Abstract
Chondroitin sulfate proteoglycan (CSPG) inhibits neurite outgrowth of various neuronal cell types, and CSPG-associated inhibition of neurite outgrowth is mediated by the Rho/ROCK pathway. Mesenchymal stromal/stem cells (MSCs) have the potential to differentiate into neuron-like cells under specific conditions and have been shown to differentiate into neuron-like cells by co-treatment with the ROCK inhibitor Y27632 and the hypoxia condition mimicking agent CoCl2. In this study, we addressed the hypothesis that a ROCK inhibitor might be beneficial to regenerate neurons during stem cell therapy by preventing transplanted MSCs from inhibition by CSPG in damaged tissues. Indeed, dose-dependent inhibition by CSPG pretreatment was observed during morphological changes of Wharton’s jelly-derived MSCs (WJ-MSCs) induced by Y27632 alone. The formation of neurite-like structures was significantly inhibited when WJ-MSCs were pre-treated with CSPG before induction under Y27632 plus CoCl2 conditions, and pretreatment with a protein kinase C inhibitor reversed such inhibition. However, CSPG treatment resulted in no significant inhibition of the WJ-MSC morphological changes into neuron-like cells after initiating induction by Y27632 plus CoCl2. No marked changes were detected in expression levels of neuronal markers induced by Y27632 plus CoCl2 upon CSPG treatment. CSPG also blocked the morphological changes of human bone marrow-derived MSCs into neuron-like cells under other neuronal induction condition without the ROCK inhibitor, and Y27632 pre-treatment blocked the inhibitory effect of CSPG. These results suggest that a ROCK inhibitor can be efficiently used in stem cell therapy for neuronal induction by avoiding hindrance from CSPG.
Keywords: Mesenchymal stromal/stem cell, ROCK inhibitor, Chondroitin sulfate proteoglycan, PKC inhibitor, Y27632
INTRODUCTION
Mesenchymal stromal cell/stem cells (MSCs) have selfregenerating ability and multipotent differentiation potential to differentiate into adipocytes, osteocytes, chondrocytes, and neuronal cells (Can and Karahuseyinoglu, 2007; Krampera et al., 2007, Datta et al., 2011). MSCs are found in various tissues, including skin, adipose tissue, bone marrow, and the umbilical cord. Differentiation of both human and rodent MSCs into neuron-like cells has been demonstrated in vitro under several specific conditions (Sanchez-Ramos et al., 2000; Woodbury et al., 2000; Mitchell et al., 2003; Fu et al., 2006). Murine bone marrow (BM)-MSCs also undergo differentiation into neuron-like cells when treated with a Rho-associated kinase (ROCK) inhibitor plus the hypoxia-mimicking agent CoCl2 (Pacary et al., 2006). We have shown that human BMMSCs differentiate into neuron-like cells by co-treatment with a highly specific ROCK inhibitor and CoCl2 (Lee et al., 2010).
Neurons in the central nervous system (CNS) do not spontaneously regenerate axons after injury. Failure to regenerate is due, in part, to the presence of axonal outgrowth inhibitors in the CNS environment (Mueller et al., 2005; Kubo et al., 2008). Three major components have been identified in the CNS myelin such as myelin-associated glycoprotein (MAG) (McKerracher et al., 1994), NOGO-A (Chen et al., 2000; Prinjha et al., 2000), and oligodendrocyte myelin glycoprotein (OMgp) (Wang et al., 2002). Chondroitin sulfate proteoglycans (CSPGs) are also a major axon growth inhibitory component of the glial scar tissue that blocks successful regeneration after damage to the nervous system (Laabs et al., 2005). The inhibitory activity of MAG and other myelin-associated inhibitors appears to require the Rho/ROCK pathway (Lehmann et al., 1999; Vinson et al., 2001). Other inhibitors such as CSPGs and semaphorin and ephrin members also activate the Rho/ ROCK pathway (Monnier et al., 2003; Sivasankaran et al., 2004; Kubo et al., 2008). Moreover, inactivating Rho or ROCK improves neurite outgrowth of primary neurons on inhibitor substrates in vitro and axon regeneration in vivo (Dergham et al., 2002; Winton et al., 2002). Both myelin inhibitors and CSPGs activate protein kinase C (PKC), and blocking PKC activity attenuates their ability to activate Rho and inhibit neurite outgrowth (Sivasankaran et al., 2004).
In this study, we addressed another possible benefit that ROCK inhibitors may protect MSCs against CSPGs during neuronal induction. Thus, we examined whether CSPGs interrupt the morphological changes of MSCs into neuron-like cells using two types of MSCs and two neuronal induction conditions and determined whether a ROCK inhibitor blocks the inhibitory effect.
MATERIALS AND METHODS
Materials
Y27632 was purchased from Calbiochem-Merck Bioscience (San Diego, CA, USA) and dissolved in ultra-pure water. CoCl2 was purchased from Sigma (St. Louis, MO, USA) and dissolved in ultra-pure water. A CSPG mixture (Chemicon, Temecula, CA, USA) was dissolved in phosphate buffered saline (PBS). Go6976 (Tocris, Bristol, UK), calphostin C (Sigma), and SB415286 (Sigma) were dissolved in dimethyl sulfoxide (DMSO).
Isolation and expansion of Wharton’s jelly-derived MSCs (WJ-MSCs) from human umbilical cords
Use of human umbilical cords was approved by the Institutional Review Board at The Catholic University of Korea College of Medicine (CUMC09U158), and cords were acquired from donors with written informed agreement. WJ-MSCs were prepared as described previously (Choi et al., 2013). Human umbilical cords were cut into 3-4 pieces after washing in 1× PBS. After removing the blood vessels and amnion, the tissues was chopped into pieces, and then incubated in 0.2% collagenase (Gibco BRL, Grand Island, NY, USA) in Dulbecco’s modified Eagle’s medium (DMEM, Gibco BRL) for 40-60 min followed by a 37℃ incubation in 0.05% trypsin for 40 min. After adding heat-inactivated fetal bovine serum (FBS, Gibco BRL), the digested mixture was mixed gently and centrifuged at 100 × g for 10 min. Separated cells were cultured in DMEM supplemented with 20% FBS, 100 U/mL penicillin, and 100 μg/mL streptomycin (Gibco BRL) in a humidified incubator at 37℃ under a 5% CO2 atmosphere.
In vitro MSC differentiation into neuron-like cells by cotreatment with Y27632 and CoCl2
WJ-MSCs (40 cell/mm2, passages 4-10) were seeded onto 24-well plates and cultured in DMEM supplemented with 10% FBS overnight. After being washed with 1×PBS, the cells were incubated in endothelial growth medium (EGM) 2 (Lonza, Walkersville, MD, USA) and co-treated with 30 μM Y27632 and 100 μM CoCl2. A 1-10 μg/mL CSPG mixture, 100 nM Go6976, 100 nM calphostin C, or 10 μM SB415286 was added at several different time points. During the ensuing 72 h, the cells were photographed at three representative fields (Leica, Inverted Microscope, Wetzlar, Germany).
Quantification of branch length
Images obtained from neuronal induction experiments were analyzed using the ImageJ (http://rsb.info.nih.gov/ij/) program to determine the lengths of cellular processes. The total length of the cellular processes was measured and divided by the number of cells to give an average total length of the cellular processes per cell. In total, 50-120 cells within four fields were assessed for each well.
Neuronal induction of BM-MSCs by the Woodbury method
Human BM-MSCs were purchased from Lonza (cat. no. PT-2501; Basel, Switzerland) and maintained in MSCGM growth medium. BM-MSCs (40 cell/mm2, passage 4) were seeded onto plates and cultured in MSCGM medium overnight. After being washed with 1× PBS, the culture medium was replaced with medium containing 10% FBS and 1 mM β-mercaptoethanol. After 24 h, the media were removed, the cells were washed with 1×PBS, and serum-free DMEM was added to the cells. Then, the cells were treated with 10 μg/mL CSPGs with/without pretreatment with 30 μM Y27632 for 1 h at 37℃. After 1 h, the cells were treated with 2% DMSO, 200 μM butylated hydroxyanisole, 25 mM KCl, 2 mM valproic acid, 10 μM forskolin, 1 μM hydrocortisone, and 5 μg/ml insulin. During the incubation, the cells were photographed at three representative fields (Zeiss, Inverted Microscope, Oberkochen, Germany).
Immunofluorescence
Following induction of in vitro differentiation into neuron-like cells for 72 h (WJ-MSC), the cells were washed with 1× PBS (pre-warmed) and fixed in 4% paraformaldehyde for 10 min at room temperature (RT). After washing the cells with 1× PBS twice, they were permeabilized using 0.2% Triton X-100 solution in 1×PBS. After being washed twice with 1× PBS, the cells were blocked in 5% bovine serum albumin (GIBCO, Auckland, NZ) at RT for 1 h and then incubated overnight (4℃) with a primary antibody against neuron-specific class III α-tubulin (Tuj1, 1:400, cat. no. MMS-435P, Covance, Richmond, CA, USA), neuron-specific enolase (NSE, 1:400, cat. no. AB951, Chemicon), or neurofilament-M (NF-M, 1:400, cat. no. MAB1615, Chemicon). After being washed with 1×PBS twice, the cells were incubated for 1 h with Cy3 conjugated anti-mouse IgG and anti-rabbit IgG (cat. no. AP186C and AP182C, Chemicon). Last, after being washed with 1×PBS, cell nuclei were stained with diamidinophenylindole (Sigma) for 3 min, and the cells were observed under a fluorescence microscope (Zeiss).
Statistical analysis
Statistical analyses were performed by one-way analysis of variance with Tukey’s post hoc test to compare differences between individual groups using GraphPad Prism (Graphpad, San Diego, CA, USA). A p<0.05 was considered significant.
RESULTS
CSPG dose-dependently inhibits morphological changes of WJ-MSCs into neuron-like cells induced by Y27632 alone
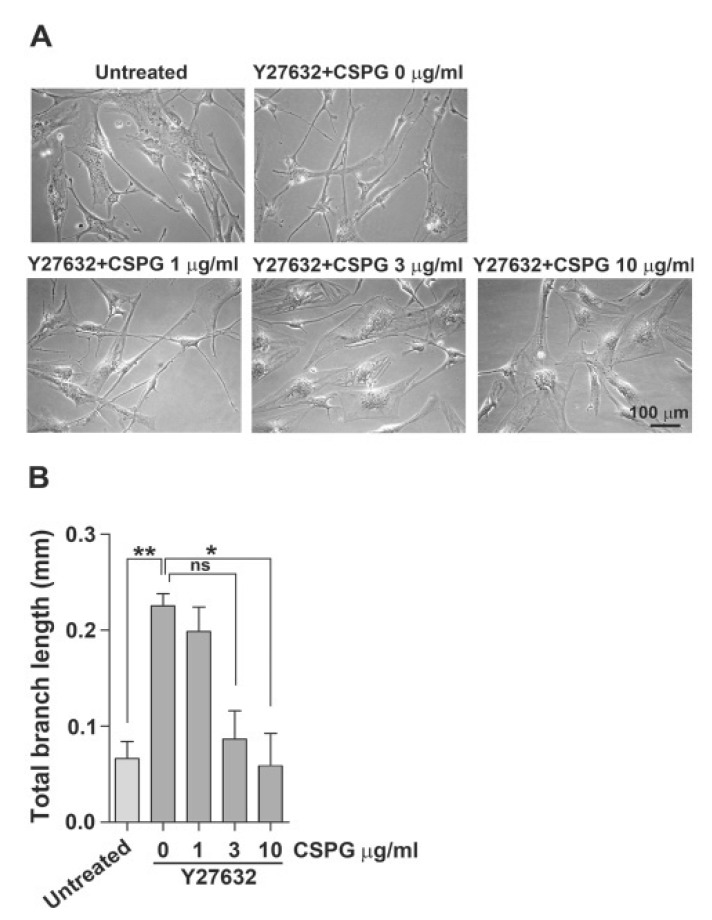
We have shown previously that human BM-MSCs undergo morphological changes into neuron-like cells in MSCGM in the presence of a ROCK inhibitor alone (Lee et al., 2010). Similarly, treatment of WJ-MSCs with Y27632 alone also showed marked morphological changes into neuron-like cells in EGM2 medium compared to those in DMEM supplemented with 10% FBS (data not shown). As shown in Fig. 1, WJ-MSCs cultured in EGM2 in the presence of Y27632 exhibited neuron-like morphology with multiple branches and long, thin processes. Thus, we tested whether pretreatment with CSPGs dose-dependently inhibits the morphological changes of WJ-MSCs into neuron-like cells in EGM2 in the presence of Y27632. When WJ-MSCs were treated with CSPGs at various concentrations prior to treatment with 30 μM Y27632, we found that CSPG dose-dependently inhibited the morphological changes into neuron-like cells. Then, we treated cells with 10 μg/ml CSPG, which resulted in maximum inhibition.
Fig. 1. CSPG dose-dependently inhibits morphological changes of WJ-MSCs induced by the ROCK inhibitor Y27632. WJ-MSCs were treated with CSPG (1, 3, or 10 μg/ml) for 1 h and then incubated in the presence of 30 μM Y27632 for 72 h. (A) The morphological changes in induced cells were examined under a microscope. Scale bar=100 μm. (B) Total branch length per cell was assessed after 72 h of treatment with CSPG and/or Y27632. Data represent mean ± standard error (n=3). *p<0.05, **p<0.01.
CSPGs inhibit morphological changes of WJ-MSCs into neuron-like cells induced by co-treatment with Y27632 and CoCl2 through mediation by PKC
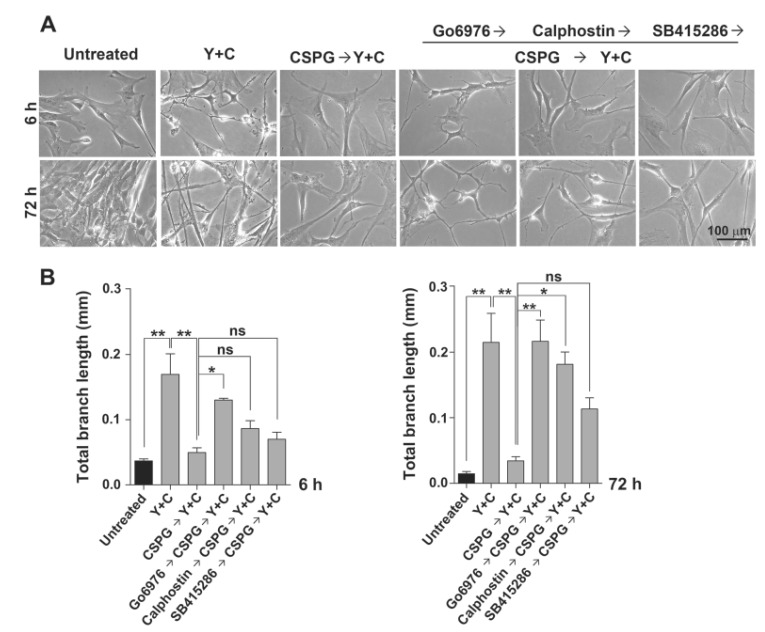
Because the synergistic inhibitory effects of CoCl2 and ROCK inhibition improve MSC differentiation into neuron-like cells (Pacary et al., 2006), we tested the inhibitory effect of CSPG under co-treatment with Y27632 and CoCl2. As shown in Fig. 2, pretreatment with CSPG before co-treatment with Y27632 and CoCl2 drastically inhibited differentiation of WJMSCs into neuron-like cells at 6 and 72 h. Myelin inhibitors and CSPGs have been shown to induce PKC activation (Sivasankaran et al., 2004). That study also showed that blocking PKC activity pharmacologically and genetically attenuates the ability of CNS myelin and CSPGs to activate Rho and inhibit neurite outgrowth. Thus, we tested whether the inhibitory effect of CSPGs on WJ-MSC differentiation into neuron-like cells is mediated by PKC. We treated cells with the PKC inhibitors Go6976 or calphostin before treatment with CSPG. At 6 h after induction by co-treatment with Y27632 and CoCl2, Go6976 showed a marked reversion of the inhibitory effect of CSPG, but calphostin showed a lesser effect. After a longer incubation, calphostin also showed a significant reversion of the inhibitory effect of CSPG at 72 h. Similar but weaker blocking of CSPG inhibition by calphostin has also been observed in neurite outgrowth of rat primary neurons (Sivasankaran et al., 2004). However, the glycogen synthase kinase 3 (GSK-3) β inhibitor SB415286 showed no reversion at 6 h but a nonsignificant reversion at 72 h. Thus, we concluded that CSPG inhibits the morphological changes of WJ-MSCs into neuronlike cells through PKC.
Fig. 2. Pretreatment of CSPG blocks morphological changes of WJ-MSCs into neuron-like cells induced by Y27632 and CoCl2 through mediation by PKC. WJ-MSCs were pre-treated with the PKC inhibitors (Go6976 or calphostin C, 100 nM) or GSK3-β inhibitor (SB415286, 10 μM) for 1 h, and then with 10 μg/mL CSPG for 1 h and incubated with 100 μM CoCl2 and 30 μM Y27632 in EGM2 for 72 h. (A) The morphological changes in induced cells were examined under a microscope. Scale bar=100 μm. (B) Total branch length per cell was assessed after 6 or 72 h of treatment with Y27632 and CoCl2. Representative data from three independent experiments performed in triplicate. Y, Y27632; C, CoCl2. Data represent mean ± standard error (n=3). *p<0.05, **p<0.01.
No significant inhibitory effects of CSPG were observed in neuronal marker expression of induced WJ-MSCs
We tested whether pretreatment with CSPG affects neuron marker expression of WJ-MSCs induced by co-treatment with Y27632 and CoCl2 for 72 h. The induced cells were stained for NSE, Tuj1, and NF-M by immunofluorescence. We have observed previously that co-treatment of WJ-MSCs with other ROCK inhibitor fasudil and CoCl2 results in decreased expression of nestin and increased expression of neuronal markers such as β-tubulin III, NF-H, and NSE (data not shown). As shown Fig. 3, no significant differences were observed in immunostaining levels between the CSPG treatment and no treatment for all three markers, although significant morphological differences were observed as described in the above experiments. Thus, we concluded that CSPG mainly affects the morphological change into neuron-like cells during neuronal induction of MSCs.
Fig. 3. Analysis of neuronal marker expression by immunofluorescence. WJ-MSCs were pre-treated with a PKC inhibitor (Go6976 or calphostin C, 100 nM) or a GSK3-β inhibitor (SB415286, 10 μM) for 1 h and then with 10 μg/mL CSPG for 1 h and incubated with 100 μM CoCl2 and 30 μM Y27632 in EGM2 for 72 h. Then, the induced cells were stained for β-tubulin III, neurofilament-M (NFM), and neurospecific enolase (NSE). The nuclei of the cells were stained with DAPI. Y, Y27632; C, CoCl2. Scale bar=50 μm.
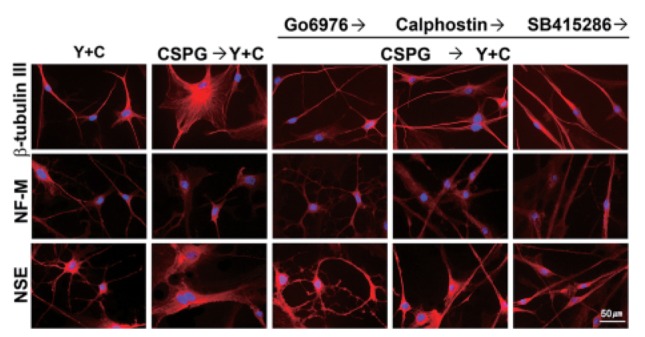
Induction of WJ-MSCs with the ROCK inhibitor and CoCl2 prior to CSPG exposure retains morphologically differentiated neuron-like cells
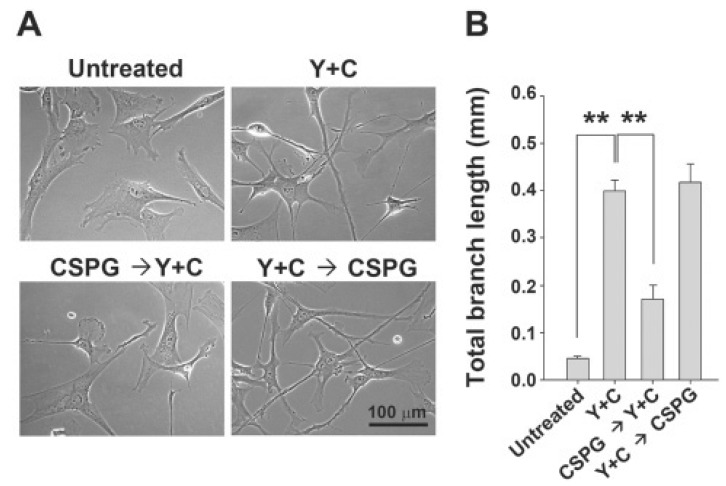
From the above experiments, we found that CSPG exposure prior to induction by co-treatment with Y27632 and CoCl2 inhibited the morphological changes into neuron-like cells with long processes. Thus, we asked whether initiating induction by co-treatment with Y27632 and CoCl2 before CSPG exposure could block the inhibitory effect of CSPG. As shown in Fig. 4, we found that CSPG treatment after co-treatment with Y27632 and CoCl2 for 1 h resulted in no inhibition of morphological changes of MSCs into neuron-like cells with processes. In contrast, CSPG treatment before co-treatment with Y27632 and CoCl2 resulted in marked inhibition of MSC morphological differentiation, suggesting that blocking the Rho/ROCK pathway prior to CSPG-mediating signaling is important for effective blockade of CSPG. Thus, we concluded that CSPG inhibits morphological differentiation into neuron-like cells by activating the of Rho/ROCK pathway similar to neuronal cells.
Fig. 4. CSPG treatment after initiation of induction by Y27632 and CoCl2 results in no significant inhibition in morphological changes into neuron-like cells. WJ-MSCs were treated with Y27632 and CoCl2 for 1 h, and then CSPG was added and incubated for 6 h. (A) Morphological changes were observed under a microscope. (B) Average total length of the branches per cell (mm) was analyzed using ImageJ software. Representative data from three independent experiments performed in triplicate. Y, Y27632; C, CoCl2. Data represent mean ± standard error (n=3). **p<0.01, Scale bar=100 μm.
CSPG also inhibits BM-MSC differentiation into neuron-like cells under a different neuronal induction condition and the ROCK inhibitor blocks the inhibitory effect of CSPG
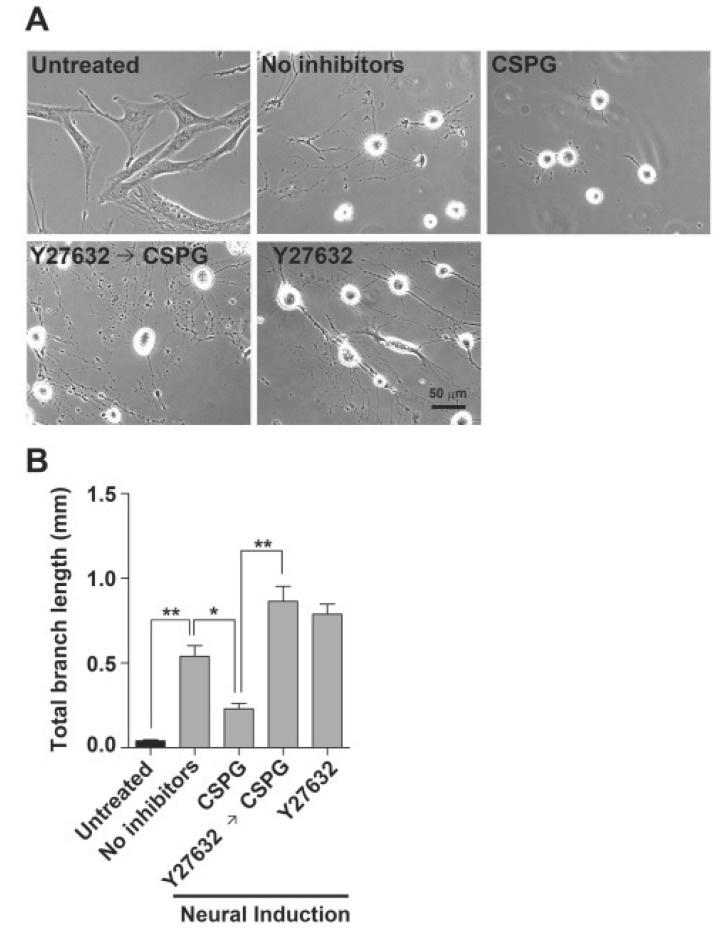
We also tested whether ROCK is involved in inhibition of BM-MSC differentiation into neuron-like cells by CSPG employing other neuronal induction condition with no ROCK inhibitor. Human BM-MSCs were induced to form neuron-like cells under conditions reported previously (Woodbury et al., 2000). As shown in Fig. 5, the induced BM-MSCs showed refractile cell bodies with extended long processes terminating in typical growth cones and filopodia. CSPG treatment drastically reduced the neuron-like cells with long processes, whereas pre-treatment of BM-MSCs with Y27632 before CSPG exposure completely blocked the inhibitory effect of CSPG. Thus, we concluded that the ROCK inhibitor efficiently blocks the inhibitory effect of CSPG during BM-MSC differentiation into neuron-like cells under different conditions. Interestingly, Y27632 pretreatment tended to increase more morphological changes of BM-MSCs into neuron-like cells with multiple branches and long processes in the presence or absence of CSPG under the tested induced condition, although it was not statistically significant.
Fig. 5. Y27632 treatment blocks the inhibitory effect of CSPG on BM-MSC differentiation into neuron-like cells by Woodbury’s method. BM-MSCs were induced to differentiate into neuron-like cells by Woodbury’s method after pre-treatment with 30 μM Y27632 (1 h), and/or then with 10 μg/mL CSPG (another 1 h). (A) After 5 h of incubation, the morphological changes were observed under a microscope. (B) Average total length of branches per cell (mm) was analyzed using ImageJ software. Images were obtained from four fields in each well. Data represent mean ± standard error (n=4). *p<0.05, **p<0.01, Scale bar=50 μm.
DISCUSSION
The efficacy of MSCs in cell therapy has been proven in preclinical and clinical studies for conditions ranging from is chemic injury to neurodegenerative diseases (Low et al., 2008; Seo and Cho, 2012). However, the efficacy of MSCs for cell transplantation must be improved to provide more feasible and efficient cell therapy. ROCK inhibitors have been shown to be useful to treat cardiovascular diseases and CNS disorders (Kubo et al., 2008; Miyamoto et al., 2010). Treatment with the ROCK inhibitor fasudil before or after acute ischemic stroke onset leads to improved patient clinical outcomes. Because cell therapy is usually applied to an injured CNS, we hypothesized that priming MSCs with a ROCK inhibitor may provide a benefit by blocking the inhibitory effect of the neurite outgrowth inhibitors. Indeed, our data show that CSPG inhibits morphological differentiation of WJ-MSCs into neuron-like cells through mediation by PKC, similar to neurite outgrowth of neuronal cells. We also showed that a ROCK inhibitor can be employed to block the inhibitory effect of CSPG on neuronal induction of MSCs.
ROCK regulates the activities of many target proteins by phosphorylation, and some of these proteins such as myosin light chain, LIM kinases, and collapsin response-mediator protein-2 regulate cell morphology (Kubo et al., 2008). Several studies have noted that changes in cell shape precommit mesenchymal lineages (McBeath et al., 2004). In a previous report, the synergistic effect of CoCl2 and Y27632 during differentiation of MSCs into neuron-like cells was suggested to act through, at least in part, activation of hypoxia inducible factor, cell cycle arrest, and ROCK inhibition (Pacary et al., 2006). They also showed that expression of the neuronal markers NSE and MAP2c increased markedly with CoCl2 treatment, but only slightly by Y-27632 treatment in a Western blot analysis. In addition, a synergistic effect on neuronal maker expression was observed by co-treatment with CoCl2 and Y27632, but did not increase drastically compared to treatment with CoCl2 alone. A similar pattern was also observed in the induction of neuronal marker expression in WJ-MSCs by co-treatment with fasudil and CoCl2 (data not shown). However, the morphological change of MSCs into neuron-like cells with long processes is drastically induced by Y27632, whereas CoCl2 treatment shows a marginal effect on these morphological changes (Lee et al., 2010). The synergistic effect on the morphological change into neuronal-like cells was not drastic (data not shown). Accordingly, CSPG, which acts by activating the Rho- ROCK pathway, had no marked effect on expression of neuronal markers, β-tubulin III, NF-H, or NSE under co-treatment with Y27632 and CoCl2 but markedly inhibited the morphological change into neuron-like cells. In addition, the inhibition by CSPG was detected in the treatment with Y27632 alone without CoCl2. However, it cannot be ruled out that CSPG affected the expression of these markers at unnoticeable levels or can affect the expression of other neuronal markers.
In this study, we used CSPG as a neurite outgrowth inhibitor for the experiments. Inhibiting ROCK increases neurite outgrowth on CSPG in Ntera-2 and PC12 cells (Lingor et al.., 2007; Gopalakrishnan et al., 2008). Similar to CSPG, the myelin-associated neurite outgrowth inhibitors such as MAG, NOGO-A, and OMgp also activate ROCK by activating PKC (Sivasankaran et al., 2004; Kubo et al., 2008) and thereby inhibit outgrowth of neurites upon axonal regeneration. Thus, we presume that a ROCK inhibitor may be used efficiently in cell therapy since it can block the inhibitory effect of most neurite outgrowth inhibitors exposed in injured tissue.
The inhibition of differentiation into neuron-like cells by CSPG was observed in BM-MSCs as well as WJ-MSCs. Similar to neurons, the inhibitory effect of CSPG was blocked by inhibitors of conventional PKC and also by the ROCK inhibitor Y27632 in BM-MSCs and WJ-MSCs. Thus, similar signaling pathways are likely to exert an inhibitory effect of CSPG on neurons and MSCs. In the future, it will be interesting to address this question in detail. Unlike PKC and ROCK inhibitors, a GSK3β inhibitor only incompletely blocked the inhibitory effect of CSPG, which corresponded with a previous report that neurite regrowth of neurons by inhibiting GSK3β is indirectly related to the Rho/ROCK pathway (Kim et al., 2011).
Interestingly, we found that Y27632 promotes formation of neuron-like structures in MSCs under neuronal induction by the Woodbury method. Pretreatment with Y27632 in this induction culture resulted in a tendency for increased level of neurite-like structures although the increase was not significant. Y27632 elicits neurite outgrowth in primary neurons, whereas PKC inhibitors do not (Sivasankaran et al., 2004). Thus, Y27632 is more beneficial than PKC inhibitors by promoting neurite outgrowth as well as blocking the inhibitory effect of neurite outgrowth inhibitors. Therefore, it will encourage us to apply ROCK inhibitors to improve the therapeutic efficacy of cell transplantation of MSCs for neuronal regeneration.
Acknowledgments
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MEST) (No. 2009-0079656).
References
- 1.Can A., Karahuseyinoglu S. Concise review: human umbilical cord stroma with regard to the source of fetus-derived stem cells. Stem Cells. (2007);25:2886–2895. doi: 10.1634/stemcells.2007-0417. [DOI] [PubMed] [Google Scholar]
- 2.Chen M. S., Huber A. B., van der Haar M. E., Frank M., Schnell L., Spillmann A. A., Christ F., Schwab M. E. Nogo-A is a myelin-associated neurite outgrowth inhibitor and an antigen for monoclonal antibody IN-1. Nature. (2000);403:434–439. doi: 10.1038/35000601. [DOI] [PubMed] [Google Scholar]
- 3.Choi M., Lee H. S., Naidansaren P., Kim H. K., O E., Cha J. H., Ahn H. Y., Yang P. I., Shin J. C., Joe Y. A. Proangiogenic features of Wharton's jelly-derived mesenchymal stromal/stem cells and their ability to form functional vessels. Int. J. Biochem. Cell Biol. (2013);45:560–570. doi: 10.1016/j.biocel.2012.12.001. [DOI] [PubMed] [Google Scholar]
- 4.Datta I., Mishra S., Mohanty L., Pulikkot S., Joshi P. G. Neuronal plasticity of human Wharton's jelly mesenchymal stromal cells to the dopaminergic cell type compared with human bone marrow mesenchymal stromal cells. Cytotherapy. (2011);13:918–932. doi: 10.3109/14653249.2011.579957. [DOI] [PubMed] [Google Scholar]
- 5.Dergham P., Ellezam B., Essagian C., Avedissian H., Lubell W. D., McKerracher L. Rho signaling pathway targeted to promote spinal cord repair. J. Neurosci. (2002);22:6570–6577. doi: 10.1523/JNEUROSCI.22-15-06570.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fu Y. S., Cheng Y. C., Lin M. Y., Cheng H., Chu P. M., Chou S. C., Shih Y. H.,, Ko M. H., Sung M. S. Conversion of human umbilical cord mesenchymal stem cells in Wharton's jelly to dopaminergic neurons in vitro: potential therapeutic application for Parkinsonism. Stem Cells. (2006);24:115–124. doi: 10.1634/stemcells.2005-0053. [DOI] [PubMed] [Google Scholar]
- 7.Gopalakrishnan S. M., Teusch N., Imhof C., Bakker M. H., Schurdak M., Burns D. J., Warrior U. Role of Rho kinase pathway in chondroitin sulfate proteoglycan-mediated inhibition of neurite outgrowth in PC12 cells. J. Neurosci. Res. (2008);86:2214–2226. doi: 10.1002/jnr.21671. [DOI] [PubMed] [Google Scholar]
- 8.Kim Y. T., Hur E. M., Snider W. D., Zhou F. Q. Role of GSK3 Signaling in Neuronal Morphogenesis. Front. Front. Mol. Neurosci. (2011);4:48. doi: 10.3389/fnmol.2011.00048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Krampera M., Franchini M., Pizzolo G., Aprili G. Mesenchymal stem cells: from biology to clinical use. Blood Transfus. (2007);5:120–129. doi: 10.2450/2007.0029-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kubo T., Yamaguchi A., Iwata N., Yamashita T. The therapeutic effects of Rho-ROCK inhibitors on CNS disorders. Ther. Clin. Risk Manag. (2008);4:605–615. doi: 10.2147/tcrm.s2907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Laabs T., Carulli D., Geller H. M., Fawcett J. W. Chondroitin sulfate proteoglycans in neural development and regeneration. Curr. Opin. Neurobiol. (2005);15:116–120. doi: 10.1016/j.conb.2005.01.014. [DOI] [PubMed] [Google Scholar]
- 12.Lee H. S., Kim K. S., O E., Joe Y. A. A Comparison of ROCK Inhibitors on Human Bone Marrow-Derived Mesenchymal Stem Cell Differentiation into Neuron-Like Cells. Biomol. Ther. (2010);18:386–395. doi: 10.4062/biomolther.2010.18.4.386. [DOI] [Google Scholar]
- 13.Lehmann M., Fournier A., Selles-Navarro I., Dergham P., Sebok A., Leclerc N., Tigyi G., McKerracher L. Inactivation of Rho signaling pathway promotes CNS axon regeneration. J. Neurosci. (1999);19:7537–7547. doi: 10.1523/JNEUROSCI.19-17-07537.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lingor P., Teusch N., Schwarz K., Mueller R., Mack H., Bahr M., Mueller B. K. Inhibition of Rho kinase (ROCK) increases neurite outgrowth on chondroitin sulphate proteoglycan in vitro and axonal regeneration in the adult optic nerve in vivo. J. Neurochem. (2007);103:181–189. doi: 10.1111/j.1471-4159.2007.04756.x. [DOI] [PubMed] [Google Scholar]
- 15.Low C. B., Liou Y. C., Tang B. L. Neural differentiation and potential use of stem cells from the human umbilical cord for central nervous system transplantation therapy. J. Neurosci. Res. (2008);86:1670–1679. doi: 10.1002/jnr.21624. [DOI] [PubMed] [Google Scholar]
- 16.McBeath R., Pirone D. M., Nelson C. M., Bhadriraju K., Chen C. S. Cell shape, cytoskeletal tension, and RhoA regulate stem cell lineage commitment. Dev. Cell. (2004);6:483–495. doi: 10.1016/S1534-5807(04)00075-9. [DOI] [PubMed] [Google Scholar]
- 17.McKerracher L., David S., Jackson D. L., Kottis V., Dunn R. J., Braun P. E. Identification of myelin-associated glycoprotein as a major myelin-derived inhibitor of neurite growth. Neuron. (1994);13:805–811. doi: 10.1016/0896-6273(94)90247-X. [DOI] [PubMed] [Google Scholar]
- 18.Mitchell K. E.,, Weiss M. L., Mitchell B. M., Martin P., Davis D., Morales L., Helwig B., Beerenstrauch M., Abou-Easa K., Hildreth T., Troyer D., Medicetty S. Matrix cells from Wharton's jelly form neurons and glia. Stem Cells. (2003);21:50–60. doi: 10.1634/stemcells.21-1-50. [DOI] [PubMed] [Google Scholar]
- 19.Miyamoto S., Del Re D. P., Xiang S. Y., Zhao X., Florholmen G., Brown J. H. Revisited and revised: is RhoA always a villain in cardiac pathophysiology? J. Cardiovasc. Transl. Res. (2010);3:330–343. doi: 10.1007/s12265-010-9192-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Monnier P. P., Sierra A., Schwab J. M., Henke-Fahle S., Mueller B. K. The Rho/ROCK pathway mediates neurite growthinhibitory activity associated with the chondroitin sulfate proteoglycans of the CNS glial scar. Mol. Cell. Neurosci. (2003);22:319–330. doi: 10.1016/S1044-7431(02)00035-0. [DOI] [PubMed] [Google Scholar]
- 21.Mueller B. K., Mack H., Teusch N. Rho kinase, a promising drug target for neurological disorders. Nat. Rev. Drug Discov. (2005);4:387–398. doi: 10.1038/nrd1719. [DOI] [PubMed] [Google Scholar]
- 22.Pacary E., Legros H., Valable S., Duchatelle P., Lecocq M., Petit E., Nicole O., Bernaudin M. Synergistic effects of CoCl(2) and ROCK inhibition on mesenchymal stem cell differentiation into neuron-like cells. J. Cell Sci. (2006);119:2667–2678. doi: 10.1242/jcs.03004. [DOI] [PubMed] [Google Scholar]
- 23.Prinjha R., Moore S. E., Vinson M., Blake S., Morrow R., Christie G., Michalovich D., Simmons D. L., Walsh F. S. Inhibitor of neurite outgrowth in humans. Nature. (2000);403:383–384. doi: 10.1038/35000287. [DOI] [PubMed] [Google Scholar]
- 24.Sanchez-Ramos J., Song S., Cardozo-Pelaez F., Hazzi C., Stedeford T., Willing A., Freeman T. B., Saporta S., Janssen W., Patel N., Cooper D. R., Sanberg P. R. Adult bone marrow stromal cells differentiate into neural cells in vitro. Exp. Neurol. (2000);164:247–256. doi: 10.1006/exnr.2000.7389. [DOI] [PubMed] [Google Scholar]
- 25.Seo J. H., Cho S. R. Neurorestoration induced by mesenchymal stem cells: potential therapeutic mechanisms for clinical trials. Yonsei Med. J. (2012);53:1059–1067. doi: 10.3349/ymj.2012.53.6.1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sivasankaran R., Pei J., Wang K. C., Zhang Y. P., Shields C. B., Xu X. M., He Z. PKC mediates inhibitory effects of myelin and chondroitin sulfate proteoglycans on axonal regeneration. Nat. Neurosci. (2004);7:261–268. doi: 10.1038/nn1193. [DOI] [PubMed] [Google Scholar]
- 27.Vinson M., Strijbos P. J., Rowles A., Facci L., Moore S. E., Simmons D. L., Walsh F. S. Myelin-associated glycoprotein interacts with ganglioside GT1b. A mechanism for neurite outgrowth inhibition. J. Biol. Chem. (2001);276:20280–20285. doi: 10.1074/jbc.M100345200. [DOI] [PubMed] [Google Scholar]
- 28.Wang K. C., Koprivica V., Kim J. A., Sivasankaran R., Guo Y., Neve R. L., He Z. Oligodendrocyte-myelin glycoprotein is a Nogo receptor ligand that inhibits neurite outgrowth. Nature. (2002);417:941–944. doi: 10.1038/nature00867. [DOI] [PubMed] [Google Scholar]
- 29.Winton M. J., Dubreuil C. I., Lasko D., Leclerc N., McKerracher L. Characterization of new cell permeable C3-like proteins that inactivate Rho and stimulate neurite outgrowth on inhibitory substrates. J. Biol. Chem. (2002);277:32820–32829. doi: 10.1074/jbc.M201195200. [DOI] [PubMed] [Google Scholar]
- 30.Woodbury D., Schwarz E. J., Prockop D. J., Black I. B. Adult rat and human bone marrow stromal cells differentiate into neurons. J. Neurosci. Res. (2000);61:364–370. doi: 10.1002/1097-4547(20000815)61:4&lt;364::AID-JNR2&gt;3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]