Abstract
Purpose
Fertility treatment in women aged ≥40 year old remains difficult and controversial. All available studies in older women report results of one specific method of ART, i.e. IUI, IVF/ICSI or oocyte donation, and success rates are always published per attempt but never per patient. Randomized studies are not available because of the obvious heterogeneity in patient populations and treatment options.
This prospective observational study aimed at analyzing the outcome in a consecutive cohort of patients above 40 undergoing various methods of ART.
Methods
A total number of 909 women older than 40 attended our fertility centre during a 3 years period. A flowchart showing the consecutive ART treatments with their respective outcome was constructed. Any delivery after 22 weeks gestation (or 500 g.) was taken as primary endpoint. Crude cumulative delivery rates (CDRs) and binomial exact 95 % confidence limits (95 % CLs) were calculated for each group of interest.
Results
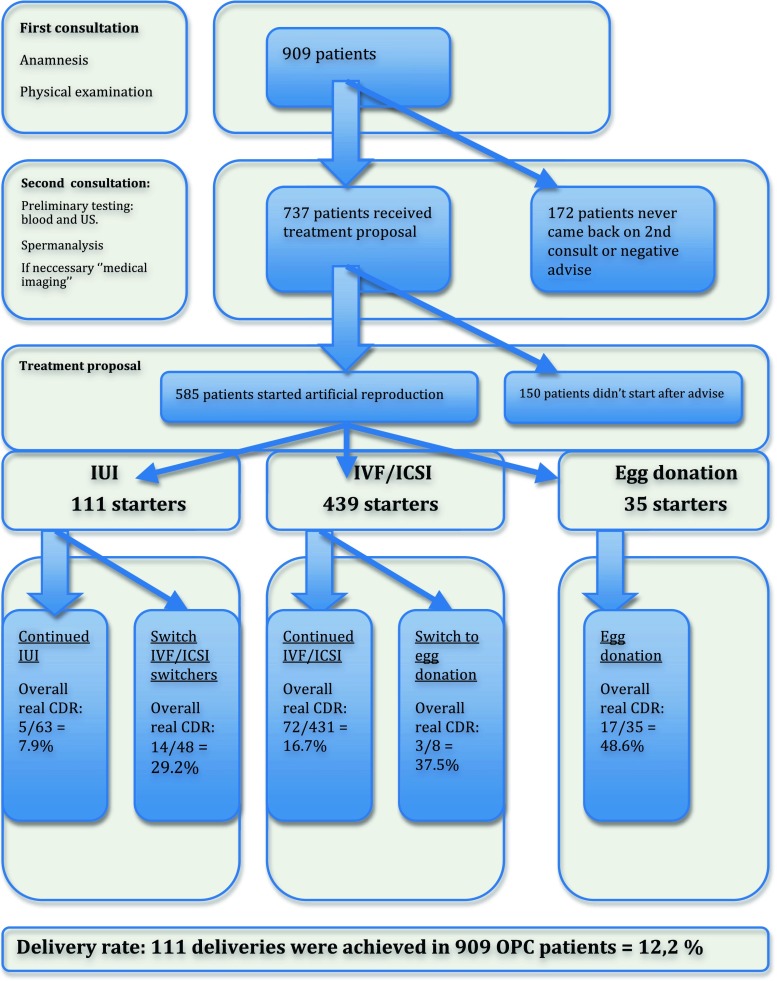
ART treatment could be proposed to 737 patients (81 %) and eventually 585 patients (64 %) started ART treatment: 111 patients started IUI, 439 patients started IVF/ICSI and 35 patients started oocyte donation as a primary approach ART. Ten patients got pregnant spontaneously and delivered before starting any treatment. In the 909 patients consulting for infertility, 111 deliveries were achieved after ART, i.e. a crude CDR of 12.2 % (95 % CL 10.1 % to 14.5 %).
Conclusion
Only 10 % of patients aged 40 and above could achieve delivery of their genetically-own child, while 1 % conceived spontaneously. More than one third of patients consulting never started any treatment for different reasons, i.e. anticipated poor prognosis, financial restrictions, illness or spontaneous pregnancy.
Keywords: Age, Cumulative delivery rates, Delivery, ART, 40 years and above
Introduction
Fecundity dramatically decreases with increasing female age. Current knowledge on this age effect is based on studies involving both natural historical series [26, 30] and contemporary populations [1], and studies in assisted reproduction technology (ART) [4, 19, 20, 27]. The availability of contraceptive methods from the 1960s onwards, together with a growing economical wealth, provided women the opportunity to increase their level of education and to participate in the labor force [14]. As a result, first childbearing has been postponed considerably [28]. In view of the current trend to postpone childbearing in Western societies, the age-related decrease in female fecundity may lead to an increased use of ART or even a permanent loss of a woman’s reproductive potential.
The normal process of reproductive ageing varies considerably among women. This implies that some women remain highly fertile until the fifth decade of life, whereas others face the loss of natural fertility already in their mid-thirties. Female reproductive aging seems to be largely based on age-related changes in ovarian reserve. Decreasing numbers of follicles, coinciding with diminished oocyte quality, dictate the gradual changes in menstrual cycle regularity and monthly fecundity. The precise mechanisms behind the observed gradual decline of the follicle pool and the reduced oocyte quality are far from being fully understood. Although recent knowledge regarding the endocrine, paracrine, genetic, and metabolic factors involved has led to a better understanding of this complex puzzle [3]. The average monthly fecundity rate of about 20 % indicates that even among healthy young couples trying to conceive, many months may be required to achieve pregnancy. With increasing female age this waiting period becomes more and more pronounced [8]. Within 1 year and under natural conditions, women aged 40 and above, will have a 44 % chance that a conception will end in a life birth. Moreover, if a patient postpones conception from age 35 to 40 years, her chances will be reduced by a further 25 %. Unfortunately, ART will only be able to compensate for the decreased natural fertility to a limited extent [13]. The UK’s latest report on IVF activity for 2010 shows that almost 20 % of all fresh IVF and ICSI cycles performed in Britain were performed in women aged 40 and above. World data gathered by ICMART (ICMART) estimated that in 2007 almost 16 % of women having ART were aged over 40 [12].
All studies in older women analyze results for one specific method of ART. For intrauterine insemination (IUI) several data are available [5]. More data are available in the literature for in-vitro fertilization, intra-cytoplasmic sperm injection [6, 7, 15, 21] and egg donation [26, 29].
Patients undergoing fertility treatment need to be clearly informed about their chances of having a baby. Although patients expect that ART will help them, ART may compensate for only half of the births lost by postponing pregnancy in women 35 to 40 years old. This leaves many couples childless even after prolonged infertility treatment. Therefore, it is important for both the candidate couples and the fertility specialists treating them, to know the cumulative probability for having a child. Which treatment should be advised to optimize this probability, is still subject to continuous debate. Moreover, many patients drop out before starting any treatment which may affect the reported success rates of a specific treatment. In the literature, success rates are often expressed per attempt limiting adequate counselling. This observational study aimed at analyzing the outcome in a consecutive cohort of patients above 40 eventually undergoing various methods of ART. However, in contrast to other studies in this field, the set-up was longitudinal since we follow each patient’s treatment pathway. Furthermore, success rates are expressed per patient and this unique approach gives a more realistic idea of the eventual success of ART in this older patient population
Material and methods
Study group
We performed a consecutive cohort study in all patients aged 40 and above who consulted our fertility centre between January 2004 and December 2006. In total, 909 women consulted for the first time. During this first appointment, a full medical history was taken and patients were asked to complete a detailed standard questionnaire. Subsequently, patients were informed about the age-related declining female fertility and their prognosis. In a second appointment, patients came back to discuss the results of the preliminary tests, including measurement of the follicle-stimulating hormone (FSH) level on day 3 of the menstrual cycle and vaginal ultrasound with antral follicle count (AFC). If indicated, additional diagnostic testing like hysterosalpingography, hysteroscopy or laparoscopy were performed. Then the potential assisted reproduction treatments were discussed, i.e. IUI, IVF/ICSI, or egg donation. The choice of treatment was left up to the fertility specialist. Often patients and their doctors immediately opted for IVF/ICSI because of the reimbursement policy of the Belgian national health insurance (similar to the British NHS), which refrains from reimbursement for the age of 43 onwards. Some patients never started the proposed treatment. Reasons for refusing treatment or discontinuation were recorded. A flowchart showing the consecutive steps in their treatments and their respective outcomes was constructed. Live birth delivery after 22 weeks of gestation (or 500 g) was taken as the primary endpoint in this study. All patients without a delivery after treatment were eligible for a subsequent treatment cycle, including patients with cancelled cycles and those with a pregnancy that did not result in a live birth.
Pregnancy follow-up was ensured by sending questionnaires to patients and their doctors or by telephone queries whenever questionnaires were incomplete or missing. The following information was obtained from patients that refrained from treatment: status with regards to treatment (no further treatment or still continuing treatment), occurrence of a pregnancy with or without fertility treatment outside of our department.
Reimbursement policy and legal restrictions
The reimbursement and legislation of the Belgian national health insurance (NHI) was important to opt immediately for a certain treatment. Patients under the Belgian NHI are reimbursed for a maximum of six treatment cycles and the patient may not be older than 42 (up to her 43rd birthday). Furthermore Belgian legislation sets an absolute age limit for fertility treatments. Retrieval of an egg can only take place up to the day before the patient’s 45th birthday. For transferring embryos, the age limit is 47. After 47, patients can’t receive further ART in Belgium. From age 40 there are no restrictions regarding the number of fresh embryos that may be transferred. By law, a maximum of two frozen-thawed embryos may be transferred at any age, even over 40.
Clinical and laboratory procedures
Intrauterine insemination
There is no evidence in older patients that adding mild ovarian hyperstimulation (MOH) in otherwise normally cycling patients would increase success rates after intrauterine insemination (IUI). Nevertheless, some patients received MOH either using clomiphene citrate 50–100 mg from Day 3 till Day 7 of the menstrual cycle or human menopausal gonadotrophins 75–150 U from Day 3 of their cycle onwards. All patients received one single IUI performed 36–44 h after injecting 5000 U hCG (Pregnyl, Merck Sharpe Dome) whenever ultrasound showed the presence of one to a maximum of three follicles measuring at least 17 mm in diameter. IUI was performed using either fresh husband sperm or frozen-thawed donor sperm with a minimum of 1 × 106 progressively motile spermatozoa being inseminated using a Frydman catheter (Laboratoires CCD, Paris, France). Sperm for insemination was prepared by a two-layer density gradient (Pure sperm™, Nidacon, Mölndal, Sweden). From the evening of the day of insemination, patients were advised to use intra-vaginal micronized progesterone 3 × 200 mg (Utrogestan, Besins, Paris France). Our patients received blocks of three cycles of insemination before returning to outpatient clinic for evaluation.
In-vitro fertilization and intra-cytoplasmic sperm injection
For controlled ovarian stimulation, a combination of long-term (40.2 %) or short term (40.7 %) desensitizing gonadotrophin-releasing hormone agonist in association with either human menopausal gonadotrophins (HMG) or recombinant FSH (rec FSH) was used in almost all patients. Up to three embryos, or in exceptional cases four, were transferred into the uterine cavity 72 h after the sperm injection procedure. Micronized progesterone (600 mg per day) was administered intravaginally in three separate doses for luteal-phase supplementation (Smitzet al. 1988).
Egg donation
Preparation of oocyte donors
For all donors, a gonadotrophin-releasing hormone (GnRH)-antagonist protocol with recombinant FSH was used as previously described [22, 23]. On day 2 of the menstrual cycle (day 1 of the stimulation) daily injections of rec FSH were initiated. On day 7 of the cycle (day 6 of the stimulation), subcutaneous (s.c.) administration of GnRH antagonist was started at a daily dose of 0.25 mg. From day 7 of the cycle onwards, ovarian ultrasound scans to assess follicular growth and blood sampling for oestradiol, progesterone, FSH and LH concentrations were performed to monitor and control follicular growth. Oocyte retrieval by transvaginal needle aspiration was performed 36 h after ovulation triggering with triptoreline 0.2 mg injection.
Preparation of oocyte acceptors
Preparation of acceptors was performed using a standard protocol of GnRH agonist, oestrogen and progesterone. Busereline (Suprefact, Sanofi-Aventis, Belgium) was started in the midluteal phase of the cycle preceding the embryo-transfer cycle, at a daily dosage of 0.6 mg. After confirming down-regulation, oestrogen was administered orally using oestradiolvalerate (Progynova, Bayer) at 2 mg twice daily for 6 days, then increasing to 2 mg three times a day for 7 days. Endometrial thickness was measured on day 13 of oestradiolvalerate administration. If the endometrial thickness reached 7 mm, a daily administration of 600 mg progesterone (Utrogestan, Bessins, Belgium) was started the day after.
Data analysis
The primary outcome of this cohort study was any delivery resulting in at least one live birth. The delivery of more than one child was given the same weight as the delivery of a singleton. Patients were not re-enrolled after having a first delivery. Each miscarriage was included in the count of the cycles, until the patients eventually reached delivery or dropped out.
In order to estimate the effectiveness of assisted reproduction technology treatment according to the number of cycles we calculated outcome by dividing the number of women achieving live birth delivery (numerator) by the total number of women who started treatment with IUI or ICSI with donor sperm (denominator). The outcome measure associated with this method is referred to as “Crude cumulative delivery rate” and provides a conservative estimate of outcome. Crude cumulative delivery rates (CDRs) and binomial exact 95 % confidence limits (95 % CLs) were calculated for each group of interest. Computational procedures were performed using STATA for Windows version 10.0 (StataCorp, College Station, Texas 77845, USA).
Results
Patient characteristics
Data were analysed in the overall population and in the 5 treatment subgroups, i.e. IUI starters, IVF/ICSI starters, oocyte donation starters, IUI to IVF/ICSI switchers and IVF/ICSI to oocyte donation switchers. Data analysed included age, FSH on day 3 and body mass index (BMI). Mean age of the whole group was 41.7 years (95 % CL [41.6–41.8]). Through the Bonferroni comparison we found a significant difference for age between the 3 primary starters groups. Patients in the IVF/ICSI starters group were younger patients than those in the IUI starters group. The egg donation group was, as expected, the oldest group. The latter was also significantly different from the 2 others with respect to FSH day 3 and BMI. Table 1 gives an overview of the patients characteristics in the different subgroups. Treatment characteristics are discussed further on in this article.
Table 1.
Patients ‘age, FSH day 3 and body mass index (BMI) in the different treatment groups. Values represent mean ± standard deviation (SD)
| Variable (mean ± SD) | IUI | IVF/ICSI | Switchers IUI to IVF/ICSI | Switcher IVF/ICSI to egg cell donation | Egg cell donation | All patients |
|---|---|---|---|---|---|---|
| Age (y) | 42.2 ± 1.9 | 41.4 ± 1.3 | 41.2 ± 1.1 | 43.5 ± 0.9 | 44.3 ± 2.1 | 41.7 ± 1.6 |
| FSH day 3 (IU/L) | 10.4 ± 4.6 | 10.2 ± 5.6 | 11.1 ± 6.1 | 26.5 ± 10.6 | 25.8 ± 17.7 | 11.4 ± 8.3 |
| BMI (kg/m2) | 23.1 ± 4.0 | 23.8 ± 5.0 | 22.9 ± 4.4 | 26.0 ± 4.6 | 26.0 ± 4.0 | 23.8 ± 4.8 |
Patient flow: continuation and discontinuation
A total of 909 women of 40 years or older, visited our fertility centre for the first time in the study period. Patients taking contact by phone yet, without taking any further appointment, were not taken into account for the analysis. After a second consultation, where evaluation of the preliminary examinations was discussed, treatment was proposed to 735 patients (81 %). Eventually, 585 patients (64 %) started an ART treatment. Nearly 80 % (585 out of 735) of the patients who received a treatment proposal actually started this treatment. Figure 1 overviews the patients in a flowchart. Reasons to discontinue treatment were various. Spontaneous pregnancy with delivery was observed in 10 patients (1.3 %). In 4 patients who opted for donation, donors dropped out because of ethical issues and the attempt was therefore canceled. Since patients in the oocyte donation group were older, it is not surprising that illness ratio was higher in this group. Three patients suffered from cancer, i.e. cervical cancer (1 patient), thyroid cancer (1 patient) and breast cancer (1 patient). A summary of these discontinuation reasons can be found in Table 2.
Fig. 1.
Flowchart: Patients pathway
Table 2.
Discontinuation reasons in 150 patients after treatment advice in 735 patients. (n = number of patients)
| Discontinuation reasons after treatment advice (n = 150) | ||||
|---|---|---|---|---|
| IUI(n = 35) | IVF/ICSI(n = 79) | Egg donation (n = 36) | All patients (n = 150) | |
| No reason | 28 (80.0 %) | 58 (73.5 %) | 24 (66.6 %) | 110 (73.3 %) |
| Illness | 1 (2.8 %) | 1 (1.2 %) | 1 (2.7 %) | 3(2 %) |
| Other centre | 2 (5.7 %) | 1 (1.2 %) | 4 (11.2 %) | 7 (4.6 %) |
| Financial | 1 (2.8 %) | 5 (6.3 %) | 3 (8.3 %) | 9 (6.0 %) |
| Egg donor problem | / | / | 4 (11.2 %) | 4 (2.7 %) |
| Spontaneous pregnancy and delivery | 3 (8.7 %) | 7 (8.9 %) | 0 | 10 (6.7 %) |
| Relational problem | 0 | 7 (8.9 %) | 0 | 7 (4.7 %) |
In most of the cases, patients were enrolled in an IVF/ICSI program (75 %) or IUI program (19 %), while only a minority opted for egg donation (6 %).
Treatment characteristics in IVF/ICSI patients
For IVF/ICSI treatment in particular, different continuous variables were analysed, i.e. stimulation, number of oocytes at pick-up, embryonic development and day of embryo transfer (see Table 3). Further information on stimulation included gonadotrophins starting dose for the first and the last treatment cycle, total dose of gonadotrophins for the first and the last treatment cycle and the number of follicles measuring more than 12 mm in diameter. The mean daily stimulation dose in the last cycle was 312.7 IU (304.3, 321.1), with an average cumulative total dose of 3197.2 IU. In the last treatment cycle a mean of 1.61 embryos was transferred either on day 3 (57.8 %) or day 5 (13.1 %) after ovum pick-up and 29.1 % of the patients had no embryo transfer.
Table 3.
Continuous treatment and treatment outcome variables (mean ± SD) in the first and the last IVF/ICSI cycles
| Continuous variables (mean ± SD) | First IVF/ICSI cycle | Last IVF/ICSI cycle |
|---|---|---|
| Gonadotrophins start dose 1st cycle (IE) | 295.6 ± 90.9 | 312.7 ± 94.2 |
| Total gonadotrophins dose (IE) | 3061.1 ± 1217.0 | 3197.3 ± 1289.3 |
| Number of follicles | 6.3 ± 5.3 | 6.0 ± 5.0 |
| Number of embryos | 4.2 ± 3.6 | 3.5 ± 3.4 |
| Number of transferred embryos | 1.52 ± 1.4 | 1.6 ± 1.5 |
Cumulative delivery rates
IUI and IVF/ICSI delivery rates: starters and switchers
Only 5 deliveries were obtained in the 63 women embarking upon IUI, which correlates with a cumulative delivery rate (CDR) of 7.9 %, (95 % CL 2.6 %, 17.6 %). None of the women had twins. Most of these patients used husband sperm (70 %). The average number of cycles per patient was 4.30. In total, 111 patients had started with an IUI treatment, though 48 of them changed after failed IUI to IVF/ICSI.
For 431 patients with a primary IVF/ICSI treatment CDR was 16.7 % (13.3 %, 20.6 %). If we included the 48 patients who switched from failed IUI to IVF/ICSI, the overall CDR was 18.0 % (14.6 %, 21.7 %) for IVF/ICSI. Overall, there were 86 deliveries; 7 deliveries were under 37 weeks gestation (12.2 %). There were 6 twin pregnancies and no triplets. The average number of cycles per patient was 1.84 (1.72 to 1.95). These success rates are shown in Fig. 1.
Egg donation delivery rates
In the 35 patients who started with oocyte donation the CDR was 48.6 % (31.4 %, 66.0 %). Including 8 patients switching from failed IVF/ICSI treatment to oocyte donation the CDR after donation was 46.5 % (31.2 %, 60.4 %). Overall, 20 deliveries were achieved, 3 deliveries were < 37 weeks gestation (6.6 %). The average number of cycles per patient was 1.26. Nearly all deliveries were achieved in the first cycle, only few patients continued up to 3 cycles. The mean age of these donors was 31.36. With exception of one donor, all donors were less than 35 years old.
Pregnancy and delivery follow up
In our group, first trimester bleeding occurred in 34 % of the patients. Pregnancy-induced hypertension (transient hypertension and pre-eclampsia) was observed in 15 %. Ten percent were hospitalized for preterm labour. PROM was recorded in 7 %. In the egg donation group two patients had an abruptio placentae. A mean birth weight of 3118.52 g (2963.64 g to 3273.40 g) was observed. Mean birth weight in the egg donation group was lower (2823.07 g [2346.79 g to 3299.35 g]) than in the other groups, although not significantly different. More girls (63.1 %) than boys (36.9 %) were born in our cohort. Overall there were 6 twins, but in 2 twins a fetal demise in-utero for one sibling occurred after 25 weeks of pregnancy. In one IVF/ICSI pregnancy a child with trisomia 21 was diagnosed and the parents opted to interrupt the pregnancy. One child was born with a 47, XXX karyotype.
Discussion
Our study gives a unique and realistic overview of the follow up of fertility treatment in women aged 40 and above. The study confirmed the impact of age on live birth delivery but provides a more accurate prediction for patient counselling. By taking all women into account visiting one of our fertility doctors for the first time, we could record how many women dropped out before actually starting a fertility treatment. This is an important fact, since eventually only 64 % of the patients started an ART treatment. We can now simply inform our patients they can expect a cumulative delivery rate of only 12 % if they continue treatment, and that they have an additional 1 % chance to achieve a spontaneous pregnancy and delivery.
Cumulative delivery rates after IVF/ICSI treatment largely influenced the overall delivery rates in our study, since nearly 75 % of the patients were advised to start with this treatment. To compare results between our study and the other published data, we did not take into account the patients who dropped out before starting any treatment. By this way, we could also evaluate the efficacy of a certain treatment in our centre: i.e. IUI, IVF/ICSI or egg donation. As mentioned in the introduction part, many data are available in literature for in vitro fertilization and intra-cytoplasmic sperm injection (ICSI). Malizia et al. [15] published data in 1,290 women aged 40 and above and reported a conservative cumulative delivery rate of 19 % after 3 IVF cycles and 23 % after 6 IVF cycles. In the paper of Osmanagaoglu et al. [21] crude cumulative delivery rates of 16 % after 6 IVF cycles was achieved in 116 patients. These cumulative success rates are comparable to the ones we are reporting in this paper. In our IVC/ICSI group, which consisted out of 487 patients, an overall crude cumulative delivery rate of nearly 18 % was reached. We can conclude that these success rates are representative for this age group and this type of treatment. Moreover these “low” success rates are confirmed in several other papers [7, 16].
For IUI several outcome data are available, though only limited cumulative data can be found for women aged 40 and more. Nearly all data about this topic are described in a review paper about the effect of age on the outcome of IUI [5]. Merviel et al. [17] published in a retrospective trial cumulative ongoing pregnancy rates of 12.5 % after six cycles in the same age category after IUI with husband sperm. This data cannot be compared with our data, since this study had a different endpoint, i.e. ongoing pregnancy instead of delivery. In this age group delivery rates per cycles were reported in several papers [2, 9, 11, 18, 24] and varied from 1.4 % up to 9.8 % per cycle. In our study 63 IUI patients achieved a low crude cumulative delivery rate of 7.8 %. A valid explanation for these low success rates in this group is a high mean age of 42.16 years. Probably due to the reimbursement policy, “younger” patients were advised to start immediately with IVF/ICSI and “ older” patients with IUI. Moreover, patients also stopped or switched earlier since we note a low average number of cycles per patient of 4.30. An additional explanation to counsel patients for IVF can be the anticipated lower success rates with IUI due to the women’s age. However, Goverde et al. [10] published a randomized trial in the Lancet: they concluded that couples with an idiopathic or male subfertility should be counseled that IUI offers the same likelihood of successful pregnancy as IVF.
In patients who weren’t able to use their own genetic material, egg donation was offered. Highest overall real cumulative delivery rate (46.5 %) was reached in this type of treatment. This high result is not a surprise, since donor age is a major determinant of success of oocyte donation/recipient program [29]. This population study concluded that the donor’s age had the largest impact on pregnancy and live delivery rates following fresh oocyte recipient cycles. Cycles with a donor age of 35–39 years and ≥ 40 years were associated with lower rates of live delivery compared with cycles using donors aged 30–34 years. Recipient age plays a limited role in success rate when a young egg donor is used. There was no significant relationship between the success rates in relation to the recipient’s age or the partner’s age. Choosing a young donor would increase the chance of pregnancy and live delivery for older recipients. Since 2010, our centre is validating a closed vitrification system in an oocyte-donation programme with a cumulative ongoing pregnancy rate per patient of 50 % [25].
Pregnancy and delivery in this age group is associated with an increased risk of complications. The incidence of hypertension during pregnancy is about 6 to 8 % in all age groups. An incidence of 15 % is reached in our population. Also the incidence of preterm labour, PROM, and abruptio placenta is higher than in younger patients. Older patients should be informed correctly on this so they are aware of potential complications.
Methodological strengths of our study include the consecutive recruitment of all patients aged 40 and above, a virtually complete patient follow-up, the choice of live birth delivery as the outcome event of primary interest. By taking into account all women who dropped out, more realistic cumulative delivery rates are presented. The overall cumulative success rates are per definition lower than in all other published papers, since drop-outs before starting any treatment were not counted in these publications. A more realistic prediction pattern is sketched for patients aged 40 and above seeking for help. The designed flow chart can easily be used to counsel patients, and moreover a more realistic prediction pattern is sketched. If patients are aware of the realistic pathway (and success rates) this flowchart can hopefully help to soften future disappointments through failed ART. This observational study has some weaknesses as well. Some patients abandoned immediately any furthers steps, i.e. first consultation, after having contact with our contact centre. Solely due to information about poor prognosis associated with advancing age some patients stopped immediately. These patients could not be taken into account. Furthermore our physicians chose independently the ART for their patients. We remark again that this has probably various reasons, i.e. reimbursement policy and an expected/anticipated higher success rate by using IVF/ICSI instead of IUI. We cannot state conclusions concerning which treatment should be offered first, since mean age in the IUI group is significantly higher than in the IVF/ICSI group. Though again, this publication is meant to sketch a realistic prediction pattern for these patients. In a recent study among patients aged 40 and above using donor sperm, we concluded that success rates after six IUI cycles were similar to that of one ICSI cycle [6]. Since time to pregnancy is an important argument in these older women we suggested to advice immediate ICSI treatment.
This large observational cohort study has an important informative value for patients aged 40 and more thanks to its unique longitudinal set-up because success rates are given per patient and not per attempt.
Acknowledgments
The authors thank Dr Stoop of the Centre for Reproductive Medicine for his literature advice concerning egg donation and results of this technique.
Footnotes
Capsule
In women aged 40 and above only 10% achieved delivery of their genetically -own child, while 1% conceived spontaneously.
References
- 1.Abma JC, Chandra A, Mosher WD, Peterson LS, Piccinino LJ. Fertility, family planning, and women’s health: new data from the 1995 National Survey of Family Growth. Vital Health Stat. 1997;23:1–114. [PubMed] [Google Scholar]
- 2.Belloc S, Cohen-Bacrie P, Benkhalifa M, Cohen-Bacrie M, De Mouzon J, Hazout A, et al. Effect of maternal and paternal age on pregnancy and miscarriage rates after intrauterine insemination. Reprod Biomed Online. 2008;17(3):392–397. doi: 10.1016/S1472-6483(10)60223-4. [DOI] [PubMed] [Google Scholar]
- 3.Broekmans FJ, Soules MR, Fauser BC. Endocr Rev. 2009;30(5):465–493. doi: 10.1210/er.2009-0006. [DOI] [PubMed] [Google Scholar]
- 4.De Brucker M, Haentjens P, Evenepoel J, Devroey P, Collins J, Tournaye H. Cumulative delivery rates in different age groups after artificial insemination with donor sperm. Hum Reprod. 2009;24(8):1891–1899. doi: 10.1093/humrep/dep085. [DOI] [PubMed] [Google Scholar]
- 5.De Brucker M, Tournaye H. The effect of age on the outcome of intrauterine insemination: a review. Facts, View & Vision In ObGyn. 2010; MONOGRAPH: 42–50.
- 6.De Brucker M, Camus M, Haentjens P, Verheyen G, Collins J, Tournaye H. Assisted reproduction using donor sperm in women aged 40 and above: the high road or the low road? Reprod Biomed Online. 2013 doi: 10.1016/j.rbmo.2013.02.008. [DOI] [PubMed] [Google Scholar]
- 7.Elizur SE, Lerner-Geva L, Levron J, Shulman A, Bider D, Dor J. Cumulative live birth rate following in vitro fertilization: study of 5,310 cycles. Gynecol Endocrinol. 2006;22(1):25–30. doi: 10.1080/09513590500453916. [DOI] [PubMed] [Google Scholar]
- 8.Evers JL. Female subfertility. Lancet. 2002;360:151–159. doi: 10.1016/S0140-6736(02)09417-5. [DOI] [PubMed] [Google Scholar]
- 9.Frederick JL, Denker MS, Rojas A, Horta I, Stone SC, Asch RH, et al. Is there a role for ovarian stimulation and intra-uterine insemination after age 40? Hum Reprod. 1994;9(12):2284–2286. doi: 10.1093/oxfordjournals.humrep.a138438. [DOI] [PubMed] [Google Scholar]
- 10.Goverde AJ, McDonnell J, Vermeiden JP, Schats R, Rutten FF, Schoemaker J. Intrauterine insemination or in-vitro fertilisation in idiopathic subfertility and male subfertility: a randomised trial and cost-effectiveness analysis. Lancet. 2000;355(9197):13–18. doi: 10.1016/S0140-6736(99)04002-7. [DOI] [PubMed] [Google Scholar]
- 11.Haebe J, Martin J, Tekepety F, Tummon I, Shepherd K. Success of intrauterine insemination in women aged 40–42 years. Fertil Steril. 2002;78(1):29–33. doi: 10.1016/S0015-0282(02)03168-0. [DOI] [PubMed] [Google Scholar]
- 12.International Committee Monitoring Assisted Reproductive Tecnologies (ICMART). http://www.icmartivf.org/current-activities.html 2007.
- 13.Leridon H. Can assisted reproduction technology compensate for the natural decline in fertility with age? A model assessment. Hum Reprod. 2004;19:1548–1553. doi: 10.1093/humrep/deh304. [DOI] [PubMed] [Google Scholar]
- 14.Leridon H. Demographic effects of the introduction of steroid contraception in developed countries. Hum Reprod Update. 2006;12:603–616. doi: 10.1093/humupd/dml025. [DOI] [PubMed] [Google Scholar]
- 15.Malizia B, Kacker MR, Penzias AS. Cumulative live-birth rates after in vitro fertilization. New Engl J Med. 2009;360:236–243. doi: 10.1056/NEJMoa0803072. [DOI] [PubMed] [Google Scholar]
- 16.Marinakis G, Nikolaou D. What is the role of assisted reproduction technology in the management of age-related infertility? Hum Fertil (Camb) 2011;14(1):8–15. doi: 10.3109/14647273.2010.549162. [DOI] [PubMed] [Google Scholar]
- 17.Merviel P, Heraud MH, Grenier N, Lourdel E, Sanguinet P, Copin H. Predictive factors for pregnancy after intrauterine insemination (IUI): an analysis of 1038 cycles and a review of the literature. Fertil Steril. 2010;93(1):79–88. doi: 10.1016/j.fertnstert.2008.09.058. [DOI] [PubMed] [Google Scholar]
- 18.Nuojua-Huttunen S, Tomas C, Bloigu R, Tuomivaara L, Martikainen H. Intrauterine insemination treatment in subfertility: an analysis of factors affecting outcome. Hum Reprod. 1999;14(3):698–703. doi: 10.1093/humrep/14.3.698. [DOI] [PubMed] [Google Scholar]
- 19.Nyboe Andersen A, Goossens V, Bhattacharya S, Ferraretti AP, Kupka MS, de Mouzon J, et al. Assisted reproductive technology and intrauterine inseminations in Europe, 2005: results generated from European registers by ESHRE: ESHRE. The European IVF Monitoring Programme (EIM), for the European Society of Human Reproduction and Embryology (ESHRE) Hum Reprod. 2009;24:1267–1287. doi: 10.1093/humrep/dep035. [DOI] [PubMed] [Google Scholar]
- 20.Osmanogaoglu K, Tournaye H, Camus M, Vandervorst M, Van Steirteghem A, Devroey P. Cumulative delivery rates after intracytoplasmic sperm injection: 5 year follow up of 498 patients. Hum Reprod. 1999;14:2651–2655. doi: 10.1093/humrep/14.10.2651. [DOI] [PubMed] [Google Scholar]
- 21.Osmanagaoglu K, Tournaye H, Kolibianakis E, Camus M, Van Steirteghem A, Devroey P. Cumulative delivery rates after ICSI in women aged 37 years. Hum Reprod. 2002;17:940–944. doi: 10.1093/humrep/17.4.940. [DOI] [PubMed] [Google Scholar]
- 22.Papanikolaou E, D’haeseleere E, Verheyen G, Van de Velde H, Camus M, Van Steirteghem A, et al. Live birth is significantly higher after blastocyst transfer than after cleavage-stage embryo transfer when at least four embryos are available on day 3 of embryo culture: a randomized prospective study. Hum Reprod. 2005;20:3198–3203. doi: 10.1093/humrep/dei217. [DOI] [PubMed] [Google Scholar]
- 23.Papanikolaou E, Bourgain C, Kolibianakis E, Tournaye H, Devroey P. Steroid receptor expression in late follicular phase endometrium in GnRH antagonist IVF cycles is already altered, indicating initiation of early luteal phase transformation in the absence of secretory changes. Hum Reprod. 2005;20:1541–1547. doi: 10.1093/humrep/deh793. [DOI] [PubMed] [Google Scholar]
- 24.Stone BA, Vargyas JM, Ringler GE, Stein AL, Marrs RP. Determinants of the outcome of intrauterine insemination: analysis of outcomes of 9963 consecutive cycles. Obstet Gynecol. 1999;180:1522–1564. doi: 10.1016/S0002-9378(99)70048-7. [DOI] [PubMed] [Google Scholar]
- 25.Stoop D, De Munck N, Jansen E, Platteau P, Van den Abbeel E, Verheyen G, et al. Clinical validation of a closed vitrification system in an oocyte-donation programme. Reprod Biomed Online. 2012;24(2):180–185. doi: 10.1016/j.rbmo.2011.10.015. [DOI] [PubMed] [Google Scholar]
- 26.Spira A. The decline of fecundity with age. Maturitas (Suppl) 1988;1:15–22. doi: 10.1016/0378-5122(88)90004-7. [DOI] [PubMed] [Google Scholar]
- 27.Templeton A, Morris JK, Parslow W. Factors that affect outcome of in vitro fertilisation treatment. Lancet. 1996;348:1402–1406. doi: 10.1016/S0140-6736(96)05291-9. [DOI] [PubMed] [Google Scholar]
- 28.teVelde ER, Pearson PL. The variability of female reproductive ageing. Hum Reprod Update. 2002;8:141–154. doi: 10.1093/humupd/8.2.141. [DOI] [PubMed] [Google Scholar]
- 29.Wang YA, Farquhar C, Sullivan EA. Donor age is a major determinant of success of oocyte donation/recipient program. Hum Reprod. 2012;27(1):118–125. doi: 10.1093/humrep/der359. [DOI] [PubMed] [Google Scholar]
- 30.Wood JW. Fecundity and natural fertility in humans. Oxf Rev Reprod Biol. 1989;11:61–109. [PubMed] [Google Scholar]