Abstract
Purpose
Determine the outcome of embryo cryopreservation in female oncology patients
Methods
The outcomes of IVF/ICSI cycles in oncology patients over 15 years in a University Teaching Hospital.
Results
Forty-two oncology patients (mean 31.9 ± 3.9 years) underwent embryo cryopreservation treatment (n = 33 IVF, n = 6 ICSI). Controlled ovarian stimulation with GnRH antagonist protocol (n = 34; 81 %) yielded fewer oocytes than GnRH agonist protocol (n = 8; 19 %) (9.4 ± 6.3 vs. 15.3 ± 8.9; p = 0.04) respectively. There was no significant difference in mean (±SD) duration of ovarian stimulation (11.6 ± 2.6 vs.10.6 ± 2.7), median gonadotrophin dose (1950 vs. 1670 IU), median day 5–6 oestradiol level (1124 vs.1129 pmol/l) or embryo yield (6.2 ± 4.1 vs. 8.8 ± 4.3; p = 0.07) between GnRH antagonist and agonist treatment cycles respectively. Thirty-nine patients cryopreserved embryos and three had their cycle cancelled. During this study period, of those who cryopreserved embryos, 5 patients underwent 9 frozen-thaw cycles (13 %), resulting in 2 live births (1 twin, 1 singleton, live birth rate 22 %). Six patients died (15 %), 3 conceived naturally (8 %) and 2 couples separated (5 %). Fourteen patients discarded their embryos (36 %). Twenty-two patients’ (56 %) have embryos remaining in storage.
Conclusions
This study demonstrates that embryo cryopreservation in female oncology patients gives a satisfactory live birth rate. However, there are concerns regarding cost-effectiveness, resulting from high disposal/non-usage of embryos, and further studies are required.
Keywords: IVF, Cancer, Embryo, Fertility Preservation, Cryopreservation
Introduction
Fertility preservation is an important part of the multidisciplinary management of cancer in reproductive-aged women, given the known gonadotoxity of various chemo/radiotherapy regimens and patients’ concern for their future fertility [11]. Although the incidence of cancer increases with age, a significant proportion of cancer diagnoses are made in young women of reproductive age. The increasing incidence of cancer, coupled with women delaying motherhood until later in life, is likely to result in more cancer diagnoses occurring in childless women. As a consequence, this may increase demand for fertility preservation treatment prior to cancer therapy.
Embryo cryopreservation is an established and successful technique. Patients are referred by oncologists for embryo cryopreservation prior to initiation of cancer treatment. However, the availability of funding and access to embryo cryopreservation treatment may limit its use worldwide.
Studies have demonstrated no correlation between embryo storage duration and the live birth rate [26], which is reassuring for oncology patients, given they often delay pregnancy to complete adjuvant chemotherapy and concerns over cancer recurrence [17].
Cryopreservation of embryos can provide women facing a cancer diagnosis with a degree of hope and reassurance about their future fertility. The fertility preservation process is not without its difficulties. Firstly, undergoing controlled ovarian stimulation may delay the initiation of cancer therapy, depending on a woman’s menstrual cycle stage and can cause anxiety amongst women [30]. Secondly, it is associated with supraphysiological oestrogen levels, up to 10 fold higher than found physiologically, which may have the potential to affect oestrogen-dependent tumours [24]. Finally, there are various ethical and legal issues associated with the creation of embryos for long-term storage; including breakdown of relationships, withdrawal of consent and death [13].
Various studies have looked at the fertility outcomes in oncology patients [27,32]. Robertson et al. [27] demonstrated a high embryo yield and pregnancy rate in patients with cancer and autoimmune diseases. However, we are not aware of any study, which have focused on the long-term outcomes of IVF in patients with cancer, in particular, the fate of cryopreserved embryos and the outcomes of frozen-thaw cycles.
Methodology
Subjects
This is a retrospective study of a cohort of women with cancer (n = 42) aged between 25 years and 41 years who had embryo cryopreservation treatment at the Edinburgh Assisted Conception Unit between July 1996–January 2011. The Edinburgh Assisted Conception unit is based at the Royal Infirmary of Edinburgh (RIE), a University Teaching Hospital. Data was extracted from the Unit’s IVF/ICSI treatment cycle database.
Patients were referred by oncologists to our clinic, and seen urgently. Before proceeding to treatment, patients underwent counselling to ensure they fully understood the potential issues associated with embryo cryopreservation.
IVF process
Controlled ovarian stimulation
Controlled ovarian suppression was achieved by using either GnRH antagonist or ‘long’ agonist protocol. GnRH agonist was used for all women, before the GnRH antagonist regimen was used for oncology patients in our centre. Currently, women with regular periods and without polycystic ovarian syndrome (PCOS) undergo GnRH antagonist protocol; Cetrorelix (n = 30) (Merck Serono, Middlesex, UK) or Ganirelix (n = 4) (Merck Sharp & Dohme Limited-MSD, Hertfordshire, UK) was started on day 6 of menses. Patients with irregular periods/PCOS undergo GnRH agonist protocol; Buserelin (Sanofi-Aventis, Surrey, UK) (n = 7) or Nafarelin (Pfizer ltd, Surrey, UK) (n = 1) was started on day 1 of menses.
Ovarian stimulation was started either on day 2 or 3 of menses in GnRH antagonist protocol or following 14 days of down-regulation in GnRH agonist protocol. Currently, a patient’s age and number of antral follicles determine the dose of gonadotrophin. Ovarian stimulation practice has changed over the study period; urinary follicle stimulating hormone (uFSH): Menogon (Ferring Pharmaceuticals, London, UK) (n = 11), Menopur (Ferring Pharmaceuticals, London, UK) (n = 15), Orgafol (MSD, Hertfordshire, UK) (n = 1) or Recombinant FSH (rFSH): Gonal F (Merck Serono, Middlesex, UK) (n = 3) or Puregon (MSD, Hertfordshire, UK) (n = 12) (Fig. 1). Ovarian stimulation was carried out until >3 follicles measured 17–18 mm on ultrasound. Current practice uses serum oestradiol (E2) levels together with ultrasound assessment of follicular growth on day 5–6 of ovarian stimulation, to monitor ovarian response and modify gonadotrophin dose.
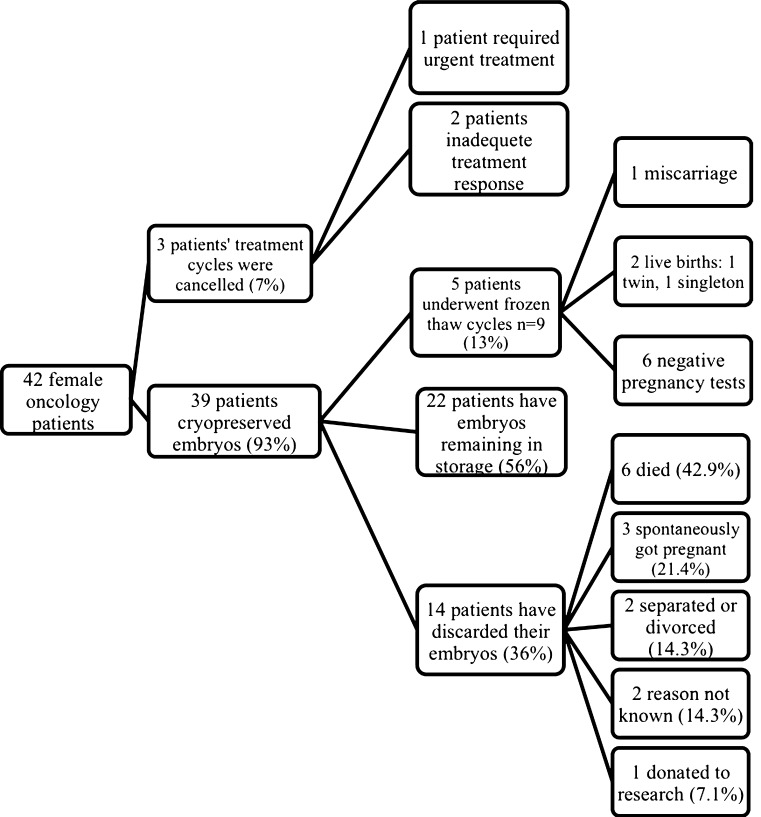
Fig. 1.
The outcome of embryos, cryopreserved by female oncology patients (n = 39) during the study period. Two patients have undergone frozen-thaw cycles, but still have embryos remaining in storage, therefore are included in both groups
Currently, a standard dose of human chorionic gonadotrophin (HCG)(5,000 IU) (Pregnyl, MSD, Hertfordshire, UK) or Ovitrelle 0.25 mg (Merck Serono, Middlesex, UK) was administered to trigger ovulation. Approximately 35 h later, oocyte recovery was carried out by transvaginal ultrasound. The mature oocytes were fertilised by IVF (n = 33) or ICSI (n = 6) depending on sperm quality. The embryos were cryopreserved at either the pronucleate (PN) or cleavage stage by a slow freezing protocol [12] using a programmable freezer, and stored for future treatment.
Frozen-thaw cycles
Patients who returned to the Centre for treatment underwent frozen-thaw cycles using their cryopreserved embryos as previously described by Kini et al. [19]. Patients who are cycling underwent down-regulation with GnRH agonists (Buserelin) for 2 weeks until the endometrial thickness was 4 mm or less on ultrasound or E2 levels < of 150pmol/l or less. Oestrogen replacement therapy (Progynova, Bayer Schering, Berkshire, UK), 6 mg daily, was then administered to patients for at least 14 days. Luteal support with progesterone pessary (Cyclogest 400 mg twice daily, Actavis, Devon, UK) was started when endometrial thickness was 8 mm or more, before a day 2 or 3 embryo transfer. A maximum of two thawed embryos were transferred under trans-abdominal ultrasound guidance in patients with a full bladder.
Embryo outcome
A member of staff accessed the hospital database (MedTRAK) to confirm the number of patients who died. Our IVF database has records of the frozen-thaw cycle outcomes, the number of embryos remaining in storage and patient’s reasons for disposal of embryos.
Statistical analysis
Normally distributed data is presented as mean ± SD. Non-parametric data was presented as median/range data. Mann–Whitney non-parametric test was used to determine significance between GnRH antagonist/agonist protocols. Statistical analysis was carried out using SPSS software version 14.
Results
Subjects
In this retrospective study, 42 female oncology patients (mean 31.9 ± 3.9 years) underwent fertility preservation treatment before initiation of chemotherapy or irradiation. Breast cancer was the most common malignancy (n = 22; 52.3 %) (Table 1). The majority were nulliparous (n = 40; 95.2 %). Three cycles were cancelled; one patient had a large mass on chest X-ray and haemoptysis, which required immediate chemotherapy treatment, and two had a poor response to ovarian stimulation, so were excluded from analysis.
Table 1.
Frequency of cancer types in patients undergoing fertility preservation
| Cancer type | n (%) | |
|---|---|---|
| Breast | E2 Receptor positive (73 %) | 22 (52.3) |
| E2 Receptor negative (27 %) | ||
| Hodgkin’s disease | 9 (21.4) | |
| Cervical | 5 (11.9) | |
| Non-Hodgkin’s lymphoma | 1 (2.4) | |
| Acute lymphoblastic leukaemia | 1 (2.4) | |
| Sarcoma (thigh) | 1 (2.4) | |
| Chronic myeloid leukaemia | 1 (2.4) | |
| Cutaneous T cell lymphoma | 1 (2.4) | |
| Endometrial carcinoma | 1 (2.4) | |
| Total | 42 (100) | |
From referral, patients waited on average 2 days (median; range 0–51) to be seen in the IVF unit. The mean (± SD) number of oocytes retrieved in women who proceeded to oocyte retrieval was 10.6 ± 7.2, and the mean (± SD) number of embryos generated was 6.7 ± 4.2. The outcome of IVF/ICSI treatment cycles is summarised in Table 2
Table 2.
Main characteristics of IVF and ICSI cycle
| Characteristic | |
|---|---|
| Age (years) (mean ± sd) | 31.9 ± 3.9 |
| Number of days to be seen (median; range) | 2.0 (0–51) |
| Number of days of stimulation (mean ± sd) | 11.4 ± 2.6 |
| Gonadotrophin dose (IU) (mean ± sd) | 2071 ± 784 |
| E2 day 5–6 (pmol/l) (median; range) | 1244 (100–3252) |
| Number of days to oocyte retrieval (mean ± sd) | 23.7 ± 10.3 |
| Number of oocytes retrieved (mean ± sd) | 10.6 ± 7.2 |
| % ICSI cycles | 15 |
| % of oocytes fertilised | 68 |
| Number of embryos stored (mean ± sd) | 6.7 ± 4.2 |
GnRH antagonist/agonist protocol
For controlled ovarian hyperstimulation, GnRH antagonist (n = 34, 81 %) was used more commonly than GnRH agonist (n = 8, 19 %). Similarly, urinary FSH (n = 27 64 %) was administered more frequently than Recombinant FSH (n = 15, 36 %).
Patients who underwent GnRH antagonist protocol had a significantly reduced oocyte yield (mean ± SD) (9.4 ± 6.3 vs. 15.3 ± 8.9; p = 0.04). There was no significant difference in mean number of embryos generated (6.2 ± 4.1 vs. 8.8 ± 4.3; p = 0.07), mean duration of ovarian stimulation (11.6 ± 2.6 vs.10.6 ± 2.7), median gonadotrophin dose [1950 (range 1200–4125) vs. 1,670 IU (range 941–3825)], median day 5–6 serum oestradiol level 1124 (range 144–3252) vs.1129 pmol/l (range 100–2956) between GnRH antagonist and agonist treatment cycles respectively.
Patient follow up
Thirty-nine patients (out of 42 patients who commenced treatment) had cryopreserved embryos by the slow-freezing protocol and stored. The median embryo storage time for all the embryos was 5 years (range 0.5–11.5 years). The outcome of the cryopreserved embryos is outlined in Fig. 1.
Currently, 22 patients (mean age 38.5) (56 %) have embryos remaining in storage for a median time of 6.1 years (range 0.5–15.1). Five patients have undergone a total of 9 frozen-thaw cycles (13 %), using a total of 21 embryos (2 were re-frozen), resulting in 2 live births (1 twin, 1 singleton, live birth rate 22 %) and 1 miscarriage (10 weeks). The singleton birth was from embryos retrieved from GnRH antagonist cycle, and the twin birth from embryos retrieved from a GnRH agonist cycle.
The median storage time of embryos prior to their use in frozen thaw cycles was 4.2 years (2.4–7.9 years). The clinical pregnancy (confirmed by USS at 6–8 weeks) rate per patient, thaw cycle and thawed embryo was 60 %, 33 %, and 14 % respectively. The live birth rate per patient, thaw cycle and thawed embryo was 40 %, 22 % and 9.5 % respectively. The live baby rate per patient, thaw cycle and thawed embryo was 60 %, 33 % and 14 % respectively.
During the study period, 6 patients died (15 %), 3 conceived spontaneously (8 %), 2 couples separated (5 %), 1 couple donated their embryos to research (3 %) and 2 couples were lost to follow-up (5 %). As a result, 14 patients chose to discard their embryos (36 %).
Discussion
The rise in cancer survival rates, coupled with improvements in cryopreservation techniques, is likely to increase demand for embryo cryopreservation treatment in the future [16]. This is the first retrospective study looking at the long-term outcome of embryo cryopreservation in a cohort of female oncology patients over a 15-year period (1996–2011).
Early referral for fertility treatment is vital, in order to maximise the likelihood that women may undergo cryopreservation of embryos, whilst avoiding lengthy delays in initiation of cancer treatment [22]. Our study demonstrated an efficient referral process, with a delay of 2 days on average to be seen in the fertility clinic following oncology referral. There was a delay of 51 days from being seen in the clinic to starting treatment in one case, this was due to a delay in the patient’s decision about whether to embark on embryo cryopreservation. However, the delay from cancer diagnosis to referral to the fertility unit was not considered in this study. Availability of funding for assisted conception treatment in the United Kingdom may restrict the eligibility for funding of embryo cryopreservation in some centres. However, in our centre, oncology patients, bypass our waiting list to access National Health Service funded cryopreservation of embryos, if they fulfil our local National Health Service (NHS) criteria for funded treatment. The requirement includes that the woman should be less than 40 years old at the commencement of the procedure.
The patients in this study may represent the tip of the iceberg of reproductive aged women who have been diagnosed with cancer. It has been reported in a US study, that 45 % of oncologists did not routinely discuss fertility preservation treatment with women of reproductive age [15] despite infertility being rated as one of the most important issues amongst women with cancer [11]. There are various factors contributing to this referral barrier. Concerns over the potential impact of fertility treatment on cancer prognosis, may influence oncologists’ referral rate. IVF and embryo cryopreservation has the potential to affect treatment for cancer in two principal ways; firstly it involves exposure to supra-physiological E2 levels, which have the potential to have an impact on the growth of oestrogen-sensitive cancers [31], secondly undergoing fertility treatment may involve a delay in the initiation of cancer therapy.
Recent studies have demonstrated promise in the use of ‘random start controlled ovarian stimulation’ cycles. Traditional ovarian stimulation protocols require women to be at the start of their menstrual cycle. The use of ‘random start cycles’ avoids this requirement and minimise the delays in initiating fertility preservation treatment [28] and ultimately ensures the prompt onset of essential cancer therapy. There is evidence to suggest that cancer survival rates were only reduced when the time from surgery to chemotherapy exceeded 12 weeks [20]. In our study, the longest time from being seen in the unit to oocyte retrieval was 47 days, which therefore is far less than the 12 weeks limit quoted in the Lohrisch study. Women referred to our clinic are counselled and may choose not to delay oncological treatment. Before initiating cryopreservation of embryos, close discussion with the Oncologists is necessary so that there is no significant delay for women undergoing chemotherapy or pelvic radiotherapy.
Recently, letrozole in combination with gonadotrophins have been used for ovarian stimulation, in women, undergoing fertility preservation of embryos with breast cancer [21]. Letrozole is not marketed for commercial use for ovulation induction in the United Kingdom and the latter precludes its use in our centre. There is evidence to suggest that letrozole with low dose gonadotrophin is better than with high dose gonadotrophin as the latter may have a lower live birth rate [21].
It is prudent that cancer treatment should be initiated as soon as embryo cryopreservation is carried out. A recent US study investigating the impact of embryo cryopreservation on the initiation of cancer therapy, found that undergoing fertility preservation did not lead to a significant delay in initiation of cancer therapy [4]. This finding is encouraging, and may reassure women who are anxious about delaying important treatment to facilitate fertility preservation treatment. Individualisation of management is necessary to avoid unnecessary delay for these patients.
There is paucity of evidence with regards to the long-term implications of undergoing fertility treatment on cancer prognosis. There have been concerns regarding the impact of exposure to supra-physiological oestrogen concentrations during controlled ovarian stimulation on the growth of oestrogen sensitive tumours. However, the long-term implications of this remain to be determined. The impact of fertility treatment on cancer growth, treatment and recurrence is not known. Furthermore, it would be ethically difficult to conduct, a large randomised controlled trial of female oncology patients to non-treatment arm versus fertility preservation treatment. An RCT would also be difficult in such a diverse study population because of different cancer type/stage, age, and parity.
The change in clinical practice over the study period is highlighted in this study. The majority of women were treated with GnRH antagonist regimen (81 %) due to the reported reduction in length of treatment cycles [1,2]. In keeping with other studies, a significantly lower oocyte yield (mean 9.4 vs. 15.3) was demonstrated in the GnRH antagonist group compared to the GnRH agonist [5,29]. However, no significant difference in the duration of ovarian stimulation or embryo yield between the GnRH agonist and antagonist groups was shown. However the small sample size, change in fertility practice over the study period and diverse study population limits the conclusions about the various treatment regimens used in this study.
The majority of patients proceeded to oocyte retrieval (n = 39; 93 %), with a mean of 6.7 ± 4.2 embryos cryopreserved. Two cycles were terminated due to poor response to ovarian stimulation; unrelated to their malignancy [9].
The principal finding of this study is the poor utilisation of frozen embryos. Currently, 22 women (mean age 38.5) (56 %) have embryos remaining in storage (mean storage duration; 6.1 years) and only five patients (13 %) have undergone frozen thaw embryo transfers (n = 9), resulting in two live births (1 twin, 1 singleton). A similar study by Robertson et al. [27] looked at the outcome of fertility preservation treatment in women undergoing chemotherapy treatment, found a slightly higher return rate with 10 women undergoing a total of 15 embryo transfers (3 fresh cycles), resulting in 5 live births, over a 6 year period. The higher embryo utilisation demonstrated may be attributable to the inclusion of women with autoimmune or cancer conditions in the latter study. It is reasonable to expect a more lengthy delay in our study of oncology patients, due to concerns of the impact of pregnancy on their treatment, prognosis and fear of cancer recurrence. Interestingly, there is recent evidence demonstrating a possible protective role of pregnancy against cancer recurrence in non-oestrogen dependent tumours [3,10,18].
Eleven patients have extended the storage of their embryos for 10 years, beyond the initial 5-year storage limit. The presence of an annual embryo storage fee has been shown to motivate patients to use/dispose their embryos [7]. Therefore, the absence of storage fees in our centre, may have contributed to the large number of stored embryos, when compared to studies in the United States of America, where storage fees are payable [27]. During the study period, a large proportion of embryos were discarded (36 %). The most common reason was patient death (n = 6), followed by spontaneous conception (n = 3) and couple separation (n = 2). In contrast, ‘family completion’ was the most common reason for embryo disposal in a study of women without cancer who underwent fertility treatment [25]. This is an expected finding given the higher risk of death in a cohort of oncology patients compared to normal women. The retrospective nature of this study restricted contact with patients, therefore, limiting the ability to determine reasons behind embryo disposition for a number of patients (n = 2).
Not all chemotherapeutic agents are gonadotoxic. A study by Forbes [14] reported, 50 % of women (<35 years old) resumed normal menses following chemotherapy. However, menses does not necessary indicate retention of fertility. In our study, four patients conceived spontaneously, resulting in three couples discarding their embryos. It is possible that more women in the study may have retained their fertility, but have yet to try to conceive or have had a pregnancy without notifying our centre. In Sweden, given the high rate of spontaneous return to fecundity, female oncology patients are required to self-fund fertility preservation treatment [6].
Fertility is an important issue for women; therefore a discussion about possible means of fertility preservation should be addressed in all cases involving gonadotoxic therapy. The success of the frozen-thaw cycles in this study has demonstrated the effectiveness of embryo cryopreservation as a means of fertility preservation in female oncology patients. However, in the current economic climate, it is important to be aware that IVF is an expensive treatment, costing approximately £4,000 per cycle. The National Health Service has invested, based on current cost per cycle, £168 000 in embryo cryopreservation for the women in this study, equating to a cost of £84,000 per live birth and £56,000 per baby born. The reason for such high costs per live baby is largely due to the large proportion of embryos that have remained in storage or been discarded. Improving embryo utilisation is the key to improving cost effectiveness. Further studies should aim to investigate the reasons why women have not used their embryos, in order to guide future development of fertility preservation strategies.
In single women, oocyte preservation can be offered for preservation of fertility. High oocyte loss following cryopreservation previously limited its use [12]. However, advances in oocyte vitrification techniques have resulted in higher oocyte survival rates from the cryopreservation process. Recent studies have demonstrated equivalent clinical pregnancy rates in IVF cycles using cryopreserved oocytes and embryos [8,23]. The success of oocyte cryopreservation provides a new dimension to the fertility preservation options available for female oncology patients.
Conclusion
This study reported on the long-term use of embryos stored in women with cancer and has shown satisfactory success rates in those women who have returned to use their embryos. In order to make a realistic assessment into the long-term usage of embryos, further long-term follow up is necessary. It is reassuring that the process of fertility preservation is efficient and is not associated with a significant delay in initiating fertility treatment. However, the delay in starting cancer treatment is not known.
Acknowledgments
Finally, we would like to thank all of the IVF staff at the Edinburgh Reproductive and Endocrine Fertility Centre for their support with this study.
Authors roles
JB contributed to the conception and design, acquisition, analysis and interpretation of data, and drafted the article. JT and ND contributed to conception and design, acquisition of data and were involved in the critical revision of the intellectual content of the manuscript.
Conflict of interest
The authors declare that they have no conflict of interest
Footnotes
Capsule Only a minority of cryopreserved embryos in female oncology patients over a fifteen year period were replaced.
References
- 1.Al-Inany H, Aboulghar M. GnRH antagonist in assisted reproduction: a cochrane review. Hum Reprod. 2002;17:874–885. doi: 10.1093/humrep/17.4.874. [DOI] [PubMed] [Google Scholar]
- 2.Al-Inany HG, Youssef MAFM, Aboulghar M, Broekmans F, Sterrenburg M, Smit J, et al. GnRH antagonists are safer than agonists: an update of a Cochrane review. Hum Reprod Update. 2011;17:435. doi: 10.1093/humupd/dmr004. [DOI] [PubMed] [Google Scholar]
- 3.Azim HA, Jr, Peccatori FA, de Azambuja E, Piccart MJ. Motherhood after breast cancer: searching for la dolce vita. Expert Rev Anticanc. 2011;11:287–298. doi: 10.1586/era.10.208. [DOI] [PubMed] [Google Scholar]
- 4.Baynosa J, Westphal LM, Madrigrano A, Wapnir I. Timing of breast cancer treatments with oocyte retrieval and embryo cryopreservation. J Am Coll Surg. 2009;209:603–607. doi: 10.1016/j.jamcollsurg.2009.08.006. [DOI] [PubMed] [Google Scholar]
- 5.Berger BM, Ezcurra D, Alper MM. Gonadotropin releasing hormone (GnRH) antagonist (cetrorelix) versus GnRH agonist treatment—an age and gonadotropin matched study in young normal responders. Fertil Steril. 2004;82:S235–S236. doi: 10.1016/j.fertnstert.2004.07.624. [DOI] [Google Scholar]
- 6.Besse D, Bellavia M, de Ziegler D and Wunder D. Psychological support in young women who contemplate emergency assisted reproductive technologies (ART) prior to chemo- and/or radiation-therapy. Swiss Med Wkly. 2010;140. [DOI] [PubMed]
- 7.Brzyski RG. Efficacy of postal communication with patients who have cryopreserved pre-embryos. Fertil Steril. 1998;70:949–951. doi: 10.1016/S0015-0282(98)00311-2. [DOI] [PubMed] [Google Scholar]
- 8.Cobo A, Kuwayama M, Pérez S, Ruiz A, Pellicer A, Remohí J. Comparison of concomitant outcome achieved with fresh and cryopreserved donor oocytes vitrified by the Cryotop method. Fertil Steril. 2008;89(6):1657–1664. doi: 10.1016/j.fertnstert.2007.05.050. [DOI] [PubMed] [Google Scholar]
- 9.Das M, Shehata F, Moria A, Holzer H, Son W-Y, Tulandi T. Ovarian reserve, response to gonadotropins, and oocyte maturity in women with malignancy. Fertil Steril. 2011;96:122–125. doi: 10.1016/j.fertnstert.2011.04.070. [DOI] [PubMed] [Google Scholar]
- 10.De Bree E, Makrigiannakis A, Askoxylakis J, Melissas J, Tsiftsis DD. Pregnancy after breast cancer. A comprehensive review. J Surg Oncol. 2010;101:534–542. doi: 10.1002/jso.21514. [DOI] [PubMed] [Google Scholar]
- 11.Duffy CM, Allen SM, Clark MA. Discussions regarding reproductive health for young women with breast cancer undergoing chemotherapy. J Clin Oncol. 2005;23:766–773. doi: 10.1200/JCO.2005.01.134. [DOI] [PubMed] [Google Scholar]
- 12.Edgar DH, Gook DA. A critical appraisal of cryopreservation (slow cooling vs vitrification) of human oocytes and embryos. Hum Reprod Update. 2012;in press. [DOI] [PubMed]
- 13.Fasouliotis SJ, Schenker JG. Cryopreservation of embryos: medical, ethical, and legal issues. J Assist Reprod Gen. 1996;13:756–761. doi: 10.1007/BF02066493. [DOI] [PubMed] [Google Scholar]
- 14.Forbes JF. Long-term effects of adjuvant chemotherapy in breast-cancer. Acta Oncol. 1992;31:243–250. doi: 10.3109/02841869209088910. [DOI] [PubMed] [Google Scholar]
- 15.Forman EJ, Anders CK, Behera MA. Pilot survey of oncologists regarding treatment-related infertility and fertility preservation in female cancer patients. 2009;54(4):203–7. [PMC free article] [PubMed]
- 16.Hery C, Ferlay J, Boniol M, Autier P. Changes in breast cancer incidence and mortality in middle-aged and elderly women in 28 countries with Caucasian majority populations. Ann Oncol. 2008;19:1009–1018. doi: 10.1093/annonc/mdm593. [DOI] [PubMed] [Google Scholar]
- 17.Holleb AI. Breast cancer and pregnancy. CA Cancer J Clin. 1965;15:182–183. doi: 10.3322/canjclin.15.4.182. [DOI] [PubMed] [Google Scholar]
- 18.Janerich DT. The fetal antigen hypothesis: cancers and beyond. Med Hypotheses. 2001;56:101–103. doi: 10.1054/mehy.2000.1119. [DOI] [PubMed] [Google Scholar]
- 19.Kini S, Li HWR, Morrell D, Pickering S, Thong KJ. Anti-mullerian hormone and cumulative pregnancy outcome in in-vitro fertilization. J Assist Reprod Gen. 2010;27:449–456. doi: 10.1007/s10815-010-9427-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lohrisch C, Paltiel C, Gelmon K, Speers C, Taylor S, Barnett J, et al. Impact on survival of time from definitive surgery to initiation of adjuvant chemotherapy for early-stage breast cancer. J Clin Oncol. 2006;24:4888–4894. doi: 10.1200/JCO.2005.01.6089. [DOI] [PubMed] [Google Scholar]
- 21.Lee S, Oktay K. Does higher starting dose of FSH stimulation with letrozole improve fertility cryopreservation outcomes in women with breast cancer? Fert Steril. 2012;98(4):961–964. doi: 10.1016/j.fertnstert.2012.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nachtigall RD, Mac Dougall K, Lee M, Harrington J, Becker G. What do patients want? Expectations and perceptions of IVF clinic information and support regarding frozen embryo disposition. Fertil Steril. 2010;94:2069–2072. doi: 10.1016/j.fertnstert.2010.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nagy ZP, Chang CC, Shapiro DB, Bernal DP, Elsner CW, Mitchell-Leef D, et al. Clinical evaluation of the efficiency of an oocyte donation program using egg cryo-banking. Fertil Steril. 2009;92(2):520–526. doi: 10.1016/j.fertnstert.2008.06.005. [DOI] [PubMed] [Google Scholar]
- 24.Oktay K, Buyuk E, Libertella N, Akar M, Rosenwaks Z. Fertility preservation in breast cancer patients: a prospective controlled comparison of ovarian stimulation with tamoxifen and letrozole for embryo cryopreservation. J Clin Oncol. 2005;23:4347–4353. doi: 10.1200/JCO.2005.05.037. [DOI] [PubMed] [Google Scholar]
- 25.Provoost V, Pennings G, De Sutter P, Gerris J, Van de Velde A, Dhont M. To continue or discontinue storage of cryopreserved embryos? Patients’ decisions in view of their child wish. Hum Reprod. 2011;26:861–872. doi: 10.1093/humrep/deq392. [DOI] [PubMed] [Google Scholar]
- 26.Riggs R, Mayer J, Dowling-Lacey D, Chi T-F, Jones E, Oehninger S. Does storage time influence postthaw survival and pregnancy outcome? An analysis of 11,768 cryopreserved human embryos. Fertil Steril. 2010;93:109–115. doi: 10.1016/j.fertnstert.2008.09.084. [DOI] [PubMed] [Google Scholar]
- 27.Robertson AD, Missmer SA, Ginsburg ES. Embryo yield after in vitro fertilization in women undergoing embryo banking for fertility preservation before chemotherapy. Fertil Steril. 2011;95:588–591. doi: 10.1016/j.fertnstert.2010.04.028. [DOI] [PubMed] [Google Scholar]
- 28.Sonmezer M, Turkcuoglu I, Coskun U, Oktay K. Random-start controlled ovarian hyperstimulation for emergency fertility preservation in letrozole cycles. Fertil Steril. 2011;95(6):2125. doi: 10.1016/j.fertnstert.2011.01.030. [DOI] [PubMed] [Google Scholar]
- 29.Stadtmauer LA, Duran H, Bocca S, Oehninger S. A comparison between gonadotropin releasing hormone (GNRH) agonist and antagonist protocols in women with good ovarian reserve undergoing IVF. Fertil Steril. 2006;86:S437. doi: 10.1016/j.fertnstert.2006.07.1204. [DOI] [Google Scholar]
- 30.Tschudin S, Bitzer J. Psychological aspects of fertility preservation in men and women affected by cancer and other life-threatening diseases. Hum Reprod Update. 2009;15:587–597. doi: 10.1093/humupd/dmp015. [DOI] [PubMed] [Google Scholar]
- 31.Venn A, Watson L, Bruinsma F, Giles G, Healy D. Risk of cancer after use of fertility drugs with in-vitro fertilisation. Lancet. 1999;354:1586–1590. doi: 10.1016/S0140-6736(99)05203-4. [DOI] [PubMed] [Google Scholar]
- 32.Yang D, Brown SE, Nguyen K, Reddy V, Brubaker C, Winslow KL. Live birth after the transfer of human embryos developed from cryopreserved oocytes harvested before cancer treatment. Fertil Steril. 2007;87(6):1469. doi: 10.1016/j.fertnstert.2006.07.1546. [DOI] [PubMed] [Google Scholar]