Abstract
We highlight recent progress in understanding cadherin and integrin function in the model organism Drosophila. New functions for these adhesion receptors continue to be discovered in this system, emphasising the importance of cell adhesion within the developing organism and showing that the requirement for cell adhesion changes between cell types. New ways to control adhesion have been discovered, including controlling the expression and recruitment of adhesion components, their posttranslational modification, recycling and turnover. Importantly, even ubiquitous adhesion components can function differently in distinct cellular contexts.
Introduction
Cell adhesion plays vital roles during the development and adult life of multicellular organisms. Two types of adhesion can be distinguished: adhesion between adjacent cells (cell-cell adhesion) and adhesion between cells and the extracellular matrix (cell-ECM adhesion, but also cell-ECM-cell adhesion). The canonical receptors for cell-cell adhesion are classical cadherins, which bind to other cadherins from neighbouring cells through homodimerization of their extracellular domains [recently reviewed in 1,2,3]. Cell-ECM adhesion occurs primarily with integrin receptors, each a heterodimer of α and β subunits, which bind specific ECM proteins [recently reviewed in 4].
In this review we describe recent advances in our understanding of these adhesion receptors as they function in the model organism Drosophila. In particular, we wish to highlight the emerging insights that arise from being able to study adhesion mechanisms in a variety of developmental and cellular contexts within the intact organism (Table 1). Using Drosophila, one can compare functions in diverse cell types, but also the same cell types in different developmental contexts, e.g. forming different organs, such as the eye or wing, or at different stages in the life cycle. Using Drosophila as a model system also has the advantage of the reduced gene number relative to vertebrates, which makes it more straightforward to remove completely the function of a particular type of protein. Thus, Drosophila has only 3 classical cadherins (E- and two N-cadherins, from a total of 17 proteins in the genome with cadherin repeats [5]), 5 integrin α subunits (αPS1-5) and 2 integrin β subunits (βPS and βν) [6]. Both cadherins and integrins recruit cytoplasmic proteins to form adhesion complexes that link their intracellular domains with the actin cytoskeleton, and each type of cytoplasmic protein is also encoded by fewer genes in Drosophila relative to vertebrates. We now focus on recent findings in Drosophila that have revealed how cell adhesion is adjusted to the requirements of different cell types and developmental events by changes in adhesion complex composition and dynamics.
Table 1.
Cadherin and Integrin functions in Drosophila
| Tissue/system | Cadherin functions | Integrin functions |
|---|---|---|
| Amnioserosa | Adhesion between cell layers (between leading edge epidermis cells and amnioserosa) E – Gorfinkiel 2007 |
Cell spreading αPS3βPS – Schock 2003 Adhesion between cell layers (yolk cell/amnioserosa cells) αPS3 and βPS – Narasimha 2004 ECM assembly βPS – Narasimha 2004 |
|
| ||
| Border cells | Cell migration E – Tepass 1999 | Collective cell movement βPS – Llense 2008 |
|
| ||
|
Embryonic
epidermis |
Cell-cell adhesion (all) E – Tepass 1999 Cell intercalation (germband elongation) E – Levayer 2011; Tamada 2012 Collective cell movement (dorsal closure) E – Gorfinkiel 2007 Modulation of signaling (Wingless) E – Sanson 1996 Organization of the actin cytoskeleton (germband elongation) E – Gorfinkiel 2007; Rauzi 2010 |
Cell migration (dorsal closure) αPS1βPS, αPS2βPS, αPS3 – Leptin 1989; Roote 1995; Stark 1997 Collective cell movement (germband retraction) αPS1βPS, αPS2βPS – Leptin 1989; Roote 1995 |
|
| ||
| Follicle cells |
Differential adhesion (follicle cells/oocyte) E – Tepass 1999 Monolayer maintenance E – Godt 1998 |
Monolayer maintenance αPS1βPS – Fernandez- Minan 2007 Organization of the actin cytoskeleton (stress fibres) βPS – Delon 2009 Organization of the microtubule cytoskeleton (mitotic spindle) αPS1βPS – Fernandez-Minan 2007 Planar cell polarity βPS – Delon 2009 Stem cell maintenance αPS1βPS – O’Reilly 2008 |
|
| ||
| Intestine | Stem cell maintenance E – Maeda 2008 Modulation of signaling (Notch) E – Maeda 2008 |
Cell migration αPS1βPS, αPS2βPS, αPS3βPS, βν – Brown 1994; Martin-Bermudo 1999; Devenport 2004 |
|
| ||
| Muscle cells | Cell fusion N – Dottermusch-Heidel 2012 | Organization of the actin cytoskeleton (sarcomeres) βPS – Volk 1990; Rui 2010 Muscle attachment αPS2βPS – Leptin 1989; Brabant 1993 ECM assembly αPS2βPS – Devenport 2007 |
|
| ||
| Nervous system |
Differential adhesion (neuron/glia) E – Slováková 2011 Tissue patterning (axons) N – Iwai 1997 |
Adhesion between cell layers (glial cell layers) αPS2βPS, αPS3βPS – Xie 2011 Axon guidance αPS2βPS, αPS3βPS – Hoang 1998 Tissue patterning (dendrites) αPS1βPS – Han 2012; Kim 2012 |
|
| ||
| Retina |
Cell geometry E, N – Hayashi 2004 Cell intercalation E – Carthew 2005 Modulation of signaling (EGF) E – Dumstrei 2002 Planar cell polarity E, N – Mirkovic 2006 Cell-cell adhesion E, N – Hayashi 2004 Tissue patterning N – Lee 2001; E – Grzeschik 2005 |
Adhesion between cell layers βPS – Zusman 1990 |
|
| ||
| Salivary gland | Cell geometry E – Pirraglia 2010 | Cell migration αPS1βPS – Bradley 2003 |
|
| ||
| Testis | Stem cell maintenance (somatic) E – Voog 2008 | Stem cell maintenance (germline) βPS – Tanentzapf 2007 |
|
| ||
| Trachea | Cell-cell adhesion E – Tepass 1999 | Cell migration αPS1βPS – Boube 2001 |
|
| ||
| Wing |
Cell death (JNK) E – Jezowska 2011 Modulation of signaling (Wingless) E – Jezowska 2011 Cell-cell adhesion E – Tepass 1999 |
Adhesion between cell layers αPS1βPS, αPS2βPS – Brower 1989, 1995; Wilcox 1989 |
|
| ||
| Malpighian tubules | Cell-cell adhesion E – Tepass 1999 | |
|
| ||
| Sensory organs | Modulation of signaling (Notch) E – Benhra 2011 | |
|
| ||
| Hemocytes |
Cell migration αPS2 – Siekhaus 2010 Phagocytosis βν – Nagaosa 2011 |
|
|
| ||
| Mesoderm |
Cell migration αPS1βPS, αPS3βPS – Urbano 2011 ECM assembly αPS2βPS – Martin-Bermudo 1999; Urbano 2011 Cell intercalation βPS – McMahon 2010 |
|
Overview of the functions discovered for cadherins and integrins in Drosophila. The specific cadherins or integrins characterized are indicated (E: E-cadherin, N: N-cadherin). Recently discovered functions are in bold and functions specific to cadherins or integrins are in italic. References: Benhra (2011) Curr. Biol. 21 87-95; Boube (2001) Genes Dev. 15 1554-1562; Brabant (1993) Dev. Biol. 157 49-59; Bradley (2003) Dev. Biol. 257 249-262; Brower (1995) Development 121 1311-1320; Brower (1989) Nature 342 285-287; Brown (1994) Development 120 1221-1231; Carthew (2005) Curr. Opin. Genet. Dev. 15 358-363; Delon (2009) J. Cell Sci. 122 4363-4374; Devenport (2004). Development 131 5405-5415; Devenport (2007) Dev. Biol. 308 294-308; Dottermusch-Heidel (2012) Dev. Biol. ahead of print; Dumstrei (2002) Development 129 3983-3994; Fernandez-Minan (2007) Curr. Biol. 17 683-688; Godt (1998) Nature 395 387-391; Gorfinkiel (2007) J. Cell Sci. 120 3289-3298; Grzeschik (2005) Development 132 2035-2045; Han (2012) Neuron 73 64-78; Hayashi (2004) Nature 431 647-652; Hoang (1998) J Neurosci. 18 7847-7855; Iwai (1997) Neuron 19 77-89; Jezowska (2011) Dev. Biol. 360 143-159; Kim (2012) Neuron 73 79-91; Lee (2001) Neuron 30 437-450; Leptin (1989) Cell 56 401-408; Levayer (2011) Nat. Cell Biol. 13 529-540; Llense (2008) Curr. Biol. 18 538-544; Maeda (2008) Gen. Cells 13 1219-1227; Martin-Bermudo (1999) Development 126 5161-5169; McMahon (2010) Development 137 2167-2175; Mirkovic (2006) Development 133 3283-3293; Nagaosa (2011) J. Biol. Chem. 286 25770-25777; Narasimha (2004) Curr. Biol. 14 381-385; O’Reilly (2008) The J. Cell Biol. 182 801-815; Pirraglia (2010) Development; Rauzi (2010) Nature 468 1110-1114; Roote (1995) Dev. Biol. 169 322-336; Rui (2010) PLoS Genet. 6 e1001208; Sanson (1996) Nature 383 627-630; Schock (2003) Genes Dev. 17 597-602; Siekhaus (2010) Nat. Cell Biol. 12 605-610; Slováková (2011) Development 138 1563-1571; Stark (1997) Development 124 4583-4594; Tamada (2012) Dev. Cell 22 309-319; Tanentzapf (2007) Nat. Cell Biol. 9 1413-1418; Tepass (1999) Curr. Opin. Cell Biol. 11 540-548; Urbano (2011) PLoS ONE 6 e23893; Volk (1990) Cell 63 525-536; Voog (2008) Nature 454 1132-1136; Wilcox (1989) Development 107 891-897; Xie (2011) Development 138 3813-3822; Zusman (1990) Development 108 391-402
Novel cadherin and integrin functions in Drosophila
New functions for cadherins and integrins continue to be discovered at a steady rate, as investigators test whether these adhesion receptors contribute to their favorite biological process. We have collated the known functions to demonstrate the breadth of activities of these receptors (Table 1, recently discovered functions are in bold). For cell biologists, these can be viewed as a range of assays that may reveal the mechanistic diversity of adhesion complexes. Just to highlight a few of the functions discovered recently: negative regulation of myoblast fusion by N-cadherin, counteracting an Arf-GEF [7]; E-cadherin-dependent proliferation and apoptosis in the absence of actin capping protein [8]; assembly of an ECM by integrins that is used by other cells as a track for their integrin-dependent migration [9]; repulsion between sensory neuron dendrites by integrins to ensure a non-overlapping field [10,11]. Not only do these discoveries aid the understanding of each developmental process, but they also provide new paradigms for the function of these receptors. Looking at Table 1 it is clear that integrins and cadherins are involved in many similar processes in the building of an organism, however, if you look at any individual tissue the two receptors are doing different things, supporting the view that they have distinct roles. The diversity of functions raises the question of whether they can be achieved by a single adhesive function for each type of receptor, or whether they require tailor-made adhesion complexes. As we shall discuss, the range of adhesive functions provided by Drosophila development and physiology has begun to reveal that their are different flavours of the adhesion machinery, and different modes of regulation of these diverse machines.
Regulation of adhesion by differential expression of the receptors
The easiest way to modulate adhesion is by controlling the expression of adhesion receptors, to control whether a cell has cadherins or integrins and also selecting the type of receptor. With integrins, 10 possible heterodimers can be formed with the 5 α subunits and 2 β subunits. While βPS is probably ubiquitously expressed, the rest show tightly controlled expression patterns, and have quite distinct functions (Table 1). A good example regulating adhesion by changing expression is in the follicular epithelium, where the cells switch from laminin-binding to RGD-binding integrins [12] (of note, a change in the composition of cadherins occurs simultaneously, with N-cadherin turned off, while E-cadherin remains on [13]). In the cases where it has been tested, the functional differences of the integrins α subunits map solely to the extracellular domains [14], even when it comes to recruiting a specific intracellular protein [12]. This suggests that the main reason different α subunits are employed is to generate heterodimers that bind particular matrix components. A number of integrin extracellular matrix ligands have been identified in Drosophila, and they also have distinct distributions [reviewed in 6]. The recruitment of many of them appears to be independent of integrins, but two require integrins for their stability and/or recruitment [9,15]. Thus, changing the expression of different integrin subunits and recruiting ligands by multiple mechanisms permits the generation of a variety of interactions with matrix proteins, creating diverse adhesion contexts throughout the developing organism.
There are multiple examples of important developmental regulation of E-cadherin. For example, elevation of E-cadherin synthesis by Src42A kinase is required for tracheal morphogenesis [16], while inhibition of E-cadherin transcription by talin ensures a differential adhesion between oocyte and follicle cells to establish the correct positioning of the oocyte and future embryo axis [17]. Actin-capping protein reduces E-cadherin synthesis in the majority of the presumptive wing cells, therefore, promoting wingless and inhibiting JNK signaling in these cells [8].
E- and the two N-cadherins have complex patterns of expression, with some cells having single receptors and others a mixture. For example, during the epithelial-mesenchymal transition of the presumptive mesoderm during gastrulation, E-cadherin transcription is downregulated, while N-cadherin is upregulated [18], and as mentioned, N-cadherin becomes downregulated in the follicular epithelium [13]. A good example of how regulation of cadherin expression in time and space can regulate cell architecture is provided by the developing eye [19,20]. Cadherin extracellular interactions can also be regulated. N-cadherin is regulated by alternative splicing, with a more adhesive isoform expressed during early developmental stages [21], suggesting that splicing is used to regulate the strength of adhesion. E-cadherin exists in different conformations in a reproducible spatial pattern in the embryo, as documented using monoclonal antibody staining of unfixed embryos during dorsal closure [22]. Although the nature of these different conformations and how they are induced is not known, it seems likely that they are different homophilic binding states with different adhesive strengths [22,23]. Finally, the degredation of cadherins can also be regulated, as the turnover of E-cadherin decreases as embryonic development progresses [24].
Regulating the synthesis and turnover of adhesion complexes
Another mechanism to modulate adhesive function is to regulate the endocytosis and recycling of the transmembrane adhesion receptors. This appears to be the main mechanism used to move E-cadherin from one membrane to another [24,25]. E-cadherin endocytosis has been found to be increased in tissues undergoing active remodelling. Src42A and Pak1 elevate E-cadherin endocytosis in trachea and salivary glands to ensure the morphogenesis of these systems [16,26]. RhoGEF2 promotes E-cadherin endocytosis specifically at the junctions that are targeted for disassembly during germ band elongation [27]. The mechanisms that control E-cadherin endocytosis can be very different between tissues; the small GTPase Cdc42 has opposite effects on E-cadherin endocytosis, promoting E-cadherin endocytosis in the pupal notum and eye, but inhibiting endocytosis in the embryonic epidermis [28-30].
In contrast to cadherins, few recent studies have analysed the endocytosis and recycling of integrins in Drosophila. The pathways targeting integrins to adhesion sites can be specific to tissues, as revealed by a novel membrane trafficking pathway in the follicular epithelium [31]. The dynamic turnover of integrins and several associated proteins at muscle attachment sites reduces as larval development proceeds [32], suggesting that stabilisation of attachment as contraction strength increases. Integrin recruitment and turnover in pupal muscles is regulated by Myotubularin, which controls the balance of phosphoinositides at the membrane [33], suggesting a link between membrane composition and integrin localization and/or turnover. Future analysis of integrin and cadherin dynamics should provide a better understanding of how these large complexes of proteins can be modulated to accommodate different requirements of cells for adhesion throughout development.
The adhesome: regulating the link between adhesion receptors and the cytoskeleton
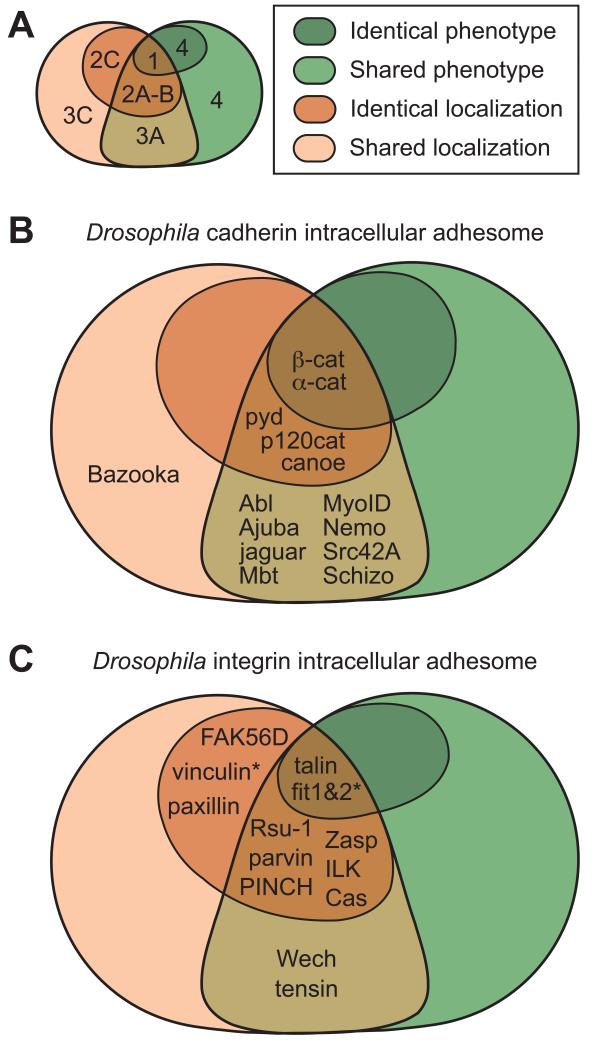
The term adhesome encapsulates the idea that it would be useful to identify the full set of proteins involved in the function of cadherins and integrins [34]. In particular, identifying all cytoplasmic proteins required for the function of these proteins is an ongoing task. Conceptually we can divide the intracellular adhesome components into 4 classes (Fig. 1 and Tables 2 and 3). Class 1 is cytoplasmic proteins that are always required for adhesion receptor function, the so-called “core” components. However, there are at least three possible ways to define such core components: 1) co-purifying with the receptor, 2) co-localising with the receptor in all types of cellular contexts, or 3) a genetic one, where the loss of core components produces the same set of defects as loss of the receptor (Fig. 1).
Figure 1.
Classes of intracellular adhesome proteins in general (A); in cadherin adhesion (B); and integrin adhesion (C). The proteins are divided in classes depending on their overlap in phenotype and colocalization with the adhesion receptor. Class 1, 2A-B and 2C proteins colocalize with receptor in all cases, and loss of class 1 proteins shares all phenotypes with the loss of receptor, loss of class 2A-B shares some phenotypes, and loss of class 2C does not share any. Class 3A and 3C proteins colocalize with receptor in some cases, and loss of class 3A proteins results in some common phenotypes with loss of receptor, while loss of class 3C proteins does not. We include a class 4 of proteins that do not colocalize with receptor, and loss of these proteins results in all or some phenotypes caused by loss of receptor. * indicates that the class of the protein was predicted based on indirect data or data from other model systems. For detailed description of the proteins and references to literature see Tables 2 and 3.
Table 2.
Cadherin-associated intracellular proteins in Drosophila
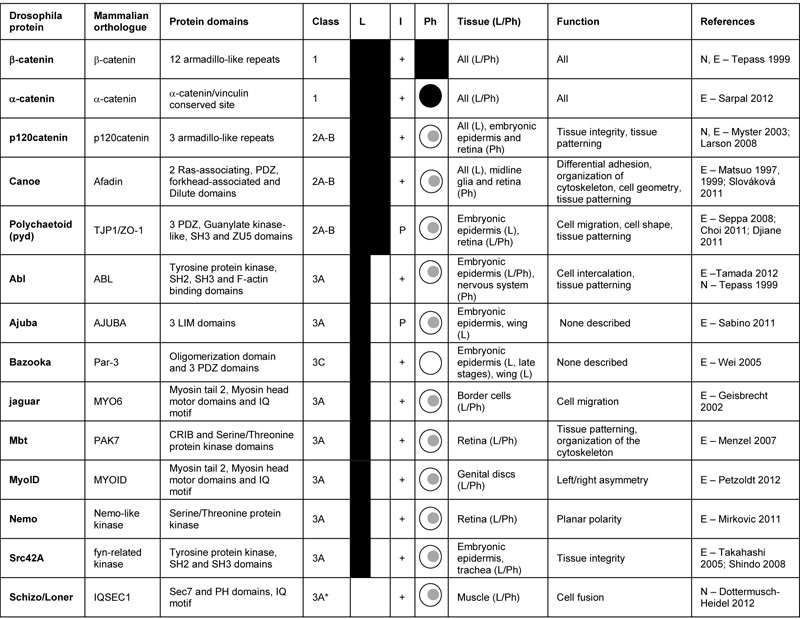
Cadherin-associated intracellular proteins in Drosophila. “Class” indicates whether the proteins: always co-localize with their receptor and are required for all functions (1 – core components) or for some functions (2A-B) of the receptor; co-localize with their receptor in certain contexts and are required for some functions (3A) or for no described function (3C) of the receptor. See text and figure 1 for more details. “L” (co-Localization) indicates whether the proteins localize with their receptors in all (full black) or some tissues (half black). “I” (Interaction) indicates whether the Drosophila proteins have been shown to (+) or are predicted to (P) interact biochemically with their receptor. “Ph” (Phenotype) indicates whether the protein is required for more functions (full black), all functions ( ), some functions (
), some functions ( ) or for no described function (
) or for no described function ( ) of its receptor. “Tissue” describes in which tissue the protein localizes (L) and shares phenotypes (Ph) with its receptor. “Function” describes the relevant processes described requiring the protein for its receptor function. References: Choi (2011) Mol. Biol. Cell 22 2010-2030; Djiane (2011) J. Cell Biol. 192 189-200; Dottermusch-Heidel (2012) Dev. Biol. ahead of print; Geisbrecht (2002) Nat. Cell Biol. 4 616-620; Larson (2008) Mech. Dev. 125 223-232; Matsuo (1997) Development 124 2671-2680; Matsuo (1999) Cell Tiss. Res. 298 397-404; Menzel (2007) Mech. Dev. 124 78-90; Mirkovic (2011) Nat. Struc. Mol. Biol. 18 665-672; Myster (2003) The J. Cell Biol. 160 433-449; Petzoldt (2012) Development 139 1874-1884; Sabino (2011) J. Cell Sci. 124 1156-1166; Sarpal (2012) J. Cell Sci. 125 233-245; Seppa (2008) Dev. Biol. 318 1-16; Shindo (2008) Development 135 1355-1364; Slováková (2011) Development 138 1563-1571; Takahashi (2005) Development 132 2547-2559; Tamada (2012) Dev. Cell 22 309-319; Tepass (1999) Curr. Opin. Cell Biol. 11 540-548; Wei (2005) Dev. Cell 8 493-504
) of its receptor. “Tissue” describes in which tissue the protein localizes (L) and shares phenotypes (Ph) with its receptor. “Function” describes the relevant processes described requiring the protein for its receptor function. References: Choi (2011) Mol. Biol. Cell 22 2010-2030; Djiane (2011) J. Cell Biol. 192 189-200; Dottermusch-Heidel (2012) Dev. Biol. ahead of print; Geisbrecht (2002) Nat. Cell Biol. 4 616-620; Larson (2008) Mech. Dev. 125 223-232; Matsuo (1997) Development 124 2671-2680; Matsuo (1999) Cell Tiss. Res. 298 397-404; Menzel (2007) Mech. Dev. 124 78-90; Mirkovic (2011) Nat. Struc. Mol. Biol. 18 665-672; Myster (2003) The J. Cell Biol. 160 433-449; Petzoldt (2012) Development 139 1874-1884; Sabino (2011) J. Cell Sci. 124 1156-1166; Sarpal (2012) J. Cell Sci. 125 233-245; Seppa (2008) Dev. Biol. 318 1-16; Shindo (2008) Development 135 1355-1364; Slováková (2011) Development 138 1563-1571; Takahashi (2005) Development 132 2547-2559; Tamada (2012) Dev. Cell 22 309-319; Tepass (1999) Curr. Opin. Cell Biol. 11 540-548; Wei (2005) Dev. Cell 8 493-504
Table 3.
Integrin-associated intracellular proteins in Drosophila.
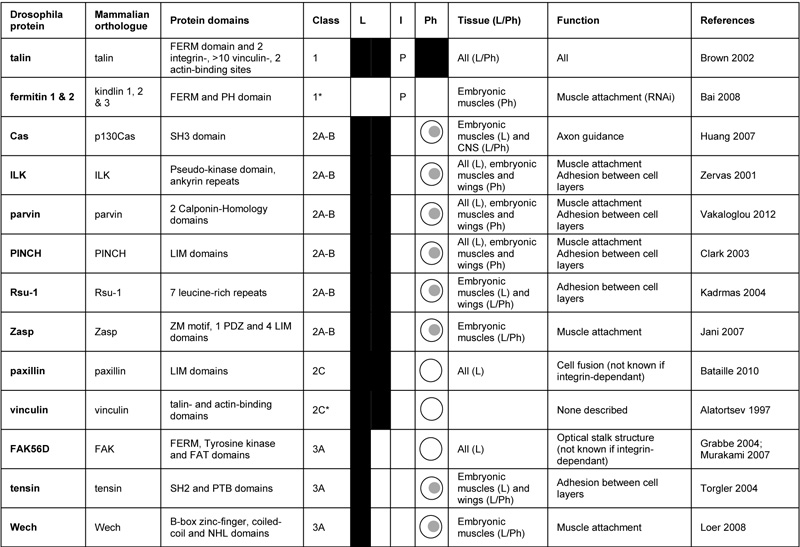
Integrin-associated intracellular proteins in Drosophila. “Class” indicates whether the proteins: always co-localize with their receptor and are required for all functions (1 – core components), for some functions (2A-B) or for no described function (2C) of the receptor; co-localize with their receptor in certain contexts and are required for some functions of the receptor (3A). See text and figure 1 for more details.
indicates that the class is predicted. “L” (co-Localization) indicates whether the proteins localize with their receptors in all (full black) or some tissues (half black). “I” (Interaction) indicates whether the Drosophila proteins have been shown to (+) or are predicted to (P) interact biochemically with their receptor. “Ph” (Phenotype) indicates whether the protein is required for more functions (full black), some functions ( ) or for no described function (
) or for no described function ( ) of its receptor. “Tissue” describes in which tissue the protein localizes (L) and shares phenotypes (Ph) with its receptor. “Function” describes the relevant processes described requiring the protein for its receptor function. “RNAi” indicates functions observed when the gene is down-regulated by RNAi in some tissues. References: Alatortsev (1997) FEBS Lett. 413 197-201; Bai (2008) Development 135 1439-1449; Bataille (2010) Dev. Cell 19 317-328; Brown (2002) Dev. Cell 3 569-579; Clark (2003) Development 130 2611-2621; Grabbe (2004) Development 131 5795-5805; Huang (2007) Development 134 2337-2347; Jani (2007) J. Cell Biol. 179 1583-1597; Kadrmas (2004) J. Cell Biol. 167 1019-1024; Loer (2008) Nat. Cell Biol. 10 422-428; Murakami (2007) Development 134 1539-1548; Torgler (2004) Dev. Cell. 6 357-369; Vakaloglou (2012) J. Cell Sci. ahead of print; Zervas (2001) J. Cell Biol. 152 1007-1018
) of its receptor. “Tissue” describes in which tissue the protein localizes (L) and shares phenotypes (Ph) with its receptor. “Function” describes the relevant processes described requiring the protein for its receptor function. “RNAi” indicates functions observed when the gene is down-regulated by RNAi in some tissues. References: Alatortsev (1997) FEBS Lett. 413 197-201; Bai (2008) Development 135 1439-1449; Bataille (2010) Dev. Cell 19 317-328; Brown (2002) Dev. Cell 3 569-579; Clark (2003) Development 130 2611-2621; Grabbe (2004) Development 131 5795-5805; Huang (2007) Development 134 2337-2347; Jani (2007) J. Cell Biol. 179 1583-1597; Kadrmas (2004) J. Cell Biol. 167 1019-1024; Loer (2008) Nat. Cell Biol. 10 422-428; Murakami (2007) Development 134 1539-1548; Torgler (2004) Dev. Cell. 6 357-369; Vakaloglou (2012) J. Cell Sci. ahead of print; Zervas (2001) J. Cell Biol. 152 1007-1018
For classical cadherins, criterion 1 has worked well as they can be purified tightly bound to three intracellular proteins: β-catenin and p120catenin bind to cadherins directly; and α-catenin binds β-catenin [for references see 1,3]. The 3 catenins also co-localise with cadherins in a wide variety of cells, fulfilling criterion 2. However, while loss of α- and β-catenin causes strong lethal phenotypes very similar to loss of cadherins, loss of p120catenin results in viable and fertile flies [Table 2, 35,36,37].
There does not appear to be a similar “core” of intracellular proteins that can be co-purified with integrins; this may due to the technical difficulties of purifying integrins bound to the insoluble extracellular matrix, or it may reflect a lower affinity in the interactions, with chemical cross-linking being required to co-purify any of the integrin-associated proteins from cultured cells [38]. A large number of proteins fulfill criterion 2 (Table 3). Comparing muscle attachment sites (the major site of integrin adhesion in the embryo) to the focal adhesion structures that form on the basal surface of the follicular epithelium revealed that 7 of the 9 components examined were present in both systems [12]. Using criterion 3, talin has emerged as the sole core component, as it is the only integrin-associated protein absolutely required for integrin adhesion [see Table 3 and 39], and it is also essential for the recruitment of many of the other associated proteins [40]. Mutants in other components have subsets of the integrin/talin phenotype, or in the case of some, no detectable phenotype.
Thus, the work characterising the cadherin and integrin adhesomes has revealed associated proteins that are always colocalised with the adhesion receptor, but not always essential for its function. We term these class 2 proteins, and divide them into 3 subgroups, A-C (Fig. 1, Table 2 and 3). Classes 2A and 2B are defined by always being present but having a mutant phenotype that only show some overlap with the cadherin or integrin mutant phenotype. The difference between 2A and 2B is that 2A is partially required for all processes, but some processes only require a partial activity of the adhesion receptor so that no defect is observed, while 2B would only be functioning in some processes. These two classes are difficult to distinguish, but one prediction is that the mutant phenotype of class 2A components should resemble that caused by a mutation that uniformly reduces adhesion receptor activity, while this would not be expected for class 2B. Class 2C consists of proteins that are always present but do not share any phenotypes with mutants in the adhesion receptors.
The class 2 components highlight the issue of redundancy: we imagine that some of the components may show a weak phenotype when removed because another protein, similar in sequence and/or function, is able to substitute fully or partially. To date, it has been difficult to identify examples of this for some of the highly conserved proteins, e.g. p120catenin, vinculin and FAK, which lack strong phenotypes, and Rsu-1 and tensin, which only contribute to integrin adhesion in the wing. The recent discovery that Rsu-1 mutants become lethal in combination with a viable site-directed mutation in PINCH, which eliminates PINCH binding to ILK [41], shows that the powerful genetic approaches in Drosophila can identify these redundant functions.
Class 3 proteins are defined as being only associated with the receptor in some cell types or developmental stages. These are of particular interest, because they suggest that the integrin adhesion complex has specific requirements in different contexts. Finally, class 4 proteins are those that do not become concentrated at sites of adhesion, and therefore do not localise or copurify, but nonetheless are required for adhesion function; these are not discussed further here.
Integrin-associated class 3 proteins are tensin and Wech. Wech is present in muscles, but not in follicle cells, while the recruitment of tensin to follicle cell focal adhesions requires the switch from αPS1βPS to αPS2βPS, with the specificity unexpectedly mapping to the extracellular domain of αPS2 [12]. The mechanism is unknown, but our favorite model is that the αPS2βPS-ECM link is a stronger attachment, and the ability to apply a stronger force uncovers binding sites for tensin.
A number of new cadherin adhesome class 3 proteins have been described recently (Table 2). Mutations in the genes encoding these proteins revealed novel and unexpected functions of cadherins that could not be uncovered by studying core components, as their absence causes severe phenotypes that mask these more subtle defects. For example, the study of MyoIB demonstrated the involvement of E-cadherin in establishing left/right asymmetry of the organism [42]; and Schizo/Loner has revealed the inhibitory role of N-cadherin on myoblast fusion [7]. Class 3 proteins also coordinate cadherin function with other pathways. Nemo kinase physically connects E-cadherin with the planar cell polarity proteins Strabismus and Prickle, contributing to ommatidial rotation [43].
While the class 3 proteins provide a clear way to regulate adhesion in different cell types or developmental stages, what has also emerged recently is that even class 1 and 2 proteins, which are always present, may function in a variety of ways. Talin is a scaffolding protein that binds the integrin β subunit cytoplasmic domain and actin, activating integrins and providing a link between integrins and the cytoskeleton. The major actin-binding domain and the two integrin-binding sites of talin are each required differently for the different integrin functions during development [44,45]. This suggests that, although talin is generally required for integrin function, the different types of cell-ECM adhesions do not rely equally on the same domains of talin. The class 2B protein Zasp has at least 13 potential splice variants and some of these are specifically expressed in muscles [46], suggesting that the apparent ubiquitous expression is in fact the tissue specific expression of multiple proteins with distinct functions, making Zasp a set of class 3 proteins. This diversity fits with data showing that point mutations in the integrins themselves can result in tissue-specific defects [47,48]. These results emphasize that even though a protein may be present in an adhesion complex at all times, we should not assume that it is molecularly or functionally identical at all times.
Diversity in the function of class 1 core components has also emerged for cadherin adhesion, with recent work emphasising the importance of β-catenin phosphorylation. In the developing eye, p21-activated kinase Mbt (D-Pak2) phosphorylates β-catenin, which destabilizes its binding to E-cadherin and reduces cell-cell adhesion strength, allowing correct cell rearrangement and morphogenesis during retina development [49,50]. A reduction in this inhibition of adhesion by Mbt could explain the observed increase in binding affinity between E-cadherin and β-catenin in the embryonic epidermis as development progresses [24], as mbt mRNA gradually decreases during embryogenesis [51]. If this is the case, it would however suggest that the Mbt-dependent reduction of affinity is not critical, as null mbt mutants are viable and fertile with rough eyes [52]. Another example is Nemo kinase, which phosphorylates β-catenin in a subset of developing photoreceptors to promote its function in ommatidial rotation [43]. Finally, Abl tyrosine kinase promotes phosphorylation of β-catenin to regulate the asymmetry of cadherin adhesion site localization and the dynamics in the epidermal cells of gastrulating embryos linked to convergent extension [53].
Conclusions
Recent work on cell adhesion in Drosophila has expanded our appreciation of the complexities of the adhesion machinery and the many possible ways to regulate its function. This model organism provides numerous adhesion events in the development and homeostasis of the animal. Each event may provide a paradigm for a particular variety of adhesive mechanism. We anticipate that advances in genetic and imaging tools will aid the elucidation of these mechanisms and reveal the importance of such variety of mechanism.
Acknowledgements
Apologies to our colleagues whose work we were not able to include due to space limitations. We are supported by grants from the Wellcome Trust (086451) and the Human Frontier Science Program (RGP2/2008)
References
- 1.Harris TJC, Tepass U. Adherens junctions: from molecules to morphogenesis. Nature Reviews Molecular Cell Biology. 2010;11:502–514. doi: 10.1038/nrm2927. [DOI] [PubMed] [Google Scholar]
- 2.Meng W, Takeichi M. Adherens junction: molecular architecture and regulation. Cold Spring Harbor Perspectives in Biology. 2009;1:a002899. doi: 10.1101/cshperspect.a002899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Niessen CM, Leckband D, Yap AS. Tissue organization by cadherin adhesion molecules: dynamic molecular and cellular mechanisms of morphogenetic regulation. Physiological reviews. 2011;91:691–731. doi: 10.1152/physrev.00004.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barczyk M, Carracedo S, Gullberg D. Integrins. Cell Tissue Res. 2010;339:269–280. doi: 10.1007/s00441-009-0834-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hill E, Broadbent ID, Chothia C, Pettitt J. Cadherin superfamily proteins in Caenorhabditis elegans and Drosophila melanogaster. Journal of molecular biology. 2001;305:1011–1024. doi: 10.1006/jmbi.2000.4361. [DOI] [PubMed] [Google Scholar]
- 6.Narasimha M, Brown NH. Integrins and Associated Proteins in Drosophila Development. In: Danen EHJ, editor. Integrins and Development. vol 1. Landes Bioscience; 2005. pp. 89–113. [Google Scholar]
- 7.Dottermusch-Heidel C, Groth V, Beck L, Önel S-F. The Arf-GEF Schizo/Loner regulates N- cadherin to induce fusion competence of Drosophila myoblasts. Developmental biology. 2012 doi: 10.1016/j.ydbio.2012.04.031. Ahead of print. [DOI] [PubMed] [Google Scholar]; * This paper demonstrates that Arf-GEF Schizo/Loner binds N-cadherin in founder cells and fusion competent myoblasts in Drosophila embryo, where it negatively regulates N-cadherin by removing it from the membrane, thus creating a protein-free zone at the membrane to allow fusion. (Note: Schizo/Loner seems to be specifically expressed in myoblasts and midline glia)
- 8.Jezowska B, Fernández BG, Amândio AR, Duarte P, Mendes C, Brás-Pereira C, Janody F. A dual function of Drosophila capping protein on DE-cadherin maintains epithelial integrity and prevents JNK-mediated apoptosis. Developmental biology. 2011;360:143–159. doi: 10.1016/j.ydbio.2011.09.016. [DOI] [PubMed] [Google Scholar]
- 9.Urbano JM, Dominguez-Gimenez P, Estrada B, Martin-Bermudo MD. PS Integrins and Laminins: Key Regulators of Cell Migration during Drosophila Embryogenesis. PLoS ONE. 2011;6:e23893. doi: 10.1371/journal.pone.0023893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Han C, Wang D, Soba P, Zhu S, Lin X, Jan LY, Jan Y-N. Integrins Regulate Repulsion-Mediated Dendritic Patterning of Drosophila Sensory Neurons by Restricting Dendrites in a 2D Space. Neuron. 2012;73:64–78. doi: 10.1016/j.neuron.2011.10.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim ME, Shrestha BR, Blazeski R, Mason CA, Grueber WB. Integrins Establish Dendrite-Substrate Relationships that Promote Dendritic Self-Avoidance and Patterning in Drosophila Sensory Neurons. Neuron. 2012;73:79–91. doi: 10.1016/j.neuron.2011.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Delon I, Brown NH. The integrin adhesion complex changes its composition and function during morphogenesis of an epithelium. Journal of Cell Science. 2009;122:4363–4374. doi: 10.1242/jcs.055996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tanentzapf G, Smith C, McGlade J, Tepass U. Apical, lateral, and basal polarization cues contribute to the development of the follicular epithelium during Drosophila oogenesis. The Journal of Cell Biology. 2000;151:891–904. doi: 10.1083/jcb.151.4.891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Martin-Bermudo MD, Dunin-Borkowski OM, Brown NH. Specificity of PS integrin function during embryogenesis resides in the [alpha] subunit extracellular domain. EMBO J. 1997;16:4184–4193. doi: 10.1093/emboj/16.14.4184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Devenport D, Bunch TA, Bloor JW, Brower DL, Brown NH. Mutations in the Drosophila alphaPS2 integrin subunit uncover new features of adhesion site assembly. Developmental biology. 2007;308:294–308. doi: 10.1016/j.ydbio.2007.02.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shindo M, Wada H, Kaido M, Tateno M, Aigaki T, Tsuda L, Hayashi S. Dual function of Src in the maintenance of adherens junctions during tracheal epithelial morphogenesis. Development (Cambridge, England) 2008;135:1355–1364. doi: 10.1242/dev.015982. [DOI] [PubMed] [Google Scholar]
- 17.Bécam IE, Tanentzapf G, Lepesant J-A, Brown NH, Huynh J-R. Integrin-independent repression of cadherin transcription by talin during axis formation in Drosophila. Nature Cell Biology. 2005;7:510–516. doi: 10.1038/ncb1253. [DOI] [PubMed] [Google Scholar]
- 18.Oda H, Tsukita S, Takeichi M. Dynamic behavior of the cadherin-based cell-cell adhesion system during Drosophila gastrulation. Developmental biology. 1998;203:435–450. doi: 10.1006/dbio.1998.9047. [DOI] [PubMed] [Google Scholar]
- 19.Hayashi T, Carthew RW. Surface mechanics mediate pattern formation in the developing retina. Nature. 2004;431:647–652. doi: 10.1038/nature02952. [DOI] [PubMed] [Google Scholar]
- 20.Gemp IM, Carthew RW, Hilgenfeldt S. Cadherin-dependent cell morphology in an epithelium: constructing a quantitative dynamical model. PLoS computational biology. 2011;7:e1002115. doi: 10.1371/journal.pcbi.1002115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yonekura S, Ting C-Y, Neves G, Hung K, Hsu S-N, Chiba A, Chess A, Lee C-H. The variable transmembrane domain of Drosophila N-cadherin regulates adhesive activity. Molecular and Cellular Biology. 2006;26:6598–6608. doi: 10.1128/MCB.00241-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mateus AM, Martinez Arias A. Patterned Cell Adhesion Associated with Tissue Deformations during Dorsal Closure in Drosophila. PLoS ONE. 2011;6:e27159. doi: 10.1371/journal.pone.0027159. [DOI] [PMC free article] [PubMed] [Google Scholar]; * This paper argues that DE-cadherin displays a reproducible spatial pattern of extracellular domain conformations and adhesive strength along Drosophila embryonic epidermis during dorsal closure. Using live embryos, the authors demonstrate that some antibodies against DE-cadherin bind their epitopes only in the amnioserosa and dorsal most epidermal cells. Reducing the total level of E-cadherin restores binding of these antibodies throughout the rest of epidermis.
- 23.Gorfinkiel N, Arias AM. Requirements for adherens junction components in the interaction between epithelial tissues during dorsal closure in Drosophila. Journal of Cell Science. 2007;120:3289–3298. doi: 10.1242/jcs.010850. [DOI] [PubMed] [Google Scholar]
- 24.Huang J, Huang L, Chen Y-J, Austin E, Devor CE, Roegiers F, Hong Y. Differential regulation of adherens junction dynamics during apical-basal polarization. Journal of Cell Science. 2011;124:4001–4013. doi: 10.1242/jcs.086694. [DOI] [PMC free article] [PubMed] [Google Scholar]; ** This paper demonstrates that E-cadherin and β-catenin biosynthetic turnover decreases, while the binding affinity of β-catenin for DE-cadherin increases in epidermal cells during embryonic development.
- 25.Cavey M, Rauzi M, Lenne P-F, Lecuit T. A two-tiered mechanism for stabilization and immobilization of E-cadherin. Nature. 2008;453:751–756. doi: 10.1038/nature06953. [DOI] [PubMed] [Google Scholar]
- 26.Pirraglia C, Walters J, Myat MM. Pak1 control of E-cadherin endocytosis regulates salivary gland lumen size and shape. Development (Cambridge, England) 2010 doi: 10.1242/dev.048827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Levayer R, Pelissier-Monier A, Lecuit T. Spatial regulation of Dia and Myosin-II by RhoGEF2 controls initiation of E-cadherin endocytosis during epithelial morphogenesis. Nature Cell Biology. 2011;13:529–540. doi: 10.1038/ncb2224. [DOI] [PubMed] [Google Scholar]; * This study demonstrates that asymmetrically regulated clathrin- and dynamin-mediated endocytosis of DE-cadherin leads to reduced DE-cadherin accumulation at the junctions that are targeted for disassembly during germband elongation of the embryo, and is required for tissue extension and cell intercalation. In addition, the authors delineate the pathway that controls the initiation of DE-cadherin endocytosis. They show that asymmetrically distributed RhoGEF2 regulates the activity of the formin Diaphanous and Myosin II, which promote lateral clustering of DE-cadherin, and recruitment of AP2 and a clathrin coat.
- 28.Georgiou M, Marinari E, Burden J, Baum B. Cdc42, Par6, and aPKC regulate Arp2/3- mediated endocytosis to control local adherens junction stability. Current biology : CB. 2008;18:1631–1638. doi: 10.1016/j.cub.2008.09.029. [DOI] [PubMed] [Google Scholar]
- 29.Warner SJ, Longmore GD. Distinct functions for Rho1 in maintaining adherens junctions and apical tension in remodeling epithelia. The Journal of Cell Biology. 2009;185:1111–1125. doi: 10.1083/jcb.200901029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Harris KP, Tepass U. Cdc42 and Par proteins stabilize dynamic adherens junctions in the Drosophila neuroectoderm through regulation of apical endocytosis. The Journal of Cell Biology. 2008;183:1129–1143. doi: 10.1083/jcb.200807020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schotman H, Karhinen L, Rabouille C. Integrins mediate their unconventional, mechanical-stress-induced secretion via RhoA and PINCH in Drosophila. Journal of Cell Science. 2009;122:2662–2672. doi: 10.1242/jcs.039347. [DOI] [PubMed] [Google Scholar]
- 32.Yuan L, Fairchild MJ, Perkins AD, Tanentzapf G. Analysis of integrin turnover in fly myotendinous junctions. J Cell Sci. 2010;123:939–946. doi: 10.1242/jcs.063040. [DOI] [PubMed] [Google Scholar]; * Analysis of the dynamics of integrins and several associated proteins at muscle attachments gives insight into the mechanisms regulating integrin turnover and shows that adhesions stabilize over time. This study focusses on integrin turnover and suggests mechanisms regulating this process.
- 33.Ribeiro Is, Yuan L, Tanentzapf G, Dowling JJ, Kiger A. Phosphoinositide Regulation of Integrin Trafficking Required for Muscle Attachment and Maintenance. PLoS Genet. 2011;7:e1001295. doi: 10.1371/journal.pgen.1001295. [DOI] [PMC free article] [PubMed] [Google Scholar]; * Myotubularin, which is important for regulating the balance of phosphoinositides at the membrane, is required for integrin function and turnover. This study suggests a connection between membrane composition and integrin dynamics.
- 34.Zaidel-Bar R, Itzkovitz S, Ma’ayan A, Iyengar R, Geiger B. Functional atlas of the integrin adhesome. Nat Cell Biol. 2007;9:858–867. doi: 10.1038/ncb0807-858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tepass U. Genetic analysis of cadherin function in animal morphogenesis. Current Opinion in Cell Biology. 1999;11:540–548. doi: 10.1016/s0955-0674(99)00025-3. [DOI] [PubMed] [Google Scholar]
- 36.Sarpal R, Pellikka M, Patel RR, Hui FYW, Godt D, Tepass U. Mutational analysis supports a core role for Drosophila α-Catenin in adherens junction function. Journal of Cell Science. 2012;125:233–245. doi: 10.1242/jcs.096644. [DOI] [PubMed] [Google Scholar]; ** The analysis of α-catenin mutant flies reveals defects similar to that of DE-cadherin loss-of-function. The mutant phenotype can be rescued by expression of a DE-cadherin-α-catenin fusion protein, arguing against a DE-cadherin-independent function of α-catenin in the cytoplasm.
- 37.Myster SH, Cavallo R, Anderson CT, Fox DT, Peifer M. Drosophila p120catenin plays a supporting role in cell adhesion but is not an essential adherens junction component. The Journal of Cell Biology. 2003;160:433–449. doi: 10.1083/jcb.200211083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Humphries JD, Byron A, Bass MD, Craig SE, Pinney JW, Knight D, Humphries MJ. Proteomic analysis of integrin-associated complexes identifies RCC2 as a dual regulator of Rac1 and Arf6. Sci Signal. 2009;2:ra51. doi: 10.1126/scisignal.2000396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Delon I, Brown NH. Integrins and the actin cytoskeleton. Curr Opin Cell Biol. 2007;19:43–50. doi: 10.1016/j.ceb.2006.12.013. [DOI] [PubMed] [Google Scholar]
- 40.Zervas CG, Psarra E, Williams V, Solomon E, Vakaloglou KM, Brown NH. A central multifunctional role of integrin-linked kinase at muscle attachment sites. Journal of Cell Science. 2011;124:1316–1327. doi: 10.1242/jcs.081422. [DOI] [PMC free article] [PubMed] [Google Scholar]; ** A comprehensive analysis of many integrin-associated proteins and their function for building integrin adhesion complexes. This study helps understand how integrin-adhesion complexes are assembled.
- 41.Elias MC, Pronovost SM, Cahill KJ, Beckerle MC, Kadrmas JL. A critical role for Ras Suppressor-1 (RSU-1) revealed when PINCH-Integrin-linked Kinase (ILK) binding is disrupted. Journal of Cell Science. 2012 doi: 10.1242/jcs.101386. [DOI] [PMC free article] [PubMed] [Google Scholar]; * Rsu-1 is a non-essential protein but becomes essential for viability when ILK and PINCH cannot interact directly, suggesting that Rsu-1 is redundant with an essential function that requires the interaction between ILK and PINCH. This study shows how Drosophila genetics can help uncover redundant functions within the integrin adhesion complex.
- 42.Petzoldt AG, Coutelis J-B, Géminard C, Spéder P, Suzanne M, Cerezo D, Noselli S. DE-Cadherin regulates unconventional Myosin ID and Myosin IC in Drosophila left-right asymmetry establishment. Development (Cambridge, England) 2012;139:1874–1884. doi: 10.1242/dev.047589. [DOI] [PubMed] [Google Scholar]; * This paper shows that DE-cadherin forms a molecular complex with MyoID, which is expressed specifically in the abdominal segment 8 of Drosophila embryos, and is required for the activity of MyoID in establishing left/right asymmetry in the embryo. In addition, DE-cadherin inhibits the closely related MyoIC, which antagonizes MyoID by preventing its binding to β-catenin.
- 43.Mirkovic I, Gault WJ, Rahnama M, Jenny A, Gaengel K, Bessette D, Gottardi CJ, Verheyen EM, Mlodzik M. Nemo kinase phosphorylates β-catenin to promote ommatidial rotation and connects core PCP factors to E-cadherin-β-catenin. Nature Structural & Molecular Biology. 2011;18:665–672. doi: 10.1038/nsmb.2049. [DOI] [PMC free article] [PubMed] [Google Scholar]; * The authors demonstrate a molecular link between the DE-cadherin complex and planar cell polarity factors in photoreceptor cells of third instar larvae eye discs. They show that Nemo kinase co-localizes and physically binds β-catenin, Strabismus and Prickle. In addition, Nemo kinase phosphorylates β-catenin and DE-cadherin while promoting ommatidial rotation.
- 44.Franco-Cea A, Ellis SJ, Fairchild MJ, Yuan L, Cheung TYS, Tanentzapf G. Distinct developmental roles for direct and indirect talin-mediated linkage to actin. Developmental biology. 2010;345:64–77. doi: 10.1016/j.ydbio.2010.06.027. [DOI] [PubMed] [Google Scholar]
- 45.Ellis SJ, Pines M, Fairchild MJ, Tanentzapf G. In vivo functional analysis reveals specific roles for the integrin-binding sites of talin. Journal of Cell Science. 2011;124:1844–1856. doi: 10.1242/jcs.083337. [DOI] [PubMed] [Google Scholar]; ** The two integrin-binding sites of talin are required differently for various integrin functions. This study shows that integrin function relies on different domains of talin from one cellular context to the other.
- 46.Katzemich A, Long JY, Jani K, Lee BR, Sch√∂ck F. Muscle type-specific expression of Zasp52 isoforms in Drosophila. Gene Expression Patterns. 2011;11:484–490. doi: 10.1016/j.gep.2011.08.004. [DOI] [PubMed] [Google Scholar]; * Zasp splice variants can be expressed in a tissue-specific pattern. This study gives insight on the potential regulation of adhesion complexes by the expression of different protein isoforms.
- 47.Pines M, Fairchild MJ, Tanentzapf G. Distinct regulatory mechanisms control integrin adhesive processes during tissue morphogenesis. Dev Dyn. 2011;240:36–51. doi: 10.1002/dvdy.22488. [DOI] [PubMed] [Google Scholar]
- 48.Bloor JW, Brown NH. Genetic Analysis of the Drosophila alphaPS2 Integrin Subunit Reveals Discrete Adhesive, Morphogenetic and Sarcomeric Functions. Genetics. 1998;148:1127–1142. doi: 10.1093/genetics/148.3.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Menzel N, Schneeberger D, Raabe T. The Drosophila p21 activated kinase Mbt regulates the actin cytoskeleton and adherens junctions to control photoreceptor cell morphogenesis. Mechanisms of Development. 2007;124:78–90. doi: 10.1016/j.mod.2006.09.007. [DOI] [PubMed] [Google Scholar]
- 50.Menzel N, Melzer J, Waschke J, Lenz C, Wecklein H, Lochnit G, Drenckhahn D, Raabe T. The Drosophila p21-activated kinase Mbt modulates DE-cadherin-mediated cell adhesion by phosphorylation of Armadillo. The Biochemical journal. 2008;416:231–241. doi: 10.1042/BJ20080465. [DOI] [PubMed] [Google Scholar]
- 51.Graveley BR, Brooks AN, Carlson JW, Duff MO, Landolin JM, Yang L, Artieri CG, van Baren MJ, Boley N, Booth BW, et al. The developmental transcriptome of Drosophila melanogaster. Nature. 2011;471:473–479. doi: 10.1038/nature09715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Melzig J, Rein KH, Schäfer U, Pfister H, Jäckle H, Heisenberg M, Raabe T. A protein related to p21-activated kinase (PAK) that is involved in neurogenesis in the Drosophila adult central nervous system. Current biology : CB. 1998;8:1223–1226. doi: 10.1016/s0960-9822(07)00514-3. [DOI] [PubMed] [Google Scholar]
- 53.Tamada M, Farrell DL, Zallen JA. Abl Regulates Planar Polarized Junctional Dynamics through β-Catenin Tyrosine Phosphorylation. Developmental Cell. 2012;22:309–319. doi: 10.1016/j.devcel.2011.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]; * The authors demonstrate that asymmetry of β-catenin localization and dynamics is required for rosette formation and tissue extension during germ band elongation in the Drosophila embryo. The junctional accumulation of β-catenin is reduced, while its dynamics are increased at the junctions that are targeted for disassembly. Abl kinase is elevated at these junctions, and promotes phosphorylation of β-catenin, consequently establishing an asymmetry in its localization and dynamics.