Abstract
Purpose
This study aimed to investigate the effect of male semen quality, occupational exposure, and lifestyle on recurrent pregnancy loss (RPL).
Methods
Information on couples’ occupational exposure and lifestyle was collected using a detailed questionnaire from 68 RPL couples and 63 randomly selected healthy controls. Semen parameters were estimated by computer-assisted sperm analysis, and sperm nuclear status was detected with aniline blue (AB) staining.
Results
Patients in the RPL group had significantly lower viability, normal morphology, and total progressive motility of sperm, and a higher mean percentage of AB staining positive sperm compared with those of controls (P < 0.05). There were no differences in sperm concentration, and motility between the groups (P > 0.05). Significant odds ratio (OR) was found when occupational exposure and unhealthy habits were superimposed (OR: 11.965, P = 0.005).
Conclusions
In addition to standard female factors for evaluating the risk for RPL, the use of male factors should also be taken into consideration. We found that sperm quality, occupational exposure, and lifestyle are factors that affect RPL. Consequently, occupational exposure and lifestyle factors should constitute an important section of questionnaires given to patients, and these factors should be evaluated by a clinician or trained staff.
Keywords: Male factors, Recurrent pregnancy loss (RPL), Semen parameters, Occupational exposure, Lifestyle, Semen quality
Introduction
Recurrent pregnancy loss (RPL) is traditionally defined as the loss of three or more consecutive pregnancies of less than 20 menstrual weeks’ duration [1]. Many factors may be involved in RPL, such as genetic, endocrinological and anatomical factors, autoimmune disorders, and infectious and systemic maternal diseases [2, 3]. In previous years, more attention has been paid to female effects on RPL rather than male effects. Recently, however, an increasing amount of researchers have investigated the effect of male factors on RPL [4, 5].
Routine semen analysis involves a series of basic tests to evaluate male fertility. Semen parameters, such as sperm concentration, motility, viability, and morphology provide useful insight into the quality of semen. However, the role and the correct interpretation of these semen parameters remain unclear and their implications on RPL are debatable [6–8].
Sperm nuclear chromatin carries the male genetic information. Sperm nuclear chromatin assay can gives us additional information on sperm quality. A recent study observed a significant increase in sperm DNA damage in male partners of RPL couples with normal sperm parameters [9]. This result leads us to speculate that sperm chromatin assay can reveal a hidden abnormality of sperm in men whose partners with RPL. Currently, different methods are employed to assay sperm chromatin status. Aniline blue (AB) staining is recommended because it is simple and inexpensive. This method can provide a specific positive reaction for lysine and visualize the DNA protein composition of sperm nuclear to evaluate the degree of residual histones.
A few studies have also suggested that occupational exposure, such as heavy metals, organic solvents, and ionizing radiation, affect semen quality, concentration, motility, viability, and morphology, as well as sperm chromatin status. These factors, together or independently, may not only result in failure of the woman to conceive, but in some cases may result in the conception of non-viable embryos [10, 11]. Recent studies using only maternal factors have shown that occupational exposure was correlated with RPL [12, 13]. However, the risk of RPL in women whose husbands/partners with a history of occupational exposure have not been evaluated, and this issue remains unclear.
This study aims to investigate whether there is an existence of association between semen quality and RPL, and whether the male partner’s occupational exposure and unhealthy lifestyle could increase the risk of RPL.
Materials and methods
Study groups
This retrospective study covered a 9-month period between August 2010 and April 2011. All subjects were from the First Hospital of Jilin University, Jilin province, China. The RPL group consisted of 68 couples who saw a doctor because of a history of three or more embryo losses during the first trimester pregnancy. All females performed examinations in chromosome karyotype, hormone, thyroid function, coagulation function, cultures for bacteria or viruses, and the anatomy of the uterus. No alterations were seen. All men were proven normality in chromosome karyotype, and collected semen samples to perform a semen analysis and sperm chromatin status assessment.
The control group consisted of 63 couples who came to the hospital for antenatal examination, and had no history of first trimester spontaneous abortion. All male partners were voluntary to collect semen samples for semen analysis and sperm chromatin status assessment. We followed up to those couples and confirmed they had healthy children.
Written consent was obtained from the couples after being given detailed explanations of the proposed study.
Questionnaire
A questionnaire was given to all subjects. This questionnaire was designed to obtain information on age, occupational exposure, smoking, and drinking of males. Occupational exposure included heat, radiation, electromagnetism and microwaves, noise, pesticides, heavy metals, organic impregnant materials. The occupational exposure history and exposure factors were evaluated according to the occupational characteristics and environment self-reported by the subjects. Tobacco consumption was defined as more than ten cigarettes every day, and drinking was defined as the quantity of Chinese spirits consumption greater than 100 ml every day or beers consumption more than two bottles every day.
Semen analysis
Semen samples were collected after 3–5 days of sexual abstinence, and they were left to liquefy for 30 min at 37 °C. Semen analysis included assessment of sperm concentration, motility, morphology, and viability. Throughout the study, all semen analyses were performed by the same technician. Concentration and motility were evaluated using computer-assisted sperm analysis (CASA; WLJY-9000, China). Sperm motility was assessed by categorization into four grades of motility at 37 °C: Grade A—rapid progressive sperm; Grade B—slow progressive sperm; Grade C—non-progressive sperm; and Grade D—immotile sperm [14]. Morphology was evaluated on air-dried smears, which were fixed and stained by the Papanicolaou stain technique [14]. WHO criteria (1999) [14] were used to determine the morphology of the spermatozoa. Sperm viability was evaluated by Eosin Y staining [14].
Aniline blue staining
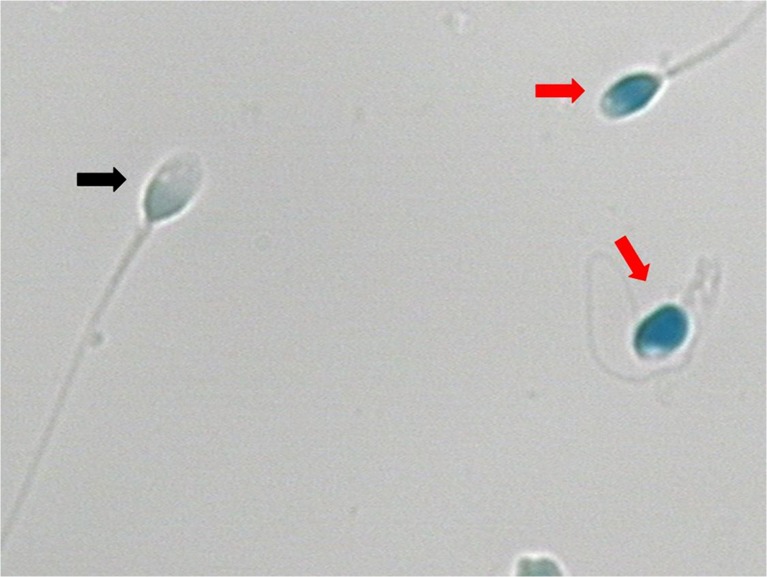
Sperm smears were fixed in 3 % buffered glutaraldehyde in 0.2 M phosphate buffer (14 mL Na2HPO4 0.2 M + 36 mL Na2HPO4 0.2 M, pH 7.2) for 30 min. The smears were stained with 5 % aqueous aniline blue in 4 % acetic acid (pH 3.5) for 5 min [15]. Under light microscopic evaluation, the head of spermatozoa with normal chromatin structure was light blue (negative spermatozoa of AB staining), and the head of spermatozoa with abnormal chromatin structure was deep blue (positive spermatozoa of AB staining) (Fig. 1).
Fig. 1.
Aniline blue (AB) staining of spermatozoa (×1000, Bright field). Red row: AB staining positive spermatozoa. Black row: AB staining negative spermatozoa
Principal component analysis
Principal component analysis was employed to evaluate the quality of semen samples. Six semen parameters were included: concentration, motility, viability, morphology, total progressive motile sperm, and AB stain. All variables were transformed through 10 logarithms of multiplying a variable by 10 and adding a value of 1 (e.g., log [10 × concentration + 1]). We acquired three principal components. The first principal component (F1) accounted for 41.70 %, the second (F2) accounted for 27.76 %, and the third (F3) accounted for 17.82 %. Based on the rotated component matrix, we found that F1 mainly explained three parameters in six semen parameters, including motility, viability, and total progressive motile sperm. F2 mainly explained AB stain and morphology. F3 mainly explained concentration. Therefore, F1 can be regarded as sperm locomotion, F2 as sperm configuration, and F3 as sperm quantity.
Statistical analysis
Comparisons of continuous variables between groups were analyzed using Student’s t test according to the results of their normality tests. Chi-square and risk analysis were used to evaluate differences between binomial variables. P < 0.05 was considered statistically significant. Statistical analyses were performed using SPSS11.5.
Results
The RPL group had significantly lower sperm viability, and a lower percentage of sperm with normal morphology and total progressive motility compared with those of the control group (P < 0.05). In addition, men in the RPL group had a significantly higher mean percentage of AB staining positive for sperm compared with that of the control group (P < 0.05). However, no differences were found in sperm concentration, and motility (Table 1). When comparing the results of principal component analysis, we found that F1, F2, and semen quality were significantly decreased in the RPL group compared with those of the control group (P < 0.05) (Table 1).
Table 1.
Comparison of semen quality parameters between the recurrent pregnancy loss (RPL) and control groups
| RPL group (n = 68) | Control group (n = 63) | P | |||
|---|---|---|---|---|---|
| Median ± SD | Range | Median ± SD | Range | ||
| AB staining (%) | 25.64 ± 11.51 | 10–65 | 19.98 ± 7.66 | 7–40 | * |
| Concentration (×106/ml) | 75.23 ± 48.08 | 9.92–263.59 | 93.03 ± 59.62 | 17.53–246.00 | |
| Motility (%) | 33.19 ± 16.40 | 3.40–68.79 | 50.79 ± 17.64 | 2.97–82.53 | |
| Viability (%) | 56.25 ± 15.18 | 18–87 | 67.87 ± 13.21 | 33–89 | * |
| Morphology (%) | 14.18 ± 7.99 | 0–43 | 17.93 ± 9.76 | 2–41 | * |
| Total progressive motile sperm (×106/ejaculate) | 91.60 ± 86.28 | 2.13–467.82 | 147.64 ± 131.49 | 6.18–645.93 | * |
| F1 | 4.12 ± 0.57 | 2.23–4.80 | 4.53 ± 0.51 | 2.77–5.25 | * |
| F2 | 3.76 ± 0.28 | 2.45–4.27 | 3.89 ± 0.25 | 3.10–4.29 | * |
| F3 | 1.56 ± 0.37 | 0.67–2.38 | 1.71 ± 0.36 | 0.92–2.25 | |
| Semen quality | 3.01 ± 0.29 | 2.09–3.41 | 3.27 ± 0.28 | 2.45–3.74 | * |
F1: The first principal component. F2: The second principal component. F3: The third principal component
AB staining (%): the percentage of aniline blue (AB) staining positive for sperm
*P < 0.05 (t-test)
The percentage of males with normal sperm concentrations was similar between the two groups (91.2 % in RPL group vs. 96.8 % in control group), as well as morphology (38.2 % in RPL group vs. 52.4 % in control group) and total progressive motility (79.4 % in RPL group vs. 85.7 % in control group). The percentage of males with normal motility (11.8 % in RPL group vs. 65.1 % in control group), viability (73.5 % in RPL group vs. 92.1 % in control group) significantly decreased and the percentage of males with abnormal AB staining(86.8 % in RPL group vs. 68.3 % in control group) significantly increased in the RPL group compared with that of the control group(P < 0.05) (Table 2).
Table 2.
Comparison between the percentages of males exposed to environmental factors, smoking and drinking in the RPL and control groups
| Factors | RPL group (n = 68) | Control group (n = 63) | OR | 95 % CI | p |
|---|---|---|---|---|---|
| Occupational exposures to environmental factors | 13.2 (9) | 19.0(12) | 1.542 | 0.601 to 3.957 | 0.821 |
| Smoking | 13.2(9) | 11.1 (7) | 0.819 | 0.286 to 2.349 | 0.138 |
| Drinking | 7.4 (5) | 3.2 (2) | 0.431 | 0.077 to 2.210 | 1.129 |
| Occupation + smoking + drinking | 16.2 (11) | 0.2 (1) | 11.965 | 1.497 to 95.626 | 0.005 |
Values in the two groups are % (n)
The odds ratios (ORs) of occupational exposure, smoking, and drinking were shown in Table 2. The ORs indicated that men just having a history of occupational exposure and smoking or drinking habit could not increase the risk of RPL. However, we combined all of these three factors and observed that the percentage of males exposed to these factors increased significantly in RPL group was observed (P < 0.05). Furthermore, the risk of RPL was significantly increased as these three factors were superimposed (OR: 11.965).
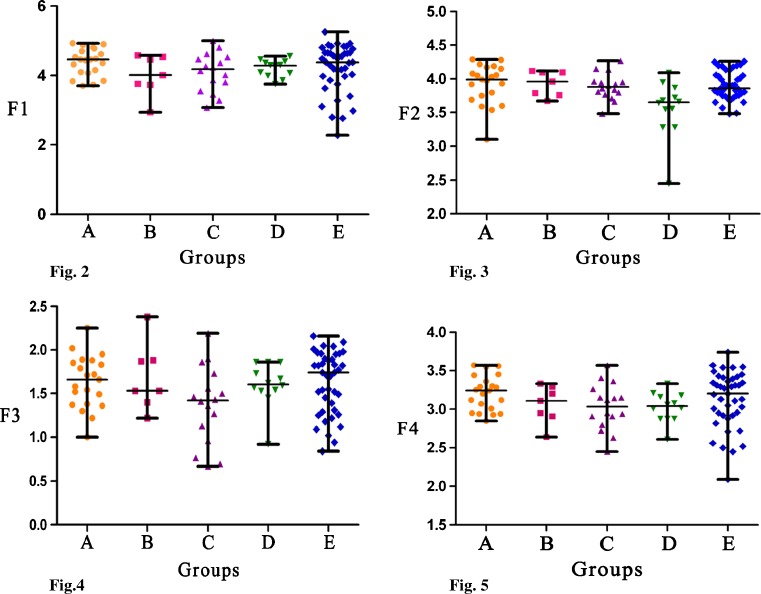
As can be seen in Table 3 and Figs. 2–5, there were no significant differences in F1, F3, and semen quality between different exposure groups, as well as between different exposure groups and non-exposure group. However, when F2 from men with occupational exposure history, smoking and drinking habits were compared with those that only had occupational exposure history or smoking habit or drinking habit, there were significant differences (P < 0.05). This significant difference was also observed between men with occupational exposure history, smoking and drinking habits and men without any exposure agents (P < 0.05).
Table 3.
Comparison of principal components between occupation group, smoking group, drinking group, occupation + smoking + drinking group and non-exposure group
| Principal component | Occupation (n = 21) | Smoking (n = 7) | Drinking (n = 16) | Occupation + smoking + drinking (n = 12) | None (n = 41) |
|---|---|---|---|---|---|
| F1 | 4.46 ± 0.38 | 4.01 ± 0.59 | 4.18 ± 0.55 | 4.29 ± 0.25 | 4.38 ± 0.69 |
| F2 | 3.98 ± 0.29* | 3.96 ± 0.19* | 3.88 ± 0.20* | 3.65 ± 0.42 | 3.87 ± 0.21* |
| F3 | 1.68 ± 0.30 | 1.53 ± 0.39 | 1.42 ± 0.44 | 1.60 ± 0.26 | 1.73 ± 0.35 |
| Semen quality | 3.25 ± 0.22 | 3.11 ± 0.24 | 3.04 ± 0.30 | 3.04 ± 0.19 | 3.19 ± 0.37 |
Values are median ± SD. *P < 0.05 compared with the occupation + smoking + drinking group
F1: The first principal component. F2: The second principal component. F3: The third principal component
Figs. 2–5.
Shown that there were no differences in F1, F3, and F4 between five groups. Figure 3 shown that F2 of D group was significantly lower when respectively comparing with A group, B group, C group and E group (P < 0.05). A group: occupational exposure group (n = 21), B group: Smoking group (n = 7), C group: Drinking group (n = 16), D group: occupational + smoking + drinking group (n = 12), E group: non-exposure group (n = 41). F1: The first principle component in occupation, F2: The second principle component in occupation, F3: The third principle component, F4: Semen quality
Discussion
Routine semen analysis still remains the cornerstone of evaluation in male fertility. The role of routine semen analysis in RPL is still under debate. In a previous study [6] it was shown that there was no significant difference in semen volume, sperm concentration, and sperm morphology in the 98 men whose partners experienced three or more spontaneous abortions, compared with 17 men fathering successful pregnancies. In contrast, some of the recent studies did not agree with this finding. Talebi et al. [7] and Brahem et al. [16] have provided evidence of an association between sperm motility and recurrent pregnancy loss. In addition, two studies carried out respectively by Absalan et al. [8] and Gil-Villa et al. [17] have also shown significantly lower sperm motility and normal morphology percentage in men with RPL history. All above observation were in accordance with our data which showed that the percentage of morphologically normal sperm and sperm motility were significantly lower in men whose partners had RPL history compared with the controls. Furthermore, we also observed a statistically significant decrease in sperm viability and total progressive motile sperm in the RPL group. We believe that the sperm motility, viability and morphology could be related to RPL. Otherwise, the data from Zhang et al. [18] also have evaluated the clinical significance of semen parameters in the management of RPL, which had shown the significant difference in sperm morphology between abortion subgroup and controls when the RPL patients were divided into three subgroups according to their reproductive outcome.
We also observed that the percentage of men with normal sperm concentration and morphology, and total progressive motile sperm in the RPL group was similar to that of the control group. Consequently, if only semen analysis is performed, it is difficult to provide a definitive diagnosis of the causes of RPL. On the one hand, the cutoff values of semen parameters, as recommended by the WHO manual, are established based on the normal male. On the other hand, semen analysis cannot cover the diverse array of biological properties per se that the spermatozoon expressed as a highly specialized cell. As a result, other relative factors should also be given a consideration. Currently, an increasing amount of studies have found an important role of sperm chromatin assessment employing different methods in RPL [17, 19]. Talebi et al. [7] used four different cytochemical tests including aniline blue (AB), chromomycin A3 (CMA3), toluidine blue (TB), acridine orange (AO), as well as nuclear chromatin stability assay, to analyze sperm. They concluded that there was significant correlation between DNA fragmentation and RPL, in agreement with our findings. Using a TUNEL assay, the results from Brahem and coworkers [16] had shown that the percentage of sperm DNA fragmentation significantly increased in men whose partners had a history of RPL. A systematic review and meta-analysis of studies including 16 cohort studies was performed by Robinson et al. [20]. Their results had shown a significantly increased miscarriage rate in men with high sperm DNA damage.
In a previous study, Agarwal et al. [21] employed principal component analysis to evaluated semen characteristics based on nine semen parameters. The results had shown that semen quality scores calculated by this method could provide meaningful information on the quality of semen for the clinician. Furthermore, Allamaneni et al. [22] also reported that semen quality scores was an effective predictor of pregnancy and live birth outcomes in couples undergoing intrauterine insemination with donor sperm. In the present study, the semen quality was significantly higher in fertile men than in men with RPL history. In addition, considering semen quality score can provide more meaningful information than individual semen parameters, therefore, we believe that semen quality score also can provide more clinical value to manage the RPL patients.
In the present study, we seek to evaluate the effect of male occupational exposure and lifestyle on RPL. Our data showed that the risk of RPL could not significantly increase in men with occupational exposures, alcohol consumption, or smoking. However, we observed that the risk score of RPL reach to 11.965, especially men with occupational exposures combined with alcohol consumption and smoking. De Fleurian et al. [23] reported the significant association between semen impairment and occupational risk factors such as exposure to heavy metals, solvents fumes and polycyclic aromatic hydrocarbons. They also observed that the mean exposure index to some chemicals was significantly higher in men with altered semen than in men with normal semen. Recent study has found exposures to pesticides at environmentally or occupationally relevant levels may be associated with decreased semen quality [11]. Concerning lifestyle, some studies reported the association between alcohol consumption, smoking and ICSI outcomes. The results from Braga et al. [24] suggested that fertilization rate could be negatively influenced by male alcohol consumption or smoking. However, Zitzmann et al. [25] demonstrated that male smokers had more risk of ICSI failure comparing with male nonsmokers. According to our data, it had shown men with occupational exposures and unhealthy lifestyle have significantly decreased sperm quality.
In the present study, there are a few limitations. On the one hand, the sample size is small, which restricted the statistical identification of the association between exposure factors and RPL. On the other hand, an outstanding problem is lack of exposure assessment of various environment factors.
In conclusion, we found that sperm quality, occupational exposure, and lifestyle are factors that affect RPL. Consequently, occupational exposure and lifestyle factors should constitute an important section of questionnaires given to patients, and these factors should be evaluated by a clinician or trained staff.
Acknowledgments
This research was generously supported by a grant from National Population and Family Planning Commission of P.R.China (No.2011-GJKJS-07).
Conflicts of interest
The authors had no conflicts of interest to declare in relation to this article.
Footnotes
Capsule Impaired sperm quality, occupational exposure, and unhealthy lifestyle are correlated with RPL. Therefore, the evaluation of male factors plays an important role in the management of RPL.
References
- 1.American Society for Reproductive Medicine. Practice Committee report: patient’s fact sheet: recurrent pregnancy loss. http://www.asrm.org/Patients/FactSheets/fact.html February 2005.
- 2.Allison JL, Schust DJ. Recurrent first trimester pregnancy loss: revised definitions and novel causes. Curr Opin Endocrinol Diabetes Obes. 2009;16(6):446–50. doi: 10.1097/MED.0b013e3283327fc5. [DOI] [PubMed] [Google Scholar]
- 3.Kiwi R. Recurrent pregnancy loss: evaluation and discussion of the causes and their management. Cleve Clin J Med. 2006;73(10):913–21. doi: 10.3949/ccjm.73.10.913. [DOI] [PubMed] [Google Scholar]
- 4.Puscheck EE, Jeyendran RS. The impact of male factor on recurrent pregnancy loss. Curr Opin Obstet Gynecol. 2007;19(3):222–8. doi: 10.1097/GCO.0b013e32813e3ff0. [DOI] [PubMed] [Google Scholar]
- 5.Barroso G, Valdespin C, Vega E, et al. Developmental sperm contributions: fertilization and beyond. Fertil Steril. 2009;92(3):835–48. doi: 10.1016/j.fertnstert.2009.06.030. [DOI] [PubMed] [Google Scholar]
- 6.Hill JA, Abbott AF, Politch JA. Sperm morphology and recurrent abortion. Fertil Steril. 1994;61(4):776–8. [PubMed] [Google Scholar]
- 7.Talebi AR, Vahidi S, Aflatoonian A, et al. Cytochemical evaluation of sperm chromatin and DNA integrity in couples with unexplained recurrent spontaneous abortions. Andrologia. 2012;44(Suppl 1):462–70. doi: 10.1111/j.1439-0272.2011.01206.x. [DOI] [PubMed] [Google Scholar]
- 8.Absalan F, Ghannadi A, Kazerooni M, et al. Value of sperm chromatin dispersion test in couples with unexplained recurrent abortion. J Assist Reprod Genet. 2012;29(1):11–4. doi: 10.1007/s10815-011-9647-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Venkatesh S, Thilagavathi J, Kumar K, et al. Cytogenetic, Y chromosome microdeletion, sperm chromatin and oxidative stress analysis in male partners of couples experiencing recurrent spontaneous abortions. Arch Gynecol Obstet. 2011;284(6):1577–84. doi: 10.1007/s00404-011-1990-y. [DOI] [PubMed] [Google Scholar]
- 10.Miranda-Contreras L, Gómez-Pérez R, Rojas G, et al. (2013) Occupational exposure to organophosphate and carbamate pesticides affects sperm chromatin integrity and reproductive hormone levels among Venezuelan farm workers. J Occup Health. doi:10.1539/joh.12-0144-FS. [DOI] [PubMed]
- 11.Martenies SE, Perry MJ. Environmental and occupational pesticide exposure and human sperm parameters: a systematic review. Toxicology. 2013;307(10):66–73. doi: 10.1016/j.tox.2013.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gardella JR, Hill JA. Environmental toxins associated with recurrent pregnancy loss. Semin Reprod Med. 2000;18(4):407–24. doi: 10.1055/s-2000-13731. [DOI] [PubMed] [Google Scholar]
- 13.Sharara FI, Seifer DB, Flaws JA. Environmental toxicants and female reproduction. Fertil Steril. 1998;70(4):613–22. doi: 10.1016/S0015-0282(98)00253-2. [DOI] [PubMed] [Google Scholar]
- 14.Laboratory manual for the examination of human semen and sperm–cervical mucus interaction. 4. Cambridge: Cambridge University Press; 1999. [Google Scholar]
- 15.Nasr-Esfahani MH, Razavi S, Mardani M. Relation between different human sperm nuclear maturity tests and in vitro fertilization. J Assist Reprod Genet. 2001;18(4):219–25. doi: 10.1023/A:1009412130417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brahem S, Mehdi M, Landolsi H, et al. Semen parameters and sperm DNA fragmentation as causes of recurrent pregnancy loss. Urology. 2011;78(4):792–6. doi: 10.1016/j.urology.2011.05.049. [DOI] [PubMed] [Google Scholar]
- 17.Gil-Villa AM, Cardona-Maya W, Agarwal A, et al. Assessment of sperm factors possibly involved in early recurrent pregnancy loss. Fertil Steril. 2010;94(4):1465–72. doi: 10.1016/j.fertnstert.2009.05.042. [DOI] [PubMed] [Google Scholar]
- 18.Zhang L, Wang L, Zhang X, et al. Sperm chromatin integrity may predict future fertility for unexplained recurrent spontaneous abortion patients. Int J Androl. 2012;35(5):752–7. doi: 10.1111/j.1365-2605.2012.01276.x. [DOI] [PubMed] [Google Scholar]
- 19.Saxena P, Misro MM, Chaki SP, et al. Is abnormal sperm function an indicator among couples with recurrent pregnancy loss? Fertil Steril. 2008;90(5):1854–8. doi: 10.1016/j.fertnstert.2007.09.031. [DOI] [PubMed] [Google Scholar]
- 20.Robinson L, Gallos ID, Conner SJ, et al. The effect of sperm DNA fragmentation on miscarriage rates: a systematic review and meta-analysis. Hum Reprod. 2012;27(10):2908–17. doi: 10.1093/humrep/des261. [DOI] [PubMed] [Google Scholar]
- 21.Agarwal A, Sharma RK, Nelson DR. New semen quality scores developed by principal component analysis of semen characteristics. J Androl. 2003;24(3):343–52. doi: 10.1002/j.1939-4640.2003.tb02681.x. [DOI] [PubMed] [Google Scholar]
- 22.Allamaneni SS, Bandaranayake I, Agarwal A. Use of semen quality scores to predict pregnancy rates in couples undergoing intrauterine insemination with donor sperm. Fertil Steril. 2004;82(3):606–11. doi: 10.1016/j.fertnstert.2004.02.113. [DOI] [PubMed] [Google Scholar]
- 23.De Fleurian G, Perrin J, Ecochard R, et al. Occupational exposures obtained by questionnaire in clinical practice and their association with semen quality. J Androl. 2009;30(5):566–79. doi: 10.2164/jandrol.108.005918. [DOI] [PubMed] [Google Scholar]
- 24.Braga DP, Halpern G, Figueira Rde C. Food intake and social habits in male patients and its relationship to intracytoplasmic sperm injection outcomes. Fertil Steril. 2012;97(1):53–9. doi: 10.1016/j.fertnstert.2011.10.011. [DOI] [PubMed] [Google Scholar]
- 25.Zitzmann M, Rolf C, Nordhoff V, et al. Male smokers have a decreased success rate for in vitro fertilization and intracytoplasmic sperm injection. Fertil Steril. 2003;79(Suppl 3):1550–4. doi: 10.1016/S0015-0282(03)00339-X. [DOI] [PubMed] [Google Scholar]