Abstract
Background
Tumor Necrosis Factor Alpha (TNF-α), is a proinflammatory cytokine in the pathogenesis of Polycystic Ovary Syndrome (PCOS). In order to investigate the role of rs1800629 and rs1799964 polymorphisms in relation to anthropometric measures, family history of complex diseases, diet and clinical features, we performed a case control study in PCOS women from South India.
Methods
A total of 589 samples comprising of 283 patients and 306 controls were enrolled in the present study. Patients were selected based on Rotterdam criteria and ultrasound scanned normal women were selected as controls. Following extraction of DNA, genotyping for rs1800629 and rs1799964 was performed by polymerase chain reaction using tetra primers and PCR-RFLP respectively.
Results
The distribution of genotypes for rs1799964 was significantly different between the groups (p = 0.001), however it was not for rs1800629. Haplotype analysis revealed a significant difference between patients and controls. The predisposing and protective role of haplotype with mutant allele at both loci (combination 3) and haplotype with mutant allele at either loci was reflected by the over representation of combination 3 in patients and combination 2 in controls respectively. In addition, rs1799964 showed an association with dietary habit, clinical hyperandrogenism and AAO. The modifying role of TT genotype on age at onset was noted in quartile analysis.
Conclusion
Replicative studies on the influence of TNF-α polymorphism in different ethnic groups may identify the potentiality of these polymorphisms as markers of inflammation and in turn may help the clinicians for the better management of the condition.
Keywords: Cytokine, Diet, Gene-environment, Haplotype, Inflammation, PCOS/Polymorphism, Tumor Necrosis Factor
Introduction
Polycystic ovary syndrome (PCOS) is a common endocrine disorder affecting 4 % to 12 % of women of reproductive age worldwide and is a major cause of female infertility; contributing to long term complications associated with oxidative stress and chronic low grade inflammation [1]. The syndrome is characterized by hyperandrogenism, polycystic ovaries and chronic anovulation. Inter-individual variation is commonly observed with respect to clinical features changing throughout the life span starting from adolescence to postmenopausal age. Thus, predisposing the individual to serious long term consequences such as type 2 diabetes, endometrial hyperplasia, thyroid dysfunction and cardiovascular diseases [2]. The aetiology of PCOS is not yet clear however genetic, biochemical, immunological and environmental factors are implicated in the aetiopathogeneis of this multifactorial condition [3]. Several candidate genes have been proposed as important contributors to PCOS but none have yet achieved acceptance as major cause [4].
Janine et al., (2001) suggested the involvement of cytokines as regulatory factors in follicular development in ovary and the frequently studied cytokine in the ovary is Tumor Necrosis Factor alpha (TNF-α) [5]. The gene for TNF-α resides within the class 3 MHC and is located on the short arm of chromosome 6 (6p21.3) [6]. Expression of TNF-α rely on various stimuli such as lipopolysaccharide, free oxygen radicals and cytokines like IL-1 and IFN-γ [7]. Its expression is regulated at both the transcriptional and post-transcriptional levels and has been suggested that variability in promoter and coding regions of TNF gene may influence the magnitude of the secretary response of this cytokine [8]. This cytokine regulates numerous cellular processes such as immune and inflammatory responses, differentiation, proliferation and cell death [9]. TNF-α also modulates several biological processes in the mammalian ovary including granulose cell proliferation, follicular development, ovulation and luteolysis, steroidogenesis, prostaglandin and proteoglycan biosynthesis [10].
Variations in the levels of immunoreactive TNF-α throughout the menstrual cycle and its secretion from the corpus luteum have been reported [11]. However, in vitro studies have shown that TNF-α may stimulate, inhibit or does not affect steroid hormone secretion in mammal ovarian cells [10]. Polymorphisms in the TNF-α promoter gene may affect transcriptional regulation by modifying the binding site of specific transcription factors [8]. Several polymorphisms in the promoter region have been associated with conditions such as insulin resistance, obesity, pre-eclampsia, endometriosis including PCOS [4, 12, 13]. More recent studies have provided evidence for a role of TNF-α in obesity and insulin resistance, indicating that perturbations of TNF-α metabolism may affect the onset of non-insulin-dependent diabetes mellitus (NIDDM) and may play a role in the development of cardiovascular disorders [8]; the conditions often considered as long term complications of PCOS (Table 1; [14–22]).
Table 1.
TNF-α polymorphisms and their associations with various disorders
| Disease | Polymorphisms studied | References |
|---|---|---|
| PCOS | −308, −850, −1031 | Milner [14], Korhonen [15], Ji-Hyun [4] |
| Insulin Resistance | −308 | Nicuad [16], C.P Day [17] |
| Obesity | −308 | Brand [18], Furuta [19] |
| Hirsutism/increased androgen secretion | −308 | Gonzalez [20] |
| Pre-eclampsia | −308 | Chen [21] |
| Endometriosis | −238, −308, −857, −863, −1031 | Ji-Hyun [4] |
| HPV/Cervical cancer | −308 | Ning Wang [22] |
Functional single nucleotide polymorphism (SNP) at positions −308 (rs1800629) and −1031 (rs1799964) of human TNF-α gene have been shown to be linked with altered promoter activity with different plasma levels of TNF-α in healthy individuals [23]. Among these SNP’s the A allele of rs1800629 and the C allele of rs1799964 polymorphisms has been shown to be associated with increasing TNF-α expression [9]. The A allele of rs1800629 polymorphism have been identified to be involved with insulin dependent diabetes, development of insulin resistance and increasing adiposity [24]. Further, Rice et al., (1998) suggested that TNF-α appears to inhibit follicle stimulating hormone (FSH) induced estradiol secretion in small follicles of human ovary leading to anovulation [25]. The rs1799964 polymorphism has been suggested to contribute to several inflammatory disorders such as Benchet’s disease, Crohn’s disease, idiopathic recurrent miscarriages, intracerebral hemorrhage, ulcerative colitis, endometriosis [9] and PCOS [4]. Hyperandrogenism was strongly associated with C allele of rs1799964 polymorphism [26].
In view of firm evidence implicating TNF-α polymorphisms in adiposity, insulin resistance, anovulation and hyperandrogenism; all of which are features of PCOS, and no reports of these polymorphisms in Indian context, the present study was aimed to screen for rs1800629 and rs1799964 polymorphisms in South Indian population.
Materials and methodology
Study population
In the present study a total of 589 women comprising of 283 patients and 306 controls were recruited from Modern Government Maternity Hospital, Hyderabad, India. Patients were selected based on Rotterdam criteria according to which a woman is said to have PCOS, if she has any two features out of three such as polycystic ovaries on ultrasound scan, menstrual irregularities and/or clinical signs or symptoms of hyperandrogenism such as acne, alopecia, hirsutism and premature pubarche. Ultrasound scanned normal fertile women with no menstrual dysfunction or history of infertility were selected as controls. Written approval was obtained from the ethics committee of Osmania University and informed consent from all subjects before peripheral blood samples were collected. Detailed information on clinical, anthropometric measures and diet was recorded through proforma. Our sample size of 589 (283 cases + 306 controls) is large enough and exceeds the estimated number of samples (~250 cases + controls) required to obtain a 90 % statistical power.
Molecular analysis
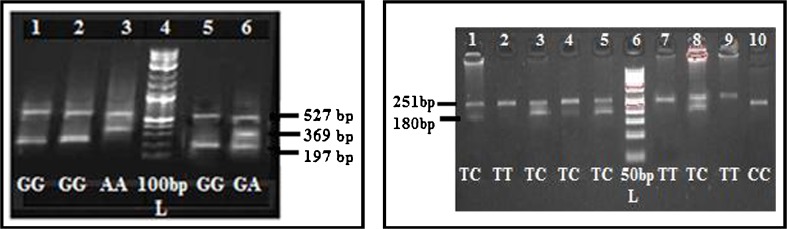
DNA was extracted from peripheral blood and for each subject; rs1800629 and rs1799964 genotyping was performed by using ARMS PCR and PCR-RFLP respectively. The rs1800629 polymorphism was amplified by tetra primers ARMS PCR designed in our lab while the rs1799964 was amplified by PCR with a set of primers [4]. Products of rs1800629 were analyzed using 2 % agarose while the products of rs1799964 was first digested with Bbs I (Fermentas, India) for 12 h at 37 °C and then genotyped using 3 % agarose gels (Fig. 1).
Fig. 1.
Gel picture showing TNF-α rs1800629 and rs1799964 genotypes
Haplotype analysis
Since haplotype provide more comprehensive information, haplotype analysis was performed to understand the nature of association of rs1800629 and rs1799964 polymorphisms with PCOS traits. Using the SNPstat software (http://bioinfo.iconcologia.net/snpstats/start.htm), four haplotypes were generated for TNF-α gene in the entire cohort of 589 individuals. In order to further understand the role of these specific haplotypes in the manifestation of the condition, haplotype combinations were made. The three haplotype combinations analyzed viz., 1. wild type alleles at both loci (G-G/T-T), 2. one mutant allele at any one locus (G-G/T-C, G-G/C-C, G-A/T-T, A-A/T-T) and 3. two mutant alleles at both loci (G-A/T-C, G-A/C-C, A-A/T-C, A-A/C-C).
Analysis of anthropometric measures: genotypes and haplotypes
The two anthropometric measures considered for the present study was Body Mass Index (BMI) and Waist to Hip ratio (WHR). Body weight, height, waist and hip circumferences were measured in all participants in order to calculate BMI and WHR. Their BMI was calculated by the formula: body mass/(height)2 [kg/m2] and waist circumference was measured at the midpoint between lower border of the rib cage and the iliac crest. Hip circumference was measured at the level of trochanter in cms. Waist hip ratio (WHR) was calculated as waist circumference divided by hip circumference. A value ≥25 kg/m2 was considered as obese and a value of ≥ 0.8 was regarded to have abdominal obesity. We considered the WHO criteria of BMI ≥25 to be the cut-off for distinguishing normal form overweight/obese Indian women to be appropriate. A study by Snehalatha et al., 2003 in South Indian women employed BMI > 23 to be the cut-off for obese cases [27]. In order to evaluate the relationship of rs1800629 and rs1799964 polymorphisms with various anthropometric measures, we compared the distribution of the rs1800629 and rs1799964 genotypes in relation to factors such as BMI and WHR in both patients and controls. They were categorized into individual groups based on BMI (cut off point 25 kg/m2), WHR (cut off point 0.80).
Family history of complex diseases and dietary habits
In view of reported incidences of complex diseases such as PCOS, menstrual disturbance, diabetes and cardiovascular diseases in the first degree relatives of PCOS patients [3], we have compared the family history of complex diseases (FHCD) in the parents, sibs and other relatives of PCOS probands. Furthermore, several studies have demonstrated that high lipid and low-fiber diet was associated with PCOS, we aimed to establish the association of these polymorphisms with dietary habits (vegetarian vs. non-vegetarians).
Clinical features
Inter-individuals variation is commonly observed with respect to clinical features changing throughout the life span starting from adolescence to post menopausal age. The clinical features represent the signs or symptoms of PCOS that had arise due to hyperandrogenism and hyperinsulinemia. The two markers acanthosis nigricans for insulin resistance; acne, hirsutism, alopecia and premature pubarche for hyperandrogenism were considered as important clinical features of PCOS. The patients were invariably asked about the onset of menstrual dysfunction/development of clinical symptoms either at adolescence or at adulthood and the age at onset (AAO) of the clinical symptoms was recorded. In order to see the association of these polymorphisms the patients were stratified based on the presence or absence of a particular clinical trait. The trait/symptoms taken into consideration for analysis are hyperandrogenism, acanthosis, hyperandogenism + acanthosis and AAO. The onset of the condition was considered as early, if they showed the signs or symptoms of PCOS at the age of ≤15 years and the onset above 15 years were referred to as late onset [2].
Quartile data analysis
In order to see whether AAO of the condition is being influenced by genotypes and haplotypes/anthropometric measures/FHCD and dietary habits/clinical features, the whole patient data was categorized into quartiles based on AAO. Quartile analysis was carried out by arranging the whole data either in ascending or descending order of AAO and were categorized into four group’s viz., quartile 1, 2, 3, 4. Finally, the above factors were analyzed in each quartile.
Statistical analysis
All the statistical analysis was performed with the help of SPSS statistical software (version17.0, IBM SPSS). Continuous data were expressed as mean ± SD. A two tailed p-value of <0.05 was considered statistically significant. The demographic characteristics of patients and controls were compared by the Student’s t-test for unpaired data. The association between genotypes and PCOS risk was evaluated by calculating the odds ratios (OR) at 95 % confidence interval. Allele and genotype frequencies were determined from observed genotype counts. Hardy Weinberg equilibrium was estimated by the χ2 test.
Results
rs1800629 and rs1799964 genotypes and haplotypes in susceptibility to PCOS
Table 2 describes the percentage distribution of rs1800629 and rs1799964 polymorphisms in patient and control groups. The overall genotype frequencies of GG, GA and AA of rs1800629 were 4 %, 95 % and 1 % in patients, while they were 3 %, 96 % and 1 % in controls respectively. The distribution of genotypes (χ2 = 0.10; p = 0.95) and alleles (χ2 = 0.02; p = 0.88) did not differ significantly between the groups.
Table 2.
Genotype and allelic frequency distribution of rs1800629 and rs1799964 polymorphisms among patients and controls
| rs1800629 | GG | GA | AA | G | A | Comparison of groups | OR (95 % CI) | P-value |
| N (%) | N (%) | N (%) | ||||||
| Controls (306) | 10 (3) | 293 (96) | 3 (1) | 0.51 | 0.49 | GG vs. GA + AA | 1.29 (0.55–3.04) | 0.55 |
| Patients (283) | 10 (4) | 270 (95) | 3 (1) | 0.52 | 0.48 | GA vs. GG + AA | 0.80 (0.37–1.72) | 0.56 |
| χ 2 = 0.1; p = 0.95 | χ 2 = 0.02;p = 0.88 | AA vs. GG + GA | 1.07 (0.21–5.35) | 0.93 | ||||
| HWE | Patients | χ 2 = 233.98; p = <0.05 | G vs. A | 1.04 (0.59–1.81) | 0.88 | |||
| Controls | χ 2 = 256.77; p = <0.05 | |||||||
| rs1799964 | TT | TC | CC | T | C | Comparison of groups | OR (95 % CI) | p-value |
| N (%) | N (%) | N (%) | ||||||
| Controls (306) | 162(53) | 139(45) | 5(2) | 0.76 | 0.24 | TT vs. TC + CC | 0.54(0.38–0.75) | <0.01* |
| Patients (283) | 107(38) | 170(60) | 6(2) | 0.68 | 0.32 | TC vs. TT + CC | 1.81 (1.30–2.51) | <0.01* |
| χ 2 = 12.79; p = 0.001* | χ 2 = 1.21;p = 0.27 | CC vs. TT + TC | 1.30 (0.39–4.32) | 0.76 | ||||
| HWE | Patients | χ 2 = 40.17; p = <0.05 | T vs. C | 0.67 (0.36–1.25) | 0.27 | |||
| Controls | χ 2 = 16.62; p = <0.05 | |||||||
OR odds ratio, CI class interval, HWE Hardy Weinberg Equilibrium
*Significant at 5 % LOS
The genotype frequencies of TT, TC and CC of rs1799964 polymorphism were 38 %, 60 % and 2 % in patients, while they were 53 %, 45 % and 2 % in controls, respectively. The distribution of genotypes showed significant difference (χ2 = 12.79; p = 0.001) between the patient and control groups but not with respect to alleles (χ2 = 1.21; p = 0.27). The TT genotype predominated in the controls (TT vs. others OR: 0.54 (CI 0.34–0.75; p = 0.0002) and TC genotype in patients (TC vs. others OR: 1.81, CI 1.30–2.51; p = 0.0003). The genotypic distributions of the two polymorphisms were not in accordance with Hardy Weinberg equilibrium in both patients and controls (p < 0.01).
Haplotype analysis proposed that none of the four haplotypes i.e. A-T, A-C, G-T and G-C were found to be significantly different between patients and controls (p > 0.05), however the haplotype combinations showed significant difference between the patients and the control group (χ2 = 15.23, p = 0.0004). Individuals with combination 2 predominated in the controls with an OR value of 0.51 (0.36–0.71, p = <0.001) compared to the patients, while Combination 3 was elevated and revealed an OR value of 1.86 (1.33–2.57, p = 0.0002) (Table 3).
Table 3.
Haplotype frequency distribution between patients and controls
| Haplotype | Patients | Controls | Comparisons of groups | OR (95 % CI) | p-value |
| A – T | 0.193 | 0.466 | A vs. T | 1.00 | – |
| G – C | 0.031 | 0.223 | G vs. C | 1.48(0.47–4.66) | 0.5 |
| G – T | 0.485 | 0.289 | G vs. T | 0.83(0.28–2.49) | 0.74 |
| A – C | 0.291 | 0.023 | A vs. C | 0.42(0.05–3.64) | 0.43 |
| Combination | Patients | Controls | Comparisons of groups | OR (95 % CI) | p-value |
| N (%) | N (%) | ||||
| 1 | 7(2.5) | 4(1.3) | 1 vs. 2 + 3 | 1.91(0.55–6.61) | 0.37 |
| 2 | 105(37.1) | 164(53.6) | 2 vs. 1 + 3 | 0.51(0.36–0.71) | <0.001* |
| 3 | 171(60.42) | 138(45.1) | 3 vs. 1 + 2 | 1.86(1.33–2.57) | <0.001* |
| χ 2 = 15.23; p = 0.00049* | |||||
1 - G-G/T-T; 2- G-G/T-C, G-G/C-C, G-A/T-T, A-A/T-T; 3 - G-A/T-C, G-A/C-C, A-A/T-C, A-A/C-C
OR odds ratio, CI Class Interval
*Significant at 5 % level of significance
Anthropometric measures: genotypes and haplotypes
The mean age of the patients was 24.48 ± 4.48 and controls it was 24.00 ± 4.84 years, while the AAO of PCOS women was 16.22 ± 4.79 years. The AAM did not differ between the groups (12.41 ± 1.28 vs. 12.43 ± 1.23). In our study, nearly 55 % of PCOS probands were with BMI ≥25 kg/m2 and 39 % had WHR ≤0.80. Both the parameters were significantly different between patients and controls (p < 0.05). Further evaluation of rs1800629 and rs1799964 polymorphisms with various anthropometric measures revealed a significant association of rs1800629 GA genotype with lower BMI (p = 0.01) and lower WHR (p = 0.01), while rs1799964 polymorphism showed involvement with lower BMI (p = 0.03) (Table 4).
Table 4.
Distribution of anthropometric and epidemiological factors with respect to rs1800629 and rs1799964 genotypes
| rs1800629 | BMI <25 kg/m2 | p-value | BMI ≥25 kg/m2 | p-value | ||||||
| N | Controls | N | PCOS | N | Controls | N | PCOS | |||
| GG | 9 | 21.88 ± 2.03 | 3 | 23.00 ± 1.73 | 0.41 | 1 | 26 ± 0.00 | 9 | 30.66 ± 4.58 | – |
| GA | 253 | 21.11 ± 1.86 | 125 | 21.63 ± 2.02 | 0.01* | 40 | 27.97 ± 4.24 | 146 | 29.30 ± 3.93 | 0.06 |
| AA | 3 | 21.33 ± 2.30 | 1 | 23.00 ± 0.00 | – | 0 | – | 2 | 29.00 ± 4.24 | – |
| rs1799964 | N | Controls | N | PCOS | p-value | N | Controls | N | PCOS | p-value |
| TT | 143 | 21.09 ± 1.83 | 44 | 21.41 ± 1.87 | 0.31 | 19 | 28.00 ± 4.24 | 63 | 29.01 ± 4.17 | 0.36 |
| TC | 118 | 21.21 ± 1.93 | 83 | 21.82 ± 2.05 | 0.03* | 21 | 27.95 ± 4.35 | 87 | 29.48 ± 3.79 | 0.11 |
| CC | 4 | 20.50 ± 1.73 | 2 | 20.00 ± 2.83 | 0.79 | 1 | 26.00 ± 0.00 | 4 | 32.00 ± 5.23 | – |
| rs1800629 | WHR <0.80 | WHR ≥0.80 | ||||||||
| N | Controls | N | PCOS | N | Controls | N | PCOS | |||
| GG | 10 | 0.74 ± 0.02 | 4 | 0.75 ± 0.02 | 0.41 | 0 | – | 8 | 0.87 ± 0.05 | – |
| GA | 262 | 0.74 ± 0.02 | 169 | 0.75 ± 0.02 | 0.0001* | 31 | 0.85 ± 0.04 | 102 | 0.84 ± 0.04 | 0.23 |
| AA | 3 | 0.75 ± 0.02 | 2 | 0.74 ± 0.05 | 0.76 | 0 | – | 1 | 0.84 ± 0.00 | – |
| rs1799964 | N | Controls | N | PCOS | N | Controls | N | PCOS | ||
| TT | 148 | 0.74 ± 0.02 | 68 | 0.74 ± 0.02 | 1.00 | 15 | 0.84 ± 0.04 | 40 | 0.85 ± 0.04 | 0.41 |
| TC | 122 | 0.75 ± 0.03 | 104 | 0.75 ± 0.03 | 1.00 | 16 | 0.85 ± 0.04 | 68 | 0.85 ± 0.04 | 1.00 |
| CC | 5 | 0.73 ± 0.03 | 3 | 0.72 ± 0.02 | 0.63 | 0 | – | 3 | 0.83 ± 0.03 | – |
| rs1800629 | Presence of FHCD | χ 2 (p-value) | Absence of FHCD | χ 2 (p-value) | ||||||
| Controls | PCOS | Controls | PCOS | |||||||
| GG | 3(30 %) | 9(75 %) | 0.158 (0.92) | 7(70 %) | 32(26 %) | 64.81 (0.00*) | ||||
| GA | 39(13 %) | 200(75 %) | 97(87 %) | 13(22 %) | ||||||
| AA | 1(33 %) | 2(67 %) | 67(86 %) | 27(26 %) | ||||||
| rs1799964 | Controls | PCOS | Controls | PCOS | ||||||
| TT | 21(13 %) | 77(72 %) | 2.27 (0.32) | 141(87 %) | 30(28 %) | 2.93 (0.23) | ||||
| TC | 20(14 %) | 129(76 %) | 119(86 %) | 41(24 %) | ||||||
| CC | 2(40 %) | 5(83 %) | 3(60 %) | 1(17 %) | ||||||
| rs1800629 | Vegetarian | χ 2 (p-value) | Non-vegetarian | χ 2 (p-value) | ||||||
| Controls | PCOS | Controls | PCOS | |||||||
| GG | 0(0 %) | 0(0 %) | 0.80 (0.37) | 10(100 %) | 12(100 %) | 0.62 (0.73) | ||||
| GA | 32(11 %) | 26(10 %) | 261(89 %) | 242(90 %) | ||||||
| AA | 1(33 %) | 0(0 %) | 2(67 %) | 3(100 %) | ||||||
| rs1799964 | Controls | PCOS | Controls | PCOS | ||||||
| TT | 0(0 %) | 13(12 %) | 21.49 (<0.01*) | 145(90 %) | 94(88 %) | 14.63 (<0.01*) | ||||
| TC | 32(21 %) | 13(8 %) | 123(79 %) | 157(92 %) | ||||||
| CC | 1(17 %) | 0(0 %) | 5(83 %) | 6(100 %) | ||||||
*Significant at 5 % level of significance
BMI body mass index, WHR waist hip ratio, FHCD family history of complex diseases
Family history of complex diseases and dietary habits
Analysis of family history of complex diseases and diet with respect to rs1800629 and rs1799964 polymorphisms are shown in Table 4. The percentage of individuals with family history of complex diseases (FHCD) in their first degree relatives was 74 % in patients whereas it was 28 % in the controls. The overall frequency of non-vegetarians (90 %) in both patients and controls were greater compared to vegetarians (10 %). The rs1800629 polymorphism did not show any involvement with these factors, however the rs1799964 demonstrated a strong association with non-vegetarian diet (p < 0.001). Analysis of these parameters in haplotype combinations revealed an elevated frequency of FHCD in PCOS women under combination 3, though did not reach statistical significance (p > 0.05) (Table 4).
Clinical features
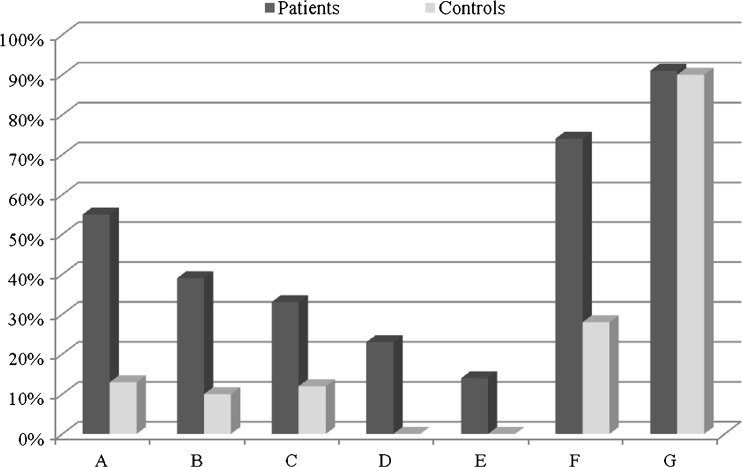
Table 5 represents the analysis of clinical features of PCOS probands with respect to rs1800629 and rs1799964 polymorphisms. Investigations suggested the presence of hyperandrogenism in 33 % of probands in the form of hirsutism, acne, alopecia and premature pubarche. Twenty three percent of individuals showed acanthosis, a marker for insulin resistance while 14 % of patients showed both hyperandrogenism and acanthosis (Fig. 2). Distribution of clinical features with respect to rs1800629 and rs1799964 genotypes, revealed a strong association of hyperandrogenism (p = 0.004) and AAO (p = 0.05) with rs1799964 polymorphism.
Table 5.
Distribution of epidemiological and clinical features with respect to rs1800629 and rs1799964 genotypes in patients
| Characteristics | GG (12) | GA (268) | AA (3) | χ 2 (p-value) | |
| Early AAO | Yes | 7(58 %) | 151(57 %) | 3(100 %) | 2.32 (0.31) |
| No | 5(46 %) | 117(43 %) | 0(0 %) | ||
| Hyperandrogenism | Yes | 4 (33 %) | 89(33 %) | 1(33 %) | 0 (1) |
| No | 8 (67 %) | 179(67 %) | 2(67 %) | ||
| Acanthosis | Yes | 4(33 %) | 59(22 %) | 2(67 %) | 2.32 (0.31) |
| No | 8(67 %) | 212(78 %) | 1(33 %) | ||
| Hyperandrogenism + Acanthosis | Yes | 4(33 %) | 35(13 %) | 0(0 %) | 4.46 (0.11) |
| No | 8(67 %) | 233(87 %) | 3(100 %) | ||
| Characteristics | TT (107) | TC (170) | CC (6) | χ 2 (p-value) | |
| Early AAO | Yes | 70(65 %) | 86(51 %) | 3(50 %) | 5.96 (0.05) |
| No | 37(35 %) | 84(49 %) | 3(50 %) | ||
| Hyperandrogenism | Yes | 47 (44 %) | 47(28 %) | 0(0 %) | 10.89 (0.004*) |
| No | 60 (56 %) | 123(72 %) | 6(100 %) | ||
| Acanthosis | Yes | 22(20 %) | 23(13 %) | 0(0 %) | 3.59 (0.17) |
| No | 86(80 %) | 149(87 %) | 6(100 %) | ||
| Hyperandrogenism + Acanthosis | Yes | 15(14 %) | 24(14 %) | 0(0 %) | 0.49 (0.78) |
| No | 92(86 %) | 146(86 %) | 3(100 %) | ||
AAO age at onset
*Significant at 5 % level of significance
Fig. 2.
Distribution of anthropometric measures, clinical features, family history of complex diseases and diet in PCOS probands and controls. a Higher BMI, b Higher WHR, c Hyperandrogenism, d Acanthosis, e Hyperandrogenism + Acanthosis, f Family history of complex diseases, g Diet (Non-vegeterian)
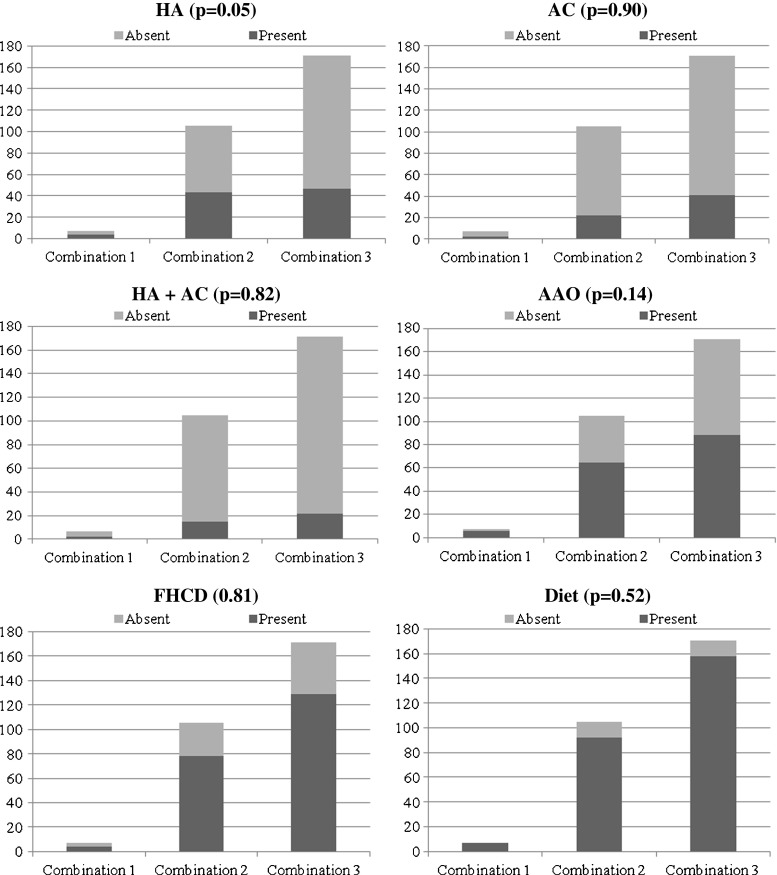
In haplotypes, PCOS women with combination 1 showed elevated frequency of cases showing hyperandrogenism, acanthosis, hyperandrogenism + acanthosis and early AAO compared to combinations 2 and 3, however, did not reach statistical significance (p > 0.05) (Fig. 3).
Fig. 3.
Analysis of haplotype combinations with different clinical and epidemiological factors. Note: Each bar compares the distribution of TNF-α combinations with respect to presence or absence of a specific trait. HA—Hyperandrogenism, AC—Acanthosis, AAO—Age at onset, FHCD—Family history of complex diseases
Quartile data analysis
Lastly, the quartile data analysis showed no involvement of rs1800629 but revealed an association of rs1799964 polymorphism on the age at onset of the condition. An elevated frequency TT genotype of rs1799964 was observed in the first two quartiles. There was a preponderance of women with higher BMI and markedly increased frequency of women with hyperandrogenic clinical features in the first two quartiles, where as other factors including rs1800629 genotypes did not differ in any of the four quartiles (Fig. 4).
Fig. 4.
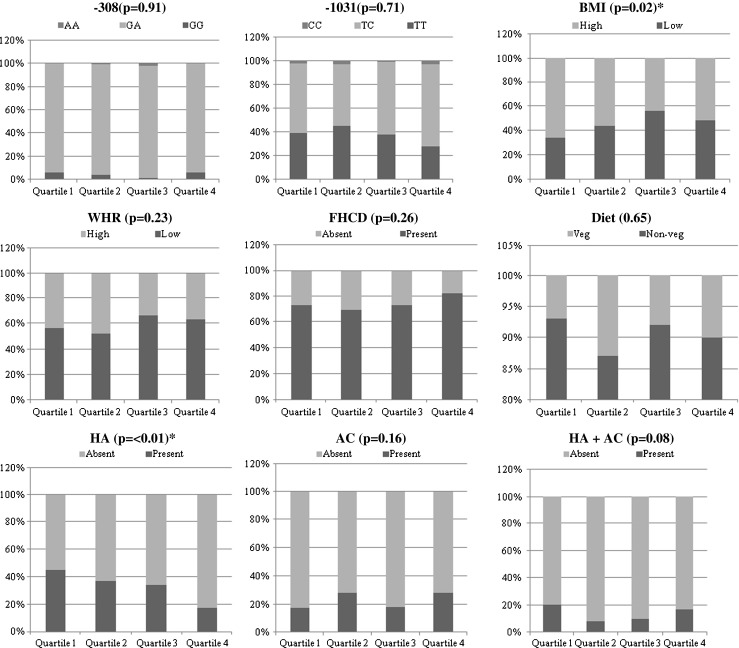
Analysis of genotypes, clinical features and epidemiological factors in quartiles with respect to AAO in patient group. Note: Each bar represents the distribution of parameters with respect to presence or absence of a specific trait. BMI—Body mass index, WHR—Waist to hip ratio, FHCD—Family history of complex diseases, HA—Hyperandrogenism, AC—Acanthosis
Discussion
TNF α is a proinflammatory cytokine present in follicular fluid of human ovary, granulosa cells and oocytes [4]; plays an important role in a wide range of diseases such as type 2 diabetes, coronary artery disease and dyslipidemia [28, 29]. It elicits inflammatory responses as a normal physiological function for ovulation and influence follicular atresia, adiposity, insulin resistance, ovarian apoptosis, increased ovarian steroid secretion, anovulation and hyperandrogenism [30]. The present study was aimed to screen for TNF-α rs1800629 and rs1799964 in PCOS, a condition associated with inflammation.
A potential physiological role of rs1800629 G > A polymorphism has been indentified in several studies [26, 30] and much recent reporter gene studies demonstrated a significant effect on its transcriptional activity [4, 31]. Variation in TNF-α gene was reported to be linked with PCOS susceptibility in Korean population. Studies on TNF-α gene in PCOS patients from Australian, Caucasian and Indian population revealed lack of association of rs1800629 polymorphism [4, 6, 13]. However, the rs1799964 T > C polymorphism suggested a strong influence on PCOS, similar to the findings of others in Asian population [4, 32]. The T allele of this polymorphism appeared to confer protection towards PCOS in our study, while it was the C allele in Korean population [4].
Specific combinations of the two promoter variants designated as “Combination 1”, “Combination 2” and “Combination 3” of TNF-α showed that combination 3 had a two fold risk for PCOS in the studied population while, combination 2 suggested a protective role towards PCOS. Since there are not many studies pertaining to these polymorphisms in relation to PCOS and no studies with respect to rs1800629 and rs1799964 haplotype, there was no scope in the current study to precisely compare the genotypes and haplotypes with clinical traits of other studies.
The increased BMI and WHR in a section of patients in our investigations, suggest the importance of obesity genes and environmental component in susceptibility to PCOS. Li S (2008) proposed that elevated TNF-α have been linked to obesity and insulin resistance in the Chinese population [12]. The mutant alleles (A and C) of rs1800629 and rs1799964 were associated with higher production of TNF-α compared to wild type (G and T) alleles. The present observation of relationship between heterozygote of rs1800629 with obesity is supported by the findings of Namita Bhagat [13]. Involvement of rs1799964 polymorphism with lower BMI was observed to be different from that of the findings of Brand et al., (2003) [33]. We reported a positive association of this polymorphism with lower WHR, whereas others have shown no association [34]. These findings of ours with respect to lower BMI and lower WHR could be explained on the basis of anti-adipogenic effects of TNF-α, that inhibits adipogenesis causing dedifferentiation of mature adipocytes thereby, reducing the expression of several adipocyte-specific genes such as PPAR γ, GLUT4 etc. [35, 36].
PCOS is a complex disorder that might result from the interaction of susceptible and protective gene variants under the influence of environmental factors [3], suggesting that the clinical phenotype of affected individuals is also influenced by gene-environment interaction. Diet was considered as the major environmental determinant of PCOS that was able to alter the rate of gene transcription and translation, which may be one mechanism that modifies disease risk [37]. It was further hypothesized that not only the quantity of food but also the quality and the type of nutrition may alter PCOS phenotype, possibly interacting with different genetic background [38]. Association of rs1799964 polymorphism with non-vegetarian diet suggests gene-environment interactions since diet is a well known environmental factor playing a role in the regulation of sex steroid metabolism. It was reported that women who were on non-vegetarian diet have increased risk of developing PCOS, which might be due to increased androgen circulating levels released due to the intake of high-lipid and low-fiber diet. Non-vegetarian diet particularly those derived from the meat of lactating animals like beef is the potential source of hormones. Uptake of such meat may lead to indirect consumption of hormones that might have used as hormonal injections for increasing the milk yield. Furthermore, studies have also demonstrated that high lipid and low-fiber diet is related to an increased androgen circulating levels leading to hyperandrogenism; a factor involved in the causation of PCOS [39].
Significant association of rs1799964 polymorphism with hyperandrogenic clinical features (p = 0.004) in the present study corresponds to the findings by Escobar-Morreale HF (2001) and Park et al., (2001) in PCOS women [26, 40].
Quartile data analysis suggested an elevated frequency of women with TT genotype, higher BMI and increased frequency of women with hyperandrogenism clinical traits in the first two quartiles than the other two. An OR value of TT vs. others (0.54, <0.01) in Table 2 indicates the protective nature of TT genotype. The presence of increased frequency of individuals with TT genotype in the early onset group led us to hypothesize that the TT genotype though being protective, were predisposed to PCOS at an early age and is due to higher BMI (p = 0.02) and hyperandrogenism (p = <0.01) in the first two quartiles than the other two, since both these conditions are often considered as factors involved in the susceptibility of this multifactorial disorder.
In conclusion, our findings suggests lack of direct involvement of rs1800629 polymorphism in the genetic predisposition to PCOS; however, rs1799964 polymorphism revealed a strong association in the manifestation of PCOS and its clinical traits. Furthermore, the rs1799964 polymorphism demonstrated the gene-environment interaction in the causation of PCOS in the studied population. Haplotype analysis showed that the T allele of rs1799964 in a single dose (combination 2) is protective over others, while the mutant allele at both loci (combination 3) confers around two fold risk of developing PCOS. To the best of our knowledge, this is the first report from India with respect to rs1799964 of TNF-α, a proinflammatory cytokine. The limitation of the present study was the inability to measure TNF-α levels for clinical correlation. Further extensive studies on the influence of TNF-α polymorphism towards PCOS in different ethnic groups may identify the potentiality of these polymorphisms as markers of inflammation and in turn may help the clinicians for the better management of the condition. Functional studies on the reporter gene assays of this gene would be required further to gain meaningful insights on the etiology of TNF-α towards PCOS phenotype.
Acknowledgments
We thank all the subjects for their active participation in the study and ICMR, India for providing financial assistance to Deepika M.L.N.
Disclosure statement
The authors declare that no competing financial interests exist.
Conflict of interest
None.
Footnotes
Capsule The TT genotype of rs1799964 confers protection in controls while the TC genotype predisposes the women to develop PCOS. The haplotypes with mutant alleles at both loci (rs1800629 and 1799964) revealed a two fold risk towards PCOS.
References
- 1.Ranjith Reddy K, Deepika MLN, Ishaq M, Jahan P. Haptoglobin: a pleiotropic marker in polycystic ovary syndrome - a study from South India. Am J Biochem Mol Biol. 2011;1:399–404. doi: 10.3923/ajbmb.2011.399.404. [DOI] [Google Scholar]
- 2.Deepika MLN, Ranjith Reddy K, Usha Rani V, Balakrishna N, Prasana Latha K, Jahan P. Do ACE I/D gene polymorphism serve as a predictive marker for age at onset in PCOS? J Assist Reprod Genet. 2013;30(1):125–30. doi: 10.1007/s10815-012-9906-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Deepika MLN, Ranjith K, Usha Rani V, Ishaq M, Jahan P. Familial background of complex diseases in PCOS probands of South Indian population. Asian J Epidemiol. 2012;5(2):50–55. [Google Scholar]
- 4.Yun J-H, Choi J-W, Lee K-J, Shin J-S, Baek K-H. The promoter −1031(T/C) polymorphism in tumor necrosis factor-alpha associated with polycystic ovary syndrome. Reprod Biol Endocrinol. 2011;9:131. doi: 10.1186/1477-7827-9-131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Prange-Kiel J, Kreutzkamm C, Wehrenberg U, Rune GM. Role of tumor necrosis factor in preovulatory follicles of swine. Biol Reprod. 2001;65:928–935. doi: 10.1095/biolreprod65.3.928. [DOI] [PubMed] [Google Scholar]
- 6.Kumar Pujhari S, Ratho RK, Prabhakar S, Mishra B, Modi M. TNF-a promoter polymorphism: a factor contributing to the different immunological and clinical phenotypes in Japanese encephalitis. BMC Infect Dis. 2012;12:23. doi: 10.1186/1471-2334-12-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ning-Bo Hao, Mu-Han Lu, Ya-Han Fan, Ya-Ling Cao, Zhi-Ren Zhang, Shi-Ming Yang. Macrophages in tumor microenvironments and the progression of tumors clinical and developmental. Immunology. 2012. doi:10.1155/2012/948098. [DOI] [PMC free article] [PubMed]
- 8.Skoog T, Van’t Hooft FM, Kallin B, Jovinge S, et al. A common functional polymorphism (C > A substitution at position-863) in the promoter region of the tumor necrosis factor-α (TNF-α) gene associated with reduced circulating levels of TNF-α. Hum Mol Genet. 1999;8(8):1143–1149. doi: 10.1093/hmg/8.8.1443. [DOI] [PubMed] [Google Scholar]
- 9.Mac ED. TNF receptor subtype signaling: differences and cellular consequences. Cell Signal. 2002;14:472–477. doi: 10.1016/s0898-6568(01)00262-5. [DOI] [PubMed] [Google Scholar]
- 10.Gonzalez-Navarrete F, Eisner V, Morales P, Castro O, Pommer R, Quiroga C, et al. Tumor necrosis factor-alpha activates nuclear factor-kappaB but does not regulate progesterone production in cultured human granulosa luteal cells. Gynecol Endocrinol. 2007;23(7):377–84. doi: 10.1080/09513590701444839. [DOI] [PubMed] [Google Scholar]
- 11.Brannstrom M, Friden BE, Jasper M, Norman R. Variations in peripheral blood levels of immunoreactive tumor necrosis factor α (TNF α) throughout the menstrual cycle and secretion of TNF α from the human corpus luteum. Eur J Obstet Gynecol Reprod Biol. 1999;83:213–217. doi: 10.1016/S0301-2115(99)00003-2. [DOI] [PubMed] [Google Scholar]
- 12.Li S, Loos RJF. Progress in the genetics of common obesity: size matters. Curr Opin Lipidol. 2008;19:113–121. doi: 10.1097/MOL.0b013e3282f6a7f3. [DOI] [PubMed] [Google Scholar]
- 13.Bhagat N, Agrawal M, Luthra K, Vikram NK, Anoop M, Rajeev G. Evaluation of single nucleotide polymorphisms of Pro12Ala in peroxisome proliferator-activated receptor-γ and Gly308Ala in tumor necrosis factor-α genes in obese Asian Indians: a population-based study. Diabetes, Metabolic Syndrome and Obesity. Targets Ther. 2010;3:349–356. doi: 10.2147/DMSOTT.S13514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Milner CR, Craig JE, Hussey ND, Norman RJ. No association between the −308 polymorphism in the tumour necrosis factor a (TNFa) promoter region and polycystic ovaries. Mol Hum Reprod. 1999;5(1):5–9. doi: 10.1093/molehr/5.1.5. [DOI] [PubMed] [Google Scholar]
- 15.Korhonen S, Romppanen EL, Hiltunen M, Mannermaa A, Punnonen K, et al. Lack of association between C-850T polymorphism of the gene encoding tumor necrosis factor-α and polycystic ovary syndrome. Gynecol Endocrinol. 2002;16:271–274. doi: 10.1080/gye.16.4.271.274. [DOI] [PubMed] [Google Scholar]
- 16.Nicaud V, Raoux S, Poirier O, Cambien F, O’Reilly DS, Tiret L. The TNF alpha/G-308A polymorphism influences insulin sensitivity in offspring of patients with coronary heart disease: the European Atherosclerosis Research Study II. Atherosclerosis. 2002;161(2):317–25. doi: 10.1016/S0021-9150(01)00648-7. [DOI] [PubMed] [Google Scholar]
- 17.Day CP, Grove J, Daly AK, Stewart MW, Avery PJ, Walker M. Tumour necrosis factor-alpha gene promoter polymorphism and decreased insulin resistance. Diabetologia. 1998;41(4):430–434. doi: 10.1007/s001250050926. [DOI] [PubMed] [Google Scholar]
- 18.Brand E, Schorr U, Kunz I, Kertmen E, Ringel J, Distler A, et al. Tumor necrosis factor-alpha–308 G/A polymorphism in obese Caucasians. Int J Obes Relat Metab Disord. 2001;25(4):581–5. doi: 10.1038/sj.ijo.0801576. [DOI] [PubMed] [Google Scholar]
- 19.Furuta M, Yano Y, Ito K, Gabazza EC, Katsuki A, Tanaka T, et al. Relationship of the tumor necrosis factor-alpha −308 A/G promoter polymorphism with insulin sensitivity and abdominal fat distribution in Japanese patients with type 2 diabetes mellitus. Diabetes Res Clin Pract. 2002;56(2):141–5. doi: 10.1016/S0168-8227(01)00358-8. [DOI] [PubMed] [Google Scholar]
- 20.Gonzalez F, Thusu K, Abdel Rahman E, et al. Elevated serum levels of tumor necrosis factor alpha in normal weight women with polycystic ovary syndrome. Metabolism. 1999;48:437–41. doi: 10.1016/S0026-0495(99)90100-2. [DOI] [PubMed] [Google Scholar]
- 21.Chen G, Wilson R, Wang SH, et al. Tumour necrosis factor-alpha (TNF-a) gene polymorphism and expression in pre-eclampsia. Clin Exp Immunol. 1996;104:154–159. doi: 10.1046/j.1365-2249.1996.d01-647.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang N, Yin D, Zhang S, Wei H, Wang S, et al. TNF-alpha rs1800629 polymorphism is not associated with HPV infection or cervical cancer in the Chinese population. PLoS ONE. 2012;7(9):e45246. doi: 10.1371/journal.pone.0045246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cuenca J, Cuchacovich M, Perez C, Ferreira L, Aguirre A, et al. The −308 polymorphism in the tumor necrosis factor (TNF) gene promoter region and ex vivo lipopolysaccharide—induced TNF expression and cytotoxic activity in Chilean patients with rheumatoid arthritis. Rheumatology. 2003;42:308–313. doi: 10.1093/rheumatology/keg092. [DOI] [PubMed] [Google Scholar]
- 24.Ovalle F, Azziz R. Insulin resistance, polycystic ovary syndrome and type 2 diabetes mellitus. Fertil Steril. 2002;77(6):1095–1105. doi: 10.1016/S0015-0282(02)03111-4. [DOI] [PubMed] [Google Scholar]
- 25.Rice VM, Limback SD, Roby KF. Differential responses of granulosa cells from small and large follicles to follicular stimulating hormone (FSH) during menstrual cycle and acyclicity: effects of tumour necrosis factor-a. Hum Reprod. 1998;13:1285–1291. doi: 10.1093/humrep/13.5.1285. [DOI] [PubMed] [Google Scholar]
- 26.Escobar-Morreale HF, Calvo RM, Sancho J, San Millán JL. TNF-alpha and hyperandrogenism: a clinical, biochemical, and molecular genetic study. J Clin Endocrinol Metab. 2001;86:3761–3767. doi: 10.1210/jc.86.8.3761. [DOI] [PubMed] [Google Scholar]
- 27.Snehalatha C, Viswanathan V, Ramachandran A. Cutoff values for normal anthropometric variables in Asian Indian adults. Diabetes Care. 2003;26(5):1380–4. [DOI] [PubMed]
- 28.Elahi MM, Asotra K, Matata BM. Tumor necrosis factor alpha – 308 gene locus promoter polymorphism: an analysis of association with health and disease. Biochim Biophys Acta. 2009;1792:163–172. doi: 10.1016/j.bbadis.2009.01.007. [DOI] [PubMed] [Google Scholar]
- 29.Saarela T, Hiltunen M, Helisalmi S. Tumor necrosis factor alpha gene haplotype is associated with preeclampsia. Mol Hum Reprod. 2005;11:437–440. doi: 10.1093/molehr/gah182. [DOI] [PubMed] [Google Scholar]
- 30.Legro RS. Polycystic ovary syndrome and cardiovascular disease: a premature association? Endocr Rev. 2003;24(3):302–312. doi: 10.1210/er.2003-0004. [DOI] [PubMed] [Google Scholar]
- 31.Espey LL. Current status of the hypothesis that mammalian ovulation is comparable to an inflammatory reaction. Biol Reprod. 1994;50:233–238. doi: 10.1095/biolreprod50.2.233. [DOI] [PubMed] [Google Scholar]
- 32.Chen Y-C, Hu F-J, Chen P, et al. Association of TNF-α gene with spontaneous deep intracerebral hemorrhage in the Taiwan population: a case control study. BMC Neurol. 2010;10:41. doi: 10.1186/1471-2377-10-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brand E, Schorr U, Kunz I, et al. Tumor necrosis factor alpha 308 G/A polymorphisms in obese Caucasians. Int J Obes Relat Metab Disord. 2001;25:581–585. doi: 10.1038/sj.ijo.0801576. [DOI] [PubMed] [Google Scholar]
- 34.Yang W, Kelly T, He J. Genetic epidemiology of obesity. Epidemiol Rev. 2007;29:49–61. doi: 10.1093/epirev/mxm004. [DOI] [PubMed] [Google Scholar]
- 35.Zhang B, Berger J, Hu E, Szalkowski D, White-Carrington S, Spiegelman BM, et al. Negative regulation of peroxisome proliferator-activated receptor-gamma gene expression contributes to the antiadipogenic effects of tumor necrosis factor-alpha. Mol Endocrinol. 1996;10(11):1457–66. doi: 10.1210/me.10.11.1457. [DOI] [PubMed] [Google Scholar]
- 36.Ruan H, Hacohen N, Golub TR, Van Parijs L, Lodish HF. Tumor necrosis factor-alpha suppresses adipocyte-specific genes and activates expression of preadipocyte genes in 3T3-L1 adipocytes: nuclear factor-kappaB activation by TNF-alpha is obligatory. Diabetes. 2002;51(5):1319–36. doi: 10.2337/diabetes.51.5.1319. [DOI] [PubMed] [Google Scholar]
- 37.Nusbaum RL, McInnes RR, Willard HF. In: Genetics in Medicine. 6. Thompson and Thompson, editor. Philadelphia: Saunders; 2004. pp. 289–309. [Google Scholar]
- 38.Uribarri J, Peppa M, Cai W. Restriction of dietary glycotoxins reduces excessive advanced glycation end products in renal failure patients. J Am Soc Nephrol. 2003;14:728–731. doi: 10.1097/01.ASN.0000051593.41395.B9. [DOI] [PubMed] [Google Scholar]
- 39.Abbott DH, Barnett DK, Bruns CM, Dumesic DA. Androgen excess fetal programming of female reproduction: a developmental aetiology for polycystic ovary syndrome? Hum Reprod Update. 2005;11:357–374. doi: 10.1093/humupd/dmi013. [DOI] [PubMed] [Google Scholar]
- 40.Park KH, Kim JY, Ahn CW, et al. Polycystic ovarian syndrome (PCOS) and insulin resistance. Int J Gynaecol Obstet. 2001;74:261–267. doi: 10.1016/S0020-7292(01)00442-8. [DOI] [PubMed] [Google Scholar]