Abstract
G-protein-coupled receptors (GPCR) are the largest superfamily of receptors responsible for signaling between cells and tissues, and because they play important physiological roles in homeostasis, they are major drug targets. New technologies have been developed for the identification of new ligands, new GPCR functions, and for drug discovery purposes. In particular, intercellular lipid mediators, such as, lysophosphatidic acid and sphingosine 1-phosphate have attracted much attention for drug discovery and this has resulted in the development of fingolimod (FTY-720) and AM095. The discovery of new intercellular lipid mediators and their GPCRs are discussed from the perspective of drug development. Lipid GPCRs for lysophospholipids, including lysophosphatidylserine, lysophosphatidylinositol, lysophosphatidylcholine, free fatty acids, fatty acid derivatives, and other lipid mediators are reviewed.
Keywords: Lipid mediator, GPCR, Lipid, Lysophospholipid, Fatty acid, Drug discovery
INTRODUCTION TO GPCRS AND NEW TECHNOLOGY
G-protein-coupled receptors
In humans and other multicellular organisms, communication systems connect cells and tissues. Endocrine, immune, and neuronal systems are representative communication methods (Im, 2002). Chemical messages, such as, hormones, autacoids, and neurotransmitters are released from cells and regulate target cells, which have receptors for first messengers. G-protein-coupled receptors (GPCR) are the largest superfamily of receptors for signaling molecules (ligands). Ligands may be amino acids, amine derivatives, peptides, proteins, lipid molecules, and even entities as small as Ca2+, the proton, and the photon (Im, 2002).
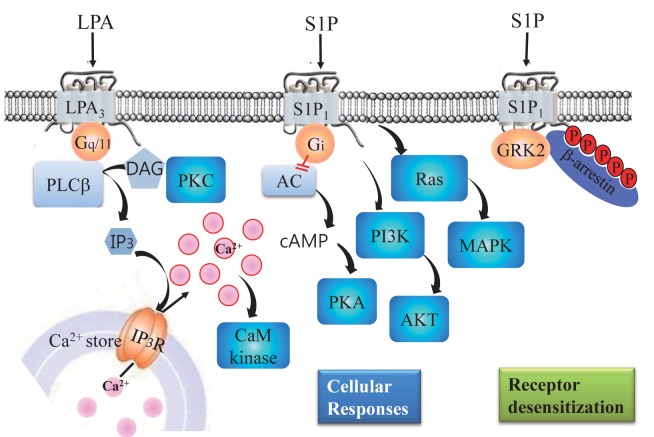
GPCRs have seven transmembrane α-helix domains (Im, 2002), and thus, are sometimes called seven transmembrane receptors (7TM receptors), because G-protein independent signaling have been found for GPCR-activated intracellular signaling (Rajagopal et al., 2010; Shukla et al., 2011). The binding and recognition of first messengers or ligands by GPCRs result in receptor conformational changes (Im, 2002), which lead to G protein activation and the subsequent modulation of effector molecules, such as, adenylyl cyclase, phospholipase C and D, and ion channels (Fig. 1) (Im, 2002). Accordingly, the levels of second messengers of cAMP, IP3, and Ca2+ are increased and/or decreased. Protein phosphorylation and dephosphorylation by kinases and phosphatases represent down-stream signaling cascades of second messengers. Furthermore, the phosphorylations of GPCRs by GRKs leads to the recruitment of β-arrestins, which results in desensitization, internalization (recycling), and G-protein-independent signaling (Rajagopal et al., 2010; Shukla et al., 2011). Representative signalings of GPCRs for lysophosphatidic acid (LPA) and sphingosine 1-phosphate (S1P) are illustrated in Fig. 1, although each GPCR may initiate unique signals in different cell types.
Fig. 1. Schematic diagram of lipid-mediated signal transduction pathways. For cellular responses, LPA activates LPA3, which leads to activation of phospholipase C (PLC). Activated PLC produces IP3 and diacylglycerol (DAG), DAG activates protein kinase C (PKC), and IP3 mobilizes Ca2+ from internal Ca2+ stores by activating IP3 receptors. The resulting increase in intracellular Ca2+ activates calmodulin-dependent protein kinases. Alternatively, S1P activates S1P1, which leads to inhibition of adenylyl cyclase and the activations of ras, MAPK, PI3K, and AKT via Gi proteins. For desensitization, S1P activates S1P1, which leads to GRK2 activation, S1P1 phosphorylation, and to the recruitment β-arrestins by S1P1. The recruited β-arrestins then inhibit S1P1-G protein coupling.
The identification of mammalian GPCR sequence β2- aderenergic receptor in 1986 opened a new era in pharmacology, the ‘receptor-hunting period’ (Dixon et al., 1986; Im, 2002). In 2012, Robert J. Lefkowitz and Brian Kobilka received the Nobel Prize in chemistry by their contribution to “studies of GPCRs”. Many receptor molecules for lipid mediators like platelet-activating factor, prostaglandins, leukotrienes, and cannabinoids, have been cloned and identified as GPCRs at the DNA level (Im, 2004). Sequencing of the human genome resulted in the identification of 865 human GPCR genes (Fredriksson et al., 2003), and 367 GPCRs, excluding olfactory receptors, are considered functional receptors (Vassilatis et al., 2003). Based on data supplied by the International Union of Basic and Clinical Pharmacology Committee on receptor nomenclature and drug classifi cation (NC-IUPHAR), 354 GPCRs are now recognized as non-chemosensory GPCRs (Sharman et al., 2011; Davenport et al., 2013). Of these 92 are still classified as ‘orphan receptors’, because their natural ligands have not been identified (Sharman et al., 2011; Davenport et al., 2013; Southern et al., 2013). GPCRs for intercellular lipid mediators have been identified and their numbers continue to increase along with the discoveries of new lipid mediators (Im, 2004, 2009).
New technologies for GPCR drug discovery
Because GPCRs play important physiological roles in homeostasis, they are major drug targets for drug discovery. About 40% of drugs on the market act on GPCRs as agonists or antagonists (Howard et al., 2001). Drug discovery based on GPCRs is an attractive project for pharmaceutical companies and academic scientists alike. However, technical problems must be overcome (Im, 2002). The traditional techniques of radioligand binding and GTPγS binding have been used along with measurement of second messengers like cAMP, IP3, and Ca2+ and the protein phosphorylation of MAPKs (Yoshida et al., 2012; Zhang and Xie, 2012). When a ligand structure is known, radioligand binding is the best way to measuring affinities with candidate compounds, but information on whether a test compound is agonist or antagonist is not available and the radiomaterials are strictly regulated, which militate against this analysis method (Yin et al., 2009; Zhang and Xie, 2012; Southern et al., 2013).
A major obstacle to GPCR drug discovery is that each GPCR utilizes different sets of G proteins, such as, Gs, Gi, Gq, or G12. Therefore, to analyze, promiscuous Gα16, or chimeric G proteins like Gqi were popularly adapted for GPCR drug discovery, like Ca2+ measurements by FLIPR (Molecular Devices), by many companies (Kostenis, 2004). Another way of overcoming this hurdle is to screen for β-arrestin recruitment, which is a later response of GPCR than Ca2+ response (Southern et al., 2013). Many other high-throughput screening methods have been devised for GPCR activation analysis, such as, the use of frog melanophores as host cells (Lerner, 1994) or of artificial cell lines transfected with promoter/reporter genes, such as, luciferase or β-lactamase (Bresnick et al., 2003). Because recent excellent review articles are available (Yoshida et al., 2012; Zhang and Xie, 2012), a new technology named TGF-α shedding would be briefly introduced, which was applied for GPCR identification for a lipid mediator, lysophosphatidylserine (LPS) (Inoue et al., 2012).
TGF-α shedding is a new technique for GPCR ligand screening, signaling, agonism and antagonism (Inoue et al., 2012). It uses an engineered plasmid encoding alkaline phosphatase and a TGF-α domain (AP-TGF-α). For example, HEK293 cells, which express endogenous TACE (also known as ADAM-17), are transfected with a candidate or testing GPCR along with the engineered AP-TGF-α. When GPCR is activated, TACE will cut off AP domain from AP-TGF-α, and thus, measures of alkaline phosphatase activities in media (AP release) and in cells (remaining AP-TGF-α) gives information on whether the activation state and degree of activation of GPCR (Inoue et al., 2012). Of the 116 GPCRs tested, 104 were detected by the TGF-α-shedding assay, the highest percentage in a single assay format achieved for GPCR (Inoue et al., 2012).
INTERCELLULAR LIPID MEDIATORS AND GPCRS
Intercellular Lipid Mediators
Intercellular lipid mediators are defined as chemical transmitters possessing water-insoluble moieties (Im, 2004). Intercellular lipid mediators are hormone-like signaling molecules, but they have the structural and physicochemical characters of lipids, such as, firt messengers of prostaglandins and leukotrienes (Im, 2004). These mediators can be divided into two groups: one with receptors in the plasma membrane and the other with intracellular nuclear receptors, such as, corticosteroids (Im, 2004). Many intercellular lipid mediators act on GPCRs in the plasma membrane, such as, cannabinoids, platelet-activating factor, prostaglandins, leukotrienes, and lysophospholipids (Im, 2004; Choi and Chun, 2013)
Certain lipid mediators have two types of receptors, that is, GPCR and nuclear receptors (Im, 2004). Estrogen and bile acids are representative examples, because they act not only through their nuclear receptors (estrogen receptor (ER) and farnesoid X receptor (FXR)) but also through GPCRs, GPR30 (now renamed GPER) for estrogen and TGR5 (now renamed GPBA) for bile acids (Prossnitz and Barton, 2009). Recently, GPR30 was found to be localized in endoplasmic reticulum (Arterburn et al., 2009) and porcine GPR3 was observed in perinuclear membrane at the germinal vesicle stage of oocytes (Yang et al., 2012). On the other hand, constitutive active rat GPR6 was shown to be localized in intracellular compartments (Padmanabhan et al., 2009). Additionally, there is a growing evidence that GPCRs function in nuclear membranes (Zhu et al., 2006; Goetzl, 2007). Therefore, it is incorrect to say that GPCRs act only in the plasma membrane. GPCR signaling in intracellular organelles, such as, endosomes and the nucleus, remains an interesting topic for future research (Irannejad et al., 2013).
GPCRs for LPA and S1P
Since the first LPA receptor was identified in 1997, three Edg-subfamily members of LPA receptors and three purinergic (non-Edg) LPA receptors have been reported (Choi and Chun, 2013; Yanagida et al., 2013). GPR23 and two additional purinergic GPCRs, that is, GPR92 and P2Y5, were confirmed as LPA receptors and renamed LPA4, LPA5, and LPA6, respectively (Chun et al., 2010; Yanagida et al., 2013). Although GPR92/ LPA5 was reported to be a receptor for farnesyl pyrophosphate and N-arachidonylglycine (Oh et al., 2008), their affinity was found to be lower than LPA in a β-arrestin assay and in a aequorin assay (Yin et al., 2009). Peroxisome proliferation-activating receptor (PPARγ) has been reported to be a non-GPCR LPA receptor (McIntyre et al., 2003), but further validation is required (Chun et al., 2010). LPA1 antagonist AM095, which is a lead compound for the treatment of idiopathic pulmonary fibrosis, systemic sclerosis, and scleroderma, is currently undergoing under clinical trials (Castelino et al., 2011; Swaney et al., 2011).
In addition, to the six LPA receptors, three orphan GPCRs have been proposed to be LPA receptors. GPR87 was reported to be an LPA receptor (Tabata et al., 2007). P2Y10 has been reported to be a GPCR for S1P and LPA (Murakami et al., 2008). P2Y10 was recently proposed to be an LPS receptor (Inoue et al., 2012). GPR35 was also suggested to be an LPA receptor, especially for 2-arachidonyl LPA (Oka et al., 2010). However, a consensus was reached in the scientific community to exclude these putative LPA receptors from the current list of LPA receptors, pending future experimental validation (Chun et al., 2010).
Five members of the Edg-subfamily GPCRs (S1P1-5) have also been reported to be S1P receptors (Chun et al., 2010). Drug research on S1P receptor has been boosted by the finding of the S1P modulation of lymphocyte egress by FTY720 (Cyster and Schwab, 2012). FTY720 is an immune modulator and is phosphorylated in vivo. In the phosphorylated state, FTY720 acts as an agonist on four S1P receptors and as a functional antagonist on S1P1 (Im, 2003). FTY-720 (fingolimod, Gilenya™; Novartis) was approved as a first-line oral treatment for relapsing-remitting multiple sclerosis by the US FDA (Obinata and Hla, 2012). Recently, a potent and selective S1P1 antagonist, NIBR-0213, was shown to suppress autoimmune inflammation in an experimental autoimmune encephalomyelitis model in mice (Quancard et al., 2012).
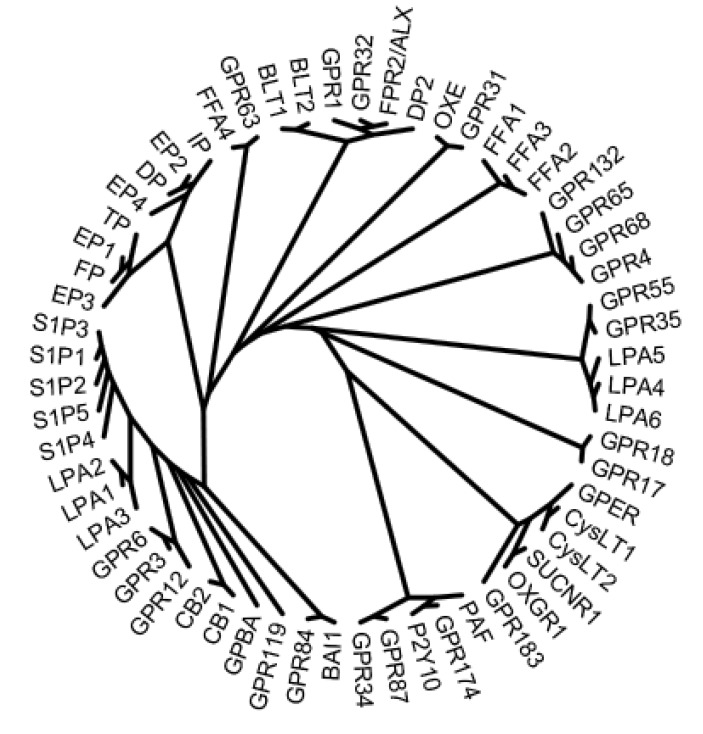
GPR3, GPR6, and GPR12 have been reported to be GPCRs for S1P and SPC. S1P was reported as a ligand for GPR3 and GPR6 and SPC for GPR12 (Im, 2004). These three GPCRs are phylogenetically similar to other GPCRs for lipid mediators, such as, LPA, S1P, and cannabinoids (Fig. 2) (Im, 2004), and were initially reported as adenylyl cyclase-activating orphan GPCRs (Eggerickx et al., 1995). The constitutive activation of adenylyl cyclase by GPCR was continuously observed in GPR3-, GPR6-, and GPR12-expressing cells, not only in over-expressing cells in vitro but also in cells in vivo (DiLuigi et al., 2008b; Padmanabhan et al., 2009; Zhang et al., 2012). Furthermore, some reports support the activation of GPR3 by S1P. More specifically, the constitutive activation of adenylyl cyclase by GPR3 and the further activation of adenylyl cyclase by S1P, resulted in the accumulation of cAMP (Hinckley et al., 2005; Zhang et al., 2012). In addition, S1P was observed to induce the internalization of GFP-tagged porcine GPR3 (Zhang et al., 2012), whereas SPC did not (Yang et al., 2012), and in the same study, SPC significantly delayed germinal vesicle breakdown in porcine oocytes. On the other hand, other did not observe any additional effect of S1P on the accumulation of cAMP, despite reproducing the increased production of cAMP in GPR3-expressing cells (Valverde et al., 2009). Furthermore, S1P was not found to be a GPCR ligand in a β-arrestin PathHunter™ assay (Yin et al., 2009; Southern et al., 2013). Therefore, NC-IUPHAR is currently classified them as constitutively active orphan GPCR (Davenport et al., 2013).
Fig. 2. Phylogenetic tree of lipid GPCRs. The phylogenetic tree was constructed using the Clustal Omega multiple sequence alignment and TreeIllustrator programs.
Nevertheless, there is growing evidence that GPR3 participates in the regulations of oocyte maturation and neurologic states. The constitutive activation of Gs proteins by GPR3 and its role in the prophase I meiotic arrest of oocytes have been shown in Xenopus, rodent, and in human oocytes and even in GPR3-knock-out mice (Freudzon et al., 2005; Hinckley et al., 2005; DiLuigi et al., 2008a). Additionally, GPR3 has been proposed to participate in neurite outgrowth (Tanaka et al., 2007), postnatal cerebellar development (Tanaka et al., 2009), emotional-like responses (Valverde et al., 2009), Alzheimer’s disease (Thathiah et al., 2009), the early phases of cocaine reinforcement (Tourino et al., 2012), and neuropathic pain therapy (Ruiz-Medina et al., 2011).
GPR63 was initially reported as a GPCR recognizing S1P and dioleoyl phosphatidic acid (Niedernberg et al., 2003), but not much progress has been made on this pairing. Like the suggestion based on phylogenetic bioinformatic analysis of GPCRs, lipid ligands might not be correct for GPR63, because sequentially related neighbor GPCRs form a large class of peptide receptors (Vassilatis et al., 2003; Im, 2004).
GPCRs for LPC and LPS
OGR1 (GPR68), GPR4, TDAG8 (GPR65), and G2A (GPR- 132), which compose a subfamily of GPCRs, were initially reported to be lipid receptors for sphingosylphosphorylcholine (SPC), lysophosphatidylcholine (LPC), and psychosine (Im, 2005; Tomura et al., 2005). Later, all four members were found to be proton-sensing GPCRs (Tomura et al., 2005), and original reports on OGR1, GPR4, and G2A were later retracted. Although NC-IUPHAR has classified all four as orphan GPCRs (Davenport et al., 2013), OGR1, GPR4, and TDAG8 continue to be reported as proton-sensing GPCRs in terms of the physiological and pathological relevances even in knockout mice (Okajima, 2013). On the other hand, G2A, a weak proton-sensing GPCR (Radu et al., 2005), has been studied in various contexts.
Firstly, 9-hydroxyoctadecadienoic acid (9-HODE) was reported to be a ligand for human G2A/GPR132, but not for mouse G2A (Obinata et al., 2005; Obinata and Izumi, 2009) and this was confirmed using the β-arrestin PathHunter™ assay (Yin et al., 2009). Additionally, oxidized free fatty acids, such as, 9(S)-HODE, 9-hydroperoxyoctadecadienoic acid, and 11-HEPE, have been reported to be potent agonists of G2A, but LPC, 13(S)-HODE, and lauric acid were found to leave G2A unaffected in the β-arrestin assay (Yin et al., 2009). Secondly, the regulatory functions of G2A have been studied in atherosclerosis, autoimmunity, and gallstone formation in G2A knockout mice (Johnson et al., 2008; Kabarowski, 2009). Thirdly, Brantton’s group suggested interesting roles for LPS in resolution of inflammation (Frasch and Bratton, 2012; Frasch et al., 2013). LPS is made in activated neutrophils and LPS in the plasma membrane of neutrophils regulates macrophage efferocytosis and phenotype changes to M2 in a G2A-dependent manner in macrophages, and this finding was supported using G2A knockout mice (Frasch et al., 2013). Previously, this group reported that LPC, LPS, and LPE mobilize neutrophil secretary vesicles and induce redundant signaling through G2A (Frasch et al., 2007). A similar LPC-induced surface redistribution of G2A was shown in a previous study (Wang et al., 2005). However, G2A involvement in lysophospholipid- induced Ca2+ signaling has been demonstrated only with antibody to G2A, such as, in experimental sepsis (Yan et al., 2004; Frasch et al., 2007).
GPR119 is largely restricted to pancreatic insulin-producing β cells and intestinal glucagon-like peptide-1-producing L-cells, and was first reported as a receptor for oleoyl-LPC, which enhances glucose-induced insulin secretion (Soga et al., 2005). The ranking order lysophospholipid was found to be 18:1-LPC, 16:0-LPC>18:0-LPC>LPE, and LPI in RH7777 rat hepatoma cells stably expressing human GPR119 (Soga et al., 2005). Later, N-oleoylethanolamide (OEA) was shown to activate GPR119 more potently than LPC (Overton et al., 2006). N-Oleoyldopamine was also reported as an agonist of GPR119 (Chu et al., 2010), and 2-oleoylglycerol was proposed as an endogenous agonist in food for GPR119 (Hansen et al., 2011). Therefore, the stimulatory effect of dietary fat on incretin secretion (GLP-1 from L cells) and insulin secretion (β cells) may be mediated through GPR119 via multiple derivatives of oleate (C18:1) (Davenport et al., 2013). Furthermore, the hypophagic effect (reduction of food intake) of GPR119 has made it a focus of anti-diabetic drug development (Overton et al., 2008). In fact, over a hundred papers have described the development of potent GPR119 agonist and preclinical and clinical data suggest that GPR119 agonists will be the next generation of compounds used to treat type 2 diabetes mellitus (Shah and Kowalski, 2010; Davenport et al., 2013).
LPS is an activator of mast cell degranulation, and has been reported to be a ligand for GPR34, which is highly expressed in mast cells (Sugo et al., 2006); however, this pairing is somewhat controversial (Iwashita et al., 2009; Liebscher et al., 2011; Ritscher et al., 2012). Kitamura et al. showed using several methods that GPR34 is a receptor of LPS with a fatty acid at the sn-2 position (Kitamura et al., 2012). Recently, additional members of GPCRs, such as, GPR174 and P2Y10, were putatively suggested using a TGF-α shedding assay (Inoue et al., 2012).
GPCRs for LPI
In addition to the classical cannabinoid receptors CB1 and CB2, cannabinoid GPCRs, have been implicated in studies on CB1-/- and CB2-/- knock-out mice and in studies using cannabinoid mimetic chemicals. Although phylogenetically distant from CB1 and CB2 receptors, several groups have reported that GPR55 is a cannabinoid receptor (Baker et al., 2006; Johns et al., 2007; Ryberg et al., 2007). However, Sugiura et al. were unable to reproduce this result and instead suggested lysophosphatidylinositol (LPI, 2-arachidonyl-sn-glycero- 3-phosphoinositol) as a ligand (Oka et al., 2007, 2009), whereas other groups suggested that GPR55 is atypical as a cannabinoid receptor and in terms of its signaling (Johns et al., 2007; Lauckner et al., 2008). In 2010, NC-IUPHAR decided not to include GPR55 as a cannabinoid receptor (Pertwee et al., 2010). However, the consensus is that LPI acts as an endogenous agonist on GPR55 (Pineiro and Falasca, 2012). In this context, Yamashita et al. proposed that phylogenetically neighboring GPR55 and GPR35 evolved to share ligand recognition properties, that is, GPR35 recognizes 2-arachidonyl LPA and GPR55 recognizes 2-arachidonyl LPI (Yamashita et al., 2013). GPR35 was initially reported to be a receptor for kynurenic acid, a metabolite of tryptophan (Wang et al., 2006a). Although kynurenic acid is able to activate GPR35, it has considerably lower potency for human GPR35 than rat GPR35 (Jenkins et al., 2011). Many surrogate ligands for GPR35, such as, zaprinast, cromolyn sodium, loop diuretics, and pamoic acid have been identified (Zhao and Abood, 2013). The importance of GPR35 in pain (spinal antinociception and inflammatory pain), heart disease, asthma, inflammatory bowel disease, and cancer has compelled scientists to find novel agonists and antagonists (Neetoo-Isseljee et al., 2013).
GPCRS FOR FREE FATTY ACIDS
GPR40, GPR43, GPR41, and GPR120 act as receptors for short chain (GPR41 and GPR43), medium long chain (GPR40), and unsaturated long chain fatty acids (GPR120) (Talukdar et al., 2011; Hara et al., 2013). IUPHAR renamed them FFA1 (GPR40), FFA2 (GPR43), FFA3 (GPR41), and FFA4 (GPR120) (Davenport et al., 2013). GPR84 has also been reported to act as a receptor for medium chain fatty acids of carbon chain lengths C9 to C14 (Wang et al., 2006b). However, it has not been nominated yet to be FFA5 (Davenport et al., 2013). Interestingly, GPR84-deficient mice showed regulation of early IL-4 gene expression in activated T cells (Venkataraman and Kuo, 2005). In a recent study, it was suggested that mediumchain fatty acids with a hydroxyl group at the 2- or 3-position are more efficacious than non-hydroxylated fatty acids, and identified 6-n-octylaminouracil as a surrogate agonist (Suzuki et al., 2013). Southern et al. confirmed that the medium chain fatty acids capric acid, undecanoic acid, and eicosatetraenoic acid evoke GPR84-mediated β-arrestin recruitment, cAMP, and calcium signaling (Southern et al., 2013). Therefore, it may only be a matter of time before it is renamed FFA5. The regulations of glucagon-like peptide-1 secretion from intestinal L cells and insulin from pancreatic β cells by free fatty acids via GPR120 and GPR40 are crucial considerations of the development of future treatments for diabetes. Because many review articles have been published on the topic of free fatty acids and their GPCRs, the reader is recommended to read (Talukdar et al., 2011; Hara et al., 2013).
CONSTITUTIVELY ACTIVE GPCRS, GPR17, GPR18, AND GPR183
GPR17 was initially reported to be a new dual uracil nucleotides/cysteinyl-leukotriens receptor (Ciana et al., 2006). Later, Maekawa et al. suggested that GPR17 is a ligand-independent, constitutive negative regulator of CysLT1 that suppresses CysLT1-mediated function at the cell membrane (Maekawa et al., 2009). Qi et al. recently confirmed this observation, that is, by lack of activation by UDP-glucose or CysLTs (Qi et al., 2013).
Another group reported the differential expressions of two isoforms of GPR17 in human brain (short) and heart and lung (long). Furthermore, although activation of GPR17 by uracil nucleotides (UDP, UDP-glucose, and UDP-galatose) was observed, but not by cysteinyl-leukotrienes (LTD4) (Benned- Jensen and Rosenkilde, 2010). Thus, the cognate ligands of GPR17 remain controversial (Davenport et al., 2013). Because GPR17 has been reported to be a regulator of many physiological and pathological processes, including brain injury, spinal cord injury, oligodendrocyte differentiation, and food intake (Ren et al., 2012; Coppi et al., 2013; Franke et al., 2013), it offers a good therapeutic target, especially for neurorepair after traumatic brain injury (Franke et al., 2013). Both short and long forms of GPR17 have been reported to be constitutively activated via Gi protein activation (Benned- Jensen and Rosenkilde, 2010), and this constitutive activity could have functional meaning, as it does for GPR3.
GPR18, an orphan GPCR in lymphoid cell lines, such as, those of the spleen and thymus, was suggested to be a receptor for N-arachidonylglycine (NAG) (Kohno et al., 2006). NAG is an endogenous metabolite of endocannabinoid anandamide (N-arachidonyl ethanolamine), and McHugh et al. found NAG and abnormal cannabidiol induced the cellular migration of BV-2 microglia, endogenously GPR18 expressing microglia, and exgenously GPR18-transfected HEK293 cells (McHugh et al., 2010). Furthermore, GPR18 was found to be the most abundantly overexpressed orphan GPCR in 40 metastatic melanomas (Qin et al., 2011). McHugh et al. reported that anandamide, ΔTHC, or NAG induced the migration of human endometrial HEC-1B cells, which express GPR18 (McHugh et al., 2012). However, the pairing of GPR18 with NAG was not reproduced in a β-arrestin PathHunter™ assay (Yin et al., 2009; Southern et al., 2013) or in GPR18-expressing neurons (Lu et al., 2013), and GPR18 was found to be constitutively active to inhibit the apoptosis (Qin et al., 2011).
EBI2 (also known as GPR183) was initially identified as one of nine up-regulated genes in Epstein-Barr virus (EBV)- infected Burkitt lymphoma cells and to show constitutive activity via Gi protein (Rosenkilde et al., 2006). Oxysterols (oxygenated cholesterol derivatives) have been shown to activate EBI2, and 7α,25-dihydroxycholesteol was the most potent (Hannedouche et al., 2011; Liu et al., 2011), which thereby, unexpectedly linked EBI2 (an orphan GPCR that controls Bcell migration) and the immunological effects of certain oxysterols, and also suggested that the EBI2-oxysterol signaling pathway play an important role in the innate and adaptive immune systems (Spann and Glass, 2013).
GPCRS FOR OTHER LIPID MEDIATORS
Brain-specific angiogenesis inhibitor-1 (BAI1), an adhesion- type GPCR with an extended extracellular region, has been reported to be a phosphatidylserine (PS) recognition receptor (Park et al., 2007). PS is known as a key “eat-me’ signal exposed on the outer leaflet of apoptotic cells, and BAI1 has been reported to function as an engulfment receptor for both the recognition and subsequent internalization of apoptotic cells (Park et al., 2007; Bratton and Henson, 2008). The roles of BAI1 in the non-opsonic phagocytosis of Gramnegative bacteria, the fusion of healthy myoblasts, synaptogenesis, and the inhibition of tumor growth and angiogenesis via proteolytically processed extracellular domains, such as, vasculostatin (Vstat120), have been investigated (Kaur et al., 2009; Cork and Van Meir, 2011; Das et al., 2011; Duman et al., 2013; Hochreiter-Hufford et al., 2013).
Bile acids are being increasingly appreciated as complex metabolic integrators and signaling factors (Thomas et al., 2008), although they have long been known to be essential in dietary lipid absorption and cholesterol catabolism (Watanabe et al., 2006). TGR5 (now renamed GPBA) is a receptor for bile acids (Maruyama et al., 2002; Kawamata et al., 2003), and thus, bile acids signal not only through nuclear hormone receptors, such as, farnesoid X receptor α (FXR-α), but also through GPBA. The administration of bile acids to mice increased energy expenditure in brown adipose tissue, preventing obesity and resistance to insulin due to GPBA activation (Watanabe et al., 2006). The targeted disruption of TGR5 in mice resulted in significant fat accumulation and body weight gain versus wild type mice when both were fed a high fat diet (Maruyama et al., 2006). In another study, high TGR5 expression in gall bladder was observed with a marked reduction in gallstone development in TGR5-/- mice on a lithogenic diet (Vassileva et al., 2006). The main indication for the development of TGR5 agonists is for the treatment of obesity, that is, to exploit the effect of the receptor on the regulating off energy expenditure (Fiorucci et al., 2009).
GPR30 (now renamed as GPER) responds to estrogen with rapid cellular signaling (Prossnitz and Barton, 2009). A GPR30 antagonist, G-15, was discovered by high throughput flow cytometry (HyperCyt®) with fluorescent estrogen ligands (both cell permeable and non-permeable), which also elegantly showed GPR30 expression in endoplasmic reticulum and not in the plasma membrane (Arterburn et al., 2009). GPERselective ligands and GPR30 knockout mice have allowed the elucidation of GPER functions in many cases, which suggests that estrogen-mediated physiological responses may be mediated by either the receptor or a combination of GPER and nuclear ER receptors (Prossnitz and Barton, 2009). Furthermore, the physiological roles of GPER have expanded from reproductive, endocrine, immune and cardiovascular systems to nervous systems, as exemplified by studies on anxiolysis (Prossnitz and Barton, 2011; Tian et al., 2013).
Serhan et al. discovered resolvin E1, resolvin D1, protectin, and maresin, which are all derivatives of omega-3 fatty acids, such as, DHA and EPA, and found they were anti-inflammatory and pro-resolving lipid mediators like lipoxin A4, a pro-resolving mediator derived from arachidonic acid that plays important roles in the resolution of inflammation (Serhan et al., 2002; Serhan et al., 2008). In addition, resolvin E1 was found to activate GPR1/ChemR23 and to inhibit BLT1. On the other hand, resolvin D1 and lipoxin A4 activated GPR32 and FPR2/ALX for their pro-resolving responses (Serhan et al., 2011). This topic has also been reviewed by experts (Serhan et al., 2011).
OXE, formerly known as TG1019, was reported to recognize eicosatetraenoic acids and polyunsaturated fatty acids, including 5-oxo-6E, 8Z, 11Z, 14Z-eicosatetraenoic acid (5-oxo-ETE) (Hosoi et al., 2002; Jones et al., 2003; Brink et al., 2004). 5-Oxo-ETE, the most potent agonist of OXE, is the most potent eosinophil chemotactic factor known (Hosoi et al., 2002; Jones et al., 2003). Because 5-oxo-ETE may be an important regulator of tissue infiltration and of the activations of eosinophils and neutrophils in diseases, such as, asthma, allergic rhinitis, arthritis, and psoriasis, OXE selective antagonists are currently under development as therapeutic agents for the treatment of asthma and other allergic diseases (Gore et al., 2013; Powell and Rokach, 2013).
Furthermore, 12-(S)-hydroxyeicosatetraenoic acid (12-SHETE), a 12-lipoxygenase metabolite of arachidonic acid has been suggested to be a high affinity ligand for GPR31, which is phylogenetically closest to OXE receptor (Guo et al., 2011; Davenport et al., 2013).
PERSPECTIVES OF INTERCELLULAR LIPID MEDIATORS AND THEIR GPCRS
Novel lipid mediators and GPCRs have been reviewed a number of times (Kostenis, 2004; Meyer zu Heringdorf and Jakobs, 2007; Grzelczyk and Gendaszewska-Darmach, 2013). In the present review, the statuses of GPCRs as intercellular lipid mediators are reviewed (Table 1). During the last decade, information on some GPCRs, such as, GPR3, GPR6, GPR12, and GPR63, in terms of ligand pairing has not progressed (Davenport et al., 2013). The status of GPR23 (now renamed LPA4) and the statuses of two additional members of purinergic LPA receptors, GPR92 (LPA5) and P2Y5 (LPA6) have been confirmed. Furthermore, GPR40, GPR43, and GPR41 are now confirmed GPCRs, and have been renamed free fatty acid receptors 1, 2, and 3 (FFA1, FFA2, and FFA3, respectively) (Davenport et al., 2013). In addition, GPR120 has been renamed fatty acid receptor 4 for long-chain, especially unsaturated fatty acids, like omega-3 DHA and EPA (FFA4) (Davenport et al., 2013). TG1019 has been confirmed as a receptor for 5-oxo-eicosatetraenoic acid (5-oxo-ETE) and renamed OXE receptor (Brink et al., 2003). Drug development targeting TGR5 (GPR131, now renamed GPBA) and GPR30 (now renamed GPER) is being actively pursued for the treatment of lipid and glucose disorders (Davenport et al., 2013). In addition, new GPCRs have been introduced, namely, GPR17, GPR18, GPR31, GPR32, GPR34, GPR35, GPR55, GPR84, GPR87, GPR119, GPR120, GPR174, GPR183, P2Y10, and BAI1 (Davenport et al., 2013).
Table 1.
Summary of recent GPCRs for intercellular lipid mediators
| GPCR | Suggested Ligand | IUPHAR name | Remark | Ref |
|---|---|---|---|---|
|
| ||||
| EDG2, EDG4, EDG7 | LPA | LPA1, LPA2, LPA3 | Chun et al., 2010 | |
| GPR23, GPR92, P2Y5 | LPA | LPA4, LPA5, LPA6 | Chun et al., 2010 | |
| EDG1, EDG5, EDG3, EDG6, EDG8 | S1P | S1P1, S1P2, S1P3, S1P4, S1P5 | Chun et al., 2010 | |
| GPR3, GPR6, GPR12 | S1P, SPC (?) | Constitutive activity | Ignatov et al., 2003; Uhlenbrock et al., 2002 | |
| GPR87 | LPA (?) | Tabata et al., 2007 | ||
| GPR35 | LPA | 2-arachidonyl LPA | Oka et al., 2010 | |
| GPR55 | LPI | 2-arachidonyl LPI | Oka et al., 2009 | |
| P2Y10 | S1P, LPA (?) LPS (?) | Murakami et al., 2008 Inoue et al., 2012 | ||
| GPR34 | LPS | 2-acyl LPS | Kitamura et al., 2012; Sugo et al., 2006 | |
| GPR174 | LPS (?) | Inoue et al., 2012 | ||
| GPR40, GPR43, GPR41, GPR120 | Free fatty acids | FFA1, FFA2, FFA3, FFA4 | Davenport et al., 2013 | |
| GPR84 | Free fatty acids | Hydroxy fatty acids | Suzuki et al., 2013; Wang et al., 2006b | |
| GPR119 | OEA, LPC | Fatty acid derivatives | Overton et al., 2006; Soga et al., 2005 | |
| G2A | LPS, LPC, H+, 9-HODE | Fatty acid derivatives (?) | Frasch et al., 2013; Obinata et al., 2005; Parks et al., 2005 | |
| GPR30 | Estrogen | GPER | Prossnitz and Barton, 2009 | |
| TGR5/BG37 | Bile acid | GPBA | Kawamata et al., 2003 | |
| TG1019 | 5-ox-ETE | OXE | oxo ETE | Grant et al., 2009 |
| GPR31 | 12-S-HETE | Hydroxy ETE | Guo et al., 2011 | |
| BAI1 | PS | Park et al., 2007 | ||
| GPR17 | CysLT (?) nucleotides (?) | Constitutive activity CysLTD4, UDP-glucose | Ciana et al., 2006; Maekawa et al., 2009; Qi et al., 2013 | |
| GPR18 | NAG (?) | Constitutive activity | Kohno et al., 2006 | |
| GPR183 | Oxysterols | 7α,25-dihydrocholesterol Constitutive activity | Hannedouche et al., 2011; Liu et al., 2011 | |
S1P: sphingosine 1-phosphate; SPC: sphingosylphosphorylcholine; LPA: lysophosphatidic acid; FFA: free fatty acid; OEA: oleoylethanolamide; 5-oxo-ETE: 5-oxo-6E,8Z,11Z,14Z-eicosatetraenoic acid; LPC: lysophosphatidylcholine; LPI: lysophosphatidylinositol; LPS: lysophosphatidylserine; PS: phosphatidylserine; NAG: N-arachidonylglycine; 9-HODE: 9-hydroxyoctadecadienoic acid; 12-S-HEPE: 12-(S)- hydroxyeicosatetraenoic acid.
GPR17, GPR18, and GPR183 have been reported to be constitutively active like GPR3, GPR6, and GPR12 (Eggerickx et al., 1995; Uhlenbrock et al., 2002; Rosenkilde et al., 2006; Benned-Jensen and Rosenkilde, 2010; Qin et al., 2011), which might be a cause of that ligand-GPCR pairings have been controversial. Care must be taken when ligand-GPCR pairing is studied in overexpressed systems (Im, 2004), especially, if GPCR overexpression leads to constitutive activity, because this can alter ligand behavior (Kenakin, 2001). The constitutive activities of GPCRs, such as, GPR3, has physiological meaning via the constitutive activity and regulation of GPCR expression in specialized tissue areas (Freudzon et al., 2005; Hinckley et al., 2005; Tanaka et al., 2007; Ruiz-Medina et al., 2011). Therefore, investigations of constitutively active GPCR expression in vivo using knockout mice should be undertaken in addition to in vitro studies on suggested ligands and GPCRoverexpressing cells.
In studies on GPR87 and P2Y10, GPCR-Gα16 fusion was used as a tool to search endogenous ligands (Tabata et al., 2007; Murakami et al., 2008). Without confirmation by other assay systems or by other laboratories, the original suggested pairing with LPA and S1P could not be supported (Chun et al., 2010; Davenport et al., 2013). Although BAI1 and GPR31 were respectively reported in a single paper, multiple assays were carefully undertaken (Park et al., 2007; Guo et al., 2011). The pairings of P2Y10 and GPR174 with LPS were found in TGF-α shedding assay (Inoue et al., 2012). Further studies are required to confirm the pairing results in the future.
Of the newly found lipid GPCRs, drug development targeting GPR119 is undoubtedly the most active field, because it is related to the treatment of diabetes (Shah and Kowalski, 2010; Ohishi and Yoshida, 2012). Fundamental studies on pathophysiologies of other GPCRs should provide bases for future GPCR drug development.
Acknowledgments
This work was supported by the Korean National Research Foundation funded by the Korean government (MSIP) (Grant no. 2009-0083538).
References
- 1.Arterburn J. B., Oprea T. I., Prossnitz E. R., Edwards B. S., Sklar L. A. Discovery of selective probes and antagonists for G-protein-coupled receptors FPR/FPRL1 and GPR30. Curr. Top. Med. Chem. (2009);9:1227–1236. doi: 10.2174/156802609789753608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baker D., Pryce G., Davies W. L., Hiley C. R. In silico patent searching reveals a new cannabinoid receptor. Trends Pharmacol. Sci. (2006);27:1–4. doi: 10.1016/j.tips.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 3.Benned-Jensen T., Rosenkilde M. M. Distinct expression and ligand-binding profiles of two constitutively active GPR17 splice variants. Br. J. Pharmacol. (2010);159:1092–1105. doi: 10.1111/j.1476-5381.2009.00633.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bratton D. L., Henson P. M. Apoptotic cell recognition: will the real phosphatidylserine receptor(s) please stand up? Curr. Biol. (2008);18:R76–79. doi: 10.1016/j.cub.2007.11.024. [DOI] [PubMed] [Google Scholar]
- 5.Bresnick J. N., Skynner H. A., Chapman K. L., Jack A. D., Zamiara E., Negulescu P., Beaumont K., Patel S., McAllister G. Identification of signal transduction pathways used by orphan g protein-coupled receptors. Assay Drug Dev. Technol. (2003);1:239–249. doi: 10.1089/15406580360545053. [DOI] [PubMed] [Google Scholar]
- 6.Brink C., Dahlen S. E., Drazen J., Evans J. F., Hay D. W., Nicosia S., Serhan C. N., Shimizu T., Yokomizo T. International Union of Pharmacology XXXVII. Nomenclature for leukotriene and lipoxin receptors. Pharmacol. Rev. (2003);55:195–227. doi: 10.1124/pr.55.1.8. [DOI] [PubMed] [Google Scholar]
- 7.Brink C., Dahlen S. E., Drazen J., Evans J. F., Hay D. W., Rovati G. E., Serhan C. N., Shimizu T., Yokomizo T. International Union of Pharmacology XLIV. Nomenclature for the oxoeicosanoid receptor. Pharmacol. Rev. (2004);56:149–157. doi: 10.1124/pr.56.1.4. [DOI] [PubMed] [Google Scholar]
- 8.Castelino F. V., Seiders J., Bain G., Brooks S. F., King C. D., Swaney J. S., Lorrain D. S., Chun J., Luster A. D., Tager A. M. Amelioration of dermal fibrosis by genetic deletion or pharmacologic antagonism of lysophosphatidic acid receptor 1 in a mouse model of scleroderma. Arthritis Rheum. (2011);63:1405–1415. doi: 10.1002/art.30262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Choi J. W., Chun J. Lysophospholipids and their receptors in the central nervous system. Biochim. Biophys. Acta. (2013);1831:20–32. doi: 10.1016/j.bbalip.2012.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chu Z. L., Carroll C., Chen R., Alfonso J., Gutierrez V., He H., Lucman A., Xing C., Sebring K., Zhou J., Wagner B., Unett D., Jones R. M., Behan D. P., Leonard J. N-oleoyldopamine enhances glucose homeostasis through the activation of GPR119. Mol. Endocrinol. (2010);24:161–170. doi: 10.1210/me.2009-0239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chun J., Hla T., Lynch K. R., Spiegel S., Moolenaar W. H. International Union of Basic and Clinical Pharmacology. LXXVIII. Lysophospholipid receptor nomenclature. Pharmacol. Rev. (2010);62:579–587. doi: 10.1124/pr.110.003111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ciana P., Fumagalli M., Trincavelli M. L., Verderio C., Rosa P., Lecca D., Ferrario S., Parravicini C., Capra V., Gelosa P., Guerrini U., Belcredito S., Cimino M., Sironi L., Tremoli E., Rovati G. E., Martini C., Abbracchio M. P. The orphan receptor GPR17 identified as a new dual uracil nucleotides/cysteinyl-leukotrienes receptor. EMBO J. (2006);25:4615–4627. doi: 10.1038/sj.emboj.7601341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Coppi E., Maraula G., Fumagalli M., Failli P., Cellai L., Bonfanti E., Mazzoni L., Coppini R., Abbracchio M. P., Pedata F., Pugliese A. M. UDP-glucose enhances outward K(+) currents necessary for cell differentiation and stimulates cell migration by activating the GPR17 receptor in oligodendrocyte precursors. Glia. (2013);61:1155–1171. doi: 10.1002/glia.22506. [DOI] [PubMed] [Google Scholar]
- 14.Cork S. M., Van Meir E. G. Emerging roles for the BAI1 protein family in the regulation of phagocytosis, synaptogenesis, neurovasculature, and tumor development. J. Mol. Med. (Berl) (2011);89:743–752. doi: 10.1007/s00109-011-0759-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cyster J. G., Schwab S. R. Sphingosine-1-phosphate and lymphocyte egress from lymphoid organs. Annu. Rev. Immunol. (2012);30:69–94. doi: 10.1146/annurev-immunol-020711-075011. [DOI] [PubMed] [Google Scholar]
- 16.Das S., Owen K. A., Ly K. T., Park D., Black S. G., Wilson J. M., Sifri C. D., Ravichandran K. S., Ernst P. B., Casanova J. E. Brain angiogenesis inhibitor 1 (BAI1) is a pattern recognition receptor that mediates macrophage binding and engulfment of Gram-negative bacteria. Proc. Natl. Acad Sci. U.S.A. (2011);108:2136–2141. doi: 10.1073/pnas.1014775108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Davenport A. P., Alexander S. P., Sharman J. L., Pawson A. J., Benson H. E., Monaghan A. E., Liew W. C., Mpamhanga C. P., Bonner T. I., Neubig R. R., Pin J. P., Spedding M., Harmar A. J. International Union of Basic and Clinical Pharmacology. LXXXVIII. G protein-coupled receptor list: recommendations for new pairings with cognate ligands. Pharmacol. Rev. (2013);65:967–986. doi: 10.1124/pr.112.007179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.DiLuigi A., Weitzman V. N., Pace M. C., Siano L. J., Maier D., Mehlmann L. M. Meiotic arrest in human oocytes is maintained by a Gs signaling pathway. Biol. Reprod. (2008a);78:667–672. doi: 10.1095/biolreprod.107.066019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.DiLuigi A. J., Maier D. B., Benadiva C. A. Ruptured ectopic pregnancy with contralateral adnexal torsion after spontaneous conception. Fertil. Steril. (2008b);90:e1–3. doi: 10.1016/j.fertnstert.2007.07.1333. [DOI] [PubMed] [Google Scholar]
- 20.Dixon R. A., Kobilka B. K., Strader D. J., Benovic J. L., Dohlman H. G., Frielle T., Bolanowski M. A., Bennett C. D., Rands E., Diehl R. E., et al. Cloning of the gene and cDNA for mammalian beta-adrenergic receptor and homology with rhodopsin. Nature. (1986);321:75–79. doi: 10.1038/321075a0. [DOI] [PubMed] [Google Scholar]
- 21.Duman J. G., Tzeng C. P., Tu Y. K., Munjal T., Schwechter B., Ho T. S., Tolias K. F. The adhesion-GPCR BAI1 regulates synaptogenesis by controlling the recruitment of the Par3/Tiam1 polarity complex to synaptic sites. J. Neurosci. (2013);33:6964–6978. doi: 10.1523/JNEUROSCI.3978-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Eggerickx D., Denef J. F., Labbe O., Hayashi Y., Refetoff S., Vassart G., Parmentier M., Libert F. Molecular cloning of an orphan G-protein-coupled receptor that constitutively activates adenylate cyclase. Biochem. J. (1995);309(Pt 3):837–843. doi: 10.1042/bj3090837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fiorucci S., Mencarelli A., Palladino G., Cipriani S. Bileacid- activated receptors: targeting TGR5 and farnesoid-X-receptor in lipid and glucose disorders. Trends Pharmacol. Sci. (2009);30:570–580. doi: 10.1016/j.tips.2009.08.001. [DOI] [PubMed] [Google Scholar]
- 24.Franke H., Parravicini C., Lecca D., Zanier E. R., Heine C., Bremicker K., Fumagalli M., Rosa P., Longhi L., Stocchetti N., De Simoni M. G., Weber M., Abbracchio M. P. Changes of the GPR17 receptor, a new target for neurorepair, in neurons and glial cells in patients with traumatic brain injury. Purinergic Signal. (2013);9:451–462. doi: 10.1007/s11302-013-9366-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Frasch S. C., Bratton D. L. Emerging roles for lysophosphatidylserine in resolution of inflammation. Prog. Lipid Res. (2012);51:199–207. doi: 10.1016/j.plipres.2012.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Frasch S. C., Fernandez-Boyanapalli R. F., Berry K. A., Murphy R. C., Leslie C. C., Nick J. A., Henson P. M., Bratton D. L. Neutrophils regulate tissue Neutrophilia in inflammation via the oxidant-modified lipid lysophosphatidylserine. J. Biol. Chem. (2013);288:4583–4593. doi: 10.1074/jbc.M112.438507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Frasch S. C., Zemski-Berry K., Murphy R. C., Borregaard N., Henson P. M., Bratton D. L. Lysophospholipids of different classes mobilize neutrophil secretory vesicles and induce redundant signaling through G2A. J. Immunol. (2007);178:6540–6548. doi: 10.4049/jimmunol.178.10.6540. [DOI] [PubMed] [Google Scholar]
- 28.Fredriksson R., Lagerstrom M. C., Lundin L. G., Schioth H. B. The G-protein-coupled receptors in the human genome form five main families. Phylogenetic analysis, paralogon groups, and fingerprints. Mol. Pharmacol. (2003);63:1256–1272. doi: 10.1124/mol.63.6.1256. [DOI] [PubMed] [Google Scholar]
- 29.Freudzon L., Norris R. P., Hand A. R., Tanaka S., Saeki Y., Jones T. L., Rasenick M. M., Berlot C. H., Mehlmann L. M., Jaffe L. A. Regulation of meiotic prophase arrest in mouse oocytes by GPR3, a constitutive activator of the Gs G protein. J. Cell Biol. (2005);171:255–265. doi: 10.1083/jcb.200506194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Goetzl E. J. Diverse pathways for nuclear signaling by G protein- coupled receptors and their ligands. FASEB J. (2007);21:638–642. doi: 10.1096/fj.06-6624hyp. [DOI] [PubMed] [Google Scholar]
- 31.Gore V., Patel P., Chang C. T., Sivendran S., Kang N., Ouedraogo Y. P., Gravel S., Powell W. S., Rokach J. 5-Oxo-ETE receptor antagonists. J. Med. Chem. (2013);56:3725–3732. doi: 10.1021/jm400480j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Grant G. E., Rokach J., Powell W. S. 5-Oxo-ETE and the OXE receptor. Prostaglandins Other Lipid Mediat. (2009);89:98–104. doi: 10.1016/j.prostaglandins.2009.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Grzelczyk A., Gendaszewska-Darmach E. Novel bioactive glycerol-based lysophospholipids: new data -- new insight into their function. Biochimie. (2013);95:667–679. doi: 10.1016/j.biochi.2012.10.009. [DOI] [PubMed] [Google Scholar]
- 34.Guo Y., Zhang W., Giroux C., Cai Y., Ekambaram P., Dilly A. K., Hsu A., Zhou S., Maddipati K. R., Liu J., Joshi S., Tucker S. C., Lee M. J., Honn K. V. Identification of the orphan G protein- coupled receptor GPR31 as a receptor for 12-(S)-hydroxyeicosatetraenoic acid. J. Biol. Chem. (2011);286:33832–33840. doi: 10.1074/jbc.M110.216564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hannedouche S., Zhang J., Yi T., Shen W., Nguyen D., Pereira J. P., Guerini D., Baumgarten B. U., Roggo S., Wen B., Knochenmuss R., Noel S., Gessier F., Kelly L. M., Vanek M., Laurent S., Preuss I., Miault C., Christen I., Karuna R., Li W., Koo D. I., Suply T., Schmedt C., Peters E. C., Falchetto R., Katopodis A., Spanka C., Roy M. O., Detheux M., Chen Y. A., Schultz P. G., Cho C. Y., Seuwen K., Cyster J. G., Sailer A. W. Oxysterols direct immune cell migration via EBI2. Nature. (2011);475:524–527. doi: 10.1038/nature10280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hansen K. B., Rosenkilde M. M., Knop F. K., Wellner N., Diep T. A., Rehfeld J. F., Andersen U. B., Holst J. J., Hansen H. S. 2-Oleoyl glycerol is a GPR119 agonist and signals GLP-1 release in humans. J. Clin. Endocrinol. Metab. (2011);96:E1409–1417. doi: 10.1210/jc.2011-0647. [DOI] [PubMed] [Google Scholar]
- 37.Hara T., Kimura I., Inoue D., Ichimura A., Hirasawa A. Free fatty acid receptors and their role in regulation of energy metabolism. Rev. Physiol. Biochem. Pharmacol. (2013);164:77–116. doi: 10.1007/112_2013_13. [DOI] [PubMed] [Google Scholar]
- 38.Hinckley M., Vaccari S., Horner K., Chen R., Conti M. The G-protein-coupled receptors GPR3 and GPR12 are involved in cAMP signaling and maintenance of meiotic arrest in rodent oocytes. Dev. Biol. (2005);287:249–261. doi: 10.1016/j.ydbio.2005.08.019. [DOI] [PubMed] [Google Scholar]
- 39.Hochreiter-Hufford A. E., Lee C. S., Kinchen J. M., Sokolowski J. D., Arandjelovic S., Call J. A., Klibanov A. L., Yan Z., Mandell J. W., Ravichandran K. S. Phosphatidylserine receptor BAI1 and apoptotic cells as new promoters of myoblast fusion. Nature. (2013);497:263–267. doi: 10.1038/nature12135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hosoi T., Koguchi Y., Sugikawa E., Chikada A., Ogawa K., Tsuda N., Suto N., Tsunoda S., Taniguchi T., Ohnuki T. Identification of a novel human eicosanoid receptor coupled to G(i/o) J. Biol. Chem. (2002);277:31459–31465. doi: 10.1074/jbc.M203194200. [DOI] [PubMed] [Google Scholar]
- 41.Howard A. D., McAllister G., Feighner S. D., Liu Q., Nargund R. P., Van der Ploeg L. H., Patchett A. A. Orphan G-proteincoupled receptors and natural ligand discovery. Trends Pharmacol. Sci. (2001);22:132–140. doi: 10.1016/S0165-6147(00)01636-9. [DOI] [PubMed] [Google Scholar]
- 42.Ignatov A., Lintzel J., Hermans-Borgmeyer I., Kreienkamp H. J., Joost P., Thomsen S., Methner A., Schaller H. C. Role of the G-protein-coupled receptor GPR12 as high-affinity receptor for sphingosylphosphorylcholine and its expression and function in brain development. J. Neurosci. (2003);23:907–914. doi: 10.1523/JNEUROSCI.23-03-00907.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Im D. S. Orphan G protein-coupled receptors and beyond. Jpn. J. Pharmacol. (2002);90:101–106. doi: 10.1254/jjp.90.101. [DOI] [PubMed] [Google Scholar]
- 44.Im D. S. Linking Chinese medicine and G-protein-coupled receptors. Trends Pharmacol. Sci. (2003);24:2–4. doi: 10.1016/S0165-6147(02)00012-3. [DOI] [PubMed] [Google Scholar]
- 45.Im D. S. Discovery of new G protein-coupled receptors for lipid mediators. J. Lipid Res. (2004);45:410–418. doi: 10.1194/jlr.R300006-JLR200. [DOI] [PubMed] [Google Scholar]
- 46.Im D. S. Two ligands for a GPCR, proton vs lysolipid. Acta Pharmacol. Sin. (2005);26:1435–1441. doi: 10.1111/j.1745-7254.2005.00237.x. [DOI] [PubMed] [Google Scholar]
- 47.Im D. S. New intercellular lipid mediators and their GPCRs: an update. Prostaglandins Other Lipid Mediat. (2009);89:53–56. doi: 10.1016/j.prostaglandins.2009.01.002. [DOI] [PubMed] [Google Scholar]
- 48.Inoue A., Ishiguro J., Kitamura H., Arima N., Okutani M., Shuto A., Higashiyama S., Ohwada T., Arai H., Makide K., Aoki J. TGFalpha shedding assay: an accurate and versatile method for detecting GPCR activation. Nat. Methods. (2012);9:1021–1029. doi: 10.1038/nmeth.2172. [DOI] [PubMed] [Google Scholar]
- 49.Irannejad R., Tomshine J. C., Tomshine J. R., Chevalier M., Mahoney J. P., Steyaert J., Rasmussen S. G., Sunahara R. K., El-Samad H., Huang B., von Zastrow M. Conformational biosensors reveal GPCR signalling from endosomes. Nature. (2013);495:534–538. doi: 10.1038/nature12000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Iwashita M., Makide K., Nonomura T., Misumi Y., Otani Y., Ishida M., Taguchi R., Tsujimoto M., Aoki J., Arai H., Ohwada T. Synthesis and evaluation of lysophosphatidylserine analogues as inducers of mast cell degranulation. Potent activities of lysophosphatidylthreonine and its 2-deoxy derivative. J. Med. Chem. (2009);52:5837–5863. doi: 10.1021/jm900598m. [DOI] [PubMed] [Google Scholar]
- 51.Jenkins L., Alvarez-Curto E., Campbell K., de Munnik S., Canals M., Schlyer S., Milligan G. Agonist activation of the G protein-coupled receptor GPR35 involves transmembrane domain III and is transduced via Galpha(1)(3) and beta-arrestin-2. Br. J. Pharmacol. (2011);162:733–748. doi: 10.1111/j.1476-5381.2010.01082.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Johns D. G., Behm D. J., Walker D. J., Ao Z., Shapland E. M., Daniels D. A., Riddick M., Dowell S., Staton P. C., Green P., Shabon U., Bao W., Aiyar N., Yue T. L., Brown A. J., Morrison A. D., Douglas S. A. The novel endocannabinoid receptor GPR55 is activated by atypical cannabinoids but does not mediate their vasodilator effects. Br. J. Pharmacol. (2007);152:825–831. doi: 10.1038/sj.bjp.0707419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Johnson L. E., Elias M. S., Bolick D. T., Skaflen M. D., Green R. M., Hedrick C. C. The G protein-coupled receptor G2A: involvement in hepatic lipid metabolism and gallstone formation in mice. Hepatology. (2008);48:1138–1148. doi: 10.1002/hep.22433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jones C. E., Holden S., Tenaillon L., Bhatia U., Seuwen K., Tranter P., Turner J., Kettle R., Bouhelal R., Charlton S., Nirmala N. R., Jarai G., Finan P. Expression and characterization of a 5-oxo-6E,8Z,11Z,14Z-eicosatetraenoic acid receptor highly expressed on human eosinophils and neutrophils. Mol. Pharmacol. (2003);63:471–477. doi: 10.1124/mol.63.3.471. [DOI] [PubMed] [Google Scholar]
- 55.Kabarowski J. H. G2A and LPC: regulatory functions in immunity. Prostaglandins Other Lipid Mediat. (2009);89:73–81. doi: 10.1016/j.prostaglandins.2009.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kaur B., Cork S. M., Sandberg E. M., Devi N. S., Zhang Z., Klenotic P. A., Febbraio M., Shim H., Mao H., Tucker-Burden C., Silverstein R. L., Brat D. J., Olson J. J., Van Meir E. G. Vasculostatin inhibits intracranial glioma growth and negatively regulates in vivo angiogenesis through a CD36-dependent mechanism. Cancer Res. (2009);69:1212–1220. doi: 10.1158/0008-5472.CAN-08-1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kawamata Y., Fujii R., Hosoya M., Harada M., Yoshida H., Miwa M., Fukusumi S., Habata Y., Itoh T., Shintani Y., Hinuma S., Fujisawa Y., Fujino M. A G protein-coupled receptor responsive to bile acids. J. Biol. Chem. (2003);278:9435–9440. doi: 10.1074/jbc.M209706200. [DOI] [PubMed] [Google Scholar]
- 58.Kenakin T. P. Quantitation in receptor pharmacology. Receptors Channels. (2001);7:371–385. [PubMed] [Google Scholar]
- 59.Kitamura H., Makide K., Shuto A., Ikubo M., Inoue A., Suzuki K., Sato Y., Nakamura S., Otani Y., Ohwada T., Aoki J. GPR34 is a receptor for lysophosphatidylserine with a fatty acid at the sn-2 position. J. Biochem. (2012);151:511–518. doi: 10.1093/jb/mvs011. [DOI] [PubMed] [Google Scholar]
- 60.Kohno M., Hasegawa H., Inoue A., Muraoka M., Miyazaki T., Oka K., Yasukawa M. Identification of N-arachidonylglycine as the endogenous ligand for orphan G-protein-coupled receptor GPR18. Biochem. Biophys. Res. Commun. (2006);347:827–832. doi: 10.1016/j.bbrc.2006.06.175. [DOI] [PubMed] [Google Scholar]
- 61.Kostenis E. A glance at G-protein-coupled receptors for lipid mediators: a growing receptor family with remarkably diverse ligands. Pharmacol. Ther. (2004);102:243–257. doi: 10.1016/j.pharmthera.2004.04.005. [DOI] [PubMed] [Google Scholar]
- 62.Lauckner J. E., Jensen J. B., Chen H. Y., Lu H. C., Hille B., Mackie K. GPR55 is a cannabinoid receptor that increases intracellular calcium and inhibits M current. Proc. Natl. Acad Sci. U.S.A. (2008);105:2699–2704. doi: 10.1073/pnas.0711278105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lerner M. R. Tools for investigating functional interactions between ligands and G-protein-coupled receptors. Trends Neurosci. (1994);17:142–146. doi: 10.1016/0166-2236(94)90087-6. [DOI] [PubMed] [Google Scholar]
- 64.Liebscher I., Muller U., Teupser D., Engemaier E., Engel K. M., Ritscher L., Thor D., Sangkuhl K., Ricken A., Wurm A., Piehler D., Schmutzler S., Fuhrmann H., Albert F. W., Reichenbach A., Thiery J., Schoneberg T., Schulz A. Altered immune response in mice deficient for the G protein-coupled receptor GPR34. J. Biol. Chem. (2011);286:2101–2110. doi: 10.1074/jbc.M110.196659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Liu C., Yang X. V., Wu J., Kuei C., Mani N. S., Zhang L., Yu J., Sutton S. W., Qin N., Banie H., Karlsson L., Sun S., Lovenberg T. W. Oxysterols direct B-cell migration through EBI2. Nature. (2011);475:519–523. doi: 10.1038/nature10226. [DOI] [PubMed] [Google Scholar]
- 66.Lu V. B., Puhl H. L., 3rd, Ikeda S. R. N-Arachidonyl glycine does not activate G protein-coupled receptor 18 signaling via canonical pathways. Mol. Pharmacol. (2013);83:267–282. doi: 10.1124/mol.112.081182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Maekawa A., Balestrieri B., Austen K. F., Kanaoka Y. GPR17 is a negative regulator of the cysteinyl leukotriene 1 receptor response to leukotriene D4. Proc. Natl. Acad Sci. U.S.A. (2009);106:11685–11690. doi: 10.1073/pnas.0905364106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Maruyama T., Miyamoto Y., Nakamura T., Tamai Y., Okada H., Sugiyama E., Itadani H., Tanaka K. Identification of membrane-type receptor for bile acids (M-BAR) Biochem. Biochem. Biophys. Res. Commun. (2002);298:714–719. doi: 10.1016/S0006-291X(02)02550-0. [DOI] [PubMed] [Google Scholar]
- 69.Maruyama T., Tanaka K., Suzuki J., Miyoshi H., Harada N., Nakamura T., Miyamoto Y., Kanatani A., Tamai Y. Targeted disruption of G protein-coupled bile acid receptor 1 (Gpbar1/M-Bar) in mice. J. Endocrinol. (2006);191:197–205. doi: 10.1677/joe.1.06546. [DOI] [PubMed] [Google Scholar]
- 70.McHugh D., Hu S. S., Rimmerman N., Juknat A., Vogel Z., Walker J. M., Bradshaw H. B. N-arachidonoyl glycine, an abundant endogenous lipid, potently drives directed cellular migration through GPR18, the putative abnormal cannabidiol receptor. BMC Neurosci. (2010);11:44. doi: 10.1186/1471-2202-11-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.McHugh D., Page J., Dunn E., Bradshaw H. B. Delta(9) -Tetrahydrocannabinol and N-arachidonyl glycine are full agonists at GPR18 receptors and induce migration in human endometrial HEC-1B cells. Br. J. Pharmacol. (2012);165:2414–2424. doi: 10.1111/j.1476-5381.2011.01497.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.McIntyre T. M., Pontsler A. V., Silva A. R., St Hilaire A., Xu Y., Hinshaw J. C., Zimmerman G. A., Hama K., Aoki J., Arai H., Prestwich G. D. Identification of an intracellular receptor for lysophosphatidic acid (LPA): LPA is a transcellular PPARgamma agonist. Proc. Natl. Acad Sci. U.S.A. (2003);100:131–136. doi: 10.1073/pnas.0135855100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Meyer zu Heringdorf D., Jakobs K. H. Lysophospholipid receptors: signalling, pharmacology and regulation by lysophospholipid metabolism. Biochim. Biophys. Acta. (2007);1768:923–940. doi: 10.1016/j.bbamem.2006.09.026. [DOI] [PubMed] [Google Scholar]
- 74.Murakami M., Shiraishi A., Tabata K., Fujita N. Identification of the orphan GPCR, P2Y(10) receptor as the sphingosine-1- phosphate and lysophosphatidic acid receptor. Biochem. Biophys. Res. Commun. (2008);371:707–712. doi: 10.1016/j.bbrc.2008.04.145. [DOI] [PubMed] [Google Scholar]
- 75.Neetoo-Isseljee Z., MacKenzie A. E., Southern C., Jerman J., Mc-Iver E. G., Harries N., Taylor D. L., Milligan G. Highthroughput identification and characterization of novel, speciesselective GPR35 agonists. J. Pharmacol. Exp. Ther. (2013);344:568–578. doi: 10.1124/jpet.112.201798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Niedernberg A., Tunaru S., Blaukat A., Ardati A., Kostenis E. Sphingosine 1-phosphate and dioleoylphosphatidic acid are low affinity agonists for the orphan receptor GPR63. Cell. Signal. (2003);15:435–446. doi: 10.1016/S0898-6568(02)00119-5. [DOI] [PubMed] [Google Scholar]
- 77.Obinata H., Hattori T., Nakane S., Tatei K., Izumi T. Identification of 9-hydroxyoctadecadienoic acid and other oxidized free fatty acids as ligands of the G protein-coupled receptor G2A. J. Biol. Chem. (2005);280:40676–40683. doi: 10.1074/jbc.M507787200. [DOI] [PubMed] [Google Scholar]
- 78.Obinata H., Hla T. Fine-tuning S1P therapeutics. Chem. Biol. (2012);19:1080–1082. doi: 10.1016/j.chembiol.2012.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Obinata H., Izumi T. G2A as a receptor for oxidized free fatty acids. Prostaglandins Other Lipid Mediat. (2009);89:66–72. doi: 10.1016/j.prostaglandins.2008.11.002. [DOI] [PubMed] [Google Scholar]
- 80.Oh D. Y., Yoon J. M., Moon M. J., Hwang J. I., Choe H., Lee J. Y., Kim J. I., Kim S., Rhim H., O'Dell D. K., Walker J. M., Na H. S., Lee M. G., Kwon H. B., Kim K., Seong J. Y. Identification of farnesyl pyrophosphate and N-arachidonylglycine as endogenous ligands for GPR92. J. Biol. Chem. (2008);283:21054–21064. doi: 10.1074/jbc.M708908200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ohishi T., Yoshida S. The therapeutic potential of GPR119 agonists for type 2 diabetes. Expert Opin. Investig. Drugs. (2012);21:321–328. doi: 10.1517/13543784.2012.657797. [DOI] [PubMed] [Google Scholar]
- 82.Oka S., Nakajima K., Yamashita A., Kishimoto S., Sugiura T. Identification of GPR55 as a lysophosphatidylinositol receptor. Biochem. Biophys. Res. Commun. (2007);362:928–934. doi: 10.1016/j.bbrc.2007.08.078. [DOI] [PubMed] [Google Scholar]
- 83.Oka S., Ota R., Shima M., Yamashita A., Sugiura T. GPR35 is a novel lysophosphatidic acid receptor. Biochem. Biophys. Res. Commun. (2010);395:232–237. doi: 10.1016/j.bbrc.2010.03.169. [DOI] [PubMed] [Google Scholar]
- 84.Oka S., Toshida T., Maruyama K., Nakajima K., Yamashita A., Sugiura T. 2-Arachidonoyl-sn-glycero-3-phosphoinositol: a possible natural ligand for GPR55. J. Biochem. (2009);145:13–20. doi: 10.1093/jb/mvn136. [DOI] [PubMed] [Google Scholar]
- 85.Okajima F. Regulation of inflammation by extracellular acidification and proton-sensing GPCRs. Cell. Signal. (2013);25:2263–2271. doi: 10.1016/j.cellsig.2013.07.022. [DOI] [PubMed] [Google Scholar]
- 86.Overton H. A., Babbs A. J., Doel S. M., Fyfe M. C., Gardner L. S., Griffin G., Jackson H. C., Procter M. J., Rasamison C. M., Tang-Christensen M., Widdowson P. S., Williams G. M., Reynet C. Deorphanization of a G protein-coupled receptor for oleoylethanolamide and its use in the discovery of small-molecule hypophagic agents. Cell Metab. (2006);3:167–175. doi: 10.1016/j.cmet.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 87.Overton H. A., Fyfe M. C., Reynet C. GPR119, a novel G protein-coupled receptor target for the treatment of type 2 diabetes and obesity. Br. J. Pharmacol. (2008);153(Suppl 1):S76–81. doi: 10.1038/sj.bjp.0707529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Padmanabhan S., Myers A. G., A. G. B. M. Constitutively active GPR6 is located in the intracellular compartments. FEBS Lett. (2009);583:107–112. doi: 10.1016/j.febslet.2008.11.033. [DOI] [PubMed] [Google Scholar]
- 89.Park D., Tosello-Trampont A. C., Elliott M. R., Lu M., Haney L. B., Ma Z., Klibanov A. L., Mandell J. W., Ravichandran K. S. BAI1 is an engulfment receptor for apoptotic cells upstream of the ELMO/Dock180/Rac module. Nature. (2007);450:430–434. doi: 10.1038/nature06329. [DOI] [PubMed] [Google Scholar]
- 90.Parks B. W., Gambill G. P., Lusis A. J., Kabarowski J. H. Loss of G2A promotes macrophage accumulation in atherosclerotic lesions of low density lipoprotein receptor-deficient mice. J. Lipid Res. (2005);46:1405–1415. doi: 10.1194/jlr.M500085-JLR200. [DOI] [PubMed] [Google Scholar]
- 91.Pertwee R. G., Howlett A. C., Abood M. E., Alexander S. P., Di Marzo V., Elphick M. R., Greasley P. J., Hansen H. S., Kunos G., Mackie K., Mechoulam R., Ross R. A. International Union of Basic and Clinical Pharmacology. LXXIX. Cannabinoid receptors and their ligands: beyond CB(1) and CB(2). Pharmacol. Rev. (2010);62:588–631. doi: 10.1124/pr.110.003004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Pineiro R., Falasca M. Lysophosphatidylinositol signalling: new wine from an old bottle. Biochim. Biophys. Acta. (2012);1821:694–705. doi: 10.1016/j.bbalip.2012.01.009. [DOI] [PubMed] [Google Scholar]
- 93.Powell W. S., Rokach J. The eosinophil chemoattractant 5-oxo-ETE and the OXE receptor. Prog. Lipid Res. (2013);52:651–665. doi: 10.1016/j.plipres.2013.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Prossnitz E. R., Barton M. Signaling, physiological functions and clinical relevance of the G protein-coupled estrogen receptor GPER. Prostaglandins Other Lipid Mediat. (2009);89:89–97. doi: 10.1016/j.prostaglandins.2009.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Prossnitz E. R., Barton M. The G-protein-coupled estrogen receptor GPER in health and disease. Nat. Rev. Endocrinol. (2011);7:715–726. doi: 10.1038/nrendo.2011.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Qi A. D., Harden T. K., Nicholas R. A. Is GPR17 a P2Y/ leukotriene receptor? Examination of uracil nucleotides, nucleotide sugars, and cysteinyl leukotrienes as agonists of GPR17. J. Pharmacol. Exp. Ther. (2013);347:38–446. doi: 10.1124/jpet.113.207647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Qin Y., Verdegaal E. M., Siderius M., Bebelman J. P., Smit M. J., Leurs R., Willemze R., Tensen C. P., Osanto S. Quantitative expression profiling of G-protein-coupled receptors (GPCRs) in metastatic melanoma: the constitutively active orphan GPCR GPR18 as novel drug target. Pigment Cell Melanoma Res. (2011);24:207–218. doi: 10.1111/j.1755-148X.2010.00781.x. [DOI] [PubMed] [Google Scholar]
- 98.Quancard J., Bollbuck B., Janser P., Angst D., Berst F., Buehlmayer P., Streiff M., Beerli C., Brinkmann V., Guerini D., Smith P. A., Seabrook T. J., Traebert M., Seuwen K., Hersperger R., Bruns C., Bassilana F., Bigaud M. A potent and selective S1P(1) antagonist with efficacy in experimental autoimmune encephalomyelitis. Chem. Biol. (2012);19:1142–1151. doi: 10.1016/j.chembiol.2012.07.016. [DOI] [PubMed] [Google Scholar]
- 99.Radu C. G., Nijagal A., McLaughlin J., Wang L., Witte O. N. Differential proton sensitivity of related G protein-coupled receptors T cell death-associated gene 8 and G2A expressed in immune cells. Proc. Natl. Acad. Sci. U.S.A. (2005);102:1632–1637. doi: 10.1073/pnas.0409415102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Rajagopal S., Rajagopal K., Lefkowitz R. J. Teaching old receptors new tricks: biasing seven-transmembrane receptors. Nat. Rev. Drug Discov. (2010);9:373–386. doi: 10.1038/nrd3024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ren H., Orozco I. J., Su Y., Suyama S., Gutierrez-Juarez R., Horvath T. L., Wardlaw S. L., Plum L., Arancio O., Accili D. FoxO1 target Gpr17 activates AgRP neurons to regulate food intake. Cell. (2012);149:1314–1326. doi: 10.1016/j.cell.2012.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Ritscher L., Engemaier E., Staubert C., Liebscher I., Schmidt P., Hermsdorf T., Rompler H., Schulz A., Schoneberg T. The ligand specificity of the G-protein-coupled receptor GPR34. Biochem. J. (2012);443:841–850. doi: 10.1042/BJ20112090. [DOI] [PubMed] [Google Scholar]
- 103.Rosenkilde M. M., Benned-Jensen T., Andersen H., Holst P. J., Kledal T. N., Luttichau H. R., Larsen J. K., Christensen J. P., Schwartz T. W. Molecular pharmacological phenotyping of EBI2. An orphan seven-transmembrane receptor with constitutive activity. J. Biol. Chem. (2006);281:13199–13208. doi: 10.1074/jbc.M602245200. [DOI] [PubMed] [Google Scholar]
- 104.Ruiz-Medina J., Ledent C., Valverde O. GPR3 orphan receptor is involved in neuropathic pain after peripheral nerve injury and regulates morphine-induced antinociception. Neuropharmacology. (2011);61:43–50. doi: 10.1016/j.neuropharm.2011.02.014. [DOI] [PubMed] [Google Scholar]
- 105.Ryberg E., Larsson N., Sjogren S., Hjorth S., Hermansson N. O., Leonova J., Elebring T., Nilsson K., Drmota T., Greasley P. J. The orphan receptor GPR55 is a novel cannabinoid receptor. Br. J. Pharmacol. (2007);152:1092–1101. doi: 10.1038/sj.bjp.0707460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Serhan C. N., Chiang N., Van Dyke T. E. Resolving inflammation: dual anti-inflammatory and pro-resolution lipid mediators. Nat. Rev. Immunol. (2008);8:349–361. doi: 10.1038/nri2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Serhan C. N., Hong S., Gronert K., Colgan S. P., Devchand P. R., Mirick G., Moussignac R. L. Resolvins: a family of bioactive products of omega-3 fatty acid transformation circuits initiated by aspirin treatment that counter proinflammation signals. J. Exp. Med. (2002);196:1025–1037. doi: 10.1084/jem.20020760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Serhan C. N., Krishnamoorthy S., Recchiuti A., Chiang N. Novel anti-inflammatory--pro-resolving mediators and their receptors. Curr. Top. Med. Chem. (2011);11:629–647. doi: 10.2174/1568026611109060629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Shah U., Kowalski T. J. GPR119 agonists for the potential treatment of type 2 diabetes and related metabolic disorders. Vitam. Horm. (2010);84:415–448. doi: 10.1016/B978-0-12-381517-0.00016-3. [DOI] [PubMed] [Google Scholar]
- 110.Sharman J. L., Mpamhanga C. P., Spedding M., Germain P., Staels B., Dacquet C., Laudet V., Harmar A. J. IUPHAR-DB: new receptors and tools for easy searching and visualization of pharmacological data. Nucleic Acids Res. (2011);39:D534–538. doi: 10.1093/nar/gkq1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Shukla A. K., Xiao K., Lefkowitz R. J. Emerging paradigms of beta-arrestin-dependent seven transmembrane receptor signaling. Trends Biochem. Sci. (2011);36:457–469. doi: 10.1016/j.tibs.2011.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Soga T., Ohishi T., Matsui T., Saito T., Matsumoto M., Takasaki J., Matsumoto S., Kamohara M., Hiyama H., Yoshida S., Momose K., Ueda Y., Matsushime H., Kobori M., Furuichi K. Lysophosphatidylcholine enhances glucose-dependent insulin secretion via an orphan G-protein-coupled receptor. Biochem. Biophys. Res. Commun. (2005);326:744–751. doi: 10.1016/j.bbrc.2004.11.120. [DOI] [PubMed] [Google Scholar]
- 113.Southern C., Cook J. M., Neetoo-Isseljee Z., Taylor D. L., Kettleborough C. A., Merritt A., Bassoni D. L., Raab W., Quinn E., Wehrman T. S., Davenport A. P., Brown A. J., Green A., Wigglesworth M. J., Rees S. Screening beta-arrestin recruitment for the identification of natural ligands for orphan G-proteincoupled receptors. J. Biomol. Screen. (2013);18:599–609. doi: 10.1177/1087057113475480. [DOI] [PubMed] [Google Scholar]
- 114.Spann N. J., Glass C. K. Sterols and oxysterols in immune cell function. Nat. Immunol. (2013);14:893–900. doi: 10.1038/ni.2681. [DOI] [PubMed] [Google Scholar]
- 115.Sugo T., Tachimoto H., Chikatsu T., Murakami Y., Kikukawa Y., Sato S., Kikuchi K., Nagi T., Harada M., Ogi K., Ebisawa M., Mori M. Identification of a lysophosphatidylserine receptor on mast cells. Biochem. Biophys. Res. Commun. (2006);341:1078–1087. doi: 10.1016/j.bbrc.2006.01.069. [DOI] [PubMed] [Google Scholar]
- 116.Suzuki M., Takaishi S., Nagasaki M., Onozawa Y., Iino I., Maeda H., Komai T., Oda T. Medium-chain fatty acid-sensing receptor, GPR84, is a proinflammatory receptor. J. Biol. Chem. (2013);288:10684–10691. doi: 10.1074/jbc.M112.420042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Swaney J. S., Chapman C., Correa L. D., Stebbins K. J., Broadhead A. R., Bain G., Santini A. M., Darlington J., King C. D., Baccei C. S., Lee C., Parr T. A., Roppe J. R., Seiders T. J., Ziff J., Prasit P., Hutchinson J. H., Evans J. F., Lorrain D. S. Pharmacokinetic and pharmacodynamic characterization of an oral lysophosphatidic acid type 1 receptor-selective antagonist. J. Pharmacol. Exp. Ther. (2011);336:693–700. doi: 10.1124/jpet.110.175901. [DOI] [PubMed] [Google Scholar]
- 118.Tabata K., Baba K., Shiraishi A., Ito M., Fujita N. The orphan GPCR GPR87 was deorphanized and shown to be a lysophosphatidic acid receptor. Biochem. Biophys. Res. Commun. (2007);363:861–866. doi: 10.1016/j.bbrc.2007.09.063. [DOI] [PubMed] [Google Scholar]
- 119.Talukdar S., Olefsky J. M., Osborn O. Targeting GPR120 and other fatty acid-sensing GPCRs ameliorates insulin resistance and inflammatory diseases. Trends Pharmacol. Sci. (2011);32:543–550. doi: 10.1016/j.tips.2011.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Tanaka S., Ishii K., Kasai K., Yoon S. O., Saeki Y. Neural expression of G protein-coupled receptors GPR3, GPR6, and GPR12 up-regulates cyclic AMP levels and promotes neurite outgrowth. J. Biol. Chem. (2007);282:10506–10515. doi: 10.1074/jbc.M700911200. [DOI] [PubMed] [Google Scholar]
- 121.Tanaka S., Shaikh I. M., Chiocca E. A., Saeki Y. The Gslinked receptor GPR3 inhibits the proliferation of cerebellar granule cells during postnatal development. PloS one. (2009);4:e5922. doi: 10.1371/journal.pone.0005922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Thathiah A., Spittaels K., Hoffmann M., Staes M., Cohen A., Horre K., Vanbrabant M., Coun F., Baekelandt V., Delacourte A., Fischer D. F., Pollet D., De Strooper B., Merchiers P. The orphan G protein-coupled receptor 3 modulates amyloid-beta peptide generation in neurons. Science. (2009);323:946–951. doi: 10.1126/science.1160649. [DOI] [PubMed] [Google Scholar]
- 123.Thomas C., Pellicciari R., Pruzanski M., Auwerx J., Schoonjans K. Targeting bile-acid signalling for metabolic diseases. Nat. Rev. Drug Discov. (2008);7:678–693. doi: 10.1038/nrd2619. [DOI] [PubMed] [Google Scholar]
- 124.Tian Z., Wang Y., Zhang N., Guo Y. Y., Feng B., Liu S. B., Zhao M. G. Estrogen receptor GPR30 exerts anxiolytic effects by maintaining the balance between GABAergic and glutamatergic transmission in the basolateral amygdala of ovariectomized mice after stress. Psychoneuroendocrinology. (2013);38:2218–2233. doi: 10.1016/j.psyneuen.2013.04.011. [DOI] [PubMed] [Google Scholar]
- 125.Tomura H., Mogi C., Sato K., Okajima F. Proton-sensing and lysolipid-sensitive G-protein-coupled receptors: a novel type of multi-functional receptors. Cell. Signal. (2005);17:1466–1476. doi: 10.1016/j.cellsig.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 126.Tourino C., Valjent E., Ruiz-Medina J., Herve D., Ledent C., Valverde O. The orphan receptor GPR3 modulates the early phases of cocaine reinforcement. Br. J. Pharmacol. (2012);167:892–904. doi: 10.1111/j.1476-5381.2012.02043.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Uhlenbrock K., Gassenhuber H., Kostenis E. Sphingosine 1-phosphate is a ligand of the human gpr3, gpr6 and gpr12 family of constitutively active G protein-coupled receptors. Cell. Signal. (2002);14:941–953. doi: 10.1016/S0898-6568(02)00041-4. [DOI] [PubMed] [Google Scholar]
- 128.Valverde O., Celerier E., Baranyi M., Vanderhaeghen P., Maldonado R., Sperlagh B., Vassart G., Ledent C. GPR3 receptor, a novel actor in the emotional-like responses. PloS one. (2009);4:e4704. doi: 10.1371/journal.pone.0004704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Vassilatis D. K., Hohmann J. G., Zeng H., Li F., Ranchalis J. E., Mortrud M. T., Brown A., Rodriguez S. S., Weller J. R., Wright A. C., Bergmann J. E., Gaitanaris G. A. The G proteincoupled receptor repertoires of human and mouse. Proc. Natl. Acad Sci. U.S.A. (2003);100:4903–4908. doi: 10.1073/pnas.0230374100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Vassileva G., Golovko A., Markowitz L., Abbondanzo S. J., Zeng M., Yang S., Hoos L., Tetzloff G., Levitan D., Murgolo N. J., Keane K., Davis H. R., Jr., Hedrick J., Gustafson E. L. Targeted deletion of Gpbar1 protects mice from cholesterol gallstone formation. Biochem. J. (2006);398:423–430. doi: 10.1042/BJ20060537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Venkataraman C., Kuo F. The G-protein coupled receptor, GPR84 regulates IL-4 production by T lymphocytes in response to CD3 crosslinking. Immunol. Lett. (2005);101:144–153. doi: 10.1016/j.imlet.2005.05.010. [DOI] [PubMed] [Google Scholar]
- 132.Wang J., Simonavicius N., Wu X., Swaminath G., Reagan J., Tian H., Ling L. Kynurenic acid as a ligand for orphan G protein-coupled receptor GPR35. J. Biol. Chem. (2006a);281:22021–22028. doi: 10.1074/jbc.M603503200. [DOI] [PubMed] [Google Scholar]
- 133.Wang J., Wu X., Simonavicius N., Tian H., Ling L. Medium-chain fatty acids as ligands for orphan G protein-coupled receptor GPR84. J. Biol. Chem. (2006b);281:34457–34464. doi: 10.1074/jbc.M608019200. [DOI] [PubMed] [Google Scholar]
- 134.Wang L., Radu C. G., Yang L. V., Bentolila L. A., Riedinger M., Witte O. N. Lysophosphatidylcholine-induced surface redistribution regulates signaling of the murine G protein-coupled receptor G2A. Mol. Biol. Cell. (2005);16:2234–2247. doi: 10.1091/mbc.E04-12-1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Watanabe M., Houten S. M., Mataki C., Christoffolete M. A., Kim B. W., Sato H., Messaddeq N., Harney J. W., Ezaki O., Kodama T., Schoonjans K., Bianco A. C., Auwerx J. Bile acids induce energy expenditure by promoting intracellular thyroid hormone activation. Nature. (2006);439:484–489. doi: 10.1038/nature04330. [DOI] [PubMed] [Google Scholar]
- 136.Yamashita A., Oka S., Tanikawa T., Hayashi Y., Nemoto-Sasaki Y., Sugiura T. The actions and metabolism of lysophosphatidylinositol, an endogenous agonist for GPR55. Prostaglandins Other Lipid Mediat.; (2013). [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 137.Yan J. J., Jung J. S., Lee J. E., Lee J., Huh S. O., Kim H. S., Jung K. C., Cho J. Y., Nam J. S., Suh H. W., Kim Y. H., Song D. K. Therapeutic effects of lysophosphatidylcholine in experimental sepsis. Nat. Med. (2004);10:161–167. doi: 10.1038/nm989. [DOI] [PubMed] [Google Scholar]
- 138.Yanagida K., Kurikawa Y., Shimizu T., Ishii S. Current progress in non-Edg family LPA receptor research. Biochim. Biophys. Acta. (2013);1831:33–41. doi: 10.1016/j.bbalip.2012.08.003. [DOI] [PubMed] [Google Scholar]
- 139.Yang C. R., Wei Y., Qi S. T., Chen L., Zhang Q. H., Ma J. Y., Luo Y. B., Wang Y. P., Hou Y., Schatten H., Liu Z. H., Sun Q. Y. The G protein coupled receptor 3 is involved in cAMP and cGMP signaling and maintenance of meiotic arrest in porcine oocytes. PloS one. (2012);7:e38807. doi: 10.1371/journal.pone.0038807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Yin H., Chu A., Li W., Wang B., Shelton F., Otero F., Nguyen D. G., Caldwell J. S., Chen Y. A. Lipid G protein-coupled receptor ligand identification using beta-arrestin PathHunter assay. J. Biol. Chem. (2009);284:12328–12338. doi: 10.1074/jbc.M806516200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Yoshida M., Miyazato M., Kangawa K. Orphan GPCRs and methods for identifying their ligands. Methods Enzymol. (2012);514:33–44. doi: 10.1016/B978-0-12-381272-8.00002-7. [DOI] [PubMed] [Google Scholar]
- 142.Zhang B. L., Li Y., Ding J. H., Dong F. L., Hou Y. J., Jiang B. C., Shi F. X., Xu Y. X. Sphingosine 1-phosphate acts as an activator for the porcine Gpr3 of constitutively active G proteincoupled receptors. Journal of Zhejiang University. J. Zhejang Univ. Sci. B. (2012);13:555–566. doi: 10.1631/jzus.B1100353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Zhang R., Xie X. Tools for GPCR drug discovery. Acta Pharmacol. Sin. (2012);33:372–384. doi: 10.1038/aps.2011.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Zhao P., Abood M. E. GPR55 and GPR35 and their relationship to cannabinoid and lysophospholipid receptors. Life Sci. (2013);92:453–457. doi: 10.1016/j.lfs.2012.06.039. [DOI] [PubMed] [Google Scholar]
- 145.Zhu T., Gobeil F., Vazquez-Tello A., Leduc M., Rihakova L., Bossolasco M., Bkaily G., Peri K., Varma D. R., Orvoine R., Chemtob S. Intracrine signaling through lipid mediators and their cognate nuclear G-protein-coupled receptors: a paradigm based on PGE2, PAF, and LPA1 receptors. Can. J. Physiol. Pharmacol. (2006);84:377–391. doi: 10.1139/y05-147. [DOI] [PubMed] [Google Scholar]