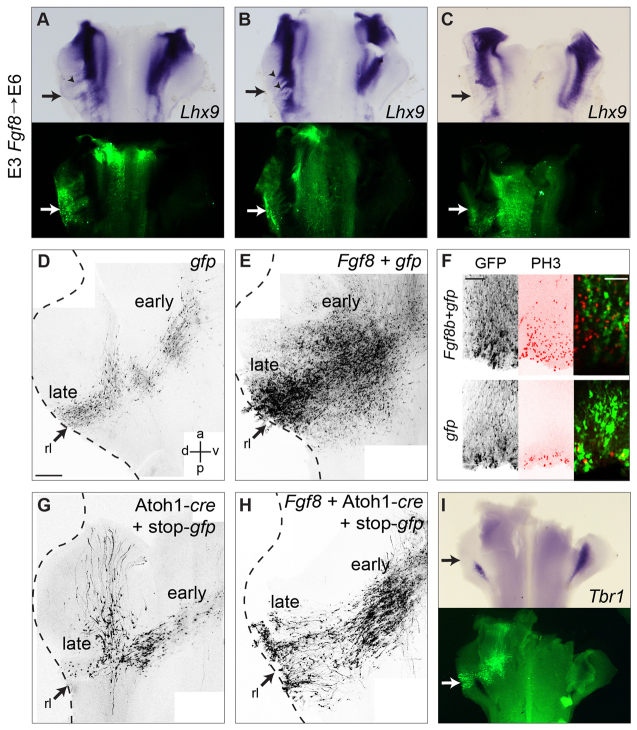
FGF overexpression supresses cerebellar cell fates. (A-C) Electroporation of Fgf8b + gfp at E3 (arrow) disrupts Lhx9 at E6, producing both ectopic expression (arrowheads) and gaps (arrows). (D,E) Electroporation of gfp (D) or Fgf8b + gfp (E) at E4 produces markedly different numbers of GFP-labelled cells. (F) Electroporation of Fgf8b + gfp at E3 cell-non-autonomously induces at E5 ectopic proliferation (PH3 in red). (G) Electroporation of Atoh1-cre + stop-gfp into rl at E3 (arrow) labels rhombic lip derivatives at E7. (H,I) Co-electroporated Fgf8b increases the proportion of ventral derivatives (H) and downregulates the deep cerebellar nucleus marker Tbr1 (I). Scale bars: 200 μm (D,E,G,H); 100 μm (F, left and middle); 50 μm (F, right). rl, rhombic lip.
A control electroporation of gfp at E4, cumulatively labels successive cohorts of derivatives of the rhombic lip when examined at E7 in flat-mounted embryos (Fig. 6D), reflecting a birth order that culminates in the production of granule cell precursors (Gilthorpe et al., 2002; Wilson and Wingate, 2006). Co-electroporation of gfp and Fgf8b into the rhombic lip at E4 results in a massive increase in GFP-labelled cells (Fig. 6E), consistent with increased proliferation (Fig. 6F) but obscuring details of cell morphology. To obtain more specific cell labelling in the presence of ectopic Fgf8, we electroporated the conditional Atoh1 reporter (Atoh1-cre and lox-stop-lox-gfp) alone (Fig. 6G) or in combination with Fgf8b (Fig. 6H) into r1 rhombic lip at E4 and looked at the cell morphology and distribution of rhombic lip derived cells at E7. Following ectopic Fgf8b expression we saw an accumulation of cells in ventral r1 in the position of early-born Lhx9-positive neurons and a concomitant loss of more dorsal, late-born cell types, such as cells that turn at the lateral edge of the EGL (Gilthorpe et al., 2002) (Fig. 6H). Furthermore, the cerebellar nucleus marker Tbr1 (Fink et al., 2006) was specifically downregulated at E6 following Fgf8b overexpression at E3 (Fig. 6I), indicating a loss of later-born cell types. This raises the possibility that, whereas FGF is required for the allocation of cerebellar territory, late-born cerebellar rhombic lip derivatives can only be produced in its absence. To test whether the specification of rhombic lip derivatives requires FGF signal transduction we next investigated the production of the lineage in a context of reduced FGF signalling in both chick and mouse.
Attenuation of FGF signalling and signal transduction does not affect the specification of rhombic lip derivatives
We examined the expression of Lhx9 following the electroporation of dn-fgfr3c into the rhombic lip at E3. At E6, the distribution of Lhx9 early-born derivatives is identical on control and electroporated sides of r1 (Fig. 7A). Analysis at E7 of the production of successive temporal cohorts following co-electroporation of dn-fgfr3c with gfp at E4 revealed no difference in cell location or morphology (Fig. 7B). Therefore, autonomous downregulation of FGF signal transduction within rhombic lip progenitors has no apparent effect on the specification of either early-born or late-born derivatives.
Fig. 7.
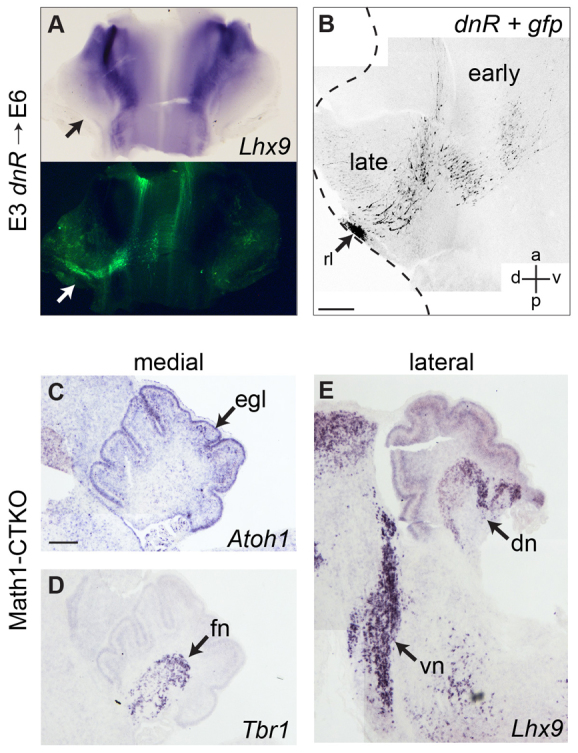
FGF signal blockade does not affect cell fate allocation at the rhombic lip. (A,B) Electroporation of dn-fgfr3c +gfp (arrow) at E3 (A) and E4 (B) does not affect either Lhx9 expression at E6 (A) or cell labelling at E7 (B). (C-E) Atoh1 (C), Tbr1 (D) and Lhx9 (E) are normal in P4 Math1-CTKO mice, seen in medial (C,D) and lateral (E) sagittal section. Scale bars: 200 μm. dn, dentate nucleus; egl, external granule layer; fn, fastigial nucleus; rl, rhombic lip; vn, ventral nuclei.
We further examined the molecular specification of rhombic lip derivatives in mice, where the precise sequence of neuronal production has been examined in a series of genetic fate maps (Machold and Fishell, 2005; Fink et al., 2006; Rose et al., 2009; Hagan and Zervas, 2012). We analysed a mutant in which cells of the Atoh1 lineage are unable to transduce FGF signals. These mice were produced by crossing mice expressing three floxed/null FGF receptor alleles: fgfr1flox/flox; fgfr2flox/flox; fgfr4-/- with mice expressing Cre recombinase under the control of the Math1/Atoh1. Loss of FGFR1 and FGFR2 in r1 is sufficient to phenocopy loss of FGF signalling in r1 (Saarimäki-Vire et al., 2007) and although FGFR3 is expressed in the most caudal region of r1 (Blak et al., 2005), it is not required for normal cerebellar development, and cannot compensate for the loss of the other FGF receptors (Blak et al., 2007). The conditional triple knockout (CTKO) mice show a superficially normal cerebellum size and developmental timecourse (Emmenegger et al., 2013). At P4, brains from these animals show normal development of the EGL (Fig. 7C) and fastigial (Fig. 7D) and dentate (Fig. 7E) cerebellar nuclei shown by expression of Atoh1, Tbr1 and Lhx9, respectively. Early-born, extra-cerebellar rhombic lip derivatives in isthmic and ventral r1 are also unchanged (Fig. 7E).
The lack of effect of cell-autonomous downregulation of FGF signal transduction in both chick and mouse cerebellum contrasts with the dramatic non-autonomous effects of attenuated isthmic signalling on cerebellar morphogenesis (Meyers et al., 1998; Chi et al., 2003). Our observations suggest that FGF is not required for cell specification at the rhombic lip. By contrast, a previous study (Basson et al., 2008) suggests that cell-non-autonomous loss of isthmic signalling leads not only to the loss of medial cerebellum (vermis) but also the most medial, fastigial cerebellar nucleus. We therefore investigated whether cell specification and morphogenesis are indeed independently regulated in the Fgf8neo/neo cerebellar hypomorph (Meyers et al., 1998). This mouse shows a pronounced loss of medial cerebellar tissue (Fig. 8A,B) (Meyers et al., 1998; Chi et al., 2003). In the residual, lateral cerebellum, in situ hybridisation for Atoh1 (Fig. 8C,D), Tbr1 (Fig. 8E,F) and Lhx9 (Fig. 8G,H), respectively, show an intact EGL, fastigial and dentate cerebellar nuclei and early-born population of isthmic and ventral non-cerebellar rhombic lip derivatives at E16.5. Thus, the sequence of cell fate allocation occurs normally at the rhombic lip despite a non-autonomous reduction in FGF signalling and accompanying cerebellar size reduction. However, it was not possible to determine whether individual nuclei within the early-born cohorts were present, owing to the lack of specific cell markers and significant changes in the anatomical structures. We conclude that ectopic FGF signalling not only prevents the production of late-born cell types from the rhombic lip, but also that FGF is not required to mediate the specification of any cell types from the cerebellar rhombic lip.
Fig. 8.
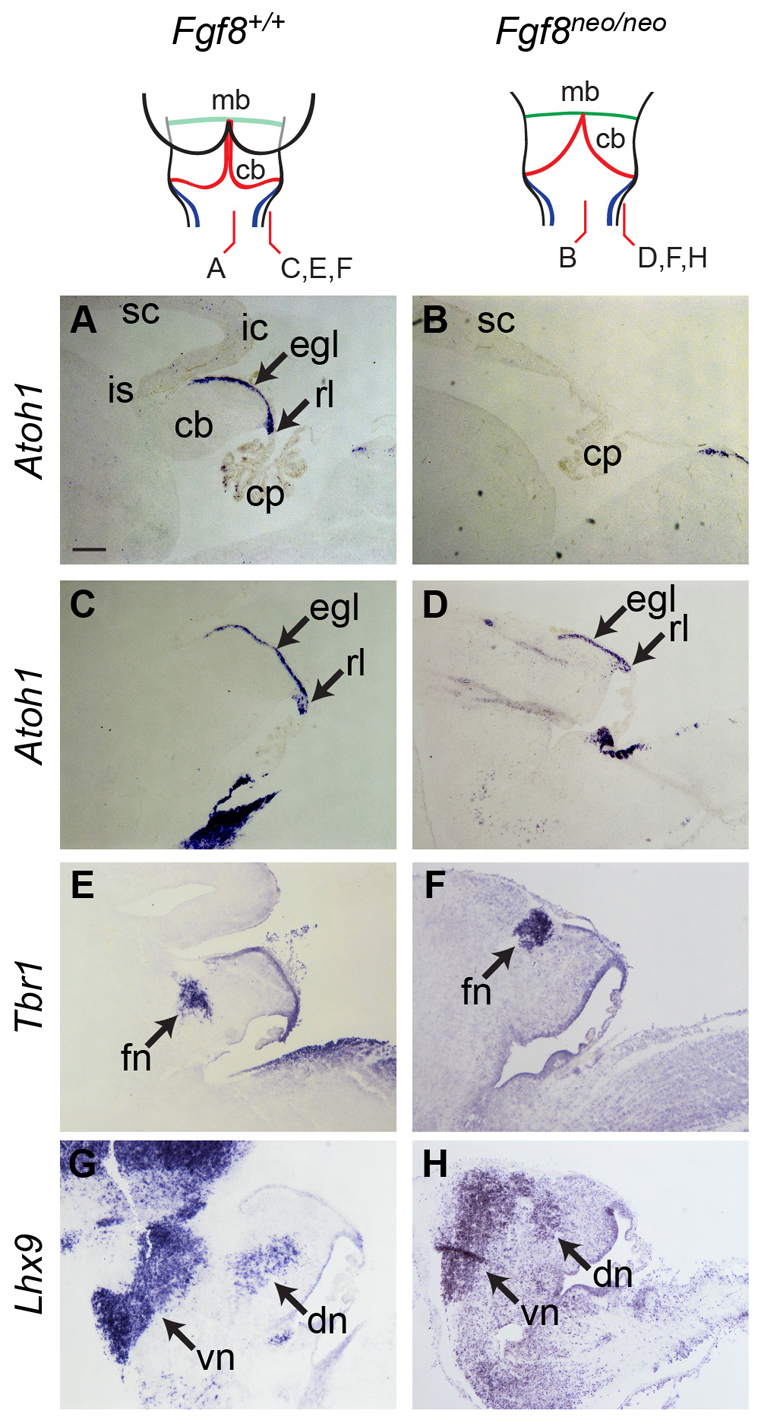
Fgf8 hypomorphs show a normal range of rhombic lip derivatives. Atoh1, Tbr1 and Lhx9 expression in sagittal sections of E16.5 Fgf8neo/neo mice (right) and wild-type littermates (left) reveals normal, if misplaced, rl derivative markers in mutants, despite the overall loss of medial cerebellar territory (B). Scale bar: 200 μm. cb, cerebellum; cp, choroid plexus; dn, dentate nucleus; egl, external granule layer; fn, fastigial nucleus; ic, inferior colliculus; is, midbrain-hindbrain isthmus; mb, midbrain; rl, rhombic lip; sc, superior colliculus; vn, ventral nuclei.
DISCUSSION
In this study, we show that the initially contiguous expression of Atoh1 in dorsal r1 in chick and mouse is composed of two cryptic progenitor domains that can be distinguished by their differential dependency on two organisers: the midbrain-hindbrain isthmus and the roof plate boundary. These domains give rise to isthmic nuclei and rhombic lip derivatives, respectively. FGF-mediated isthmic signalling is required for the maintenance of a rostral Atoh1-positive progenitor pool, independent of roof plate signals. FGF signalling also influences the size of the cerebellar anlage by increasing the length of the rhombic lip in r1. However, FGF signalling is not required for the patterning of rhombic lip derivatives and inhibits their production when ectopically upregulated. Differently regulated Atoh1 domains thus give rise to discrete functional elements of the proprioceptive/interoceptive network.
Isthmic function and cerebellar development
The isthmus has been shown to have a profound influence on cerebellum development by both regulating the boundaries of midbrain territory (Sato et al., 2001; Sato and Nakamura, 2004; Foucher et al., 2006) and the size of dorsal r1, from which the cerebellum will develop (Irving and Mason, 2000; Sato and Joyner, 2009). Our results support a model of declining influence of isthmic-derived FGF signals on cerebellum growth (Sato and Joyner, 2009) and we show that the interface between Otx2- and Gbx2-expressing cells at the isthmus becomes progressively dorsalised (Fig. 4A-C) and, correspondingly, responses to isthmic signalling (indicated by Sprouty2) become restricted to rostral r1 (Fig. 4F,G). Despite this reduced influence, blockade of FGF responses using a dominant-negative receptor fgfr3c, in chick, results in significant changes in cerebellar size (Fig.4K-N), independent of any change to the integrity or position of the isthmus itself. This indicates a role for FGF in regulating proliferation and specifically the linear extent of the rhombic lip, beyond the initial establishment of r1 territory.
That a normal cerebellar morphology and complement of rhombic lip derivatives is displayed in the Math1-CTKO mice suggests that the effects of FGF signalling occur before the allocation of cells to the Math1-positive lineage from an as yet unidentified stem cell pool (Machold and Fishell, 2005; Wingate, 2005). This observation is confirmed by the fact that alterations to rhombic lip ‘length’ appear to be independent of a normal orderly sequence of cell production in chick dn-fgfr3 electroporated embryos and in a mouse Fgf8 hypomorph. However, whilst FGF is required for the correct establishment of r1 territory and size, altered gene expression and cell fate following Fgf8 overexpression suggest that FGF signalling, although not required at the rhombic lip, can override its normal programme of cell specification when ectopically expressed. Therefore the declining influence of isthmic FGF8 is permissive for the generation of cerebellar cell types. This is supported by evidence that FGF signalling must be suppressed to allow ectopic cerebellar differentiation following the transformation of the mesencephalon to a metencephalic fate (Suzuki-Hirano et al., 2010).
When combined with previous studies, our observations suggest a three-stage model for avian and mammalian cerebellar growth: an FGF-dependent regulation and maintenance of r1 territorial boundaries (Irving and Mason, 2000; Sato et al., 2001; Sato and Nakamura, 2004; Foucher et al., 2006; Sato and Joyner, 2009), an extended intermediate FGF-dependent phase of expansion of the progenitor pool independent of cell specification, and finally a late Shh-dependent expansion of the cerebellar cell surface through the FGF-independent transit amplification of granule cell precursors (Dahmane and Ruiz i Altaba, 1999; Wallace, 1999; Wechsler-Reya and Scott, 1999; Klein et al., 2005; Corrales et al., 2006), which is dependent on sustained Atoh1 expression (Ben-Arie et al., 1997; Flora et al., 2009). The intermediate, FGF-dependent growth phase, explored in this study, may be particularly significant for cerebellar development in non-mammalian vertebrates where Shh-responsive granule cell precursors are absent (Chaplin et al., 2010; Kani et al., 2010). The developing cerebellum in embryonic basal vertebrates such as the embryonic shark (Chaplin et al., 2010) and basal ray-finned fish (T. Butts and R.J.T.W., unpublished observations) display a highly elongated cerebellar rhombic lip. In the zebrafish, similarly, cerebellar growth is driven by cell division at the isthmic apex of the cerebellar rhombic lip: the valvulus (Kaslin et al., 2009; Chaplin et al., 2010; Kani et al., 2010).
Two different modes of Atoh1 regulation define different progenitor territories
Just as the teleost proliferative node or valvulus remains juxtaposed to the isthmus as the cerebellum grows, we describe a distinct isthmic Atoh1 domain that is dynamically repositioned with respect to, and ultimately excluded from, the growing cerebellum. In contrast to Atoh1 expression at the rhombic lip, which is both induced (Alder et al., 1996; Lee et al., 2000) and maintained (Broom et al., 2012) by roof plate signals, maintenance of the isthmic domain is exclusively dependent on the isthmic organiser via FGF signalling. Fate mapping in chick using targeted electroporation of a Atoh1-Cre enhancer reveals this domain as the origin of the nucleus isthmi pars parvocellularis, which projects to the tectum, and the presumptive isthmo-optic nucleus, which are born at E5 and (Clarke, 1982; Puelles and Martinez-de-la-Torre, 1987; Hellmann et al., 2001) and E6/E7 (Clarke, 1982), respectively. Mammals lack a late-born isthmo-optic nucleus and correspondingly, the isthmic domain of Atoh1 expression is smaller in mouse than in chick. However, the parabigeminal nucleus, which genetic fate maps have identified as a product of Atoh1-positive progenitors and hence a presumptive rhombic lip derivative (Machold and Fishell, 2005; Rose et al., 2009), is functionally homologous to the earlier-born isthmic nuclei of the chick. Both have reciprocal topographic projections to the visual midbrain and monitor and modulate bilateral sensory representation (Butler and Hodos, 1996). Early-born neurons of the dorsal nucleus of the lateral lemniscus fulfil a similar anatomical and functional role in the auditory system. Again, their neurons are also attributed to the different progenitor pools in chick (isthmus) and mouse (rhombic lip). This developmental homology between nuclei in mouse and chick demonstrates that previous genetic fate maps of this region in mouse (Machold and Fishell, 2005; Wang et al., 2005) failed to discriminate between cryptic isthmic and rhombic lip domains of Atoh1 expression. Thus, a diversity that has previously been explained in terms of homoplasy (multiple different developmental origins coupled with functional convergence) (Butler and Hodos, 1996) may be resolved by the presence of a conserved isthmic Atoh1 compartment that is contiguous with the rhombic lip.
Our experimental demonstration in chick of different regulatory constraints on Atoh1 expression suggests that FGF signalling is involved in its maintenance. A possible mechanism is the blockade of the auto-inhibitory feedback loop that normally attenuates Atoh1 expression via ATOH1 protein (Gazit et al., 2004). This negative feedback is uncoupled in specific developmental contexts: in the EGL to promote transit amplification (Gazit et al., 2004) and in Zic1-mediated regulation of the rhombic lip progenitor pool (Ebert et al., 2003). Such a model is consistent with our observations in chick that isthmic signalling can alter the breadth of Atoh1 expression in the rhombic lip but is not required for its induction. Correspondingly, Fgf8b overexpression maintains only a relatively weak expression of Atoh1 in the absence of roof plate. A parsimonious model for the origin of an Atoh1 isthmic domain is that FGF selectively and regionally sustains Atoh1 expression in a progressively rostralised pool of Atoh1 progenitors induced by the roof plate.
We show that an absence of FGF signalling at the rhombic lip does not alter the generation of successive temporal cell fates. Although it is not clear what specific instructive cues determine cell fate, particularly of the late born cerebellar nuclei and cerebellar granule cells, the fourth ventricle roof plate is a possible source for these patterning cues (Wilson and Wingate, 2006; Broom et al., 2012). Furthermore, we show that the lack of FGF signalling in caudal r1 at later stages is permissive in generating cerebellar cell types and accordingly the prolonged presence of FGF signalling at the isthmus is likely to be important in reserving this territory as non-cerebellar. This region is comparatively larger in chick, which displays an additional isthmic nucleus but lacks a cerebellar vermis. Correspondingly, the mouse has a smaller isthmic domain and a rostrally extended rhombic lip. This suggests that FGF signalling determines the proportion of rostral r1 that is allocated to cerebellum. It also implies that the cerebellar vermis, which is derived from rostral rhombic lip (Sgaier et al., 2005), may have evolved through a reduction in the influence of the isthmus. This is an unexpected inference given that FGF8 mutants display vermal hypoplasia (Meyers et al., 1998; Chi et al., 2003) and highlights the importance of separating early territorial readjustments of the midbrain-hindbrain boundary (resulting in loss of rostral tissue) from the later roles of isthmic signalling that we demonstrate here. At these later stages of cerebellar specification, discrete domains of Atoh1 expression are defined by their differential dependence on discrete local organisers, with FGF signalling both influencing the size of cerebellum and sub-regionalisation of rhombomere 1.
MATERIALS AND METHODS
In ovo and in vitro manipulations
Fertilised wild-type eggs (Henry Stewart, UK) were incubated at 38°C for 2 to 6 days. For explant cultures, embryos were removed from eggs and the midbrain/hindbrain region of the neural tube was dissected in Tyrode’s solution. Tissue was bisected along the dorsal midline and roof plate or midbrain tissue was removed by dissection. Explants were cultured pial surface uppermost on 0.4 μm inserts (Millicell-CM, Millipore) for 2 days (37°C/6% CO2) as per Broom et al. (Broom et al., 2012). For electroporations, the neural tube in the region of r1 was injected with ∼100-200 nl of DNA plasmids mixed to equal concentrations, giving a final concentration of 0.8-2 μg/μl of each plasmid: Atoh1-cre (Kohl et al., 2012), pFlox-pA-EGFP (lox-stop-lox-GFP) and pCX-Cre (Morin et al., 2007), pEFX-dn-fgfr3c and pEFX-Fgf8 (Toyoda et al., 2010), pCAβ-eGFP-m5 (Yaneza et al., 2002). Electroporated embryos were incubated for a further 1-3 days at 38°C.
Mouse embryos
CTKO mice (Math1-cre; fgfr1flox/flox; fgfr2flox/flox; fgfr4-/-) were generated and genotyped as per Emmenegger et al. (Emmenegger et al., 2013). Fgf8neo/neo embryos were donated by Albert Basson (King’s College London, UK) and obtained from crosses of Fgf8neo/+ mice as previously described (Meyers et al., 1998). Wild-type littermates were used as controls.
Histology and photomicroscopy
Embryos were fixed in 4% (w/v) paraformaldehyde (in phosphate-buffered saline) and either dissected or processed for cryostat sectioning. Tissue was stained by in situ hybridisation (Myat et al., 1996) with digoxygenin- or fluorescein-labelled (Roche) riboprobes for: Atoh1 (Wilson and Wingate, 2006), Otx2 (Millet et al., 1996), Ptf1a (ChEST1028o4), Lhx9 (Alessio Delogu, King’s College London, UK), Gdf7 (Broom et al., 2012), Gbx2 (Kiecker and Lumsden, 2004), Fgf8 and Sprouty2 (Chambers et al., 2000); and mouse probes for: Atoh1 (Albert Basson, King’s College London, UK), Lhx9 and Tbr1 (Alessio Delogu, King’s College London, UK). Following in situ hybridisation, GFP signal was amplified immunohistochemically with an anti-GFP antibody (IgG 1:100, Invitrogen) and mitotic cells were detected with an anti-phospho-histone H3 antibody (1:100, NEB). Some whole-mounts were further processed for vibratome sectioning. Digital brightfield and fluorescence images were acquired on either stereo (Leica MZFLIII) or compound (Nikon Elipse80i) microscopes equipped with epifluorescence or by laser scanning confocal microscopy (Olympus AX70).
Supplementary Material
Acknowledgments
We are grateful to Dalit Sela-Donenfeld and Avihu Klar for their generous gift of reagents and to Albert Basson for supplying Fgf8neo/neo mouse embryos.
Footnotes
Competing interests
The authors declare no competing financial interests.
Author contributions
M.J.G. and R.J.T.W. planned the study and prepared the manuscript. M.J.G., A.M.M. and L.J.W. characterised gene expression. M.J.G. carried out chick experiments. B.A.E. and R.J.W.-R. generated the CTKO mouse line.
Funding
This work was supported by a Medical Research Council studentship (to M.J.G.); and a Wellcome Trust project grant [grant number WT080019AIA to R.J.T.W.]. Deposited in PMC for immediate release.
Supplementary material
Supplementary material available online at http://dev.biologists.org/lookup/suppl/doi:10.1242/dev.099119/-/DC1
References
- Alder J., Cho N. K., Hatten M. E. (1996). Embryonic precursor cells from the rhombic lip are specified to a cerebellar granule neuron identity. Neuron 17, 389–399 [DOI] [PubMed] [Google Scholar]
- Alder J., Lee K. J., Jessell T. M., Hatten M. E. (1999). Generation of cerebellar granule neurons in vivo by transplantation of BMP-treated neural progenitor cells. Nat. Neurosci. 2, 535–540 [DOI] [PubMed] [Google Scholar]
- Basson M. A., Echevarria D., Ahn C. P., Sudarov A., Joyner A. L., Mason I. J., Martinez S., Martin G. R. (2008). Specific regions within the embryonic midbrain and cerebellum require different levels of FGF signaling during development. Development 135, 889–898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Arie N., Bellen H. J., Armstrong D. L., McCall A. E., Gordadze P. R., Guo Q., Matzuk M. M., Zoghbi H. Y. (1997). Math1 is essential for genesis of cerebellar granule neurons. Nature 390, 169–172 [DOI] [PubMed] [Google Scholar]
- Blak A. A., Naserke T., Weisenhorn D. M., Prakash N., Partanen J., Wurst W. (2005). Expression of Fgf receptors 1, 2, and 3 in the developing mid- and hindbrain of the mouse. Dev. Dyn. 233, 1023–1030 [DOI] [PubMed] [Google Scholar]
- Blak A. A., Naserke T., Saarimäki-Vire J., Peltopuro P., Giraldo-Velasquez M., Vogt Weisenhorn D. M., Prakash N., Sendtner M., Partanen J., Wurst W. (2007). Fgfr2 and Fgfr3 are not required for patterning and maintenance of the midbrain and anterior hindbrain. Dev. Biol. 303, 231–243 [DOI] [PubMed] [Google Scholar]
- Broom E. R., Gilthorpe J. D., Butts T., Campo-Paysaa F., Wingate R. J. (2012). The roof plate boundary is a bi-directional organiser of dorsal neural tube and choroid plexus development. Development 139, 4261–4270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler A., Hodos W. (1996). Comparative Vertebrate Neuroanatomy: Evolution and Adaptation. New York, NY: Wiley-Liss; [Google Scholar]
- Chambers D., Medhurst A. D., Walsh F. S., Price J., Mason I. (2000). Differential display of genes expressed at the midbrain - hindbrain junction identifies sprouty2: an FGF8-inducible member of a family of intracellular FGF antagonists. Mol. Cell. Neurosci. 15, 22–35 [DOI] [PubMed] [Google Scholar]
- Chaplin N., Tendeng C., Wingate R. J. (2010). Absence of an external germinal layer in zebrafish and shark reveals a distinct, anamniote ground plan of cerebellum development. J. Neurosci. 30, 3048–3057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chi C. L., Martinez S., Wurst W., Martin G. R. (2003). The isthmic organizer signal FGF8 is required for cell survival in the prospective midbrain and cerebellum. Development 130, 2633–2644 [DOI] [PubMed] [Google Scholar]
- Clarke P. G. (1982). The generation and migration of the chick’s isthmic complex. J. Comp. Neurol. 207, 208–222 [DOI] [PubMed] [Google Scholar]
- Corrales J. D., Blaess S., Mahoney E. M., Joyner A. L. (2006). The level of sonic hedgehog signaling regulates the complexity of cerebellar foliation. Development 133, 1811–1821 [DOI] [PubMed] [Google Scholar]
- Cowan W. M., Clarke P. G. H. (1976). The development of the isthmo-optic nucleus. Brain Behav. Evol. 13, 345–359 [DOI] [PubMed] [Google Scholar]
- Dahmane N., Ruiz i Altaba A. (1999). Sonic hedgehog regulates the growth and patterning of the cerebellum. Development 126, 3089–3100 [DOI] [PubMed] [Google Scholar]
- Ebert P. J., Timmer J. R., Nakada Y., Helms A. W., Parab P. B., Liu Y., Hunsaker T. L., Johnson J. E. (2003). Zic1 represses Math1 expression via interactions with the Math1 enhancer and modulation of Math1 autoregulation. Development 130, 1949–1959 [DOI] [PubMed] [Google Scholar]
- Eddison M., Toole L., Bell E., Wingate R. J. (2004). Segmental identity and cerebellar granule cell induction in rhombomere 1. BMC Biol. 2, 14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emmenegger B. A., Hwang E. I., Moore C., Markant S. L., Brun S. N., Dutton J. W., Read T. A., Fogarty M. P., Singh A. R., Durden D. L., et al. (2013). Distinct roles for fibroblast growth factor signaling in cerebellar development and medulloblastoma. Oncogene 32, 4181–4188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fink A. J., Englund C., Daza R. A., Pham D., Lau C., Nivison M., Kowalczyk T., Hevner R. F. (2006). Development of the deep cerebellar nuclei: transcription factors and cell migration from the rhombic lip. J. Neurosci. 26, 3066–3076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flora A., Klisch T. J., Schuster G., Zoghbi H. Y. (2009). Deletion of Atoh1 disrupts Sonic Hedgehog signaling in the developing cerebellum and prevents medulloblastoma. Science 326, 1424–1427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foucher I., Mione M., Simeone A., Acampora D., Bally-Cuif L., Houart C. (2006). Differentiation of cerebellar cell identities in absence of Fgf signalling in zebrafish Otx morphants. Development 133, 1891–1900 [DOI] [PubMed] [Google Scholar]
- Gazit R., Krizhanovsky V., Ben-Arie N. (2004). Math1 controls cerebellar granule cell differentiation by regulating multiple components of the Notch signaling pathway. Development 131, 903–913 [DOI] [PubMed] [Google Scholar]
- Gilthorpe J. D., Papantoniou E. K., Chédotal A., Lumsden A., Wingate R. J. (2002). The migration of cerebellar rhombic lip derivatives. Development 129, 4719–4728 [DOI] [PubMed] [Google Scholar]
- Guo Q., Li K., Sunmonu N. A., Li J. Y. (2010). Fgf8b-containing spliceforms, but not Fgf8a, are essential for Fgf8 function during development of the midbrain and cerebellum. Dev. Biol. 338, 183–192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagan N., Zervas M. (2012). Wnt1 expression temporally allocates upper rhombic lip progenitors and defines their terminal cell fate in the cerebellum. Mol. Cell. Neurosci. 49, 217–229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harkmark W. (1954). Cell migrations from the rhombic lip to the inferior olive, the nucleus raphe and the pons; a morphological and experimental investigation on chick embryos. J. Comp. Neurol. 100, 115–209 [DOI] [PubMed] [Google Scholar]
- Hellmann B., Manns M., Güntürkün O. (2001). Nucleus isthmi, pars semilunaris as a key component of the tectofugal visual system in pigeons. J. Comp. Neurol. 436, 153–166 [PubMed] [Google Scholar]
- Helms A. W., Abney A. L., Ben-Arie N., Zoghbi H. Y., Johnson J. E. (2000). Autoregulation and multiple enhancers control Math1 expression in the developing nervous system. Development 127, 1185–1196 [DOI] [PubMed] [Google Scholar]
- Hunt S. P., Künzle H. (1976). Observations on the projections and intrinsic organization of the pigeon optic tectum: an autoradiographic study based on anterograde and retrograde, axonal and dendritic flow. J. Comp. Neurol. 170, 153–172 [DOI] [PubMed] [Google Scholar]
- Irving C., Mason I. (2000). Signalling by FGF8 from the isthmus patterns anterior hindbrain and establishes the anterior limit of Hox gene expression. Development 127, 177–186 [DOI] [PubMed] [Google Scholar]
- Joyner A. L. (1996). Engrailed, Wnt and Pax genes regulate midbrain—hindbrain development. Trends Genet. 12, 15–20 [DOI] [PubMed] [Google Scholar]
- Kani S., Bae Y. K., Shimizu T., Tanabe K., Satou C., Parsons M. J., Scott E., Higashijima S., Hibi M. (2010). Proneural gene-linked neurogenesis in zebrafish cerebellum. Dev. Biol. 343, 1–17 [DOI] [PubMed] [Google Scholar]
- Kaslin J., Ganz J., Geffarth M., Grandel H., Hans S., Brand M. (2009). Stem cells in the adult zebrafish cerebellum: initiation and maintenance of a novel stem cell niche. J. Neurosci. 29, 6142–6153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiecker C., Lumsden A. (2004). Hedgehog signaling from the ZLI regulates diencephalic regional identity. Nat. Neurosci. 7, 1242–1249 [DOI] [PubMed] [Google Scholar]
- Klein C., Butt S. J., Machold R. P., Johnson J. E., Fishell G. (2005). Cerebellum- and forebrain-derived stem cells possess intrinsic regional character. Development 132, 4497–4508 [DOI] [PubMed] [Google Scholar]
- Kohl A., Hadas Y., Klar A., Sela-Donenfeld D. (2012). Axonal patterns and targets of dA1 interneurons in the chick hindbrain. J. Neurosci. 32, 5757–5771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K. J., Dietrich P., Jessell T. M. (2000). Genetic ablation reveals that the roof plate is essential for dorsal interneuron specification. Nature 403, 734–740 [DOI] [PubMed] [Google Scholar]
- Machold R., Fishell G. (2005). Math1 is expressed in temporally discrete pools of cerebellar rhombic-lip neural progenitors. Neuron 48, 17–24 [DOI] [PubMed] [Google Scholar]
- Martinez S., Crossley P. H., Cobos I., Rubenstein J. L., Martin G. R. (1999). FGF8 induces formation of an ectopic isthmic organizer and isthmocerebellar development via a repressive effect on Otx2 expression. Development 126, 1189–1200 [DOI] [PubMed] [Google Scholar]
- McGill J. I., Powell T. P., Cowan W. M. (1966). The retinal representation upon the optic tectum and isthmo-optic nucleus in the pigeon. J. Anat. 100, 5–33 [PMC free article] [PubMed] [Google Scholar]
- Meyers E. N., Lewandoski M., Martin G. R. (1998). An Fgf8 mutant allelic series generated by Cre- and Flp-mediated recombination. Nat. Genet. 18, 136–141 [DOI] [PubMed] [Google Scholar]
- Millet S., Bloch-Gallego E., Simeone A., Alvarado-Mallart R. M. (1996). The caudal limit of Otx2 gene expression as a marker of the midbrain/hindbrain boundary: a study using in situ hybridisation and chick/quail homotopic grafts. Development 122, 3785–3797 [DOI] [PubMed] [Google Scholar]
- Morin X., Jaouen F., Durbec P. (2007). Control of planar divisions by the G-protein regulator LGN maintains progenitors in the chick neuroepithelium. Nat. Neurosci. 10, 1440–1448 [DOI] [PubMed] [Google Scholar]
- Myat A., Henrique D., Ish-Horowicz D., Lewis J. (1996). A chick homologue of Serrate and its relationship with Notch and Delta homologues during central neurogenesis. Dev. Biol. 174, 233–247 [DOI] [PubMed] [Google Scholar]
- Puelles L., Martinez-de-la-Torre M. (1987). Autoradiographic and Golgi study on the early development of n. isthmi principalis and adjacent grisea in the chick embryo: a tridimensional viewpoint. Anat. Embryol. (Berl.) 176, 19–34 [DOI] [PubMed] [Google Scholar]
- Reifers F., Böhli H., Walsh E. C., Crossley P. H., Stainier D. Y., Brand M. (1998). Fgf8 is mutated in zebrafish acerebellar (ace) mutants and is required for maintenance of midbrain-hindbrain boundary development and somitogenesis. Development 125, 2381–2395 [DOI] [PubMed] [Google Scholar]
- Rodriguez C. I., Dymecki S. M. (2000). Origin of the precerebellar system. Neuron 27, 475–486 [DOI] [PubMed] [Google Scholar]
- Rose M. F., Ahmad K. A., Thaller C., Zoghbi H. Y. (2009). Excitatory neurons of the proprioceptive, interoceptive, and arousal hindbrain networks share a developmental requirement for Math1. Proc. Natl. Acad. Sci. USA 106, 22462–22467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saarimäki-Vire J., Peltopuro P., Lahti L., Naserke T., Blak A. A., Vogt Weisenhorn D. M., Yu K., Ornitz D. M., Wurst W., Partanen J. (2007). Fibroblast growth factor receptors cooperate to regulate neural progenitor properties in the developing midbrain and hindbrain. J. Neurosci. 27, 8581–8592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato T., Nakamura H. (2004). The Fgf8 signal causes cerebellar differentiation by activating the Ras-ERK signaling pathway. Development 131, 4275–4285 [DOI] [PubMed] [Google Scholar]
- Sato T., Joyner A. L. (2009). The duration of Fgf8 isthmic organizer expression is key to patterning different tectal-isthmo-cerebellum structures. Development 136, 3617–3626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato T., Araki I., Nakamura H. (2001). Inductive signal and tissue responsiveness defining the tectum and the cerebellum. Development 128, 2461–2469 [DOI] [PubMed] [Google Scholar]
- Sgaier S. K., Millet S., Villanueva M. P., Berenshteyn F., Song C., Joyner A. L. (2005). Morphogenetic and cellular movements that shape the mouse cerebellum; insights from genetic fate mapping. Neuron 45, 27–40 [DOI] [PubMed] [Google Scholar]
- Shamim H., Mahmood R., Logan C., Doherty P., Lumsden A., Mason I. (1999). Sequential roles for Fgf4, En1 and Fgf8 in specification and regionalisation of the midbrain. Development 126, 945–959 [DOI] [PubMed] [Google Scholar]
- Suzuki-Hirano A., Harada H., Sato T., Nakamura H. (2010). Activation of Ras-ERK pathway by Fgf8 and its downregulation by Sprouty2 for the isthmus organizing activity. Dev. Biol. 337, 284–293 [DOI] [PubMed] [Google Scholar]
- Toyoda R., Assimacopoulos S., Wilcoxon J., Taylor A., Feldman P., Suzuki-Hirano A., Shimogori T., Grove E. A. (2010). FGF8 acts as a classic diffusible morphogen to pattern the neocortex. Development 137, 3439–3448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace V. A. (1999). Purkinje-cell-derived Sonic hedgehog regulates granule neuron precursor cell proliferation in the developing mouse cerebellum. Curr. Biol. 9, 445–448 [DOI] [PubMed] [Google Scholar]
- Walshe J., Mason I. (2000). Expression of FGFR1, FGFR2 and FGFR3 during early neural development in the chick embryo. Mech. Dev. 90, 103–110 [DOI] [PubMed] [Google Scholar]
- Wang V. Y., Rose M. F., Zoghbi H. Y. (2005). Math1 expression redefines the rhombic lip derivatives and reveals novel lineages within the brainstem and cerebellum. Neuron 48, 31–43 [DOI] [PubMed] [Google Scholar]
- Wechsler-Reya R. J., Scott M. P. (1999). Control of neuronal precursor proliferation in the cerebellum by Sonic Hedgehog. Neuron 22, 103–114 [DOI] [PubMed] [Google Scholar]
- Wilson L. J., Wingate R. J. (2006). Temporal identity transition in the avian cerebellar rhombic lip. Dev. Biol. 297, 508–521 [DOI] [PubMed] [Google Scholar]
- Wingate R. (2005). Math-Map(ic)s. Neuron 48, 1–4 [DOI] [PubMed] [Google Scholar]
- Wingate R. J. (2001). The rhombic lip and early cerebellar development. Curr. Opin. Neurobiol. 11, 82–88 [DOI] [PubMed] [Google Scholar]
- Wingate R. J., Hatten M. E. (1999). The role of the rhombic lip in avian cerebellum development. Development 126, 4395–4404 [DOI] [PubMed] [Google Scholar]
- Yaneza M., Gilthorpe J. D., Lumsden A., Tucker A. S. (2002). No evidence for ventrally migrating neural tube cells from the mid- and hindbrain. Dev. Dyn. 223, 163–167 [DOI] [PubMed] [Google Scholar]
- Zervas M., Millet S., Ahn S., Joyner A. L. (2004). Cell behaviors and genetic lineages of the mesencephalon and rhombomere 1. Neuron 43, 345–357 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.