Abstract
Purpose
The aim was to culture primordial follicles in vitro to reach preantral stage in vitrified human ovarian tissue.
Methods
Ovarian tissue samples were obtained from six women. Tissue strips were vitrified by infiltration with a cryoprotectant followed by mounting on a stainless steel carrier. After culturing for 7 days the morphology and developmental stages of follicles enclosed in fresh and vitrified groups were analyzed.
Results
High proportion of viable follicles in vitrified ovarian strips was obtained. After culturing for 7 days the percentage of secondary and preantral follicles increased significantly (P < 0.05) whereas primordial and transitory follicles showed a significant decrease (P < 0.05) compared to their respective counterparts at day 0 of culture.
Conclusions
Vitrification of ovarian strips with an improved carrier device and culturing of follicles in ovarian strips after warming yielded developed follicles with high viability and morphological integrity that may be suitable for use in fertility preservation among cancer patients.
Keywords: Carrier device, In vitro culture, Preantral follicles, Preservation of fertility, Primordial follicles
Introduction
Cryopreservation of ovarian tissue before cancer therapy to provide opportunities for future fertility is one of the most challenging procedures in assisted reproductive techniques (ART) [1]. With the improved rate of cancer survivors in recent years, there is an increasing demand for cryopreservation of fertility [2]. The need for this approach is particularly eminent among young women without a male partner and in women who cannot postpone their cancer therapies or in pre-pubertal girls to whom embryo cryopreservation is not applicable. Presently, two techniques are commonly employed for cryopreservation: a conventional one which involves slow freezing and the other which involves vitrification. To date 24 live births have been reported after cryopreservation and transplantation of ovarian tissue strips with the slow freezing procedure [3]. In slow freezing lower concentrations of cryoprotectant are used. Slow freezing had been the preferred method for cryopreservation of human ovarian tissue until now. There are conflicting results on vitrification versus slow freezing in studies carried out by various groups. With the slow freezing procedure, some authors have noted the negative effects of ice crystal formation on certain ovarian components [4, 5]. On the other hand the vitrification procedure requires more efficient cooling rate and higher concentrations of cryoprotectant that may cause osmotic damage. During vitrification fluid in the cell is converted to the solid state, avoiding cellular damage caused by the formation of intracellular ice crystals [6]. Vitrification also does not induce apoptosis in ovarian tissue after warming [7, 8]. Recent studies reported that the use of vitrification yielded higher oocyte survival rates compared to slow freezing [4, 9]. Vitrification is a relatively inexpensive procedure and does not require any special instrument [10]. Ovarian tissue vitrification has been performed in mice [11–13], domestic animals [14–17], non-human primates [18–20], and in humans [21–24]. To date, no pregnancy has been reported in humans after transplantation of vitrified tissue [3]. The carrier system is considered one of the most important factors that can influence the cooling rate and vitrification outcome [25]. Various carrier tools have been used for ovarian tissue vitrification such as Pasteur pipettes [18, 26], copper grids [27], cryovials [28], acupuncture needles [29], 4 fine stainless needle [17, 19] and the solid surface method [25].
The ovarian cortex contains a large number of follicles in various stages of development. Primordial follicles are the most populated ovarian follicles each of which contains an immature oocyte in meiotic arrest [30]. There is evidence that primordial follicles are more tolerant to cryopreservation owing to their small size, less differentiation, quiescence of their oocyte, and the lack of zona pellucida and cortical granules [31]. In vitro culture of cryopreserved primordial follicles embedded in ovarian strips to developed and growing follicles would allow the transfer of developed follicles for transplantation, eliminating the potential risk of reseeding cancer with ovarian tissue grafting [32].
There are different ways to evaluate the quality of the cryopreservation technique such as assessment of follicles immediately after thawing [33] and after post-thawing in vitro culture [34, 35], evaluation after post-thawing xenotransplantation [22, 36], and culture of follicles on embryonic chorio-allantoic membrane [37, 38]. Other investigators have studied the effect of human ovarian cryopreservation on viability and development of follicles and on xenotransplantation [39–42]. Different strategies have been used to culture immature follicles in vitro using fresh or cryopreserved cortical tissue in situ [43–46]. It has been shown in a recent study that, after 6 days in culture, the number of primordial follicles decreased and the number of preantral follicles increased in cortical strips [46].
Recently the neutral red (NR) dye has been employed as a tool to quantify viable follicles in situ without compromising viability and development of follicles before and after tissue cryopreservation [47, 48]. However for assessment of viability of stromal cells in ovarian strips fluorescent staining such as Calcein AM has been used [29]. In view of the information mentioned above, the aim of the present study was to assess the in vitro development of primordial follicles to preantral stage in ovarian fragments after vitrification using an improved carrier device made of stainless steel mesh and to compare the results with those obtained with the fresh (non-vitrified) group.
Materials and methods
Use of human ovarian tissue for this study was approved by the Human Ethics Board of Queen’s University, Kingston, Ontario, Canada. Ovarian tissue samples were obtained with consent from six women aged 27–42 years old who were candidates for oophorectomy for benign gynecologic conditions. The reason of oophorectomy for the 27 years old woman was due to sex reassignment. All chemicals used in this study were purchased from Sigma-Aldrich unless stated otherwise. Freshly collected ovarian tissue was immediately placed at room temperature in a 50-ml sterile falcon tube containing 10 ml of Leibovitz medium (GIBCO) supplemented with sodium pyruvate (2 mM), glutamine (2 mM), BSA (3 mg/ml), penicillin G (75 mg/ml), streptomycin (50 mg/ml) and ascorbic acid (50 mg/ml) [46]. The tissue sample was then transferred within a few minutes to a Petri dish containing fresh Leibovitz medium with supplements as described above and the cortical ovarian tissue was trimmed with a scalpel into small pieces of about 0.5 × 2 × 2 mm in size under a dissecting microscope (Leica). Two ovarian strips prepared from each cortical tissue were fixed in Bouin’s solution for histological evaluation. All the cortical tissues were divided randomly in two groups designated, respectively, as the vitrified group and the fresh or non-vitrified group which served as the control.
Vitrification
The vitrification procedure was performed according to the method of Kagawa et al. (17) with minor modifications. Ovarian strips were equilibrated for 15 min at room temperature in a mixture solution containing 7.5 % dimethyl sulphoxide (DMSO) and 7.5 % ethylene glycol (EG) in HTCM (TCM-199 with HEPES-buffer, GIBCO) supplemented with 10 % human serum albumin (HSA) (Life Global). This was followed by immersion in a vitrification solution containing 15 % DMSO, 15 % EG, 2.5 % polyvinylpyrrolidone (PVP) [22], 10 % HSA with 0.5 mol/L sucrose in HTCM for 7 min or until all strips descended to the bottom of the falcon tube.
Ovarian strips prepared from each ovary were placed on a stainless steel mesh (Fig. 1) which was vitrified by plunging into liquid nitrogen. Individual pieces of stainless steel mesh carrying the ovarian strips were transferred into a 1.8 ml-cryovial pre-filled with liquid nitrogen and were stored in a liquid nitrogen tank for at least 1 week before they were further processed.
Fig. 1.
Photographs showing the actual dimensions of a stainless steel mesh for carrying ovarian strips for vitrification (a) and how they appear in a petri-dish (b)
Warming
After opening the cryovial the stainless steel mesh was immediately transferred into 20 ml of pre-warmed (37 °C) HTCM solution supplemented with 10 % HSA and 1.0 mol/L sucrose for 3 min. Then the samples were transferred into 10 ml HTCM solution supplemented with 10 % HSA and 0.5, 0.25, 0.125 mol/L sucrose, respectively, for 5 min each at room temperature. Afterwards they were washed twice for 10 min with HTCM supplemented with 10 % HSA. Two ovarian strips were fixed in Bouin’s solution and processed for histological evaluation. The warmed strips were incubated in fresh medium at 37 °C in 5 % CO2.
In vitro culture (IVC)
Fresh and vitrified tissue samples were cultured for 7 days in 24-well culture plates containing 300 μl of McCoy’s 5a culture medium supplemented with bicarbonate (GIBCO), HEPES (20 mM), HSA (0.1 %), glutamine (3 mM), penicillin G (0.1 mg/ml), streptomycin (0.1 mg/ml), transferrin (2.5 mg/ml), selenium (5 ng/ml), insulin (10 ng/ml) and ascorbic acid (50 mg/ml). The plates were incubated in 5 % CO2 at 37 °C. Half of the culture medium was replaced with fresh one every other day [46]. After 7 days two ovarian strips from each group (fresh and vitrified) were fixed in Bouin’s solution and processed for histological evaluation.
Evaluation of follicles in situ with neutral red
Neutral Red (NR) is a weak cationic dye which penetrates the plasma membrane and accumulates in the lysosomes of viable cells [49]. It has been previously used to assess the viability of porcine oocytes, granulosa and theca cells [50, 51]. In the present study, the viability of follicular cells in tissue strips was assessed, respectively, at day 0 and at day 7 of in vitro culture in both fresh and vitrified-warmed groups. The strips were incubated for 5 h at 37 °C in 5 % CO2 in 10 ml of pre-warmed culture medium supplemented with 50 μg/ml of neutral red solution [47, 48].
Isolation of follicles
Ovarian strips (n = 120) of both fresh and vitrified groups after 7 days of IVC were transferred to 50-ml falcon tubes containing 10 ml of McCoy’s 5a culture medium supplemented with 50 μg/ml of NR solution and incubated for 5 h. Thereafter, for enzymatic digestion of the strips, a mixture of Liberase TM (0.08 mg/ml) (Roche) and Collagenase IV (0.2 mg/ml) was added to the culture medium and the ovarian strips were incubated for 90 min at 37 °C in 5 % CO2. The strips were agitated every 15 min by gentle pipetting. The enzymatic digestion was completed by the addition of 10 ml of cold (4 °C) PBS containing 10 % FBS (GIBCO) [48]. The enzyme-digested strips were mechanically disrupted with a thin pasture pipette to release follicles from the stroma into the medium. Under a stereomicroscope NR-positive follicles with red color and unstained follicles from each group were transferred with a thin pasture pipette into a culture dish containing fresh culture medium. The number of reddish-staining, viable follicles and their corresponding stages of follicular development were recorded using an inverted microscope (IX 70 Olympus).
Evaluation of viability of stromal cells and isolated follicles
Vitrified ovarian strips after IVC were evaluated for viability of stromal cells and follicles using fluorescent double staining with Calcein-AM and Ethidium homodimer-1(Invitrogen) [14, 52]. Briefly, ovarian strips and isolated follicles were incubated for 40 min in an incubator at 37 °C in 5 % CO2 in 1 ml of McCoy’s 5a medium containing 1 % HSA, 2 μM Calcein AM and 5 μM Ethidium homodimer-1. Non-fluorescent Calcein-AM enters follicles and is cleaved by esterase in live follicles to become fluorescent Calcein. Ethidium homodimer-1 enters follicles with disrupted membranes producing red fluorescence in dead follicles. Ovarian strips and isolated follicles were washed with McCoy’s 5a culture medium containing 1 % HSA. Images were taken on an inverted fluorescent microscope (IX 70 Olympus).
Histological procedure and evaluation
Ovarian strips after 24 h of fixation in Bouin’s solution were dehydrated in ascending concentrations of ethanol (70 %–100 %) and then embedded in paraffin wax. Five micron-thick serial sections were cut. Every sixth section was stained with hematoxylin and eosin and then examined under a light microscope (Nikon). The follicles were classified into 6 categories according to their stage of development based on morphology of the granulosa cells surrounding the oocyte [30, 46, 53, 54].
Primordial follicles: a single layer of flattened granulosa cells
Transitory follicles: appearance of one or more cuboidal granulosa cells
Primary follicles: a complete layer of cuboidal granulosa cells
Secondary follicles: two complete layers of cuboidal granulosa cells
Preantral follicles: more than two layers of cuboidal granulosa cells
Atretic follicles: oocyte with a pyknotic nucleus and pyknotic granulosa cells.
To avoid double counting of the follicles, follicles were counted when focused on one plane and only follicles displaying an oocyte were counted. A follicle was considered normal when the follicle displayed well organized granulosa cells of spherical shape, an intact layer of theca interna, and a spherical oocyte with a normal nucleus. Atretic follicles were characterized by the presence of a pyknotic nucleus in the oocyte and an uneven distribution and disorganization of granulosa cells. The percentage of various stages of follicular development was determined for both fresh (non-vitrified) and vitrified ovarian strips.
Statistical analysis
Statistical analyses were carried out using the SPSS 16 program (SPSS 16, Chicago, IL). Number and percentage of follicles in different stages were expressed as mean ± SD. Mean values of measurements were compared using t-test. Values of p < 0.05 were considered statistically significant.
Results
Histological analysis
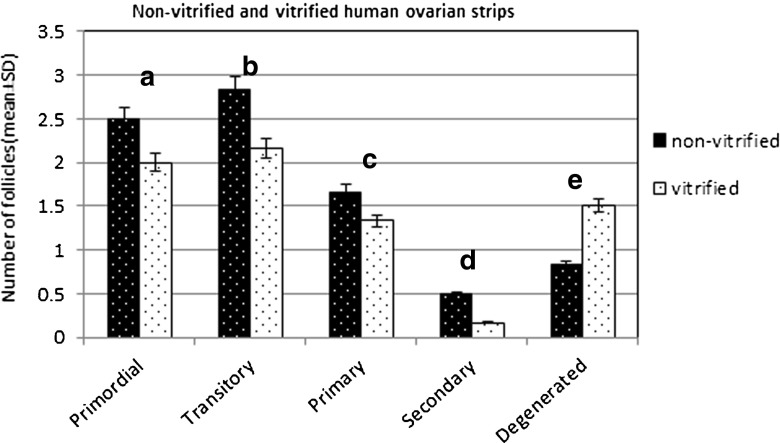
Ovarian strips in fresh (non-vitrified) (n = 12) and vitrified groups (n = 12) after fixation were compared and the morphology and developmental stages of follicles in both groups were evaluated (Fig. 2). A total of fifty-two follicles in the non-vitrified strips and forty-three follicles in the vitrified strips were counted. Atretic follicles were found to be 9.61 % in the fresh strips and 20.93 % in the vitrified group. In the fresh strips 65.39 % of the normal follicles were primordial and transitory follicles while 25 % of the follicles were at primary and secondary stages. In the vitrified strips the percentage of primordial and transitory follicles was 58.14 % and the percentage of primary and secondary follicles was 20.93 % (Table 1 and Fig. 3).
Fig. 2.
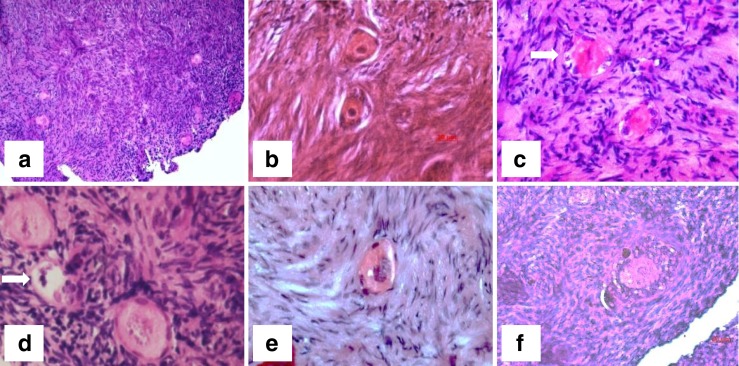
Light photomicrographs showing hematoxylin and eosin stained human ovarian follicles in fresh ovarian strip and after vitrification/warming. a and b: fresh follicles; c: follicles after IVC; d and e: primordial follicle after vitrification; f: preantral follicle after vitrification and 7 days of IVC. Abnormal follicles are indicated by arrows in c and d. Bars: a = 100 μm, b and f = 20 μm, c-e = 50 μm
Table 1.
Histological analysis showing the percentage of follicles in different stages of development in ovarian strips of the fresh and vitrified groups
| Primordial | Transitory | Primary | Secondary | Atretic | |
|---|---|---|---|---|---|
| Non-vitrified (n = 12 strips) |
28.85 | 36.54 | 19.23 | 5.77 | 9.61 |
| Vitrified (n = 12 strips) |
27.91 | 30.23 | 18.60 | 2.33 | 20.93 |
Fig. 3.
Histogram showing the number of follicles (mean ± SD) in non-vitrified and vitrified ovarian strips. There is no significant difference between the vitrified and fresh (non-vitrified) groups. a p = 0.503, b p = 0.140, c p = 0.496, d p = 0.260 and e p = 0.11
Histological evaluation after IVC
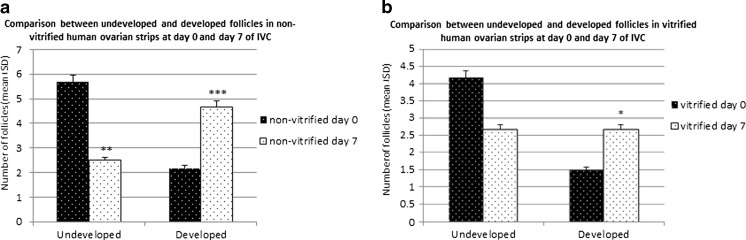
After 7 days of in vitro culture (IVC) the morphology and developmental stages of follicles in fresh (n = 12) and vitrified strips (n = 12) were compared (Fig. 2). Fifty follicles in the fresh strips and 44 follicles in the vitrified fragments were counted. After 7 days of IVC the percentage of normal primordial and transitory follicles (undeveloped follicles) in fresh ovarian strips decreased to 30 % compared to 65.39 % before IVC and the percentage of developed follicles (primary, secondary and preantral) increased to 56 % compared to 25 % before IVC. In the vitrified group developed follicles increased to 38.64 % compared to 20.93 % before IVC and undeveloped follicles decreased to 36.36 % compared to 58.14 % before IVC. After 7 days of IVC the percentage of atretic follicles in the non-vitrified group was 14 % compared to 9.61 % at day 0. In the vitrified group the percentage of atretic follicles increased from 20.93 % at day 0 to 25 % at day 7 of IVC (Table 2 and Fig. 4).
Table 2.
Histological analysis showing the percentage of follicles in different stages of development in ovarian strips of the fresh and vitrified groups after in vitro culture for 7 days
| Primordial | Transitory | Primary | Secondary | Preantral | |
|---|---|---|---|---|---|
| Non-vitrified (n = 12 strips) |
14 | 16 | 24 | 20 | 12 |
| Vitrified (n = 12 strips) |
15.91 | 20.45 | 18.18 | 13.64 | 6.82 |
Fig. 4.
Histogram showing the number of developed and undeveloped follicles (mean ± SD) at day 0 and day 7 after IVC. a: fresh (non- vitrified group); b: vitrified group. * indicates significant difference (p < 0.05), **indicates significant difference (p < 0.01) and ***indicates significant difference (p < 0.001) between day 0 and day 7
Evaluation of isolated follicles with NR
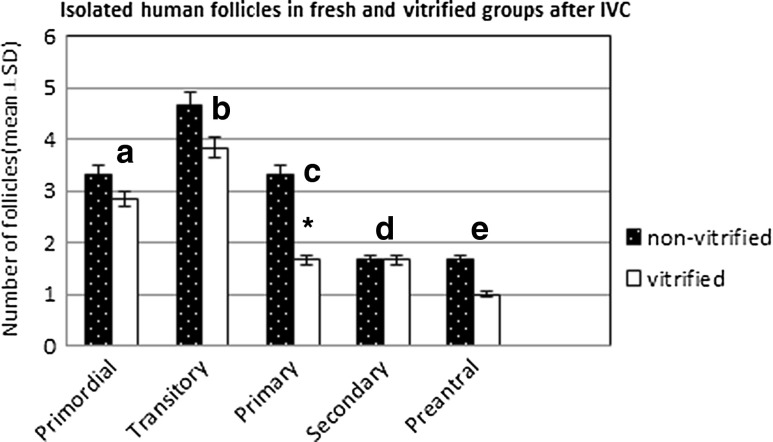
Follicles from fresh (non-vitrified) (n = 60) and vitrified (n = 60) ovarian strips after in vitro culture were stained with NR and then isolated. Only well-organized, spherical follicles with an intact basement membrane were considered. A total of 120 follicles in non-vitrified and 100 follicles in vitrified strips were counted. 81.64 % of the isolated follicles in the fresh strips and 72 % in the vitrified strips after IVC were positively stained with Neutral Red. The developmental stages of live follicles in both fresh and vitrified strips were also evaluated. There were more primary, secondary and preantral follicles in fresh IVC strips than those found in the vitrified group after IVC. 41.65 % of the follicles in the fresh group were developed follicles as compared to 32 % in the vitrified group (Table 3 and Fig. 5).
Table 3.
Percentage of isolated live follicles (neutral red stained)
| IVC of human ovarian strips | |||||
|---|---|---|---|---|---|
| Primordial | Transitory | Primary | Secondary | Preantral | |
| Non-vitrified (n = 60 strips) |
16.66 | 23.33 | 16.66 | 16.66 | 8.33 |
| Vitrified (n = 60 strips) |
17 | 23 | 17 | 9 | 6 |
Fig. 5.
Histogram showing the number of isolated follicles (mean ± SD) in non-vitrified and vitrified ovarian strips after 7 days of IVC. There is no difference between the isolated follicles in non-vitrified and vitrified groups except for primary follicles (c p = 0.011). a p = 0.360, b p = 0.096, d p = 1.000 and e p = 0.206
Staining of viable follicles in situ with NR
Two ovarian strips each from the fresh group and the vitrified group and both after 7 days of culture were placed in NR dye for 5 h. The reddish-staining viable follicles at different stages of development were detected in both groups although the numbers of viable follicles after vitrification decreased significantly. After vitrification more live primordial and transitory follicles were detected as compared to primary and secondary follicles. Dead follicles were unstained but they could be visualized under the inverted microscope in thinner regions of the ovarian strips. After 7 days of culture there was a decrease in the number of NR-positive primordial and transitory follicles in both fresh and vitrified groups while the proportion of NR-positive secondary follicles was found to be higher than that of the follicles in other stages of development (Fig. 6).
Fig. 6.
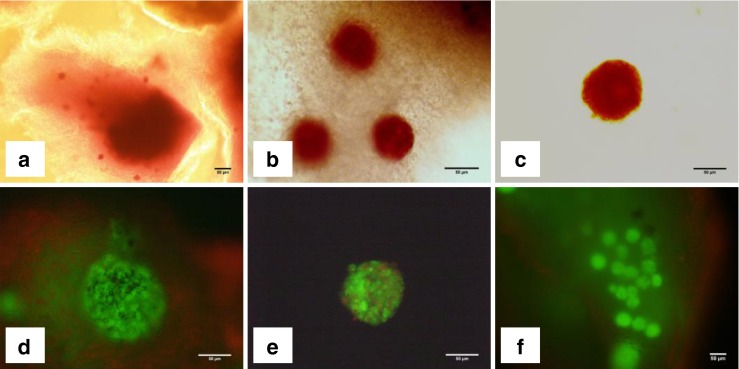
Light photomicrographs showing ovarian strips (a and b) and isolated follicle (c) stained with Neutral Red after 7 days of IVC, and isolated follicles (d and e) as well as vitrified/warmed ovarian strips (e) stained with Calcein AM and Ethidium homodimer-1 after 7 days of IVC. a: fresh ovarian strips; b: vitrified/warmed ovarian strips; c: isolated follicle; d: viable isolated follicle (green) and dead stromal cells (red); e: an isolated follicle with several dead granulosa cells (red); f: ovarian strip showing numerous live stromal cells and follicles within the strip. Bars = 50 μm
Assessment of viability of ovarian stromal cells and isolated follicles
Fluorescent staining was performed in vitrified ovarian strips after 7 days of IVC. Live follicles and stromal cells indicated by green fluorescence were observed in most of the ovarian strips. Statistical analysis was not performed in this particular experiment as the thickness of ovarian strips varied and the location of follicles was not in the same level among different ovarian strips making it difficult to carry out a meaningful statistical analysis. However, the number of viable isolated follicles was recorded after vitrification and IVC for a qualitative assessment. The follicles which displayed green fluorescence in their entirety were counted as viable and follicles with red fluorescence counted as dead ones. Based on this premise, 65 % of the isolated follicles following vitrification and IVC were found to be viable (Fig. 6). As shown in Fig. 6d, most of the follicles and the surrounding stroma did not show any damage but displayed green fluorescence in full.
Discussion
In recent years ovarian tissue cryopreservation especially with the slow freezing method and transplantation have become a promising approach with great potential to preserve fertility among cancer patients [1, 55]. In spite of this potential, transplantation of ovarian tissue suffers from the risk of reintroducing malignant cells of certain types of cancer [32]. In addition, a large number of grafted follicles could be lost due to ischemia that might occur in post-grafted fragments [56]. An alternative option is cryopreservation of ovarian tissue whereby immature follicles can be isolated from the thawed tissue and then allowed to grow in vitro to the desirable developmental stage for subsequent transplantation. As follicles in earlier stages of development do not contain any small blood vessels, capillaries or white blood cells, grafting would be much safer [57]. In vitro culture has been generally used to evaluate the viability of vitrified ovarian follicles [58].
In the present study we used a simple carrier device made of stainless steel mesh which is inexpensive and suitable for carrying relatively large number of ovary fragments. The stainless steel mesh consists of criss-crossed strands of metal wires with large pores allowing drainage of excess vitrification solution. It also provides a roughened surface for the support of ovarian tissue fragments, and increases the cooling rate. For vitrification we added PVP as an additional non-permeable cryoprotectant to the vitrification solution to increase glass formation [22] and viscosity [59]. Hashimoto et al.[20] reported that adding PVP to the vitrification solution increases the number of follicles with normal morphology in ovarian tissues obtained from monkeys. An optimal exposure of ovarian strips to the cryoprotectant solution is an important factor for successful vitrification [60]. The exposure time and the size of ovarian tissue strips are closely related to each other in the vitrification procedure.
Various sizes of human ovarian strips and different exposure times were employed in previous studies. Thus, it would be difficult to compare results of the present study with those of previous studies. For example Chang et al. (2011)[60] reported that optimal exposure time for vitrification of human ovarian fragments with 1 mm cube thickness is 10 min [60]. On the other hand Kagawa et al. (2009) [21] used 1 × 10 × 10 mm ovarian fragments with 15 min exposure time and reported this method as a successful procedure for human vitrification [21]. Our histological evaluation showed that the proportion of developed follicles (primary, secondary and preantral) increased and undeveloped follicles (primordial and transitory) decreased significantly in both fresh and vitrified groups albeit there were more growing follicles in the fresh group than the vitrified group. These results showed that after vitrification follicles have the potential to grow from undeveloped towards developmental stages. In contrast to our results Choi et al. (2007) [61] reported that in vitrified mouse ovarian samples after in vitro culture for 5 days the percentage of primordial follicles was significantly higher than that of developing follicles. They concluded that cryopreservation caused the death of ovarian cells through apoptosis and thus inhibiting further development of primordial follicles [61]. However a study by Mazoochi et al.(2008) [62] carried out in the mouse showed that there was no sign of apoptosis in vitrified and cultured follicles [62]. A study carried out with human ovarian tissue also reported that vitrification has no effect on apoptosis of follicles [63, 64]. In the present study, the number of viable isolated follicles obtained with fluorescent staining in the vitrified/IVC group appeared to be smaller than that obtained with NR staining in the same group (65 % versus 72 %). This discrepancy may be due to the criteria for evaluation of viable follicles between the two staining methods. In the fluorescent staining method, we considered follicles that displayed green fluorescence without any red coloration of granulosa cells as viable and those with any red coloration of granulosa cells as dead follicles. This evaluation method might have skewed the viable follicles to a smaller number as determined by the fluorescent staining method compared to NR staining. To the best of our knowledge only a few studies on cryopreservation of ovarian tissue have been carried out culturing tissue strips in vitro for more than 24 h after thawing to evaluate the survival and development of follicles [25, 44, 61, 65, 66]. Finally, results of the present study showed that stainless steel mesh can be used as a carrier device in the vitrification procedure. This procedure makes it possible to restore folliculogenesis in in vitro culture allowing immature follicles to further develop to preantral stage after 7 days in organ culture. This provides a new and potential avenue, in the future, for transplanting a suspension of developed follicles in patients who, otherwise, may be at risk of transmission of malignant cells after transplantation of ovarian fragments. Clearly, more investigations will be necessary to evaluate the feasibility of transplanting these follicles in patients who opt for the preservation of fertility. For example, it will be important to demonstrate whether the developed follicles obtained from vitrified human ovarian tissue strips can further grow and become mature eggs in an appropriate milieu such as the three-dimensional in vitro culture system. The latter is currently being investigated in our laboratory.
Acknowledgments
This research was supported, in part, by a grant from the Canadian Institutes of Health Research to F.W.K.K and by a research grant (No.17764) from the Tehran University of Medical Sciences to F.A.
Footnotes
Capsule
In vitro culture of vitrified ovarian strips yielded follicles with high morphological integrity and viability suitable for fertility preservation in cancer patients.
References
- 1.Donnez J, Jadoul P, Squifflet J, Van Langendonckt A, Donnez O, Van Eyck AS, et al. Ovarian tissue cryopreservation and transplantation in cancer patients. Best Pract Res Clin Obstet Gynaecol. 2010;24:87–100. doi: 10.1016/j.bpobgyn.2009.09.003. [DOI] [PubMed] [Google Scholar]
- 2.Abir R, Fisch B, Raz A, Nitke S, Ben-Rafael Z. Preservation of fertility in women undergoing chemotherapy: current approach and future prospects. J Assist Reprod Genet. 1998;15:469–477. doi: 10.1023/A:1022578303272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Donnez J, Dolmans MM, Pellicer A, Diaz-Garcia C, Sanchez Serrano M, Schmidt KT, et al. Restoration of ovarian activity and pregnancy after transplantation of cryopreserved ovarian tissue: a review of 60 cases of reimplantation. Fertil Steril. 2013;99:1503–1513. doi: 10.1016/j.fertnstert.2013.03.030. [DOI] [PubMed] [Google Scholar]
- 4.Silber S, Kagawa N, Kuwayama M, Gosden R. Duration of fertility after fresh and frozen ovary transplantation. Fertil Steril. 2010;94:2191–2196. doi: 10.1016/j.fertnstert.2009.12.073. [DOI] [PubMed] [Google Scholar]
- 5.Keros V, Xella S, Hultenby K, Pettersson K, Sheikhi M, Volpe A, et al. Vitrification versus controlled-rate freezing in cryopreservation of human ovarian tissue. Hum Reprod. 2009;24:1670–1683. doi: 10.1093/humrep/dep079. [DOI] [PubMed] [Google Scholar]
- 6.Liebermann J, Nawroth F, Isachenko V, Isachenko E, Rahimi G, Tucker MJ. Potential importance of vitrification in reproductive medicine. Biol Reprod. 2002;67:1671–1680. doi: 10.1095/biolreprod.102.006833. [DOI] [PubMed] [Google Scholar]
- 7.Rahimi G, Isachenko E, Sauer H, Isachenko V, Wartenberg M, Hescheler J, et al. Effect of different vitrification protocols for human ovarian tissue on reactive oxygen species and apoptosis. Reprod Fertil Dev. 2003;15:343–349. doi: 10.1071/RD02063. [DOI] [PubMed] [Google Scholar]
- 8.Mazoochi T, Salehnia M, Valojerdi MR, Mowla SJ. Morphologic, ultrastructural, and biochemical identification of apoptosis in vitrified-warmed mouse ovarian tissue. Fertil Steril. 2008;90:1480–1486. doi: 10.1016/j.fertnstert.2007.07.1384. [DOI] [PubMed] [Google Scholar]
- 9.Silber SJ. Ovary cryopreservation and transplantation for fertility preservation. Mol Hum Reprod. 2012;18:59–67. doi: 10.1093/molehr/gar082. [DOI] [PubMed] [Google Scholar]
- 10.Amorim CA, Curaba M, Van Langendonckt A, Dolmans MM, Donnez J. Vitrification as an alternative means of cryopreserving ovarian tissue. Reprod Biomed Online. 2011;23:160–186. doi: 10.1016/j.rbmo.2011.04.005. [DOI] [PubMed] [Google Scholar]
- 11.Hasegawa A, Hamada Y, Mehandjiev T, Koyama K. In vitro growth and maturation as well as fertilization of mouse preantral oocytes from vitrified ovaries. Fertil Steril. 2004;81(Suppl 1):824–830. doi: 10.1016/j.fertnstert.2003.08.028. [DOI] [PubMed] [Google Scholar]
- 12.Hasegawa A, Mochida N, Ogasawara T, Koyama K. Pup birth from mouse oocytes in preantral follicles derived from vitrified and warmed ovaries followed by in vitro growth, in vitro maturation, and in vitro fertilization. Fertil Steril. 2006;86:1182–1192. doi: 10.1016/j.fertnstert.2005.12.082. [DOI] [PubMed] [Google Scholar]
- 13.Wang X, Catt S, Pangestu M, Temple-Smith P. Successful in vitro culture of pre-antral follicles derived from vitrified murine ovarian tissue: oocyte maturation, fertilization, and live births. Reproduction. 2011;141:183–191. doi: 10.1530/REP-10-0383. [DOI] [PubMed] [Google Scholar]
- 14.Santos RR, Tharasanit T, Van Haeften T, Figueiredo JR, Silva JR, Van den Hurk R. Vitrification of goat preantral follicles enclosed in ovarian tissue by using conventional and solid-surface vitrification methods. Cell Tissue Res. 2007;327:167–176. doi: 10.1007/s00441-006-0240-2. [DOI] [PubMed] [Google Scholar]
- 15.Carvalho AA, Faustino LR, Silva CM, Castro SV, Luz HK, Rossetto R, et al. Influence of vitrification techniques and solutions on the morphology and survival of preantral follicles after in vitro culture of caprine ovarian tissue. Theriogenology. 2011;76:933–941. doi: 10.1016/j.theriogenology.2011.04.024. [DOI] [PubMed] [Google Scholar]
- 16.Bao RM, Yamasaka E, Moniruzzaman M, Hamawaki A, Yoshikawa M, Miyano T. Development of vitrified bovine secondary and primordial follicles in xenografts. Theriogenology. 2010;74:817–827. doi: 10.1016/j.theriogenology.2010.04.006. [DOI] [PubMed] [Google Scholar]
- 17.Hashimoto S, Suzuki N, Amo A, Yamochi T, Hosoi Y, Morimoto Y. Good thermally conducting material supports follicle morphologies of porcine ovaries cryopreserved with ultrarapid vitrification. J Reprod Dev. 2013;in press. [DOI] [PMC free article] [PubMed]
- 18.Yeoman RR, Wolf DP, Lee DM. Coculture of monkey ovarian tissue increases survival after vitrification and slow-rate freezing. Fertil Steril. 2005;83(Suppl 1):1248–1254. doi: 10.1016/j.fertnstert.2004.11.036. [DOI] [PubMed] [Google Scholar]
- 19.Suzuki N, Hashimoto S, Igarashi S, Takae S, Yamanaka M, Yamochi T, et al. Assessment of long-term function of heterotopic transplants of vitrified ovarian tissue in cynomolgus monkeys. Hum Reprod. 2012;27:2420–2429. doi: 10.1093/humrep/des178. [DOI] [PubMed] [Google Scholar]
- 20.Hashimoto S, Suzuki N, Yamanaka M, Hosoi Y, Ishizuka B, Morimoto Y. Effects of vitrification solutions and equilibration times on the morphology of cynomolgus ovarian tissues. Reprod Biomed Online. 2010;21:501–509. doi: 10.1016/j.rbmo.2010.04.029. [DOI] [PubMed] [Google Scholar]
- 21.Kagawa N, Silber S, Kuwayama M. Successful vitrification of bovine and human ovarian tissue. Reprod Biomed Online. 2009;18:568–577. doi: 10.1016/S1472-6483(10)60136-8. [DOI] [PubMed] [Google Scholar]
- 22.Amorim CA, Dolmans MM, David A, Jaeger J, Vanacker J, Camboni A, et al. Vitrification and xenografting of human ovarian tissue. Fertil Steril. 2012;98:1291–8. [DOI] [PubMed]
- 23.Sheikhi M, Hultenby K, Niklasson B, Lundqvist M, Hovatta O. Clinical grade vitrification of human ovarian tissue: an ultrastructural analysis of follicles and stroma in vitrified tissue. Hum Reprod. 2011;26:594–603. doi: 10.1093/humrep/deq357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bian J, Li T, Ding C, Xin W, Zhu B, Zhou C. Vitreous cryopreservation of human preantral follicles encapsulated in alginate beads with mini mesh cups. J Reprod Dev. 2013;59:288–295. doi: 10.1262/jrd.2012-157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huang L, Mo Y, Wang W, Li Y, Zhang Q, Yang D. Cryopreservation of human ovarian tissue by solid-surface vitrification. Eur J Obstet Gynecol Reprod Biol. 2008;139:193–198. doi: 10.1016/j.ejogrb.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 26.Li YB, Zhou CQ, Yang GF, Wang Q, Dong Y. Modified vitrification method for cryopreservation of human ovarian tissues. Chin Med J (Engl) 2007;120:110–114. [PubMed] [Google Scholar]
- 27.Isachenko E, Isachenko V, Rahimi G, Nawroth F. Cryopreservation of human ovarian tissue by direct plunging into liquid nitrogen. Eur J Obstet Gynecol Reprod Biol. 2003;108:186–193. doi: 10.1016/S0301-2115(02)00465-7. [DOI] [PubMed] [Google Scholar]
- 28.Salehnia M, Abbasian Moghadam E, Rezazadeh Velojerdi M. Ultrastructure of follicles after vitrification of mouse ovarian tissue. Fertil Steril. 2002;78:644–645. doi: 10.1016/S0015-0282(02)03287-9. [DOI] [PubMed] [Google Scholar]
- 29.Wang Y, Xiao Z, Li L, Fan W, Li SW. Novel needle immersed vitrification: a practical and convenient method with potential advantages in mouse and human ovarian tissue cryopreservation. Hum Reprod. 2008;23:2256–2265. doi: 10.1093/humrep/den255. [DOI] [PubMed] [Google Scholar]
- 30.Gougeon A. Regulation of ovarian follicular development in primates: facts and hypotheses. Endocr Rev. 1996;17:121–155. doi: 10.1210/edrv-17-2-121. [DOI] [PubMed] [Google Scholar]
- 31.Depalo R, Loverro G, Selvaggi L. In vitro maturation of primordial follicles after cryopreservation of human ovarian tissue: problems remain. Med Pediatr Oncol. 2002;38:153–157. doi: 10.1002/mpo.10053. [DOI] [PubMed] [Google Scholar]
- 32.Dolmans MM, Luyckx V, Donnez J, Andersen CY, Greve T. Risk of transferring malignant cells with transplanted frozen-thawed ovarian tissue. Fertil Steril. 2013;99:1514–1522. doi: 10.1016/j.fertnstert.2013.03.027. [DOI] [PubMed] [Google Scholar]
- 33.Poirot C, Vacher-Lavenu MC, Helardot P, Guibert J, Brugieres L, Jouannet P. Human ovarian tissue cryopreservation: indications and feasibility. Hum Reprod. 2002;17:1447–1452. doi: 10.1093/humrep/17.6.1447. [DOI] [PubMed] [Google Scholar]
- 34.Hovatta O. Cryopreservation and culture of human ovarian cortical tissue containing early follicles. Eur J Obstet Gynecol Reprod Biol. 2004;113(Suppl 1):S50–S54. doi: 10.1016/j.ejogrb.2003.11.012. [DOI] [PubMed] [Google Scholar]
- 35.Ting AY, Yeoman RR, Lawson MS, Zelinski MB. In vitro development of secondary follicles from cryopreserved rhesus macaque ovarian tissue after slow-rate freeze or vitrification. Hum Reprod. 2011;26:2461–2472. doi: 10.1093/humrep/der196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gook DA, Edgar DH, Borg J, Archer J, McBain JC. Diagnostic assessment of the developmental potential of human cryopreserved ovarian tissue from multiple patients using xenografting. Hum Reprod. 2005;20:72–78. doi: 10.1093/humrep/deh550. [DOI] [PubMed] [Google Scholar]
- 37.Martinez-Madrid B, Donnez J, Van Eyck AS, Veiga-Lopez A, Dolmans MM, Van Langendonckt A. Chick embryo chorioallantoic membrane (CAM) model: a useful tool to study short-term transplantation of cryopreserved human ovarian tissue. Fertil Steril. 2009;91:285–292. doi: 10.1016/j.fertnstert.2007.11.026. [DOI] [PubMed] [Google Scholar]
- 38.Isachenko V, Mallmann P, Petrunkina AM, Rahimi G, Nawroth F, Hancke K, et al. Comparison of in vitro- and chorioallantoic membrane (CAM)-culture systems for cryopreserved medulla-contained human ovarian tissue. PLoS One. 2012;7:e32549. doi: 10.1371/journal.pone.0032549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Amorim CA, David A, Dolmans MM, Camboni A, Donnez J, Van Langendonckt A. Impact of freezing and thawing of human ovarian tissue on follicular growth after long-term xenotransplantation. J Assist Reprod Genet. 2011;28:1157–1165. doi: 10.1007/s10815-011-9672-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rahimi G, Isachenko V, Kreienberg R, Sauer H, Todorov P, Tawadros S, et al. Re-vascularisation in human ovarian tissue after conventional freezing or vitrification and xenotransplantation. Eur J Obstet Gynecol Reprod Biol. 2010;149:63–67. doi: 10.1016/j.ejogrb.2009.11.015. [DOI] [PubMed] [Google Scholar]
- 41.Nisolle M, Casanas-Roux F, Qu J, Motta P, Donnez J. Histologic and ultrastructural evaluation of fresh and frozen-thawed human ovarian xenografts in nude mice. Fertil Steril. 2000;74:122–129. doi: 10.1016/S0015-0282(00)00548-3. [DOI] [PubMed] [Google Scholar]
- 42.Van den Broecke R, Liu J, Handyside A, Van der Elst JC, Krausz T, Dhont M, et al. Follicular growth in fresh and cryopreserved human ovarian cortical grafts transplanted to immunodeficient mice. Eur J Obstet Gynecol Reprod Biol. 2001;97:193–201. doi: 10.1016/S0301-2115(00)00507-8. [DOI] [PubMed] [Google Scholar]
- 43.Hovatta O, Silye R, Abir R, Krausz T, Winston RM. Extracellular matrix improves survival of both stored and fresh human primordial and primary ovarian follicles in long-term culture. Hum Reprod. 1997;12:1032–1036. doi: 10.1093/humrep/12.5.1032. [DOI] [PubMed] [Google Scholar]
- 44.Isachenko V, Montag M, Isachenko E, van der Ven K, Dorn C, Roesing B, et al. Effective method for in-vitro culture of cryopreserved human ovarian tissue. Reprod Biomed Online. 2006;13:228–234. doi: 10.1016/S1472-6483(10)60620-7. [DOI] [PubMed] [Google Scholar]
- 45.Picton HM, Gosden RG. In vitro growth of human primordial follicles from frozen-banked ovarian tissue. Mol Cell Endocrinol. 2000;166:27–35. doi: 10.1016/S0303-7207(00)00294-X. [DOI] [PubMed] [Google Scholar]
- 46.Telfer EE, McLaughlin M, Ding C, Thong KJ. A two-step serum-free culture system supports development of human oocytes from primordial follicles in the presence of activin. Hum Reprod. 2008;23:1151–1158. doi: 10.1093/humrep/den070. [DOI] [PubMed] [Google Scholar]
- 47.Chambers EL, Gosden RG, Yap C, Picton HM. In situ identification of follicles in ovarian cortex as a tool for quantifying follicle density, viability and developmental potential in strategies to preserve female fertility. Hum Reprod. 2010;25:2559–2568. doi: 10.1093/humrep/deq192. [DOI] [PubMed] [Google Scholar]
- 48.Kristensen SG, Rasmussen A, Byskov AG, Andersen CY. Isolation of pre-antral follicles from human ovarian medulla tissue. Hum Reprod. 2011;26:157–166. doi: 10.1093/humrep/deq318. [DOI] [PubMed] [Google Scholar]
- 49.Allison AC, Young MR. Uptake of dyes and drugs by living cells in culture. Life Sci. 1964;3:1407–1414. doi: 10.1016/0024-3205(64)90082-7. [DOI] [PubMed] [Google Scholar]
- 50.Brankin V, Mitchell MR, Webb B, Hunter MG. Paracrine effects of oocyte secreted factors and stem cell factor on porcine granulosa and theca cells in vitro. Reprod Biol Endocrinol. 2003;1:55. doi: 10.1186/1477-7827-1-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shores EM, Picton HM, Hunter MG. Differential regulation of pig theca cell steroidogenesis by LH, insulin-like growth factor I and granulosa cells in serum-free culture. J Reprod Fertil. 2000;118:211–219. [PubMed] [Google Scholar]
- 52.Martinez-Madrid B, Dolmans MM, Van Langendonckt A, Defrere S, Donnez J. Freeze-thawing intact human ovary with its vascular pedicle with a passive cooling device. Fertil Steril. 2004;82:1390–1394. doi: 10.1016/j.fertnstert.2004.06.036. [DOI] [PubMed] [Google Scholar]
- 53.Hansen KR, Craig LB, Zavy MT, Klein NA, Soules MR. Ovarian primordial and nongrowing follicle counts according to the Stages of Reproductive Aging Workshop (STRAW) staging system. Menopause. 2012;19:164–171. doi: 10.1097/gme.0b013e31823b0b2e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rodgers RJ, Irving-Rodgers HF. Morphological classification of bovine ovarian follicles. Reproduction. 2010;139:309–318. doi: 10.1530/REP-09-0177. [DOI] [PubMed] [Google Scholar]
- 55.Amorim CA, Dolmans MM, David A, Jaeger J, Vanacker J, Camboni A, et al. Vitrification and xenografting of human ovarian tissue. Fertil Steril. 2012;98:1291–1298. doi: 10.1016/j.fertnstert.2012.07.1109. [DOI] [PubMed] [Google Scholar]
- 56.Donnez J, Martinez-Madrid B, Jadoul P, Van Langendonckt A, Demylle D, Dolmans MM. Ovarian tissue cryopreservation and transplantation: a review. Hum Reprod Update. 2006;12:519–535. doi: 10.1093/humupd/dml032. [DOI] [PubMed] [Google Scholar]
- 57.Dolmans MM, Michaux N, Camboni A, Martinez-Madrid B, Van Langendonckt A, Nottola SA, et al. Evaluation of Liberase, a purified enzyme blend, for the isolation of human primordial and primary ovarian follicles. Hum Reprod. 2006;21:413–420. doi: 10.1093/humrep/dei320. [DOI] [PubMed] [Google Scholar]
- 58.Vanacker J, Camboni A, Dath C, Van Langendonckt A, Dolmans MM, Donnez J, et al. Enzymatic isolation of human primordial and primary ovarian follicles with Liberase DH: protocol for application in a clinical setting. Fertil Steril. 2011;96:379–383. doi: 10.1016/j.fertnstert.2011.05.075. [DOI] [PubMed] [Google Scholar]
- 59.Pegg DE. The role of vitrification techniques of cryopreservation in reproductive medicine. Hum Fertil (Camb) 2005;8:231–239. doi: 10.1080/14647270500054803. [DOI] [PubMed] [Google Scholar]
- 60.Chang HJ, Moon JH, Lee JR, Jee BC, Suh CS, Kim SH. Optimal condition of vitrification method for cryopreservation of human ovarian cortical tissues. J Obstet Gynaecol Res. 2011;37:1092–1101. doi: 10.1111/j.1447-0756.2010.01496.x. [DOI] [PubMed] [Google Scholar]
- 61.Choi J, Lee JY, Lee E, Yoon BK, Bae D, Choi D. Cryopreservation of the mouse ovary inhibits the onset of primordial follicle development. Cryobiology. 2007;54:55–62. doi: 10.1016/j.cryobiol.2006.11.003. [DOI] [PubMed] [Google Scholar]
- 62.Mazoochi T, Salehnia M, Pourbeiranvand S, Forouzandeh M, Mowla SJ, Hajizadeh E. Analysis of apoptosis and expression of genes related to apoptosis in cultures of follicles derived from vitrified and non-vitrified ovaries. Mol Hum Reprod. 2009;15:155–164. doi: 10.1093/molehr/gap002. [DOI] [PubMed] [Google Scholar]
- 63.Salehnia M, Sheikhi M, Pourbeiranvand S, Lundqvist M. Apoptosis of human ovarian tissue is not increased by either vitrification or rapid cooling. Reprod Biomed Online. 2012;25:492–9. [DOI] [PubMed]
- 64.Depalo R, Lorusso F, Bettocchi S, Selvaggi L, Cavallini A, Valentini AM, et al. Assessment of estrogen receptors and apoptotic factors in cryopreserved human ovarian cortex. Syst Biol Reprod Med. 2009;55:236–243. doi: 10.3109/19396360903046761. [DOI] [PubMed] [Google Scholar]
- 65.Paynter SJ, Cooper A, Fuller BJ, Shaw RW. Cryopreservation of bovine ovarian tissue: structural normality of follicles after thawing and culture in vitro. Cryobiology. 1999;38:301–309. doi: 10.1006/cryo.1999.2170. [DOI] [PubMed] [Google Scholar]
- 66.Tsuribe PM, Gobbo CA, Landim-Alvarenga FC. Viability of primordial follicles derived from cryopreserved ovine ovarian cortex tissue. Fertil Steril. 2009;91:1976–1983. doi: 10.1016/j.fertnstert.2008.03.031. [DOI] [PubMed] [Google Scholar]