Abstract
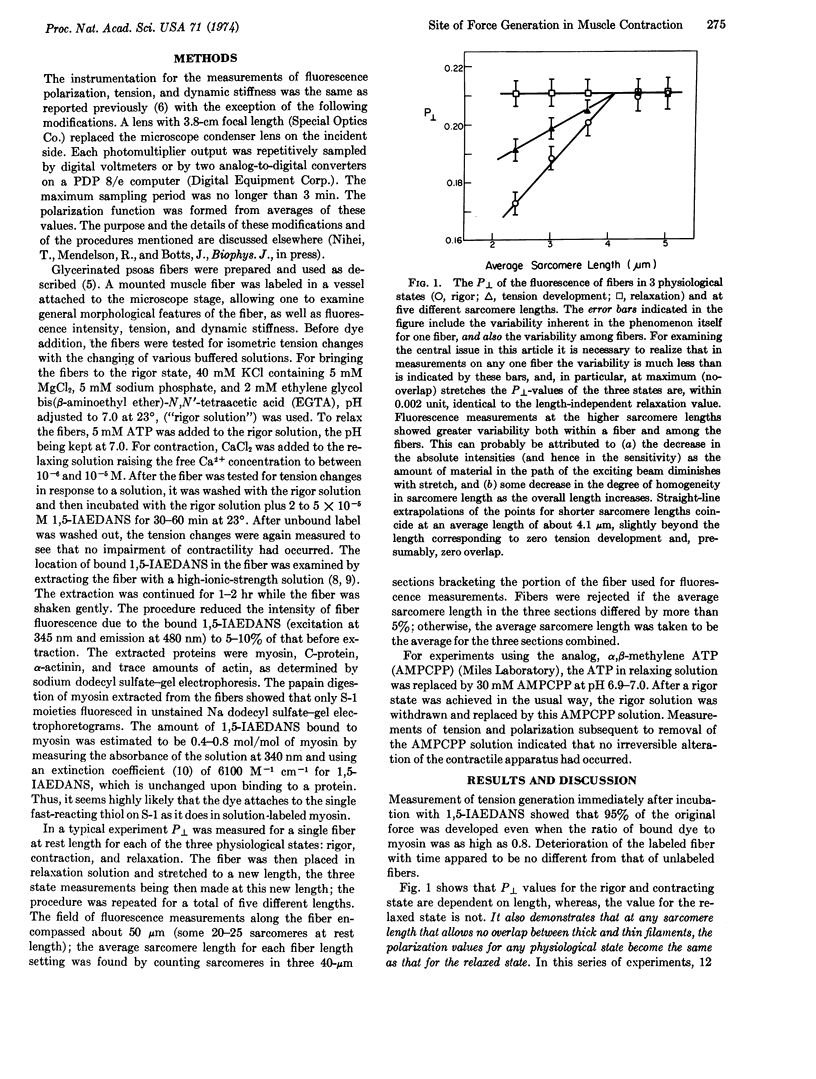
The fluorescent dye, N(-iodoacetylamino)-l-naphthylanine-5-sulfonic acid, labeled exclusively the myosin cross-bridges in rabbit glycerinated psoas muscle fibers, without impairing their function. Fluorescence polarization was used to study cross-bridge orientation in rigor, relaxation, and contraction, as a function of sarcomere length. At a length where no overlap between thick and thin filaments occurs, rigor-inducing, relaxation-inducing, and contraction-inducing solutions all induced the relaxation attitude. At lengths where overlap does exist, the slowly-hydrolyzing ATP analog, “α,β-methylene ATP,” induced the relaxation attitude. The data were consistent with the A. F. Huxley-Simmons model of force generation. Combined with our earlier results, the data indicated that torque was generated at the actin-myosin interface.
Keywords: psoas, cross-bridge, myofilament, overlap, myosin
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aronson J. F., Morales M. F. Polarization of tryptophan fluorescence in muscle. Biochemistry. 1969 Nov;8(11):4517–4522. doi: 10.1021/bi00839a044. [DOI] [PubMed] [Google Scholar]
- BARANY M., BARANY K. Studies on "active centers" of L-myosin. Biochim Biophys Acta. 1959 Oct;35:293–309. doi: 10.1016/0006-3002(59)90378-6. [DOI] [PubMed] [Google Scholar]
- Dos Remedios C. G., Millikan R. G., Morales M. F. Polarization of tryptophan fluorescence from single striated muscle fibers. A molecular probe of contractile state. J Gen Physiol. 1972 Jan;59(1):103–120. doi: 10.1085/jgp.59.1.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dos Remedios C. G., Yount R. G., Morales M. F. Individual states in the cycle of muscle contraction. Proc Natl Acad Sci U S A. 1972 Sep;69(9):2542–2546. doi: 10.1073/pnas.69.9.2542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HANSON J., HUXLEY H. E. Quantitative studies on the structure of cross-striated myofibrils. II. Investigations by biochemical techniques. Biochim Biophys Acta. 1957 Feb;23(2):250–260. doi: 10.1016/0006-3002(57)90326-8. [DOI] [PubMed] [Google Scholar]
- Huxley A. F., Simmons R. M. Proposed mechanism of force generation in striated muscle. Nature. 1971 Oct 22;233(5321):533–538. doi: 10.1038/233533a0. [DOI] [PubMed] [Google Scholar]
- Huxley H. E. The mechanism of muscular contraction. Science. 1969 Jun 20;164(3886):1356–1365. doi: 10.1126/science.164.3886.1356. [DOI] [PubMed] [Google Scholar]
- Mannherz H. G., Leigh J. B., Holmes K. C., Rosenbaum G. Identification of the transitory complex myosin-ATP by the use of , -methylene-ATP. Nat New Biol. 1973 Feb 21;241(112):226–229. doi: 10.1038/newbio241226a0. [DOI] [PubMed] [Google Scholar]
- Mendelson R. A., Morales M. F., Botts J. Segmental flexibility of the S-1 moiety of myosin. Biochemistry. 1973 Jun 5;12(12):2250–2255. doi: 10.1021/bi00736a011. [DOI] [PubMed] [Google Scholar]
- Offer G., Moos C., Starr R. A new protein of the thick filaments of vertebrate skeletal myofibrils. Extractions, purification and characterization. J Mol Biol. 1973 Mar 15;74(4):653–676. doi: 10.1016/0022-2836(73)90055-7. [DOI] [PubMed] [Google Scholar]





