Abstract
Cytochrome P450 4A11 (CYP4A11) is a fatty acid hydroxylase enzyme expressed in human liver. It catalyzes not only the hydroxylation of saturated and unsaturated fatty acids, but the conversion of arachidonic acid to 20-hydroxyeicosatetraenoic acid (20-HETE), a regulator of blood pressure. In this study, we performed a directed evolution analysis of CYP4A11 using the luminogenic assay system. A random mutant library of CYP4A11, in which mutations were made throughout the entire coding region, was screened with luciferase activity to detect the demethylation of luciferin-4A (2-[6-methoxyquinolin-2-yl]-4,5-dihydrothiazole-4-carboxylic acid) of CYP4A11 mutants in Escherichia coli. Consecutive rounds of random mutagenesis and screening yielded three improved CYP4A11 mutants, CP2600 (A24T/T263A), CP2601 (T263A), and CP2616 (A24T/T263A/V430E) with ~3-fold increase in whole cells and >10-fold increase in purified proteins on the luminescence assay. However, the steady state kinetic analysis for lauric acid hydroxylation showed the significant reductions in enzymatic activities in all three mutants. A mutant, CP2600, showed a 51% decrease in catalytic efficiency (kcat/Km) for lauric acid hydroxylation mainly due to an increase in Km. CP2601 and CP2616 showed much greater reductions (>75%) in the catalytic efficiency due to both a decrease in kcat and an increase in Km. These decreased catalytic activities of CP2601 and CP2616 can be partially attributed to the changes in substrate affinities. These results suggest that the enzymatic activities of CYP4A11 mutants selected from directed evolution using a luminogenic P450 substrate may not demonstrate a direct correlation with the hydroxylation activities of lauric acid.
Keywords: P450, CYP4A11, Luciferin, Lauric acid, GC-mass spectrometry
INTRODUCTION
Cytochrome P450s (CYPs, P450s) comprise the superfamily of heme-containing oxidoreductase enzymes found in various organisms including animals, plants, fungi and bacteria (Ortiz de Montellano, 2005). In humans, CYPs are responsible for the metabolism of various endogenous and exogenous chemicals including drugs (Guengerich, 2005). Among 57 human CYPs, CYP4A11 is a major fatty acid hydroxylase found in human liver (Guengerich, 2005). It catalyzes the hydroxylation of saturated and unsaturated fatty acids mainly at the terminal carbon position and therefore converts lauric acid to 12-hydroxylauric acid (Okita and Okita, 2001; He et al., 2005). This terminal hydroxylation of fatty acids is considered to maintain the balance of lipids in human liver (Adas et al., 1999). It is also involved in the terminal oxidation reaction of arachidonic acid to produce 20-hydroxyeicosatetraenoic acid (20-HETE), which known as a regulator of blood pressure (Gainer et al., 2005). The production of 20-HETE is relatively unique among the human P450s and therefore, the regulation of CYP4A11 activity can suggest a new therapeutic approach to control hypertension.
Random mutagenesis and subsequent screening (socalled directed evolution) is an effective tool to engineer enzymes or to investigate structure-function relationships of proteins. CYP enzymes have been gaining particular attention for their applications in biotransformation and in the study of structure-function relationships (Kim et al., 2005). In previous studies, the formations of dye products and fluorescent metabolite were used for efficient screening of interesting clones from CYP2A6 random mutant libraries (Nakamura et al., 2001; Kim et al., 2005). Also, CYP-mediated mutagenic bioactivation was applied to select CYP1A2 mutants with enhanced catalytic activity (Kim and Guengerich, 2004b). Three consecutive rounds of random mutagenesis and screening yielded a highly improved CYP1A2 mutant with a >10-fold increase in the bioactivation of aromatic amine based on the genotoxicity assay and a 12-fold enhanced catalytic efficiency in steady-state analysis (Kim and Guengerich, 2004b).
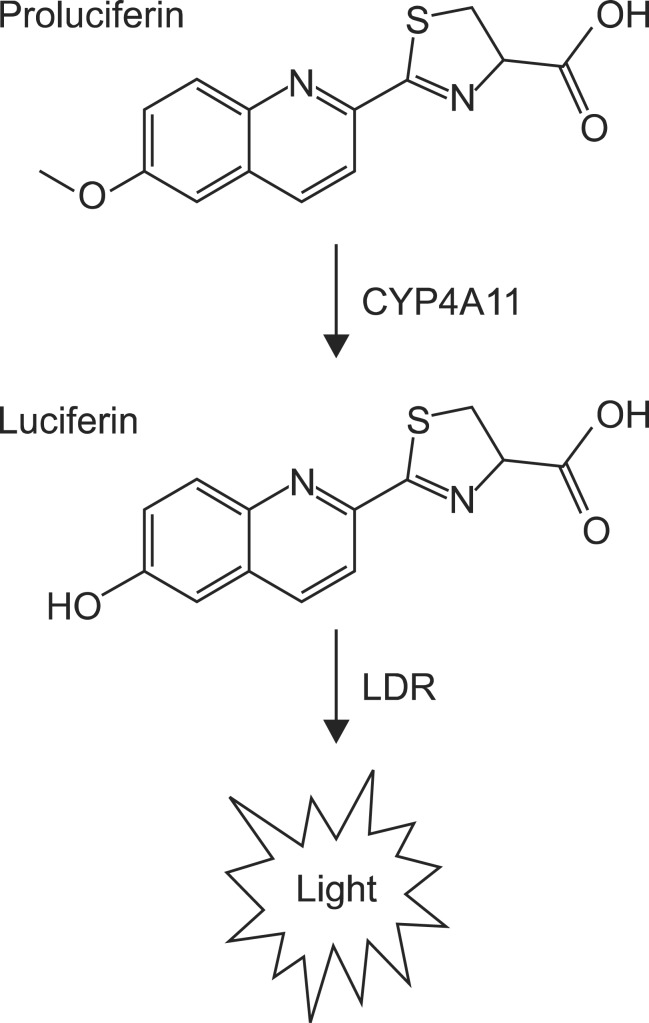
Recently, many luminogenic CYP assays were introduced using various prosubstrates for the light-generating reaction of firefly luciferase. The general principle is that CYPs convert prosubstrates to an active luciferin, which produces light in the second reaction with a luciferase (Fig. 1) (Cali et al., 2006). The amount of light generated is proportional to the amount of luciferin produced by the CYP enzyme and its activity. It was shown that CYP4A11 selectively converts a luciferin derivative, 2-(6-methoxyquinolin-2-yl)-4,5-dihydrothiazole-4-carboxylic acid (Luciferin-4A), to quinolylluciferin, an active alternative to native beetle luciferin (Branchini et al., 1989).
Fig. 1. Generation of luminescence by CYP4A11 and Luciferin Detection Reagent (LDR). LDR includes luciferase enzyme to produce the luminescent light using luciferin.
In this study, we applied a luminogenic CYP substrate and identified human CYP4A11 mutants with enhanced catalytic activity using random mutagenesis and high-throughput screening. The selected mutants were evaluated for their fatty acid hydroxylation and luminescence production activities.
MATERIALS AND METHODS
Chemicals
Lauric acid, 3-[(3-cholamidopropyl)dimethylammonio]-1-propanesulfonate (CHAPS), 1,2-dilauroyl-sn-glycero-3-phosphocholine (DLPC), N, O-bis(trimethylsilyl)triflueroacetamide (BSTFA), NADP+, and NADPH were purchased from SigmaAldrich (St. Louis, MO, USA). Luciferin-4A (2-(6-methoxyquinolin-2yl)-4,5-dihydrothiazole-4-carboxylic acid), and luciferin detection reagent (LDR) were purchased from Promega (Madison, WI, USA). Ni2+-nitrilotriacetate (NTA) agarose was purchased from Qiagen, (Valencia, CA, USA). Other chemicals were of the highest commercially available grade. Escherichia coli DH10b and DH5α cells were purchased from Invitrogen (Life Technologies, Grand Island, NY, USA). Rat NADPH-P450 reductase (NPR) was heterolously expressed in E. coli (TOPP3 strain) and purified as described elsewhere (Han et al., 2009; Park et al., 2010; Lee et al., 2012)
Construction of CYP4A11 random mutant library plasmids
The open reading frame of human CYP4A11 was randomly mutated using the low-fidelity polymerase PCR method as previously described (Kim and Guengerich, 2004b). Random mutagenesis PCR reactions were conducted using Gene- Morph II Random Mutagenesis Kit (Stratagene, La Jolla, CA, USA) with the following primers: 5’-AACCACATATGGCTCTGTTA-3’ (N-terminal primer) and 5’-GGTAGTCTAGATTAATGGTG-3’ (C-terminal primer). The amplified PCR library fragment was purified and cloned into the pCW4A11 bicistronic vector (Lim et al., 2010), which also expresses NADPH-P450 reductase. The ligation mixture was transformed into E. coli DH10b ultracompetent cells, and then the library plasmid DNA was purified.
Luminescence high-throughput screening
Constructed library plasmids of CYP4A11 were transformed into E. coli DH5α, and individual colonies were picked up and transferred to single wells of 96-microtiter plates filled with 200 μl of Terrific Broth (TB) expression media including ampicillin and supplements (0.5 mM 5-aminolevulinic acid, 1.0 mM isopropyl β-d-thiogalactoside, 1.0 mM thiamine, and trace elements). For each generation of library screening, ~1.5×103 colonies were picked and, the 96-well plates were incubated at 28℃ for 48 hr. For the measurement of luminescent activity of mutants, M9 medium including the Luciferin-4A substrate (and glucose) was added after TB medium was removed with centrifugation. After 20 min of incubation at 37℃, the reaction was stopped with the addition of Luciferin Detection Reagent (including luciferase, CYP-GloTM Screening System) and further incubated at room temperature for 20 min to stabilize the luminescent signal. Luminescence was detected using a 96-microtiter luminometer (Veritas, Promega).
Protein expression and purification
Expression and purification of CYP4A11 mutants were carried out as previously described with some modifications (Kim et al., 2005). The E. coli DH5α strains transformed with pCW vectors were inoculated into LB medium containing 50 μg/ml ampicillin and incubated overnight at 37℃. LB cultures were then seeded into 500 ml of Terrific broth (TB) expression medium containing 50 μg/ml ampicillin. The expression cultures were grown at 37℃ with shaking at 250 rpm. When the OD600 of the cultures reached 0.5, supplements were added, and the expression cultures were further grown at 28℃ with shaking at 200 rpm for 48 hr. Bacterial inner membrane fractions containing CYP4A11 wild type and mutants were isolated and prepared from TB expression cultures, by the method described previously (Han et al., 2009). The prepared membrane fractions were solubilized overnight at 4℃ in 100 mM potassium phosphate buffer (pH 7.4) including 20% glycerol, 0.1 mM EDTA, 10 mM β-mercaptoethanol, and 1.5% CHAPS (w/v). Purification of enzymes using a Ni2+-nitrilotriacetate column eluted with 300 mM imidazole, was also performed using a previously described method (Yun et al., 2000).
Analysis of lauric acid hydroxylation using GC-mass spectrometry
Lauric acid hydroxylation activity was determined by GCmass spectrometry as described (Han et al., 2009). The reaction mixtures consisted of 0.1 mM purified P450 protein in 100 mM potassium phosphate buffer (pH 7.4) including 0.2 mM rat NADPH-P450 reductase, DLPC (0.1 mg/ml), 0.1 mM cytochrome b5 and 500 μM of dithiothreitol (DTT), and varying concentrations of lauric acid in a total volume of 0.5 ml. The reaction was initiated by adding an NADPH-generating system [0.5 mM NADP+, 10 mM glucose 6-phosphate, and 1.0 IU glucose 6-phosphate dehydrogenase mL-1] at 37℃ and terminated with addition of 1 ml of CH2Cl2 after 20 min. The CH2Cl2 extract was dried under N2 and then converted to trimethylsilyl derivatives by incubation with 50 μl of N,O-bis(trimethylsilyl)trifluoroacetamide at 70℃ for 10 min. The derivatized samples were analyzed on the Varian 240 ion-trap gas chromatograph mass spectrometry system.
Substrate binding spectral titration
Purified CYP4A11 mutant enzymes were diluted to 2 μM in 100 mM potassium phosphate buffer (pH 7.4) and then divided between two glass cuvettes. Spectral changes (from 350 to 500 nm) were recorded with subsequent addition of lauric acid in methanol using a CARY 100 spectrophotometer (Varian). Binding affinities were estimated by fitting the data of absorbance difference to the quadratic equation using nonlinear regression analysis with Graph-Pad Prism software (Graph- Pad, San Diego, CA, USA).
RESULTS
Random mutagenesis and selection of CYP4A11 mutants
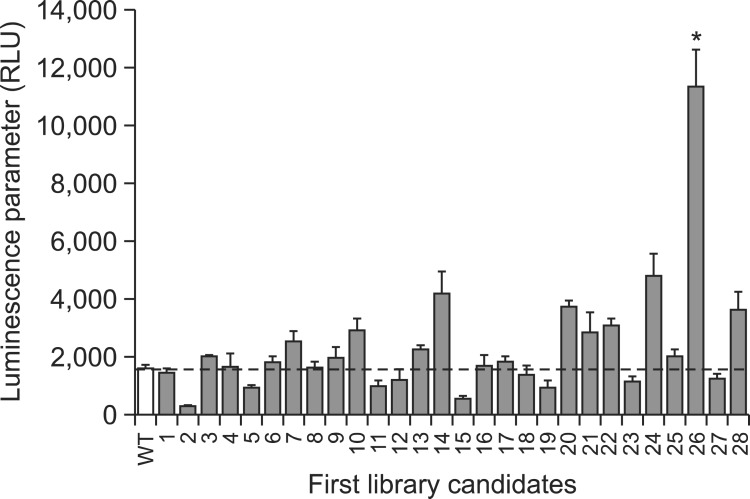
Random mutant library of CYP4A11 in pCW bicistronic vectors was successfully generated in the error-prone PCR reaction. The constructed mutant library was screened for mutant clones with activity to produce increased luminescence (Fig. 1). About 1.5×103 clones were screened in the 1st generation mutant library, and putative 28 mutants were selected and verified as positive clones with the enhanced activity (Fig. 2). CP2600 mutant showed highly enhanced activity to produce luminescence among the selected 1st generation mutants. CP2600 mutant had double mutations, A24T and T263A. Interestingly, Ala24 was not the native sequence of CYP4A11 but a part of N-terminal modified sequence to increase the expression level of recombinant proteins (Han et al., 2009). CP2601 mutant which has only T263A mutation was generated using the site-directed mutation of CP2600 mutant. CP2616 mutant was selected from the consecutive screening analysis of additional random mutant library generated using CP2600 mutant as a PCR template. It contained triple mutations V430E as well as A24T and T263A (which were found in CP2600). Enhanced luminescence was no longer observed in the consecutive screening of random mutant library generated from CP2616 mutant.
Fig. 2. Comparison of luminescence activities of CYP4A11 wild type (WT) and selected mutants. Twenty eight clones were selected in the first generation screening of mutant library and verified for their enhanced enzyme activities.
Luminescence production by selected CYP4A11 mutants
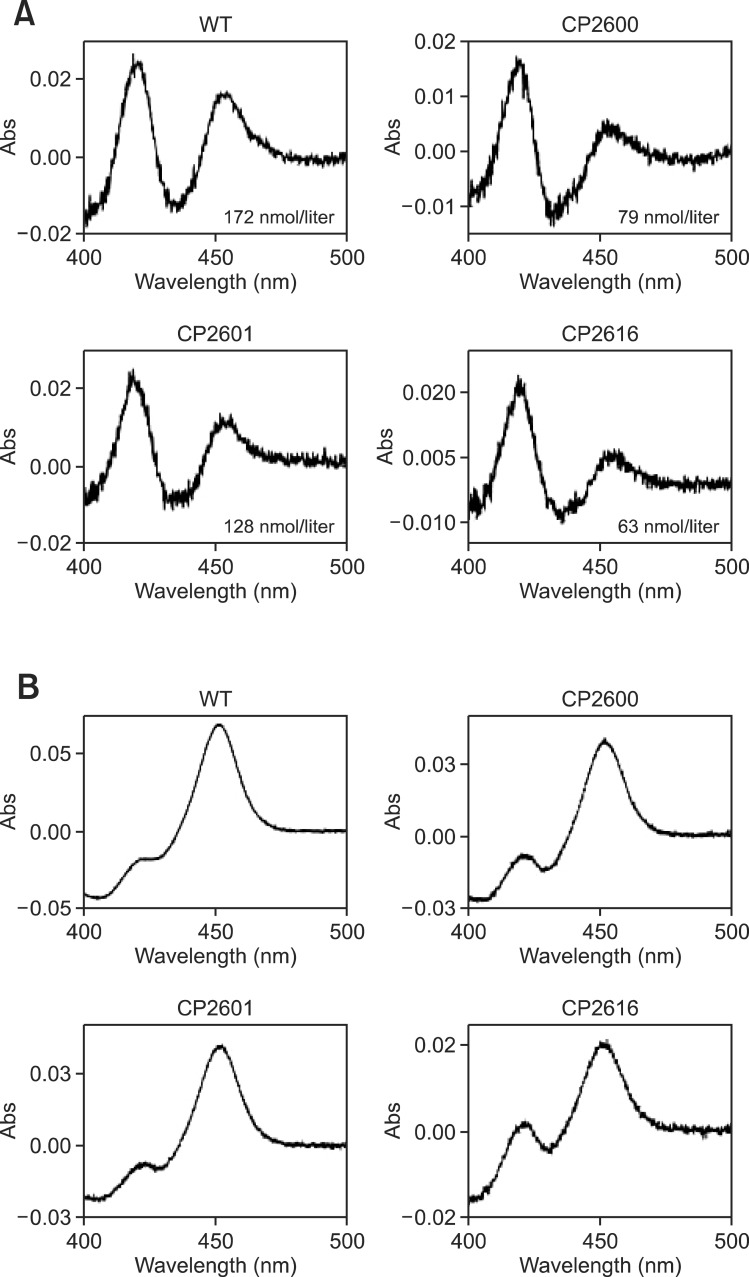
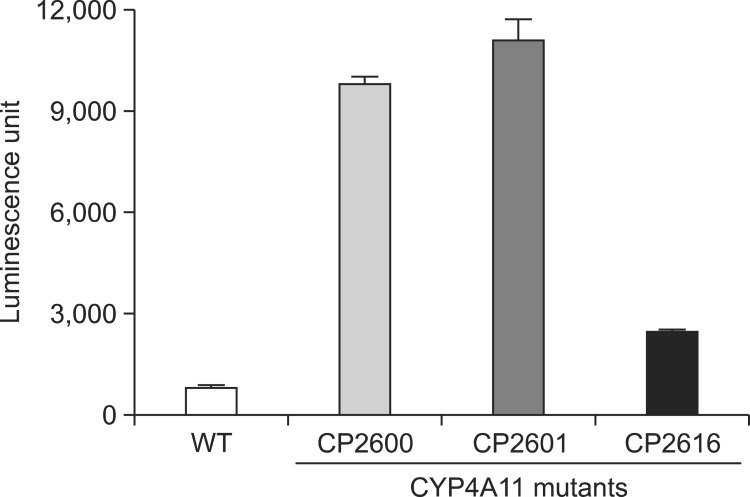
CYP4A11 wild type and the selected mutants were expressed in large scale. The expression levels ranged from 63 to 172 nmol/liter culture (Fig. 3A). Recombinant proteins of CYP4A11 wild type and three mutants were purified using a Ni2+-NTA affinity chromatography column. The concentrations of all purified proteins were spectroscopically determined to be in the range of 4.3-15 μM (Fig. 3B). These results suggested that three mutations partially affected the structural stability of the recombinant CYP4A11 protein. Demethylation activity of Luciferin-4A [2-(6-methoxyquinolin-2-yl)-4, 5-dihydrothiazole-4-carboxylic acid] was measured using purified CYP4A11 mutant proteins after normalizing the CYP holoenzyme levels (based on CO-binding spectrum). Purified CP2600 and CP- 2601 mutants showed >10-fold increases, but CP2616 mutant increased only ~3 fold (Fig. 4).
Fig. 3. Expression of CYP4A11 wild type and selected mutants in E.coli. The CO-binding spectra of whole cell level (A) and purified protein (B) were measured.
Fig. 4. Luminescence activities of purified wild type and mutants of CYP4A11. Results are presented as means ± SD (range) of quadruplicate assays.
Lauric acid hydroxylation activities of selected CYP4A11 mutants
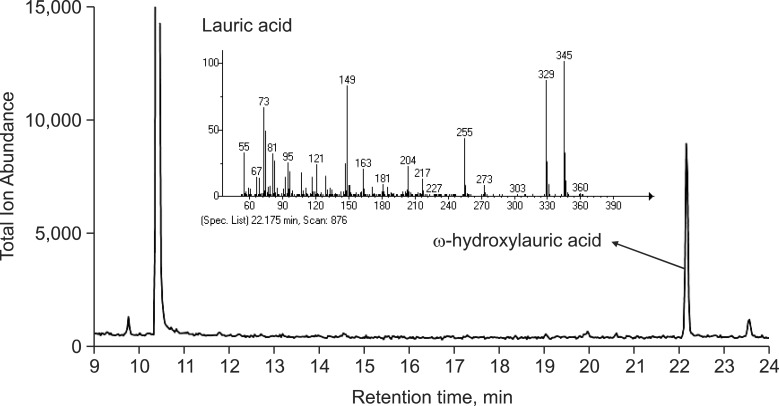
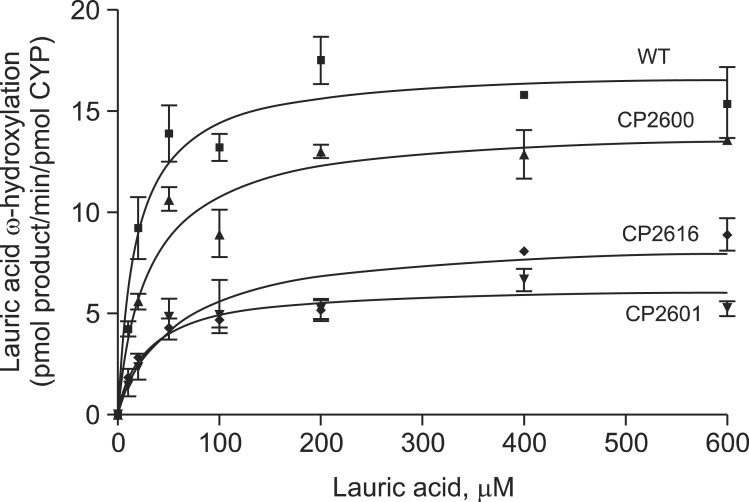
Hydroxylation of lauric acid is a typical enzymatic activity of CYP4A11. In GC-mass spectrometry analysis, CYP4A11 produced mainly ω-hydroxylated lauric acid as a major metabolite at 22 min (Fig. 5). All three mutants showed significant reductions of lauric acid hydroxylation activities in the steady state kinetic analysis (Fig. 6, Table 1). CP2600 mutant showed a 51% decrease in catalytic efficiency (kcat/Km) for lauric acid hydroxylation mainly due to an increase in Km value. CP2601 and CP2616 showed much greater reductions in catalytic efficiency (17-25% of wild-type) due to both decreased kcat and increased Km (Fig. 6, Table 1).
Fig. 5. GC-mass spectrometry analysis of lauric acid hydroxylation by CYP4A11. Lauric acid and ω-hydroxy lauric acid were detected at 10 and 22 min, respectively in the chromatogram of total ion abundance. Mass fragmentation of ω-hydroxy lauric acid was indicated.
Fig. 6. Steady-state kinetics of lauric acid hydroxylation by CYP4A11 wild type and mutants. Each point represents a mean ± SD (range) of duplicate assays. The steady-state kinetic parameters were calculated in Table 1.
Table 1.
Steady-state kinetic parameters of hydroxylation of lauric acid by purified CYP4A11 wild type and mutants
| CYP4A11 mutants | Mutations | ω-Hydroxylation of lauric acid | |||
|---|---|---|---|---|---|
|
| |||||
| kcat (min-1) | Km (μM) | kcat/Km | Folds | ||
|
| |||||
| WT | - | 17.1 ± 0.8 | 19.1 ± 4.5 | 0.89 | 1 |
| CP2600 | A24T/T263A | 14.2 ± 0.8 | 32.6 ± 7.5 | 0.44 | 0.49 |
| CP2601 | T263A | 6.3 ± 0.4 | 29.0 ± 9.0 | 0.22 | 0.25 |
| CP2616 | A24T/T263A/V430E | 8.7 ± 0.7 | 58.0 ± 17.2 | 0.15 | 0.17 |
Results are presented as means ± SD of duplicate assays.
Lauric acid binding affinities of selected CYP4A11 mutants
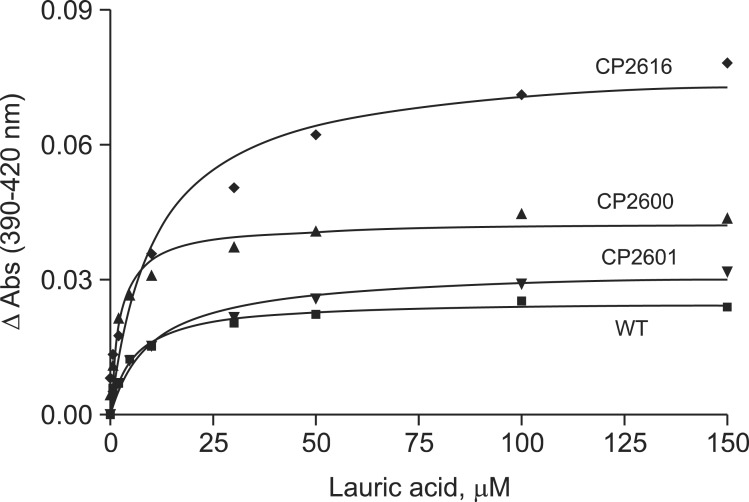
Binding titration of lauric acid to CYP4A11 wild type and mutants showed a typical type I spectral change (data not shown). Binding affinities of purified CYP4A11 wild type and mutants for lauric acid were determined (Fig. 7 and Table 2). The binding of lauric acid to CYP4A11 wild type yielded a Kd value of 5.6 ± 0.6 μM (Table 2). CP2600 mutant showed stronger binding with a Kd value of 2.5 ± 0.4 μM, but CP2601 and CP2616 mutants exhibited ~2 fold increases in Kd values (Table 2).
Fig. 7. Substrate binding of CYP4A11 wild type and mutants. The binding affinities of lauric acid were calculated in Table 2.
Table 2.
Estimated lauric acid binding affinities of CYP4A11 wild type and mutants
| CYP4A11 mutants | Mutations | Kd, μM |
|---|---|---|
|
| ||
| WT | - | 5.6 ± 0.6 |
| CP2600 | A24T/T263A | 2.5 ± 0.4 |
| CP2601 | T263A | 12.6 ± 1.9 |
| CP2616 | A24T/T263A/V430E | 13.2 ± 2.9 |
DISCUSSION
Although the directed evolution approach is a powerful tool for analyzing the structure and function relationships of proteins, no attempt has been made regarding the study of CYP4A11 due to the lack of its screening method possessing efficiency and validity. In this study, bioluminescence was used for the high-throughput screening method of CYP4A11 random mutagenesis library. Sobol et al. reported that luciferin-4A is a selective substrate for CYP4A11 among human CYPs, which showed time dependence and a saturated hyperbolic dose response with a Km value of 80 μM (Sobol et al., 2008). This approach differs from that previously used for human CYP1A2 and CYP2A6, in which the fluorescence of specific CYP reactions was used for screening (Kim and Guengerich, 2004a; Kim et al., 2005). The current study identified three CYP4A11 mutants with enhanced luminescence producing activity, CP2600 (A24T/T263A), CP2601 (T263A), and CP2616 (A24T/T263A/V430E), with a ~3-fold increase in whole cells and a >10-fold increase in purified proteins.
Lauric and arachidonic acid are typical substrates for human CYP4A11. Enhancement of catalytic activity in selected mutants was not observed for the hydroxylation of lauric acid. Increased catalytic activity is selective for the luciferin-4A substrate. Luciferin-4A is a quinoline structure and this significant structural difference from lauric acid (or arachidonic acid) may contribute to the changing catalytic activity of selected mutants for lauric acid. This finding supports the previous caveat that the effects of any particular mutation are relatively unique to enzyme-substrate complementarity and may diverge with different substrates (Kim and Guengerich, 2004b). Weaker affinities of lauric acid to CP2601 and CP2616 mutants may have an effect on the decreased catalytic activity to some extent albeit the stronger binding to CP2600 mutant (Table 2).
All of mutations appeared to be located in the regions remote from the proposed substrate binding site based on the sequence alignment of CYP4A11 (data not shown). The T263A mutation, present in all mutants, is located in the Ghelix region. The helix G region contains helical structures rich in hydrophobic residues and therefore it can penetrate into the deep depth of the membrane (Pylypenko and Schlichting, 2004). Loss of possible hydrogen bonds mediated by Thr residue in the membrane may indicate lower expression levels of all mutants due to structural instability (Fig. 3).
In conclusion, we selected and analyzed mutant enzymes from CYP4A11 through directed evolution using a luminescent substrate. The enhanced enzymatic activity of CYP4A11 mutants for the luminescent substrate was not in accordance with lauric acid hydroxylation. Further investigation of CYP4A11 luminescent probe for application to directed evolution is required to warrant the correlation with its physiological substrate. Possibly, the fatty acid derived luminescent substrate with a comparative activity of lauric acid hydroxylation can be an alternative substrate for the high-throughput screening of CYP4A11 mutant library.
Acknowledgments
This work was supported by the National Research Foundation of Korea (NRF) Grants funded by the Korea government (NRF-2010-0021785).
References
- 1.Adas F., Salaun J. P., Berthou F., Picart D., Simon B., Amet Y. Requirement for omega and (omega-1)-hydroxylations of fatty acids by human cytochromes P450 2E1 and 4A11. J. Lipid Res. (1999);40:1990–1997. [PubMed] [Google Scholar]
- 2.Branchini B. R., Hayward M. M., Bamford S., Brennan P. M., Lajiness E. J. Naphthyl- and quinolylluciferin: green and red light emitting firefly luciferin analogues. Photochem. Photobiol. (1989);49:689–695. doi: 10.1111/j.1751-1097.1989.tb08442.x. [DOI] [PubMed] [Google Scholar]
- 3.Cali J. J., Ma D., Sobol M., Simpson D. J., Frackman S., Good T. D., Daily W. J., Liu D. Luminogenic cytochrome P450 assays. Expert Opin. Drug Metab. Toxicol. (2006);2:629–645. doi: 10.1517/17425255.2.4.629. [DOI] [PubMed] [Google Scholar]
- 4.Gainer J. V., Bellamine A., Dawson E. P., Womble K. E., Grant S. W., Wang Y., Cupples L. A., Guo C. Y., Demissie S., O'Donnell C. J., Brown N. J., Waterman M. R., Capdevila J. H. Functional variant of CYP4A11 20-hydroxyeicosatetraenoic acid synthase is associated with essential hypertension. Circulation. (2005);111:63–69. doi: 10.1161/01.CIR.0000151309.82473.59. [DOI] [PubMed] [Google Scholar]
- 5.Guengerich F. P. Human cytochrome P450 enzymes. In Cytochrome P450: Structure, Mechanism, and Biochemistry (P. R. Ortiz de Montellano, Ed.) Plenum Press; New York: (2005). pp. 377–551. [Google Scholar]
- 6.Han S., Eun C.-Y., Han J.-S., Chun Y.-J., Kim D.-H., Yun C.-H., Kim D. Self-sufficient catalytic system of human cytochrome P450 4A11 and NADPH-P450 reductase. Biomol. Ther. (2009);17:156–161. doi: 10.4062/biomolther.2009.17.2.156. [DOI] [Google Scholar]
- 7.He X., Cryle M. J., De Voss J. J., de Montellano P. R. Calibration of the channel that determines the omega-hydroxylation regiospecificity of cytochrome P4504A1: catalytic oxidation of 12-halododecanoic acids. J. Biol. Chem. (2005);280:22697–22705. doi: 10.1074/jbc.M502632200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim D., Guengerich F. P. Enhancement of 7-methoxyresorufin O-demethylation activity of human cytochrome P450 1A2 by molecular breeding. Arch. Biochem. Biophys. (2004a);432:102–108. doi: 10.1016/j.abb.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 9.Kim D., Guengerich F. P. Selection of human cytochrome P450 1A2 mutants with enhanced catalytic activity for heterocyclic amine N-hydroxylation. Biochemistry. (2004b);43:981–988. doi: 10.1021/bi035593f. [DOI] [PubMed] [Google Scholar]
- 10.Kim D., Wu Z. L., Guengerich F. P. Analysis of coumarin 7-hydroxylation activity of cytochrome P450 2A6 using random mutagenesis. J. Biol. Chem. (2005);280:40319–40327. doi: 10.1074/jbc.M508171200. [DOI] [PubMed] [Google Scholar]
- 11.Lee H., Park H. G., Lim Y. R., Lee I. S., Kim B. J., Seong C. H., Chun Y. J., Kim D. Functional expression and characterization of recombinant NADPH-P450 reductase from Malassezia globosa. J. Microbiol. Biotechnol. (2012);22:141–146. doi: 10.4014/jmb.1107.07058. [DOI] [PubMed] [Google Scholar]
- 12.Lim Y. R., Eun C. Y., Park H. G., Han S., Han J. S., Cho K. S., Chun Y. J., Kim D. Regioselective oxidation of lauric acid by CYP119, an orphan cytochrome P450 from Sulfolobus acidocaldarius. J. Microbiol. Biotechnol. (2010);20:574–578. [PubMed] [Google Scholar]
- 13.Nakamura K., Martin M. V., Guengerich F. P. Random mutagenesis of human cytochrome p450 2A6 and screening with indole oxidation products. Arch. Biochem. Biophys. (2001);395:25–31. doi: 10.1006/abbi.2001.2569. [DOI] [PubMed] [Google Scholar]
- 14.Okita R. T., Okita J. R. Cytochrome P450 4A fatty acid omega hydroxylases. Curr. Drug Metab. (2001);2:265–281. doi: 10.2174/1389200013338423. [DOI] [PubMed] [Google Scholar]
- 15.Ortiz de Montellano P. R. Cytochrome P450: Structure, Mechanism, and Biochemistry. Plenum Press; New York: (2005). [Google Scholar]
- 16.Park H. G., Lim Y. R., Eun C. Y., Han S., Han J. S., Cho K. S., Chun Y. J., Kim D. Candida albicans NADPH-P450 reductase: expression, purification, and characterization of recombinant protein. Biochem. Biophys. Res. Commun. (2010);396:534–538. doi: 10.1016/j.bbrc.2010.04.138. [DOI] [PubMed] [Google Scholar]
- 17.Pylypenko O., Schlichting I. Structural aspects of ligand binding to and electron transfer in bacterial and fungal P450s. Annu. Rev. Biochem. (2004);73:991–1018. doi: 10.1146/annurev.biochem.73.011303.073711. [DOI] [PubMed] [Google Scholar]
- 18.Sobol M., Ma D., Cali J. J. Available from: http://ita.promega.com/resources/pubhub/enotes/selective-cytochrome-p450-4a11-enzymeassay-using-a-novel-bioluminescent-probe-substrate/ Selective cytochrome P450 4A11 enzyme assay using a novel bioluminescent probe substrate. Promega. [Internet] (2008)
- 19.Yun C.-H., Miller G. P., Guengerich F. P. Rate-determining steps in phenacetin oxidations by human cytochrome P450 1A2 and selected mutants. Biochemistry. (2000);39:11319–11329. doi: 10.1021/bi000869u. [DOI] [PubMed] [Google Scholar]