Abstract
Eucommia ulmoides Oliv. Bark (EUE) is commonly used for the treatment of hypertension, rheumatoid arthritis, lumbago, and ischialgia as well as to promote longevity. In this study, we tested the effects of EUE aqueous extract in graded doses to protect and enhance cognition in scopolamine-induced learning and memory impairments in mice. EUE significantly improved the impairment of short-term or working memory induced by scopolamine in the Y-maze and significantly reversed learning and memory deficits in mice as measured by the passive avoidance and Morris water maze tests. One day after the last trial session of the Morris water maze test (probe trial session), EUE dramatically increased the latency time in the target quadrant in a dose-dependent manner. Furthermore, EUE significantly inhibited acetylcholinesterase (AChE) and thiobarbituric acid reactive substance (TBARS) activities in the hippocampus and frontal cortex in a dose-dependent manner. EUE also markedly increased brain-derived neurotrophic factor (BDNF) and phosphorylation of cAMP element binding protein (CREB) in the hippocampus of scopolamine-induced mice. Based on these findings, we suggest that EUE may be useful for the treatment of cognitive deficits, and that the beneficial effects of EUE are mediated, in part, by cholinergic signaling enhancement and/or protection.
Keywords: Eucommia ulmoides Oliv. Bark, Scopolamine, Learning and memory, Brain-derived neurotrophic factor, cAMP element binding protein, Alzheimer’s disease
INTRODUCTION
Alzheimer’s disease (AD) has been estimated to account for 50-60% of dementia cases in persons over 65 years of age (Francis et al., 1999). AD is a progressive neurodegenerative disease and a major and increasing public health concern. The characteristic pathological features of the central nervous system (CNS) in AD are senile plaques, neurofibrillary tangle formation, aberrant oxidative and inflammatory processes, and neurotransmitter disturbances. Moreover, a cholinergic deficit is a consistent neuropathological occurrence associated with memory loss and has been correlated with the severity of AD (Collerton, 1986; Bierer et al., 1995). The restoration of cholinergic function remains a rational target for the treatment of AD symptoms.
Scopolamine, a muscarinic cholinergic receptor antagonist, impairs learning and memory in rodents and humans, especially the processes of learning acquisition and short-term memory (Beatty et al., 1986; Collerton, 1986; Kopelman and Corn, 1988). Cholinergic neurons in the CNS are involved in learning and memory in both humans and animals (Bartus et al., 1982). In this context, scopolamine triggers reactive oxygen species (ROS) formation and induces free radical injury (El-Sherbiny et al., 2003; Kwon et al., 2010). A complex anti-oxidant defense system has evolved to regulate oxidative stress, and natural anti-oxidants participate in this regulatory process. It is therefore of interest to identify anti-oxidant compounds in natural products and herbal preparations for therapeutic applications (Singh et al., 2003; Wang et al., 2009).
Eucommia ulmoides Oliv. Bark (EUE) is a traditional tonic medicine used in Korea, China, and Japan. As a folk medicine, EUE is used to fortify the muscles and lungs, lower blood pressure, prevent miscarriage, improve the tone of liver and kidneys, and promote longevity (Lee et al., 2005). Moreover, the aqueous extract of EUE, commonly known as Du-zhong tea, is a popular folk drink in Japan and is used as a functional health food (Singh et al., 2003; Wang et al., 2009). In a previous report, we demonstrated that EUE had protective effects on amyloid beta25-35-induced cognitive dysfunction in mice (Kwon et al., 2011). Additionally, our studies revealed that EUE has neuroprotective activities against hydrogen peroxide-induced neuronal cell death via mitochondrial membrane dysfunction and oxidative stress, as well as phosphorylation of mitogenactivated protein kinase and phosphatidylinositol 3-kinase/Akt (Kwon et al., 2012). However, little is known about the role of EUE in the brain or its therapeutic role in dementia. Therefore, in this study, we investigated the possible mechanism underlying the effects of EUE on scopolamine-induced cognitive deficits in mice using behavioral and biochemical tests.
MATERIALS AND METHODS
Animals
Male ICR mice (4-weeks-old, 18-20 g) were purchased from Koatech Co., Ltd. (Pyongtaek, Korea). Mice were housed 10 per cage, allowed access to water and food ad libitum, and maintained in constant temperature (23 ± 1℃) and humidity (55 ± 5%) conditions under a 12 h light/dark cycle (lights on 07:00 to 19:00 h). All experiments were conducted in accord with the NIH Guide for the Care and Use of Laboratory Animals and with the approval of the Institutional Animal Care and Use Committee of Sungkyunkwan University.
Preparation of EUE extract
Dried stem bark of EUE was purchased from Kyung-Dong Oriental medicine market (Seoul, Korea). The stem bark of EUE was originally collected in Andong, South Korea in June 2008 and was identified by professor Sun Yeou Kim (College of Pharmacy, Gachon University, Incheon, Korea). A voucher specimen (KSYHP-EC-002) was deposited at the herbarium of the Graduate School of East-West Medical Science at Kyung Hee University. Dried EUE was cut into small pieces and extracted three times in hot water using reflux extraction equipment (70℃) with a cooling system (40℃) for 1 h, and then the aqueous extract was filtered through paper (Whatman No. 2, USA). The supernatants were concentrated under reduced pressure with a vacuum rotary evaporator (EYELA, N-1000, Japan), followed by lyophilization. From 1 kg of dried EUE, 100 g of dried extract was obtained (yield 10%) and stored at -20℃ until use.
Drugs and chemicals
Acetylthiocholine iodide, 5,5'-dithiobis(2-nitrobezoic acid) (DTNB), scopolamine hydrobromide, and anti-β-actin antibody were purchased from Sigma Chemical Co. (St. Louis, MO, USA). Donepezil hydrochloride monohydrate (donepezil, DNZ) were provided by CJ Pharmaceutical Co., Ltd. polyclonal anti-brain-derived neurotrophic factor (BDNF) antibody was obtained from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA, USA). Rabbit monoclonal anti-phospho cAMP element binding protein (CREB) and anti-CREB antibodies were purchased from Epitomics (Burlingame, CA, USA). All other materials were of the highest grade available.
Spontaneous alternation behavior Y-maze test
The spontaneous alternation behavior Y-maze test is a horizontal maze (30 cm long and 5 cm wide, with walls 12 cm high) with three arms (labeled A, B, and C). The maze floor and walls are constructed of dark grey, polyvinyl plastic. Mice were initially placed within one arm, and the number of alternations (i.e., consecutive entry sequences of ABC, CAB, or BCA but not BAB) and the number of arm entries were manually recorded for each mouse over an 8 min period. One hour before each test, the mice were given EUE (5, 10, or 20 mg/kg, p.o.) or DNZ (5 mg/kg, p.o.). After 30 min, memory impairment was induced by administering scopolamine (0.5 mg/kg, i.p.). The control group received distilled water instead of EUE. The percentage alternation was calculated according to the following equation: Percentage alternation=[(Number of alternations)/(Total arm entries-2)]×100. The number of arm entries per trial was used as an indicator of locomotor activity. The Y-maze arms were cleaned with 10% ethanol between tests to remove odors and residues.
Step-through passive avoidance test
Assessment of the training or test trials of the passive avoidance test was carried out in identical illuminated and non-illuminated compartments (12×10×12 cm) containing 2-mm stainless steel rods spaced 0.5 cm apart. A 50 W lamp positioned 1 meter above both chambers illuminated the apparatuses. Briefly, the mice underwent two separate trials, a training trial and a test trial 24 h later. For the training trial, mice were initially placed in the clear chamber. When they entered the dark chamber, the door closed and a 20-sec electrical foot shock (0.4 mA) was delivered through the stainless steel rods. One hour before each training trial, mice were given EUE (5, 10, or 20 mg/kg, p.o.) or donepezil (5 mg/kg, p.o.). After 30 min, memory impairment was induced by administering scopolamine (0.5 mg/kg, i.p.). Twenty-four hours after the training trial, mice were placed in the illuminated chamber for the test trial. The time for the mouse to enter the dark compartment after the door opening was defined as latency for both training and test trials. Latencies were recorded for up to 300 s. To avoid a ceiling effect in unimpaired animals, EUE alone was administered 1 h before the training trial without scopolamine treatment. The intensity of electrical foot shock was set at 0.25 mA for 2 s. This lower intensity shock allowed for a behavioral window through which to detect any enhancing effect of EUE.
Morris water maze test
The Morris water maze test was performed as described in our previous reports (Kwon et al., 2009; Kwon et al., 2011). The Morris water maze is a circular pool (100 cm in diameter and 35 cm in height) with a featureless inner surface. The circular pool was filled with water and nontoxic water-soluble black dye (20 ± 1℃). The pool was divided into four quadrants of equal area. A black platform (8 cm in diameter and 10 cm in height) was centered in one of the four quadrants of the pool and submerged 1 cm below the water surface so that it was invisible at water level. The location of each swimming mouse, from the start position to the platform, was monitored by a video tracking system (Ethovision, Noldus, Wageningen, Netherlands). The first experimental day was dedicated to swim training for 60 s in the absence of the platform. The mice were then given two trial sessions each day for four consecutive days, with an inter-trial interval of 15 min, and the escape latencies were recorded. This parameter was averaged for each session of trials and for each mouse. The point of entry of the mouse into the pool and the location of the platform for escape remained unchanged between trials 1 and 2 but was changed each day thereafter. Once the mouse located the platform, it was permitted to remain on it for 10 s. If the mouse did not locate the platform within 120 s, it was placed on the platform for 10 s and then removed from the pool. On the day after the last training trial session, the mice were subjected to a probe trial session in which the platform was removed from the pool, and mice were allowed to search for 60 s. A record was kept of the time each mouse spent swimming in the pool quadrant where the platform had been previously located.
Thiobarbituric acid reactive substance activity assay
Thiobarbituric acid reactive substance (TBARS) activity was determined using a TBARS assay kit (Cayman Chemical Co., Ann Arbor, MI, USA) according to the manufacturer’s instructions. Mice were euthanized 60 min after treatment, and their brains were removed following the Morris water maze test. Three mouse brains were used per assay. Malondialdehyde (MDA) levels were calculated as micromole per brain tissue weight.
Acetylcholinesterase activity assay
Acetylcholinesterase (AChE) activity was measured using the method of Ellman et al. (1961) with slight modifications (Cheng and Tang, 1998). Mice were euthanized 60 min after treatment, and the brains were removed following the Morris water maze test. Three mouse brains were used per assay. The frontal cortex and hippocampus were dissected from each brain and rapidly homogenized using a rotary homogenizer (MagNa Lyser, Roche Diagnostics, GmbH, Germany) containing sodium phosphate buffer (0.1 mM, pH 7.4); the homogenates were centrifuged at 14,000×g for 15 min at 4℃. The supernatant was the enzyme source for the assay. Acetylthiocholine iodide solution (25 μl, 75 mM) and 100 μl of buffered Ellman's reagent (10 mM DTNB and 15 mM sodium bicarbonate) were reacted at room temperature for 10 min. Absorbance was measured at 412 nm using a UV spectrometer (UV-1700 PharmaSpec, SHIMADZU Co. Ltd., Japan) immediately after adding the enzyme source (8 μl) to the reaction mixture. AChE activity was calculated as nanomole per reaction minutes per brain tissue weight.
Western blot analysis
Mice were sacrificed by cervical dislocation 60 min after treatment, and their brains were removed following the Morris water maze test. Isolated hippocampal tissues from both hemispheres were promptly excised and homogenized using a rotary homogenizer with 200 μl of ice-cold lysis T-per tissue protein extraction buffer (Thermo Scientific, Rockford, IL, USA) containing protease and phosphatase inhibitor cocktails (Roche Diagnostics, GmbH, Mannheim, Germany) and incubated on ice for 30 min. After centrifugation at 10,000×g for 15 min, the supernatant was separated and stored at -70℃. The protein concentration was determined using a protein assay kit (Thermo Scientific, Rockford, IL, USA). Proteins were subjected to 12.5% SDS-polyacrylamide gel separation under reducing conditions, transferred onto a polyvinylidene difluoride transfer membrane (Pall Corporation, Pensacola, FL, USA) in transfer buffer [25 mM Tri-HCl buffer (PH .74) containing 192 mM glycine and 20% v/v methanol] for 1 h at 4℃, and blocked with 5% non-fat milk in 0.5 mM Tris-HCl (pH 7.5), 150 mM NaCl, and 0.1% Tween-20 for 1 h at room temperature. The membrane was subsequently incubated with one of the primary antibodies overnight at 4℃ (each of the following antibodies at a dilution of 1:500; anti-BDNF, 1:1000; phospho-CREB, and 1:500; CREB). After three washes with TBST (Tris-buffered saline with 0.1% Tween-20), the blots were incubated with horseradish-peroxidase-conjugated secondary antibodies (Jackson ImmunoResearch Laboratories, Inc., West Grove, PA, USA) in TBST with 5% non-fat milk at a 1:5000 dilution for 1 h at room temperature. The blots were then again washed five times in TBST buffer. Blots were developed using the enhanced chemiluminescence detection method by immersing them for 5 min in a mixture of ECL reagents A and B (Anigen, Hwaseong, Korea) at the ratio 1:1 and exposing them to photographic film. Protein bands were quantified by densitometric analysis using Image Gauge 4.0 software (Fujifilm, Stamford, CT, USA).
Statistical analyses
Data were expressed as mean ± S.E.M. and analyzed with Prism 5.0 software. Data from the Y-maze, passive avoidance, and probe trial of the Morris water maze tests, DPPH free radical scavenging, ABTS radical cation scavenging, AChE, TBARS activity assays, and Western blot analysis were analyzed by one-way analysis of variance (ANOVA) followed by Newman-Keuls test. In the Morris water maze test, escape latency values were analyzed by two-way ANOVA followed by the Bonferroni post-test using Prism 5.0 (Graphpad Software, Inc). Statistical significance was set at p<0.05.
RESULTS
Effects of EUE on the spontaneous alternation behavior Y-maze test
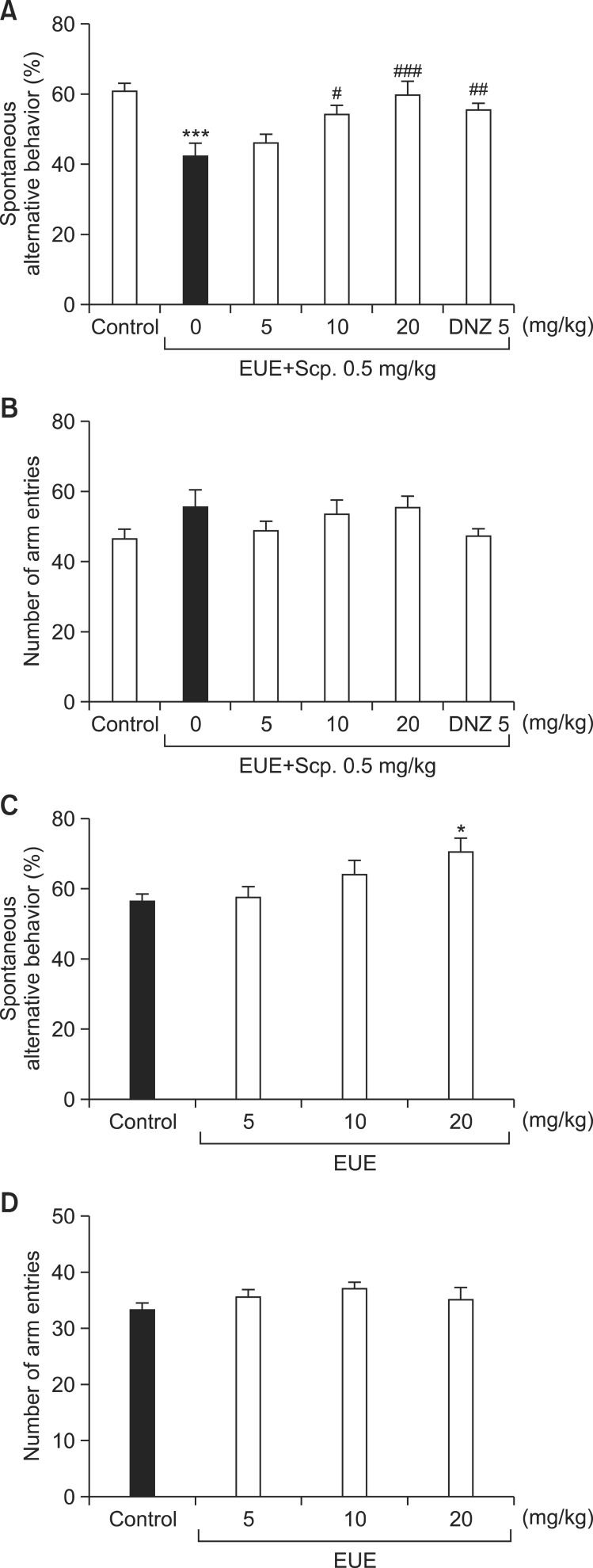
Scopolamine significantly decreased the spontaneous alternation behavior compared with the control group (Fig. 1A, p<0.001). However, this decreased spontaneous alternation behavior induced by scopolamine was significantly inhibited by EUE (10 and 20 mg/kg) or DNZ (5 mg/kg) (p<0.05, p<0.01, and p<0.001, respectively). Scopolamine did not significantly increase the number of arm entries compared to the control group (Fig. 1B). Interestingly, EUE alone increased spontaneous alternation behavior at dose of 20 mg/kg without changing the number of arm entries (Fig. 1C, p<0.05 and Fig. 1D).
Fig. 1. Effects of EUE on the spontaneous alternation behavior Y-maze test. EUE (5, 10, and 20 mg/kg, p.o.) or donepezil (DNZ, 5 mg/kg, p.o.) was administered to mice 60 min before the tests. Thirty minutes later, the mice were treated with scopolamine (Scp., 0.5 mg/kg, i.p.) and tested in the Y-maze (A, B). To assess the effect of EUE (5, 10, and 20 mg/kg, p.o.) in the Y-maze, EUE (5, 10, and 20 mg/kg, p.o.) was administered to mice 60 min before the tests (C, D). Data are presented as the mean ± S.E.M. (n=10/group). *p<0.05 and ***p<0.001 compared with the control group. #p<0.05, ##p<0.01, and ###p<0.001 compared with the scopolamine-treated group.
Effects of EUE on the step-through passive avoidance test
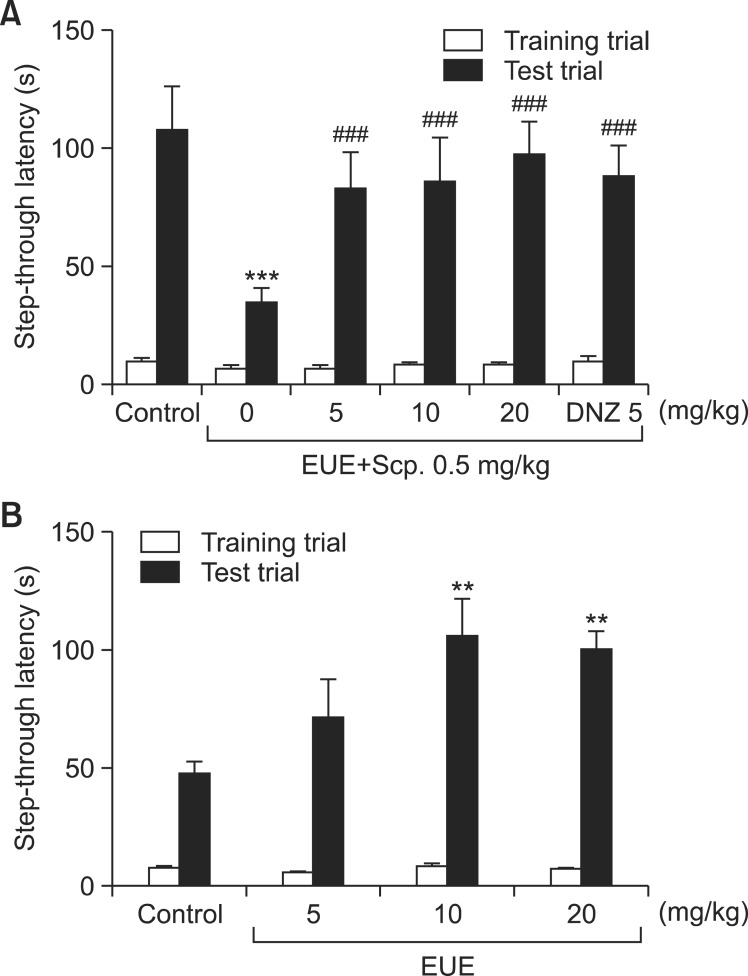
Scopolamine decreased the step-through latency time (Fig. 2A, p<0.001), and EUE (5, 10, and 20 mg/kg) or DNZ (5 mg/kg) blocked this decrease (p<0.001, respectively). The step-through latency time during the training trial was not affected by any drug treatment. Furthermore, the groups treated with EUE (10 and 20 mg/kg) had significantly improved stepthrough latency times compared with the control group (Fig. 2B, p<0.01, respectively).
Fig. 2. Effects of EUE on the step-through passive avoidance test. EUE (5, 10, and 20 mg/kg, p.o.) or donepezil (DNZ, 5 mg/kg, p.o.) was administered to mice 60 min before the test. Thirty minutes later, the mice were treated with scopolamine (Scp., 0.5 mg/kg, i.p.) and tested for passive avoidance (A). To assess the effect of EUE (5, 10, and 20 mg/kg, p.o.) on passive avoidance, EUE (5, 10, and 20 mg/kg, p.o.) was administered to mice 60 min before the tests (B). Data are presented as the mean ± S.E.M. (n=19-21/group). **p<0.01 and ***p<0.001 compared with the control group. ###p<0.001 compared with the scopolamine-treated group.
Effects of EUE on the Morris water maze test
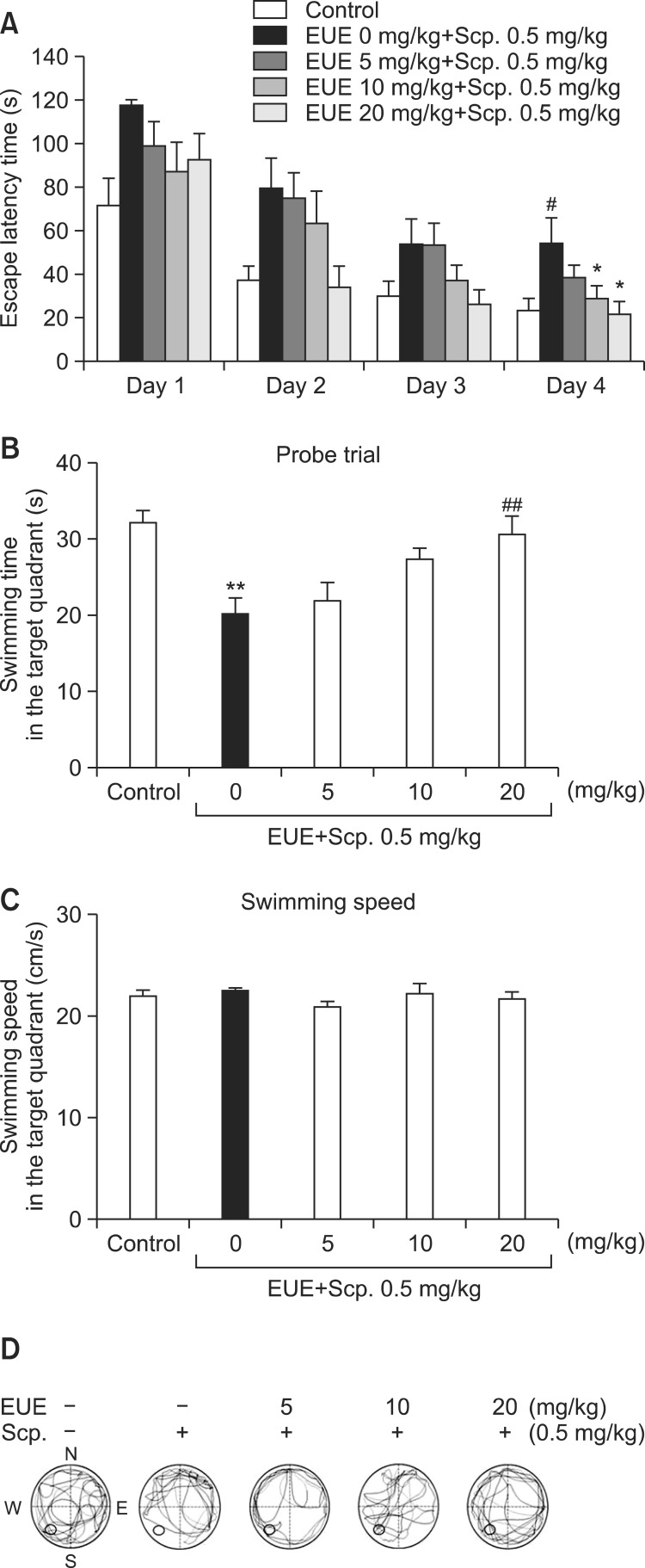
The control group rapidly learned the location of the platform and would swim quickly across the pool to reach it. Scopolamine significantly delayed the escape latency time on day 4 compared with the control group (Fig. 3A, p<0.05). Moreover, EUE (10 and 20 mg/kg) significantly decreased escape latency time on day 4 (p<0.05, respectively). Following the last day of trial sessions, EUE (20 mg/kg) significantly increased the time in the target quadrant after the platform was removed (Fig. 3B, p<0.01). Thus, EUE did not cause any marked effects on swimming speed in the target quadrant among the groups (Fig. 3C).
Fig. 3. Effect of EUE on average latency time (A), probe trial (B), and swimming speed (C) in trial sessions of the Morris water maze test. At 60 min before the first trial session and probe trial session, EUE (5, 10, and 20 mg/kg, p.o.) was administered to the mice. Thirty minutes later, the mice were treated with scopolamine (Scp., 0.5 mg/kg, i.p.) and tested in the Morris water maze test. Probe trial sessions were performed for 60 sec. Representative swimming paths of mice from each group in the Morris water maze test on the training trial day 4 (D). Data represent mean ± S.E.M. (n=10/group). *p<0.05 and **p<0.01 compared with the control group. #p<0.05 and ##p<0.01 compared with the scopolamine-treated group.
Effects of EUE on MDA levels in the hippocampus and frontal cortex
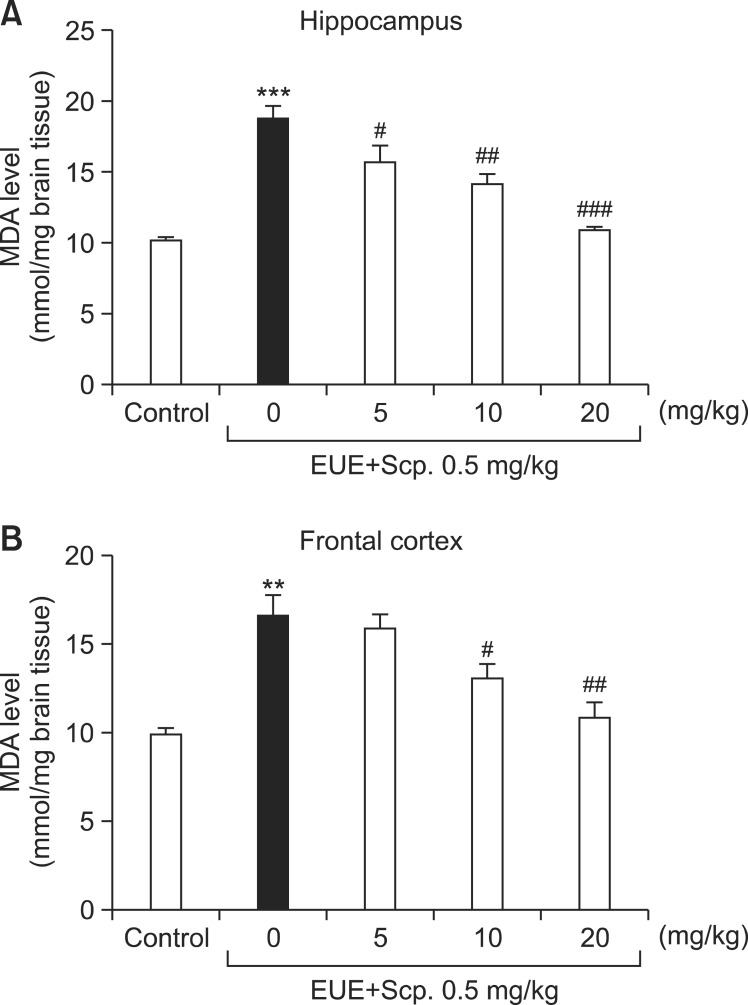
Scopolamine-treated mice experienced significantly increased MDA levels in the hippocampus and frontal cortex compared with control mice (Fig. 4A, p<0.001 and Fig. 4B, p<0.01, respectively). EUE (5, 10, and 20 mg/kg) significantly reduced MDA levels in the hippocampus by 83.97%, 75.61%, and 58.36%, compared with scopolamine-treated mice (p<0.05, p<0.01, and p<0.001, respectively). EUE (10 and 20 mg/kg) also significantly reduced MDA levels in the frontal cortex by 79.10% and 65.68%, compared with scopolamine-treated mice (p<0.05 and p<0.01, respectively).
Fig. 4. Effects of EUE on MDA levels in the hippocampus (A) and frontal cortex (B). Animals were decapitated 60 min after probe trial sessions of the Morris water maze test, and the hippocampus and frontal cortex were dissected to assay TBARS activity. Data represent mean ± S.E.M. (n=3/group). **p<0.01 and ***p<0.001 compared with the control group. #p<0.05, ##p<0.01, and ###p<0.001 compared with the scopolamine-treated group.
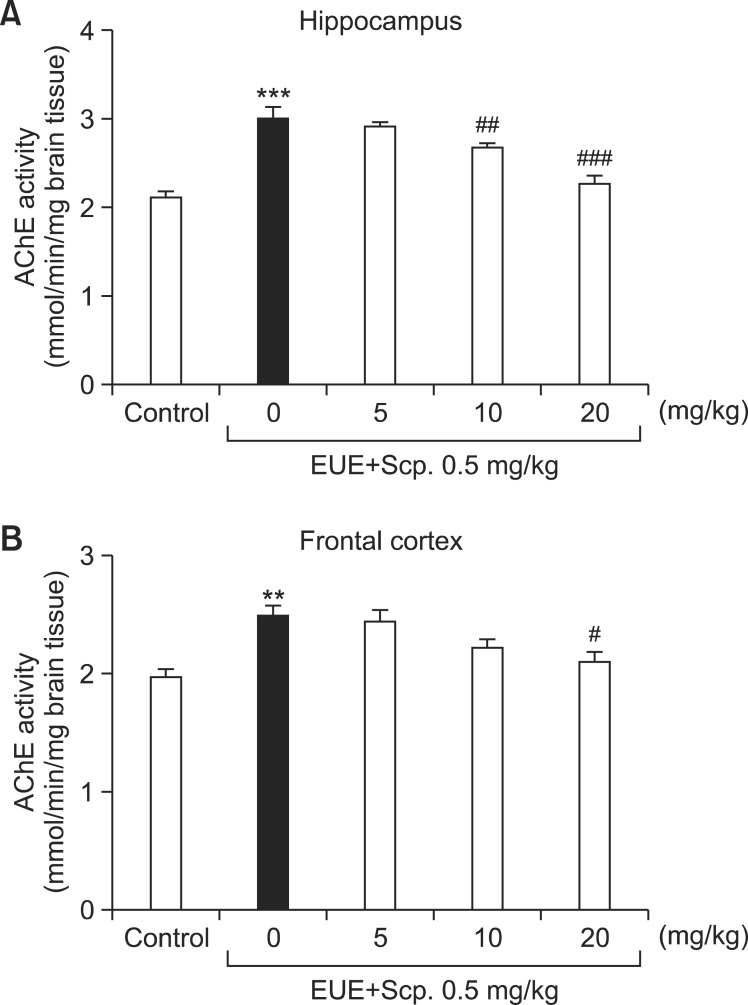
Effects of EUE on AChE activity in the hippocampus and frontal cortex
Scopolamine-treated mice showed significantly increased AChE activity in the hippocampus and frontal cortex compared with control mice (Fig. 5A, p<0.001 and Fig. 5B, p<0.01, respectively). EUE (10 and 20 mg/kg) significantly inhibited AChE activity in the hippocampus by 87.86% and 74.42%, compared with the scopolamine-treated mice (p<0.05 and p<0.01, respectively). EUE (20 mg/kg) also significantly inhibited AChE activity in the frontal cortex by 83.93% compared with the scopolamine-treated mice (p<0.05).
Fig. 5. Effects of EUE on AChE activity in the hippocampus (A) and frontal cortex (B). Animals were decapitated 60 min after probe trial sessions of the Morris water maze test, and the hippocampus (A) and frontal cortex (B) were dissected to assay AChE activity. Data represent mean ± S.E.M. (n=3/group). **p<0.01 and ***p<0.001 compared with the control group. #p<0.05, ##p<0.01, and ###p<0.001 compared with the scopolamine-treated group.
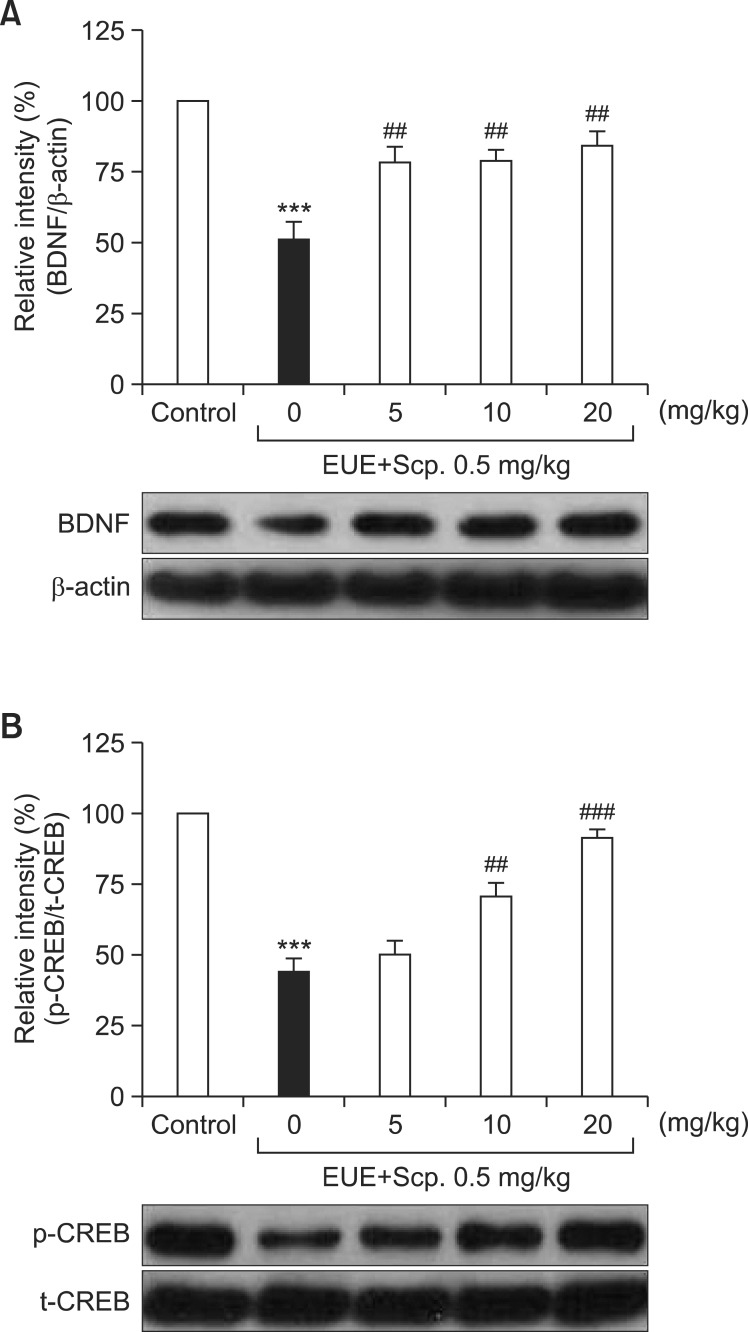
Effects of EUE on BDNF and phosphorylation of CREB expression levels in the hippocampus
Statistical analysis revealed that a significant group effect of EUE was observed on the level of memory-related proteins in the hippocampus. The scopolamine-treated group had significantly decreased BDNF and phosphorylation of CREB in the hippocampus to 50.96% and 44.04%, compared to that in the control group (Fig. 6, p<0.001, respectively). EUE (5 mg/kg) significantly increased BDNF levels in the hippocampus to 78.24% compared to that of the control group (p<0.01). EUE (10 mg/kg) also significantly increased BDNF and phosphorylation of CREB levels in the hippocampus to 78.78% and 70.89% compared to control groups (p<0.01, respectively). Finally, EUE (20 mg/kg) significantly inhibited down-regulation of BDNF and phosphorylated CREB levels in the hippocampus to 84.35% and 91.95% compared to control groups (p<0.01 and p<0.001, respectively).
Fig. 6. Effects of EUE on BDNF (A) and phosphorylation of CREB (B) expression levels in the hippocampus. Animals were decapitated 60 min after probe trial sessions of the Morris water maze test. The hippocampus was dissected for Western blot analysis. Data represent mean ± S.E.M. (n=3/group). ***p<0.001 compared with the control group. ##p<0.01 and ###p<0.001 compared with the scopolamine-treated group.
DISCUSSION
In the present study, we investigated the neuroprotective effects of EUE on learning and memory impairments in mice induced by scopolamine using Y-maze and passive avoidance and Morris water maze tests. We also investigated the effects of EUE on AChE and TBARS activity assays. Moreover, we confirmed for the first time the effects of EUE on the levels of cognitive-related biochemical parameters, including BDNF and CREB in the hippocampus.
Spontaneous alternation behavior in the Y-maze is a surrogate measure of short-term and working memory (Kim et al., 2006; Kwon et al., 2009). EUE administration (10 and 20 mg/kg) or DNZ (5 mg/kg) significantly increased spontaneous alternation behavior in mice and attenuated the scopolamineinduced decrease in spontaneous alternation behavior. EUE alone at 20 mg/kg significantly increased spontaneous alternation behavior without changing the number of arm entries. This result indicates that EUE may improve short-term and working memory by rescuing and/or enhancing the acetylcholine system.
The passive avoidance test is an indicator of long-term memory (LeDoux, 1993). Here, EUE administration (5, 10, and 20 mg/kg) or DNZ (5 mg/kg) ameliorated scopolamineinduced reductions in step-through latency time but did not change latencies during the training trials. EUE alone at 5, 10, and 20 mg/kg also increased the spontaneous step-through latency time. These results suggest that EUE reduces scopolamine- induced long-term memory impairments through rescue of the acetylcholine system.
In our Morris water maze tests, mice in the control group rapidly reduced the daily escape latency time to find the location of the platform from day 2 and achieved a stable escape latency time. By contrast, scopolamine-treated mice did not reduce escape latencies times from day 1 to day 4, indicating long-term memory impairment induced in the scopolaminetreated group. EUE (10 and 20 mg/kg) significantly shortened the escape latency time prolonged by scopolamine on day 4. During the probe trial session, EUE (20 mg/kg) dramatically improved the swimming time within the target quadrant compared with the scopolamine-treated group. The swimming speed during the probe trial session showed no significant difference in the placebo group. Consequently, these results suggest that EUE improves long-term and reference memory impairments induced by scopolamine treatment, and that the ameliorative effect of EUE on long-term and reference memory impairment is due to rescue of the acetylcholine system from deficits caused by scopolamine treatment.
In order to elucidate the underlying mechanism of action of EUE, we assessed the effect of EUE on scopolamine-induced TBARS activity. TBARS activity represents an important marker for lipid peroxidation in the learning and memorydeficient mouse brains. Because oxidative stress contributes significantly to the perturbation of calcium homeostasis and subsequent apoptosis, as seen in AD patients (Joseph et al., 1997; Yu et al., 2001; Annunziato et al., 2003), many clinical studies have reported strong evidence that oxidative stress is involved in the pathogenesis of AD (Lovell et al., 1995; Marcus et al., 1998). Scopolamine significantly increases MDA levels in the hippocampus and frontal cortex (Ben-Barak and Dudai, 1980; Sakurai et al., 1998; Fan et al., 2005; Jeong et al., 2008). These results are in agreement with our findings that brain MDA levels were increased in the scopolamine-treated group. We found that EUE significantly inhibited MDA levels in the hippocampus and frontal cortex, suggesting that the neuroprotective effects of EUE may be due to anti-oxidant action. Based on the results of behavioral and biochemical studies, we hypothesize that EUE may act directly as a free radical scavenger and/or regulator to inhibit oxidative stress in brains treated with scopolamine. These results are supported by our recent report demonstrating that the anti-oxidant capacity of EUE inhibits ROS production induced by oxidative stress in SH-SY5Y cells (Kwon et al., 2012).
To investigate the underlying mechanism of EUE in learning and memory impairments induced by scopolamine, the activities of cholinergic marker enzyme were determined following the Morris water maze test. Loss of cholinergic cells, particularly in the basal forebrain, is accompanied by loss of the neurotransmitter acetylcholine (Selkoe, 1994). A decrease in acetylcholine in the brains of patients with AD appears to be a critical element in producing dementia (Becker et al., 1988). In chronic dementia, re-uptake of acetylcholine decreases in neurons of the frontal cortex and hippocampus, and changes in increasing AChE activity occur. In this experiment, we assessed the effects of EUE on scopolamine-induced AChE activity in the hippocampus and frontal cortex. Scopolamine significantly increased AChE activity in the hippocampus and frontal cortex. These results are in agreement with those of two previous studies (Becker et al., 1988; Sakurai et al., 1998). We also found that EUE (10 or 20 mg/kg) significantly inhibited AChE activity in the hippocampus and frontal cortex. These findings agree with our behavioral data and together suggest that EUE inhibits scopolamine-induced learning and memory impairments, in part, by mediating AChE activity inhibition.
To investigate the molecular mechanism of the neurogenesis- modulating action of the cholinergic system, the levels of BDNF and phosphorylation of CREB expression in the hippocampus after EUE treatment with scopolamine were investigated. Brain plasticity is regulated by several factors including BDNF, a member of the neurotrophin family of growth factors that is widely expressed throughout the mammalian brain and plays a crucial role in development, maintenance and function of the CNS (Komulainen et al., 2008). Accumulating data have suggested that neuronal activity regulates BDNF expression, which, in turn, modulates synaptogenesis, synaptic plasticity, and memory formation (Yamada and Nabeshima, 2003). The activation of CREB is also well linked to neuronal survival and synaptic plasticity. In addition, CREB is recognized as a very solid molecular marker of memory processing in the hippocampus for spatial leaning, and the activation of the CREB signaling pathway plays an important role in spatial memory formation (Mizuno et al., 2002; Alberini, 2009). Furthermore, in the AD post-mortem brain, there is a decrease in the levels of CREB-regulated BDNF (Phillips et al., 1991). Our Western blot analysis data showed that scopolamine significantly reduced BDNF and activation of CREB expression levels in the hippocampus, and their reductions were found to be proportional to memory deficit. However, treatment with EUE significantly prevented scopolamine-induced reduction of BDNF and activation of CREB expression levels. Our studies have shown altered BDNF and CREB expression levels in the hippocampus with dementia, which might be attributed to the reduction of cholinergic activity. On the other hand, considering the enhancing effects of EUE on learning and memory in Y-maze and passive avoidance tests, we suggest that research on the mechanisms underlying learning and memory formation and enhancement has revealed that certain key molecules are involved in these processes. For example, the phosphorylation of CREB is required for memory formation and storage in the hippocampus and BDNF contributes to long-term potentiation (O’Connell et al., 2000; Jia et al., 2010). Moreover, recent findings regarding neurogenesis are currently being widely applied in the field of learning and memory (Kempermann, 2008). In this study, we have been observed increased pCREB and BDNF expression in the hippocampus due to the treatment of EUE, suggesting that EUE-induced increases of BDNF expression could be mediated by phosphorylation of CREB. However, further studies are needed to clarify this issue.
To our knowledge, this is the first report providing evidence that the potent neuroprotective effects of EUE are linked to inhibition of AChE and TBARS activities in the hippocampus and frontal cortex of mice with scopolamine-induced amnesia. Moreover, EUE might improve learning and memory deficits induced by scopolamine by increasing BDNF and CREB expression in the hippocampus.
In conclusion, our results suggest that EUE has anti-amnesic activity, and that it may hold significant therapeutic value for alleviating cognitive deficits. Moreover, the mechanism(s) of the anti-amnesic effects of EUE may be in part involved in the activation of the cholinergic system via inhibition AChE and TBARS activities, as well as protection of BDNF and activation of CREB expression. Therefore, the neuroprotective actions of EUE may potentially be applied in the treatment of neurodegenerative diseases such as AD.
Acknowledgments
This research was supported by a grant from Basic Science Research Program through the National Research Foundation of Korea (MRC, 2012-0009851) funded by the Ministry of Education, Science and Technology, Republic of Korea.
References
- 1.Alberini C. M. Transcription factors in long-term memory and synaptic plasticity. Physiol. Rev. (2009);89:121–145. doi: 10.1152/physrev.00017.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Annunziato L., Amoroso S., Pannaccione A., Cataldi M., Pignataro G., D'Alessio A., Sirabella R., Secondo A., Sibaud L., Di Renzo G. F. Apoptosis induced in neuronal cells by oxidative stress: role played by caspases and intracellular calcium ions. Toxicol. Lett. (2003);139:125–133. doi: 10.1016/S0378-4274(02)00427-7. [DOI] [PubMed] [Google Scholar]
- 3.Bartus R. T., Dean R. L., 3rd, Beer B., Lippa A. S. The cholinergic hypothesis of geriatric memory dysfunction. Science. (1982);217:408–414. doi: 10.1126/science.7046051. [DOI] [PubMed] [Google Scholar]
- 4.Beatty W. W., Butters N., Janowsky D. S. Patterns of memory failure after scopolamine treatment: implications for cholinergic hypotheses of dementia. Behav. Neural Biol. (1986);45:196–211. doi: 10.1016/S0163-1047(86)90772-7. [DOI] [PubMed] [Google Scholar]
- 5.Becker R., Giacobini E., Elble R., McIlhany M., Sherman K. Potential pharmacotherapy of Alzheimer disease. A comparison of various forms of physostigmine administration. Acta Neurol. Scand. Suppl. (1988);116:19–32. doi: 10.1111/j.1600-0404.1988.tb07983.x. [DOI] [PubMed] [Google Scholar]
- 6.Ben-Barak J., Dudai Y. Scopolamine induces an increase in muscarinic receptor level in rat hippocampus. Brain Res. (1980);193:309–313. doi: 10.1016/0006-8993(80)90973-7. [DOI] [PubMed] [Google Scholar]
- 7.Bierer L. M., Haroutunian V., Gabriel S., Knott P. J., Carlin L. S., Purohit D. P., Perl D. P., Schmeidler J., Kanof P., Davis K. L. Neurochemical correlates of dementia severity in Alzheimer's disease: relative importance of the cholinergic deficits. J. Neurochem. (1995);64:749–760. doi: 10.1046/j.1471-4159.1995.64020749.x. [DOI] [PubMed] [Google Scholar]
- 8.Cheng D. H., Tang X. C. Comparative studies of huperzine A, E2020, and tacrine on behavior and cholinesterase activities. Pharmacol. Biochem. Behav. (1998);60:377–386. doi: 10.1016/S0091-3057(97)00601-1. [DOI] [PubMed] [Google Scholar]
- 9.Collerton D. Cholinergic function and intellectual decline in Alzheimer's disease. Neuroscience. (1986);19:1–28. doi: 10.1016/0306-4522(86)90002-3. [DOI] [PubMed] [Google Scholar]
- 10.El-Sherbiny D. A., Khalifa A. E., Attia A. S., Eldenshary Eel D. Hypericum perforatum extract demonstrates antioxidant properties against elevated rat brain oxidative status induced by amnestic dose of scopolamine. Pharmacol. Biochem. Behav. (2003);76:525–533. doi: 10.1016/j.pbb.2003.09.014. [DOI] [PubMed] [Google Scholar]
- 11.Ellman G. L., Courtney K. D., Andres V., Jr., Feather-Stone R. M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. (1961);7:88–95. doi: 10.1016/0006-2952(61)90145-9. [DOI] [PubMed] [Google Scholar]
- 12.Fan Y., Hu J., Li J., Yang Z., Xin X., Wang J., Ding J., Geng M. Effect of acidic oligosaccharide sugar chain on scopolamine- induced memory impairment in rats and its related mechanisms. Neurosci. Lett. (2005);374:222–226. doi: 10.1016/j.neulet.2004.10.063. [DOI] [PubMed] [Google Scholar]
- 13.Francis P. T., Palmer A. M., Snape M., Wilcock G. K. The cholinergic hypothesis of Alzheimer's disease: a review of progress. J. Neurol. Neurosurg. Psychiatry. (1999);66:137–147. doi: 10.1136/jnnp.66.2.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jeong E. J., Lee K. Y., Kim S. H., Sung S. H., Kim Y. C. Cognitive-enhancing and antioxidant activities of iridoid glycosides from Scrophularia buergeriana in scopolamine-treated mice. Eur. J. Pharmacol. (2008);588:78–84. doi: 10.1016/j.ejphar.2008.04.015. [DOI] [PubMed] [Google Scholar]
- 15.Jia Y., Gall C. M., Lynch G. Presynaptic BDNF promotes postsynaptic longterm potentiation in the dorsal striatum. J. Neurosci. (2010);30:14440–14445. doi: 10.1523/JNEUROSCI.3310-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Joseph J. A., Strain J. G., Jimenez N. D., Fisher D. Oxidant injury in PC12 cells--a possible model of calcium "dysregulation" in aging: I. Selectivity of protection against oxidative stress. J. Neurochem. (1997);69:1252–1258. doi: 10.1046/j.1471-4159.1997.69031252.x. [DOI] [PubMed] [Google Scholar]
- 17.Kempermann G. The neurogenic reserve hypothesis: what is adult hippocampal neurogenesis good for? Trends Neurosci. (2008);31:163–169. doi: 10.1016/j.tins.2008.01.002. [DOI] [PubMed] [Google Scholar]
- 18.Kim D. H., Hung T. M., Bae K. H., Jung J. W., Lee S., Yoon B. H., Cheong J. H., Ko K. H., Ryu J. H. Gomisin A improves scopolamine-induced memory impairment in mice. Eur. J. Pharmacol. (2006);542:129–135. doi: 10.1016/j.ejphar.2006.06.015. [DOI] [PubMed] [Google Scholar]
- 19.Komulainen P., Pedersen M., Hanninen T., Bruunsgaard H., Lakka T. A., Kivipelto M., Hassinen M., Rauramaa T. H., Pedersen B. K., Rauramaa R. BDNF is a novel marker of cognitive function in ageing women: the DR's EXTRA Study. Neurobiol. Learn. Mem. (2008);90:596–603. doi: 10.1016/j.nlm.2008.07.014. [DOI] [PubMed] [Google Scholar]
- 20.Kopelman M. D., Corn T. H. Cholinergic 'blockade' as a model for cholinergic depletion. A comparison of the memory deficits with those of Alzheimer-type dementia and the alcoholic Korsakoff syndrome. Brain. (1988);111(Pt 5):1079–1110. doi: 10.1093/brain/111.5.1079. [DOI] [PubMed] [Google Scholar]
- 21.Kwon S. H., Kim H. C., Lee S. Y., Jang C. G. Loganin improves learning and memory impairments induced by scopolamine in mice. Eur. J. Pharmacol. (2009);619:44–49. doi: 10.1016/j.ejphar.2009.06.062. [DOI] [PubMed] [Google Scholar]
- 22.Kwon S. H., Kim M. J., Ma S. X., You I. J., Hwang J. Y., Oh J. H., Kim S. Y., Kim H. C., Lee S. Y., Jang C. G. Eucommia ulmoides Oliv. Bark. protects against hydrogen peroxide-induced neuronal cell death in SH-SY5Y cells. J. Ethnopharmacol. (2012);142:337–345. doi: 10.1016/j.jep.2012.04.010. [DOI] [PubMed] [Google Scholar]
- 23.Kwon S. H., Lee H. K., Kim J. A., Hong S. I., Kim H. C., Jo T. H., Park Y. I., Lee C. K., Kim Y. B., Lee S. Y., Jang C. G. Neuroprotective effects of chlorogenic acid on scopolamineinduced amnesia via anti-acetylcholinesterase and anti-oxidative activities in mice. Eur. J. Pharmacol. (2010);649:210–217. doi: 10.1016/j.ejphar.2010.09.001. [DOI] [PubMed] [Google Scholar]
- 24.Kwon S. H., Lee H. K., Kim J. A., Hong S. I., Kim S. Y., Jo T. H., Park Y. I., Lee C. K., Kim Y. B., Lee S. Y., Jang C. G. Neuroprotective effects of Eucommia ulmoides Oliv. Bark on amyloid beta(25-35)-induced learning and memory impairments in mice. Neurosci. Lett. (2011);487:123–127. doi: 10.1016/j.neulet.2010.10.042. [DOI] [PubMed] [Google Scholar]
- 25.LeDoux J. E. Emotional memory systems in the brain. Behav. Brain Res. (1993);58:69–79. doi: 10.1016/0166-4328(93)90091-4. [DOI] [PubMed] [Google Scholar]
- 26.Lee M. K., Cho S. Y., Kim D. J., Jang J. Y., Shin K. H., Park S. A., Park E. M., Lee J. S., Choi M. S., Kim M. J. Duzhong (Eucommia ulmoides Oliv.) cortex water extract alters heme biosynthesis and erythrocyte antioxidant defense system in leadadministered rats. J. Med. Food. (2005);8:86–92. doi: 10.1089/jmf.2005.8.86. [DOI] [PubMed] [Google Scholar]
- 27.Lovell M. A., Ehmann W. D., Butler S. M., Markesbery W. R. Elevated thiobarbituric acid-reactive substances and antioxidant enzyme activity in the brain in Alzheimer's disease. Neurology. (1995);45:1594–1601. doi: 10.1212/WNL.45.8.1594. [DOI] [PubMed] [Google Scholar]
- 28.Marcus D. L., Thomas C., Rodriguez C., Simberkoff K., Tsai J. S., Strafaci J. A., Freedman M. L. Increased peroxidation and reduced antioxidant enzyme activity in Alzheimer's disease. Exp. Neurol. (1998);150:40–44. doi: 10.1006/exnr.1997.6750. [DOI] [PubMed] [Google Scholar]
- 29.Mizuno M., Yamada K., Maekawa N., Saito K., Seishima M., Nabeshima T. CREB phosphorylation as a molecular marker of memory processing in the hippocampus for spatial learning. Behav. Brain Res. (2002);133:135–141. doi: 10.1016/S0166-4328(01)00470-3. [DOI] [PubMed] [Google Scholar]
- 30.O’Connell C., Gallagher H. C., O’Malley A., Bourke M., Regan C. M. CREB phosphorylation coincides with transient synapse formation in the rat hippocampal dentate gyrus following avoidance learning. Neural Plast. (2000);7:279–289. doi: 10.1155/NP.2000.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Phillips H. S., Hains J. M., Armanini M., Laramee G. R., Johnson S. A., Winslow J. W. BDNF mRNA is decreased in the hippocampus of individuals with Alzheimer's disease. Neuron. (1991);7:695–702. doi: 10.1016/0896-6273(91)90273-3. [DOI] [PubMed] [Google Scholar]
- 32.Sakurai T., Kato T., Mori K., Takano E., Watabe S., Nabeshima T. Nefiracetam elevates extracellular acetylcholine level in the frontal cortex of rats with cerebral cholinergic dysfunctions: an in vivo microdialysis study. Neurosci. Lett. (1998);246:69–72. doi: 10.1016/S0304-3940(98)00244-4. [DOI] [PubMed] [Google Scholar]
- 33.Selkoe D. J. Alzheimer's disease: a central role for amyloid. J. Neuropathol. Exp. Neurol. (1994);53:438–447. doi: 10.1097/00005072-199409000-00003. [DOI] [PubMed] [Google Scholar]
- 34.Singh B., Bhat T. K., Singh B. Potential therapeutic applications of some antinutritional plant secondary metabolites. J. Agric. Food Chem. (2003);51:5579–5597. doi: 10.1021/jf021150r. [DOI] [PubMed] [Google Scholar]
- 35.Wang W., Sun F., An Y., Ai H., Zhang L., Huang W., Li L. Morroniside protects human neuroblastoma SH-SY5Y cells against hydrogen peroxide-induced cytotoxicity. Eur. J. Pharmacol. (2009);613:19–23. doi: 10.1016/j.ejphar.2009.04.013. [DOI] [PubMed] [Google Scholar]
- 36.Yamada K., Nabeshima T. Brain-derived neurotrophic factor/TrkB signaling in memory processes. J. Pharmacol. Sci. (2003);91:267–270. doi: 10.1254/jphs.91.267. [DOI] [PubMed] [Google Scholar]
- 37.Yu S. P., Canzoniero L. M., Choi D. W. Ion homeostasis and apoptosis. Curr. Opin. Cell Biol. (2001);13:405–411. doi: 10.1016/S0955-0674(00)00228-3. [DOI] [PubMed] [Google Scholar]