Abstract
Objective
To study implications of psychological distress on in vitro fertilization (IVF) outcome of an infertile couple.
Methods
Prospective study in an academic infertility practice setting. Couples undergoing embryo transfer (ET) following IVF were offered participation. Female patient (n = 89) and partner (n = 77) completed questionnaires reflecting dysphoria (POMS) and pessimism (LOT) after undergoing ET. Relationship between dysphoria and pessimism and implications of individual and couple’s psychological distress on IVF cycle parameters and outcomes were assessed using multivariable analyses.
Results
Statistically significant correlations between dysphoria and pessimism were observed within the individual and between partners, (p < 0.01). Higher couple pessimism correlated with longer duration of controlled ovarian hyperstimulation (COH, p = 0.02); higher partner psychological distress related to lower fertilization rate (FR, p = 0.03). On adjusted analyses, partner’s depression score was an independent predictor of reduced likelihood of clinical pregnancy (p = 0.03).
Conclusions
Our data validate the concept of a “stressed couple”. Adverse implications of a couple’s psychological distress for gamete biology (longer duration of COH and lower FR with increasing distress) are suggested. Partner’s depressive scores negatively correlated with IVF success. These findings suggest the importance of including partner’s evaluation in studies that focus on effects of psychological stress on IVF outcome; future studies should examine whether interventions aimed at reducing psychological stress for the infertile couple may improve IVF cycle success.
Electronic supplementary material
The online version of this article (doi:10.1007/s10815-013-0098-7) contains supplementary material, which is available to authorized users.
Keywords: Stress, Mood, Dysphoria, Pessimism, POMS, LOT, Infertility, IVF
Introduction
While stress relating to infertility and fertility treatment is well recognized [1–4], a cause-effect relationship is far from clear [5–7]. Psychological stressors are suggested to negatively impact the success of in vitro fertilization (IVF) [8–18]. Evidence to this effect however is equivocal at best [19–22], and some have even implied positive effects of stress on IVF outcome [23].
The periods of egg retrieval (ER), embryo transfer (ET) and pregnancy test following IVF are all recognized as vulnerable times linked with high levels of stress [10, 24–29]. Behavioral modification, psychological support and acupuncture have been shown to positively impact on success of fertility treatments [28, 29], albeit inconsistently [30]. A relevance of psychological distress proximate to the timing of ET for IVF outcome is suggested as improvements in pregnancy rates following IVF are described when acupuncture was administered as a stress reduction strategy on the day of ET compared to when instituted on the day of ER [31]. Indeed, an accruing body of literature is supportive of potential for benefit for infertile women undergoing IVF [28, 29, 31]. Detrimental effects of “stress” on semen quality have also been described [32–34] and a single study reported a correlation between male partner stress and IVF failure [9]. Overall, however, the effect of male stress on ART outcome has been poorly explored. We hypothesized a cumulative detriment of psychological stress experienced by either and both partners in couples undergoing ART.
While the mechanisms are unclear, psychological stress may affect reproductive competence through a variety of pathways including the hypothalamic-pituitary-adrenal and hypothalamic-pituitary-gonadal axes, oxytocin, immunologic mechanisms [1, 5, 26, 31] and possibly through adversely influencing uterine receptivity [35].
We report the results of a prospective study in which we have explored reproductive implications of dysphoric mood and pessimism in infertile couples undergoing IVF.
Materials and methods
Couples undergoing fresh ET (11/2006–11/2007) at the Montefiore Institute for Reproductive Medicine and Health (MIRMH) were prospectively offered participation. The study was approved by the institutional review board at Montefiore Medical Center and participants provided written consent.
Methods
Consecutive patients were offered recruitment after successful completion of ET with a target enrollment of 100. The patient and her partner (if present) individually completed two validated questionnaires (Profile of Mood States –POMS, and Life Orientation Test- LOT). POMS measures 6 mood dimensions (tension-anxiety; depression-dejection; anger-hostility; vigor-activity; fatigue-inertia and confusion-bewilderment) whereas LOT is a measure of dispositional optimism [36, 37]. The questionnaires were scored by a trained psychologist (KB) who was blinded to participant gender and cycle outcome. Use of a sedative or relaxant prior to ET was an exclusion criterion.
Patient and cycle data were collected from medical records including age, body mass index (BMI), early follicular (days 1–3) serum FSH and estradiol (E2) levels, GnRH agonist versus antagonist use, gonadotropin dose, duration of controlled ovarian hyperstimulation (COH), serum E2 and progesterone (P) levels and endometrial thickness (EMT in mm) on the day of hCG trigger, number of eggs retrieved, insemination method (IVF, intracytoplasmic sperm injection [ICSI], or split), fertilization rate (FR, %), day of ET (day#3 or blastocyst), cryopreservation of surplus embryos (yes/no) and IVF cycle outcome (implantation rate [IR] and clinical pregnancy [CP]). Data on partner’s age and semen parameters on day of egg retrieval were also assessed.
Statistics
Recruitment goal was set at n = 100 for this pilot study. Post hoc power analysis demonstrated that with a sample size of 90, and an anticipated correlation coefficient of −0.3 between distress score and CP, the study was powered at 0.82 for an alpha of 0.05.
Dysphoria and pessimism scores were calculated for the patient and partner; summation of respective individual scores reflected totaldysphoria and pessimism for the couple. A compositepsychological distress score (summation of dysphoria and pessimism scores) was calculated for the individuals and the couple. Higher scores indicate greater dysphoria and pessimism respectively.
Data distributions were analyzed; Student’s t test compared normally distributed data across categories by cycle outcome (CP versus not pregnant). Skewed data (gonadotropin dose and IR) were log transformed for similar analyses if normal distribution was achieved; otherwise nonparametric tests were used (Mann Whitney U Rank Sum Test). Clinical pregnancy and IR were primary outcomes and IVF cycle parameters were secondary outcomes of interest. Relationship of specified outcomes with dysphoria, pessimism and composite psychological distress scores (i.e. independent variables of interest) were assessed utilizing appropriate univariate analyses (Pearson or Spearman correlation, Student’s T test or Mann U Whitney for continuous data, and Chi-square test for categorical data). Seasons were defined by month of ET: Winter: December–February, Spring: March–May, Summer: June–August, and Fall: September–November. Seasonal variation in mood was similarly assessed. Kruskall Wallis rank test was used to compare continuous data across more than 2 categories (i.e. mood scores across infertility diagnoses and by season). Multivariable regression analyses (logistic or linear as appropriate) identified independent correlates to the outcomes of interest after adjusting for potential confounders that were recognized to influence IVF success (age, method of insemination (IVF vs. ICSI), use of GnRH agonist, EMT, number of ET, P on the day of hCG); season and partner depression score; variables demonstrating p value of < 2.0 for association with CP were additionally examined for confounding. Continuous data are presented as mean (standard deviation) and associations as odds ratio (OR) and 95 % confidence interval (CI). STATA Intercooled 12.0 (StataCorp, College Station, TX) was utilized and two tailed p-value <0.05 was considered significant.
Results
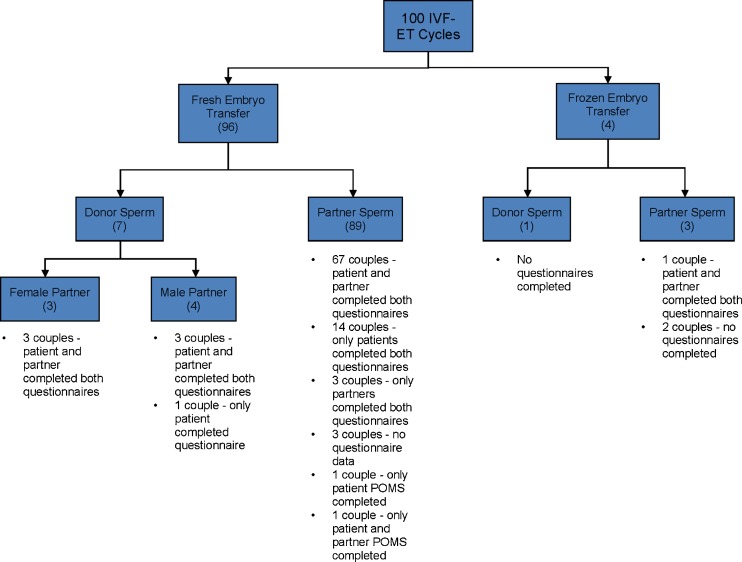
Figure 1 outlines enrollment details. Of the 100 consenting patients, both questionnaires were completed by 89 patients and 77 partners.
Fig. 1.
Flow diagram reflecting enrollment details
Table 1 provides patient characteristics, cycle parameters and mood and pessimism scores by cycle outcome. Younger age, use of a GnRH agonist, lower gonadotropin dose, increased EMT, and cryopreservation of surplus embryos were associated with an increased likelihood of CP following IVF-ET. Infertility diagnoses did not relate to the likelihood of cycle success (data not shown).
Table 1.
Participant characteristics, IVF cycle parameters and aspects of psychological distress (higher scores on POMS and LOT screening reflect worsening dysphoria and pessimism respectively) are presented according to the outcome of embryo transfer cycle (clinical pregnancy versus not pregnant)
| Characteristic | Clinical pregnancy (N = 38) | Not pregnant (N = 62) | P- value | |
|---|---|---|---|---|
| Demographics | Patient age (years)a | 32.6 ± 4.33 | 35.7 ± 4.25 | <0.01 |
| Partner age (years)a | 35.2 ± 5.36 | 37.6 ± 5.96 | 0.05 | |
| Patient BMI (kg/m2)a | 26.3 ± 4.30 | 26.4 ± 5.55 | 0.59 | |
| Smoker (patient) (%) | 10.5 % | 8.5 % | 0.73 | |
| Smoker (partner) (%) | 27 % | 12.7 % | 0.08 | |
| Cycle parameters | 1st ART cycle (%) | 58 % | 53 % | 0.65 |
| IVF cycles (%) | 26.3 % | 27.4 % | 0.90 | |
| ICSI cycles (%) | 50 % | 58 % | 0.43 | |
| IVF-ICSI split cycles (%) | 21 % | 9.7 % | 0.11 | |
| GnRH agonist (Lupron) (%) | 86.8 % | 66.1 % | 0.02 | |
| Cycle day 3 FSH (mIU/mL)a | 7.1 ± 2.12 | 7.7 ± 2.56 | 0.97 | |
| Gonadotropin dose (IU) (log transformed)a | 7.7 ± 0.45 | 8.0 ± 0.50 | 0.01 | |
| Duration of COH (days)a | 10.4 ± 1.54 | 10.4 ± 1.78 | 1.00 | |
| Endometrial thickness day of hCG (mm)a | 11.8 ± 2.80 | 10.7 ± 2.13 | 0.03 | |
| Estradiol day of hCG (pg/ml) (log transformed)a | 7.6 ± 0.44 | 7.4 ± 0.55 | 0.10 | |
| Serum progesterone day of hCG (ng/ml)b | 0.9 (0.6–1.21) | 1.1 (0.7–1.41) | 0.16 | |
| # of eggs retrieved | 13.5 (8–16) | 11 (6–15) | 0.19 | |
| # of embryos transferredb | 2 (2–3) | 2 (2–3) | 0.98 | |
| # embryos cryopreserved | 12/38 (32 %) | 6/62 (10 %) | <0.01 | |
| Mood parameters | POMS score (dysphoria)b | |||
| Patient | 18 (−2 to 45) | 13.5 (0.5 to 35) | 0.80 | |
| Partner | 15 (−3 to 31) | 17.5 (−6.5 to 35) | 0.87 | |
| Couple | 30 (2 to 69) | 34 (−4 to 59) | 0.68 | |
| LOT score (pessimism)b | ||||
| Patient | 5 (3 to 11) | 6 (2 to10) | 0.57 | |
| Partner | 6 (4 to 14) | 8 (3 to 12) | 0.83 | |
| Couple | 15 (9 to19) | 13 (8 to 18) | 0.35 | |
| LOT + POMS (composite psychological distress)b | ||||
| Patient | 23 (2 to52) | 22 (5 to 45) | 0.85 | |
| Partner | 25 (4 to 42) | 22 (0 to 45) | 0.77 | |
| Couple | 37 (17 to 90) | 44 (2 to 86) | 0.58 | |
Continuous data are presented as mean (SD)a or as median (inter-quartile range)b
Statistically significant linear correlations were observed between dysphoria and pessimism scores for patients (r = 0.42, p < 0.001) and for partners (r = 0.33, p = 0.004). Statistically significant correlations were observed between patient and partner dysphoria scores (r = 0.32, p = 0.005) and between patient and partner composite psychological distress scores (r = 0.34, p = 0.003) (Supplemental Figure). The relationship between patient and partner pessimism scores was not statistically significant (r = 0.16, p = 0.169).
Seasonal differences in dysphoric mood and pessimism were observed. Dysphoria and composite psychological distress scores for the partner as well as dysphoria, pessimism and composite psychological distress scores for the couple were significantly higher for those undergoing ET in the winter compared to other seasons (Table 2).
Table 2.
Worsening mood and pessimism were observed in couples undergoing ART cycles during winter months, compared to other seasons
| Wintera | Other seasonsa | P-value | |
|---|---|---|---|
| POMS score (dysphoria) | |||
| Patient | 21 (0 to 40) | 13 (−3 to 35) | 0.26 |
| Partner | 27 (11 to 48) | 11 (−7.5 to 32) | 0.04 |
| Couple | 53 (23 to 83) | 24 (−5 to 48) | 0.02 |
| LOT score (pessimism) | |||
| Patient | 7 (4 to 11) | 5 (2 to 9) | 0.13 |
| Partner | 8 (4 to 14) | 7 (3 to 11) | 0.24 |
| Couple | 17 (13 to 20) | 11 (7 to 18) | 0.03 |
| LOT + POMS (composite psychological distress) | |||
| Patient | 27 (6 to 52) | 18 (2 to 45) | 0.21 |
| Partner | 33 (13 to 55) | 20 (−2 to 41) | 0.02 |
| Couple | 71 (34 to 98) | 32 (2 to 79) | 0.03 |
Continuous data presented as median (inter-quartile range)a
Higher scores indicate worsening parameter
On univariate analyses, neither total dysphoria nor pessimism scores related to cycle outcome (Table 1). An inverse relationship between partner depression score and likelihood of CP was noted; partners of women achieving CP following ET scored lower on the depression domain of POMS compared to partners of those with failed outcome (log transformed respective depression scores 1.46 ± 1.03 vs. 1.97 ± 0.93, p = 0.054).
After adjustment for patient age, insemination method (IVF versus ICSI), COH protocol, EMT, #ET, serum P on the day of hCG and season, partner’s depression score emerged as an independent determinant of CP following ET. Each unit increase in partner’s depression score was associated with a 17 % decreased likelihood of CP (p = 0.030). Advancing patient age (Adjusted OR 0.65, 95 % CI 0.49–0.88) and higher serum P levels (AOR 0.14, 95 % CI 0.02–0.91) were independent negative predictors of CP whereas GnRH agonist (vs. antagonist) (AOR 22.29, 95 % CI 1.95–254.95), higher EMT (AOR 1.79, 95 % CI 1.2–2.66) and IVF-ICSI split cycles (AOR 64.39, 95 % CI 2.37–1751.28) related to a significantly increased likelihood of CP following IVF. The statistical model exhibited 89 % sensitivity for predicting CP. No relationship between IR and psychological parameters for the patient, partner or couple was appreciated (data not shown).
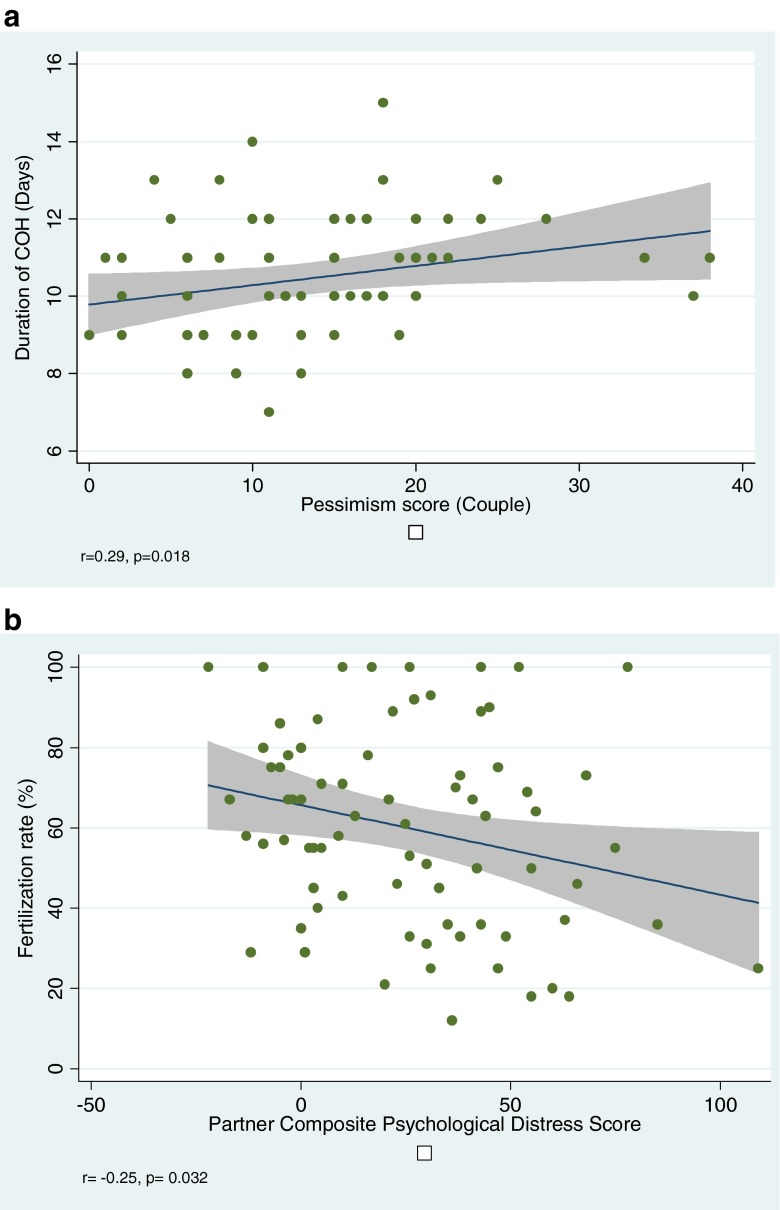
While no association was observed between patient or partner dysphoria and IVF cycle parameters, higher couple pessimism scores correlated with longer duration of COH (Fig. 2a). A significant negative correlation was observed between partner dysphoria and composite psychological distress scores and FR (Fig. 2b); of note, three same gender couples were excluded from analyses assessing relationship between partner/couple’s distress and FR. No significant relationship was observed between FR and patient or partner dysphoria or pessimism (data not shown). Sensitivity analyses failed to demonstrate any relationship between cycle outcome and partner’s presence or absence at ET (data not shown).
Fig. 2.
Relationship between psychological stress and gamete biology is suggested by the observed positive correlation between couple’s pessimism score and duration of controlled ovarian stimulation (a), and by the observed inverse correlation between fertilization rate and partner’s psychological distress (b)
Multivariable linear regression analysis confirmed partner’s composite psychological distress score as a negative predictor of FR (β coefficient −0.18, SE 0.09, p = 0.049) after adjusting for female partner age, ICSI (vs. IVF) and % motile sperm on the day of ER; 20 % variability in FR was explained by this model (adjusted R2 0.20). Sperm motility (%) was identified as an independent predictor of FR (β coefficient 0.53, SE 1.4, p < 0.001). Sensitivity analyses excluding couples who utilized donor sperm and adjusting for ICSI confirmed persistence of the negative relationship between partner psychological distress and FR (data not shown).
No significant differences were observed in patient, partner or couples’ dysphoric (p = 0.33, p = 0.61 and p = 0.38 respectively) or pessimism scores across various categories of infertility diagnosis (p = 0.84, p = 0.46 and p = 0.61 respectively, by Kruskal Wallis Rank test). Similarly, POMS AND LOT scores were no worse in women with a known diagnosis of diminished ovarian response (n = 14) or in male partners amongst couples with a known male factor contribution to infertility (n = 31, p = 0.20 and p = 0.57 respectively, by student’s T test). Neither patient nor partner dysphoric or pessimism scores demonstrated any correlation with baseline FSH (p >0.05).
Discussion
We assessed concordance of dysphoria and pessimism between partners in couples undergoing fresh ET following IVF. Our data suggest that while dysphoria and overall psychological distress (reflected by the composite score) are concordant between patients and partners, pessimism is not. Prior research suggests that discordance in the emotional experience of infertility within heterosexual couples may relate to differential coping styles of men versus women [38, 39]. While our data validate the concept of a “stressed couple”, both individual and gender-related factors must be considered when assessingcouples’ psychological needs.
Our study design does not allow causative interpretation to the observations; however, the noted association between couples’ psychological distress and COH parameters may be construed as negative influence of stress on folliculogenesis. Similarly, the observed relationship between partners’ psychological distress and FR can be interpreted as reflecting adverse influences of stress on gamete biology. Partner’s depressive mood is seen as a negative predictor of CP following ET. In light of our findings, access to psychological assessment and support should be considered for both partners undergoing IVF.
Some of the heterogeneity in the literature regarding the effect of psychological distress on ART success may be attributable to the wide variety of tools utilized to assess psychological state. The choice of psychological tools (POMS and LOT) allowed us to assess a range of dysphoric moods (anxiety, tension, depression, anger and confusion) as well as a couple’s expectations regarding cycle outcome. To our knowledge, this is the first study to assess implications of optimism/pessimism for cycle outcome in infertile couples undergoing IVF.
The observed inverse association between male partner’s psychological distress and FR, independent of insemination methodology, suggests implications of psychological wellbeing on male gamete physiology. Clarke et al. measured anxiety, stressfulness, and perceived importance of producing a semen sample prior to IVF initiation and again at egg retrieval in males undergoing first IVF cycles. Higher anxiety and a significant increase in the perceived importance of producing a sample at egg retrieval compared to an earlier collection were observed and were significantly correlated with decreased sperm concentration, total motile sperm, and motile sperm concentration [34]. In keeping with this latter observation, a non-significant trend was observed in our study between both decreasing sperm concentration and motility and increasing partner dysphoria. While our findings are in agreement with Clarke et al. that psychological distress may adversely influence semen parameters in infertile couples undergoing IVF, it is important to appreciate that these associations may not translate to healthy and fertile populations [40, 41].
The prospective design, relatively robust questionnaire completion rate, blinded scoring of questionnaires and the adjusted analytic strategies are strengths of our study. Given the emergence of partner depression as mood domain predictive of CP, consideration might be given to using a more specific depression screen for partners of women anticipating IVF treatment in future studies. While the timing of questionnaire administration limited confounding from earlier stressors, this single time point also limits our ability to draw broad conclusions from the observed relationships. A higher proportion of partners acknowledged smoking in the group attaining CP, an observation that is difficult to rationalize as others have identified passive exposure to tobacco as a detriment to reproductive success [42]; in the absence of any plausible explanation, this observed association may indeed reflect an alpha error.
Our goal in assessing psychological distress following ET was to better understand the implications of the couple’s psychological wellbeing on the outcomes of interest while eliminating individual and couple’s concerns regarding fertilization, embryo development and achieving ET. Our study design does not allow us to comment on modulation of psychological stress in either partner during the period between cycle start and ET or while awaiting cycle outcome. Although sensitivity analyses failed to demonstrate a relationship between cycle outcome and partner’s presence or absence, our study is not powered in this context. While the observed relationship between IVF-ICSI split cycles and CP is of interest, our study design does not allow an elaboration on this finding. The decision to offer IVF-ICSI split cycle was based on the supervising clinician’s judgment and individualized concerns regarding fertilization potential [43]. Implications of advancing age, COH protocol and EMT for cycle outcome as evident in our population are consistent with existing data [44–46].
Prior IVF experience may relate to stress levels in subsequent attempts [16, 27]. The proportion of patients in our study undergoing first ART attempt was comparable between cycles achieving CP versus failed cycles (58 % versus 53 %, p 0.649); our data fail to relate first versus repeat ART cycle with either psychological distress or outcome.
While partner depression may impact the ability to achieve CP, of interest are the associations between patient distress and duration of COH, and between partner distress and FR without an apparent impact on CP. Studies of first pregnancy planners have correlated positive psychological parameters with increased fertility [47], and increasing psychological distress with lower odds of conception per cycle [48]. While further studies are needed to delineate mechanisms to explain how stress may impact reproductive competence, our findings suggest that ET following IVF may overcome a plausible biologic hurdle imposed by psychological distress on natural conception (as suggested by prolonged COH and decreased FR with increasing psychological burden in the couple).
Our results highlight that the psychological well-being of each partner undergoing IVF is intertwined and may have implications for cycle outcome. Although a causative role for psychological stress to the observed associations cannot be assigned, improved cycle outcomes are described with de-stressing interventions including cognitive behavioral therapy and acupuncture [28, 29], albeit inconsistently [30]. Far fewer studies have investigated the effectiveness of stress reduction strategies in partners of women undergoing fertility treatment. Increased “healthy” sperm and decreased chromosomal aberrations were observed in men treated with Conveyer of Modulating Radiance (CRM) therapy (a radiofrequency instrument used to treat stress and anxiety) [49] and others report improved sperm parameters after acupuncture [40, 41, 50, 51].
In summary, our data validate the concept of a stressed couple. Adverse implications of psychological distress for folliculogenesis and fertilization are suggested without a demonstrable impact on IVF cycle success. We propose that ART may overcome any hurdles that psychological distress may impose on reproductive competence. Controlled studies are needed to further investigate the impact of psychological distress in the female patient, her partner and in the couple on ART cycles to enable us to better appreciate when and how to intervene so as to maximize reproductive success in couples undergoing ART.
Electronic supplementary material
Linear correlation was observed between psychological distress scores of the female patients and their partners. (DOC 26 kb)
Acknowledgments
The authors wish to thank the patients and the staff at MIRMH for their participation in making this work a reality.
Declaration of interest statement
The authors report no declarations of interest.
Footnotes
Capsule In couples undergoing in vitro fertilization (IVF), psychological distress may have implications for gamete biology and for IVF success.
This work was in part supported by NIH 5K12 RR17672 (LP). This work was presented at the 63rd and 64th Annual Meetings of ASRM.
References
- 1.Seibel MM, Taymor ML. Emotional aspects of infertility. Fertil Steril. 1982;37:137–145. doi: 10.1016/s0015-0282(16)46029-2. [DOI] [PubMed] [Google Scholar]
- 2.Freeman EW, Boxer AS, Rickels K, Tureck R, Mastroianni L., Jr Psychological evaluation and support in a program of in vitro fertilization and embryo transfer. Fertil Steril. 1985;43:48–53. doi: 10.1016/s0015-0282(16)48316-0. [DOI] [PubMed] [Google Scholar]
- 3.Chen TH, Chang SP, Tsai CF, Juang KD. Prevalence of depressive and anxiety disorders in an assisted reproductive technique clinic. Hum Reprod. 2004;19:2313–2318. doi: 10.1093/humrep/deh414. [DOI] [PubMed] [Google Scholar]
- 4.Kee BS, Jung BJ, Lee SH. A study on psychological strain in IVF patients. J Assist Reprod Genet. 2000;17:445–448. doi: 10.1023/A:1009417302758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cwikel J, Gidron Y, Sheiner E. Psychological interactions with infertility among women. Eur J Obstet Gynecol Reprod Biol. 2004;117:126–131. doi: 10.1016/j.ejogrb.2004.05.004. [DOI] [PubMed] [Google Scholar]
- 6.Wasser SK, Sewall G, Soules MR. Psychosocial stress as a cause of infertility. Fertil Steril. 1993;59:685–689. [PubMed] [Google Scholar]
- 7.Stoleru S, Teglas JP, Spira A, Magnin F, Fermanian J. Psychological characteristics of infertile patients: discriminating etiological factors from reactive changes. J Psychosom Obstet Gynaecol. 1996;17:103–118. doi: 10.3109/01674829609025670. [DOI] [PubMed] [Google Scholar]
- 8.Boivin J, Takefman JE. Stress level across stages of in vitro fertilization in subsequently pregnant and nonpregnant women. Fertil Steril. 1995;64:802–810. doi: 10.1016/s0015-0282(16)57858-3. [DOI] [PubMed] [Google Scholar]
- 9.Boivin J, Schmidt L. Infertility-related stress in men and women predicts treatment outcome 1 year later. Fertil Steril. 2005;83:1745–1752. doi: 10.1016/j.fertnstert.2004.12.039. [DOI] [PubMed] [Google Scholar]
- 10.Csemiczky G, Landgren BM, Collins A. The influence of stress and state anxiety on the outcome of IVF-treatment: psychological and endocrinological assessment of Swedish women entering IVF-treatment. Acta Obstet Gynecol Scand. 2000;79:113–118. doi: 10.1034/j.1600-0412.2000.079002113.x. [DOI] [PubMed] [Google Scholar]
- 11.Demyttenaere K, Bonte L, Gheldof M, Vervaeke M, Meuleman C, Vanderschuerem D, et al. Coping style and depression level influence outcome in in vitro fertilization. Fertil Steril. 1998;69:1026–1033. doi: 10.1016/S0015-0282(98)00089-2. [DOI] [PubMed] [Google Scholar]
- 12.Klonoff-Cohen H, Chu E, Natarajan L, Sieber W. A prospective study of stress among women undergoing in vitro fertilization or gamete intrafallopian transfer. Fertil Steril. 2001;76:675–687. doi: 10.1016/S0015-0282(01)02008-8. [DOI] [PubMed] [Google Scholar]
- 13.Klonoff-Cohen H, Natarajan L. The concerns during assisted reproductive technologies (CART) scale and pregnancy outcomes. Fertil Steril. 2004;81:982–988. doi: 10.1016/j.fertnstert.2003.08.050. [DOI] [PubMed] [Google Scholar]
- 14.Sanders KA, Bruce NW. Psychosocial stress and treatment outcome following assisted reproductive technology. Hum Reprod. 1999;14:1656–1662. doi: 10.1093/humrep/14.6.1656. [DOI] [PubMed] [Google Scholar]
- 15.Smeenk JM, Verhaak CM, Eugster A, van Minnen A, Zielhuis GA, Braat DD. The effect of anxiety and depression on the outcome of in-vitro fertilization. Hum Reprod. 2001;16:1420–1423. doi: 10.1093/humrep/16.7.1420. [DOI] [PubMed] [Google Scholar]
- 16.Thiering P, Beaurepaire J, Jones M, Saunders D, Tennant C. Mood state as a predictor of treatment outcome after in vitro fertilization/embryo transfer technology (IVF/ET) J Psychosom Res. 1993;37:481–491. doi: 10.1016/0022-3999(93)90004-Y. [DOI] [PubMed] [Google Scholar]
- 17.Ebbesen SM, Zachariae R, Mehlsen MY, Thomsen D, Hojgaard A, Ottosen L, et al. Stressful life events are associated with a poor in-vitro fertilization (IVF) outcome: a prospective study. Hum Reprod. 2009;24:2173–2182. doi: 10.1093/humrep/dep185. [DOI] [PubMed] [Google Scholar]
- 18.Gurhan N, Akyuz A, Atici D, Kisa S. Association of depression and anxiety with oocyte and sperm numbers and pregnancy outcomes during in vitro fertilization treatment. Psychol Rep. 2009;104:796–806. doi: 10.2466/pr0.104.3.796-806. [DOI] [PubMed] [Google Scholar]
- 19.Anderheim L, Holter H, Bergh C, Moller A. Does psychological stress affect the outcome of in vitro fertilization? Hum Reprod. 2005;20:2969–2975. doi: 10.1093/humrep/dei219. [DOI] [PubMed] [Google Scholar]
- 20.Harlow CR, Fahy UM, Talbot WM, Wardle PG, Hull MG. Stress and stress-related hormones during in-vitro fertilization treatment. Hum Reprod. 1996;11:274–279. doi: 10.1093/HUMREP/11.2.274. [DOI] [PubMed] [Google Scholar]
- 21.Lovely LP, Meyer WR, Ekstrom RD, Golden RN. Effect of stress on pregnancy outcome among women undergoing assisted reproduction procedures. South Med J. 2003;96:548–551. doi: 10.1097/01.SMJ.0000054567.79881.E9. [DOI] [PubMed] [Google Scholar]
- 22.Slade P, Emery J, Lieberman BA. A prospective, longitudinal study of emotions and relationships in in-vitro fertilization treatment. Hum Reprod. 1997;12:183–190. doi: 10.1093/humrep/12.1.183. [DOI] [PubMed] [Google Scholar]
- 23.Cooper BC, Gerber JR, McGettrick AL, Johnson JV. Perceived infertility-related stress correlates with in vitro fertilization outcome. Fertil Steril. 2007;88:714–717. doi: 10.1016/j.fertnstert.2006.11.158. [DOI] [PubMed] [Google Scholar]
- 24.Cheong YC, Hung Yu Ng E, Ledger WL. Acupuncture and assisted conception. Cochrane Database Syst Rev. 2008:CD006920. [DOI] [PubMed]
- 25.El-Toukhy T, Sunkara SK, Khairy M, Dyer R, Khalaf Y, Coomarasamy A. A systematic review and meta-analysis of acupuncture in in vitro fertilisation. BJOG. 2008;115:1203–1213. doi: 10.1111/j.1471-0528.2008.01838.x. [DOI] [PubMed] [Google Scholar]
- 26.Barrell GK. Immunological influences on reproductive neuroendocrinology. Soc Reprod Fertil Suppl. 2007;64:109–122. doi: 10.5661/rdr-vi-109. [DOI] [PubMed] [Google Scholar]
- 27.Lancastle D, Boivin J. Dispositional optimism, trait anxiety, and coping: unique or shared effects on biological response to fertility treatment? Health Psychol. 2005;24:171–178. doi: 10.1037/0278-6133.24.2.171. [DOI] [PubMed] [Google Scholar]
- 28.Domar AD, Clapp D, Slawsby EA, Dusek J, Kessel B, Freizinger M. Impact of group psychological interventions on pregnancy rates in infertile women. Fertil Steril. 2000;73:805–811. doi: 10.1016/S0015-0282(99)00493-8. [DOI] [PubMed] [Google Scholar]
- 29.Ng EH, So WS, Gao J, Wong YY, Ho PC. The role of acupuncture in the management of subfertility. Fertil Steril. 2008;90:1–13. doi: 10.1016/j.fertnstert.2008.02.094. [DOI] [PubMed] [Google Scholar]
- 30.Domar AD, Meshay I, Kelliher J, Alper M, Powers RD. The impact of acupuncture on in vitro fertilization outcome. Fertil Steril. 2009;91:723–726. doi: 10.1016/j.fertnstert.2008.01.018. [DOI] [PubMed] [Google Scholar]
- 31.Schenker JG, Meirow D, Schenker E. Stress and human reproduction. Eur J Obstet Gynecol Reprod Biol. 1992;45:1–8. doi: 10.1016/0028-2243(92)90186-3. [DOI] [PubMed] [Google Scholar]
- 32.Harrison KL, Callan VJ, Hennessey JF. Stress and semen quality in an in vitro fertilization program. Fertil Steril. 1987;48:633–636. [PubMed] [Google Scholar]
- 33.Ragni G, Caccamo A. Negative effect of stress of in vitro fertilization program on quality of semen. Acta Eur Fertil. 1992;23:21–23. [PubMed] [Google Scholar]
- 34.Clarke RN, Klock SC, Geoghegan A, Travassos DE. Relationship between psychological stress and semen quality among in-vitro fertilization patients. Hum Reprod. 1999;14:753–758. doi: 10.1093/humrep/14.3.753. [DOI] [PubMed] [Google Scholar]
- 35.Kondoh E, Okamoto T, Higuchi T, Tatsumi K, Baba T, Murphy SK, et al. Stress affects uterine receptivity through an ovarian-independent pathway. Hum Reprod. 2009;24:945–953. doi: 10.1093/humrep/den461. [DOI] [PubMed] [Google Scholar]
- 36.Lorr M, McNair D, Heuchert J, Droppleman L. Profile of mood states. Canada: Multi-Health Systems Inc.; 2003. [Google Scholar]
- 37.Scheier MF, Carver CS. Optimism, coping, and health: assessment and implications of generalized outcome expectancies. Health Psychol. 1985;4:219–247. doi: 10.1037/0278-6133.4.3.219. [DOI] [PubMed] [Google Scholar]
- 38.Pellicer A, Ruiz M. Fertilization in vitro of human oocytes by spermatozoa collected in different stressful situations. Hum Reprod. 1989;4:817–820. doi: 10.1093/oxfordjournals.humrep.a136992. [DOI] [PubMed] [Google Scholar]
- 39.Poland ML, Giblin PT, Ager JW, Moghissi KS. Effect of stress on semen quality in semen donors. Int J Fertil. 1986;31:229–231. [PubMed] [Google Scholar]
- 40.Siterman S, Eltes F, Wolfson V, Zabludovsky N, Bartoov B. Effect of acupuncture on sperm parameters of males suffering from subfertility related to low sperm quality. Arch Androl. 1997;39:155–161. doi: 10.3109/01485019708987914. [DOI] [PubMed] [Google Scholar]
- 41.Zhang M, Huang G, Lu F, Paulus WE, Sterzik K. Influence of acupuncture on idiopathic male infertility in assisted reproductive technology. J Huazhong Univ Sci Technolog Med Sci. 2002;22:228–230. doi: 10.1007/BF02896780. [DOI] [PubMed] [Google Scholar]
- 42.Meeker JD, Benedict MD. Infertility, pregnancy loss and adverse birth outcomes in relation to maternal secondhand tobacco smoke exposure. Curr Womens Health Rev. 2013;9(1):41–49. [DOI] [PMC free article] [PubMed]
- 43.Hershlag A, Paine T, Kvapil G, Feng H, Napolitano B. In vitro fertilization-intracytoplasmic sperm injection split: an insemination method to prevent fertilization failure. Fertil Steril. 2002;77:229–232. doi: 10.1016/S0015-0282(01)02978-8. [DOI] [PubMed] [Google Scholar]
- 44.Kolibianakis EM, Venetis CA, Bontis J, Tarlatzis BC. Significantly lower pregnancy rates in the presence of progesterone elevation in patients treated with GnRH antagonists and gonadotrophins: a systematic review and meta-analysis. Curr Pharm Biotechnol. 2011. [DOI] [PubMed]
- 45.Orvieto R, Meltzer S, Rabinson J, Zohav E, Anteby EY, Nahum R. GnRH agonist versus GnRH antagonist in ovarian stimulation: the role of endometrial receptivity. Fertil Steril. 2008;90:1294–1296. doi: 10.1016/j.fertnstert.2007.10.022. [DOI] [PubMed] [Google Scholar]
- 46.van Loendersloot LL, van Wely M, Limpens J, Bossuyt PM, Repping S, van der Veen F. Predictive factors in in vitro fertilization (IVF): a systematic review and meta-analysis. Hum Reprod Update. 2010;16:577–589. doi: 10.1093/humupd/dmq015. [DOI] [PubMed] [Google Scholar]
- 47.Vartiainen H, Saarikoski S, Halonen P, Rimon R. Psychosocial factors, female fertility and pregnancy: a prospective study–part I: fertility. J Psychosom Obstet Gynaecol. 1994;15:67–75. doi: 10.3109/01674829409025631. [DOI] [PubMed] [Google Scholar]
- 48.Hjollund NH, Jensen TK, Bonde JP, Henriksen TB, Andersson AM, Kolstad HA, et al. Distress and reduced fertility: a follow-up study of first-pregnancy planners. Fertil Steril. 1999;72:47–53. doi: 10.1016/S0015-0282(99)00186-7. [DOI] [PubMed] [Google Scholar]
- 49.Collodel G, Moretti E, Fontani V, Rinaldi S, Aravagli L, Sarago G, et al. Effect of emotional stress on sperm quality. Indian J Med Res. 2008;128:254–261. [PubMed] [Google Scholar]
- 50.Siterman S, Eltes F, Wolfson V, Lederman H, Bartoov B. Does acupuncture treatment affect sperm density in males with very low sperm count? A pilot study. Andrologia. 2000;32:31–39. [PubMed] [Google Scholar]
- 51.Pei J, Strehler E, Noss U, Abt M, Piomboni P, Baccetti B, et al. Quantitative evaluation of spermatozoa ultrastructure after acupuncture treatment for idiopathic male infertility. Fertil Steril. 2005;84:141–147. doi: 10.1016/j.fertnstert.2004.12.056. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Linear correlation was observed between psychological distress scores of the female patients and their partners. (DOC 26 kb)