Abstract
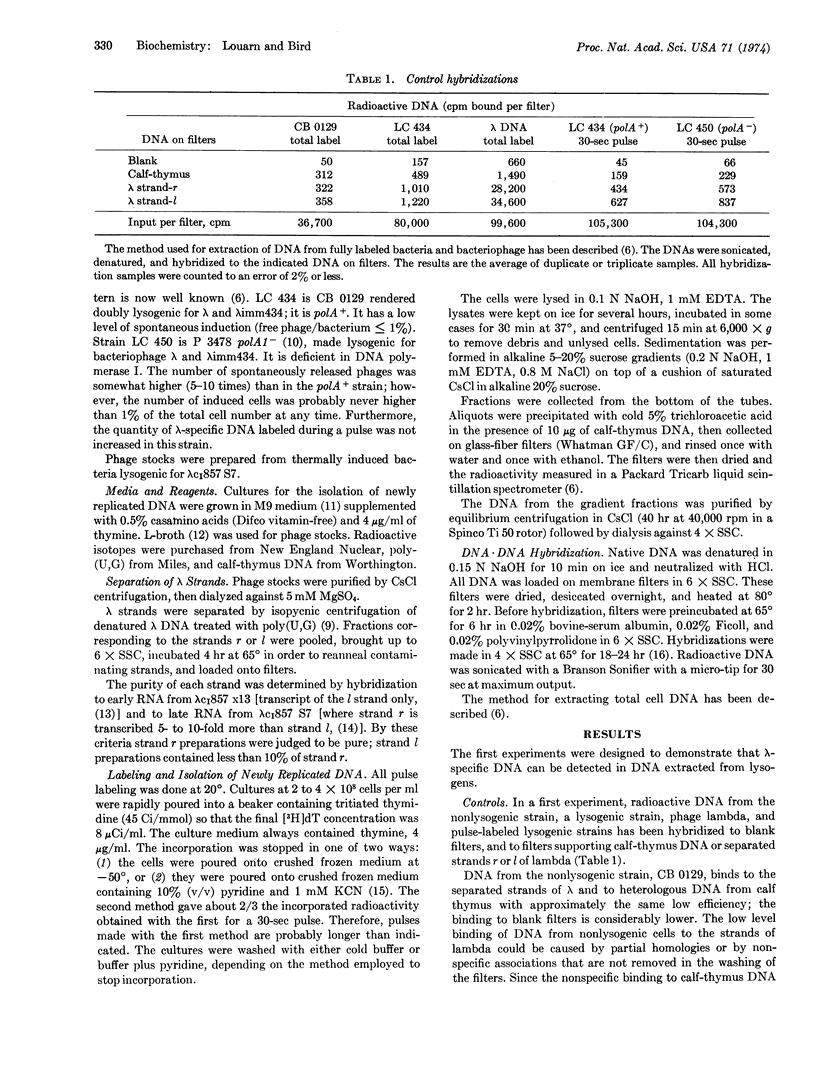
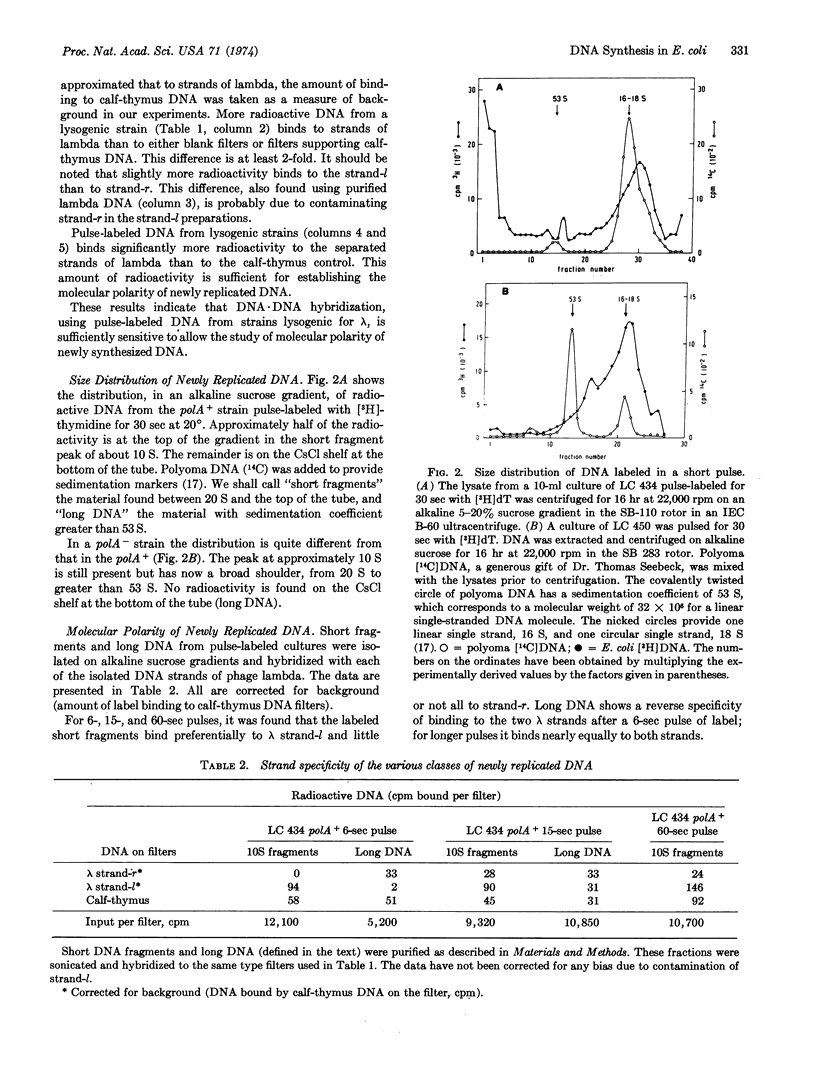
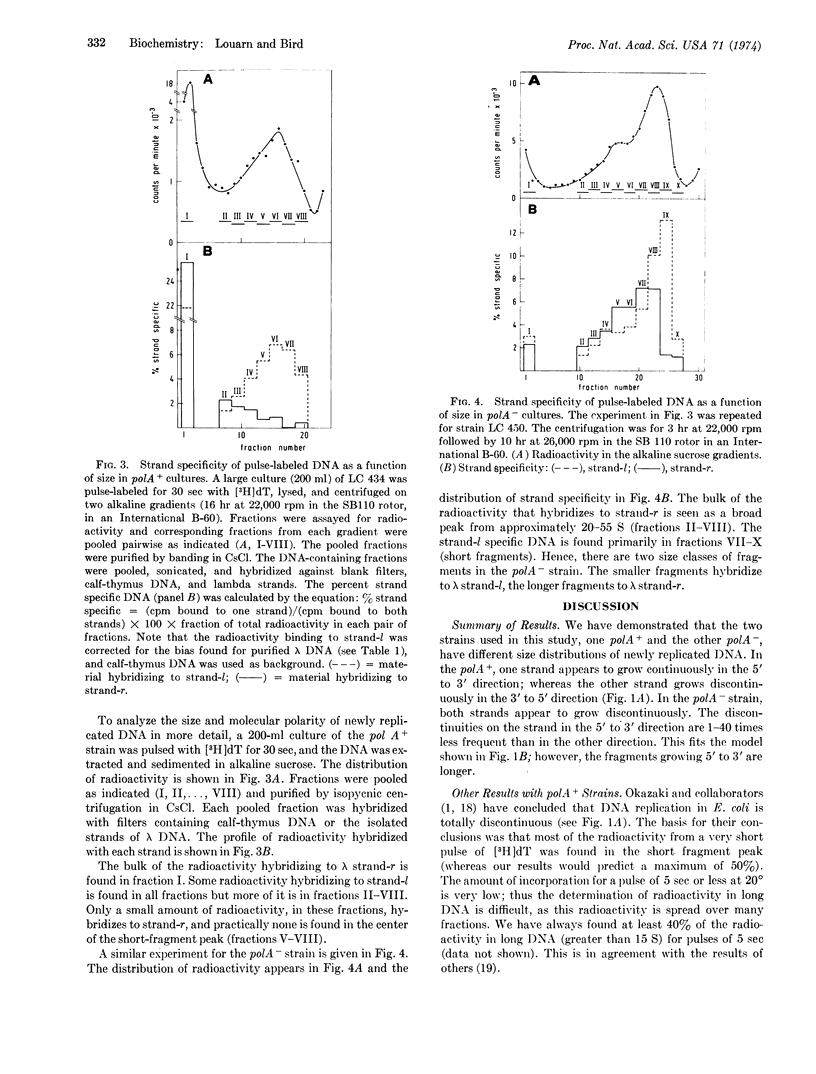
Newly synthesized DNA, in E. coli lysogenic for the phage λ, was labeled by short pulses of [3H]-thymidine, isolated, and separated on the basis of size by alkaline sucrose density gradient centrifugation. The molecular polarity of this DNA was determined by hybridization with each of the separated strands of λ DNA. The results show that, in the 3′ to 5′ direction, replication proceeds by synthesis of short chains that are subsequently joined to long DNA. This is true for both a polA+ and a polA- strain. (The polA locus codes for DNA polymerase I.) In the 5′ to 3′ direction, replication proceeds continuously, by addition of nucleotides to long DNA, for the polA+ strain. In the polA- strain, however, replication in the 5′ to 3′ direction is also discontinuous, but the discontinuities are 1-40 times less frequent than in the other direction.
Keywords: DNA biosynthesis, discontinuous replication, DNA·DNA hybridization, polA- mutant, nascent DNA
Full text
PDF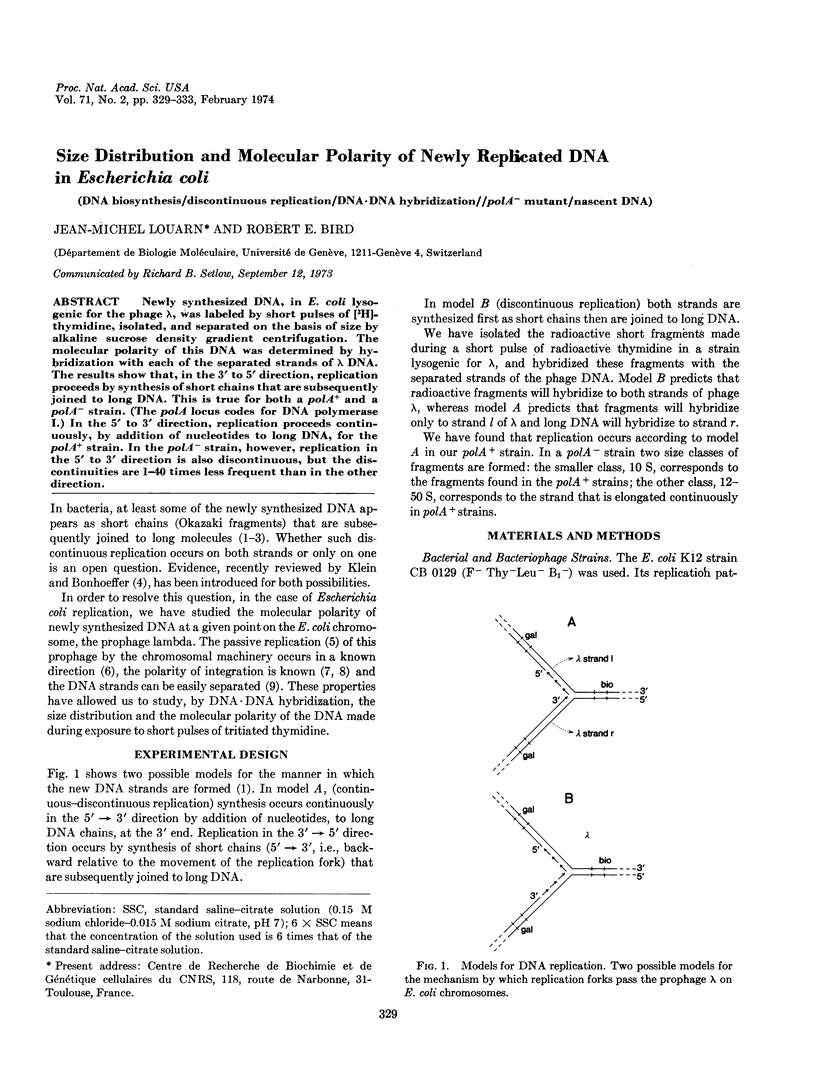

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bird R. E., Louarn J., Martuscelli J., Caro L. Origin and sequence of chromosome replication in Escherichia coli. J Mol Biol. 1972 Oct 14;70(3):549–566. doi: 10.1016/0022-2836(72)90559-1. [DOI] [PubMed] [Google Scholar]
- De Lucia P., Cairns J. Isolation of an E. coli strain with a mutation affecting DNA polymerase. Nature. 1969 Dec 20;224(5225):1164–1166. doi: 10.1038/2241164a0. [DOI] [PubMed] [Google Scholar]
- Delius H., Howe C., Kozinski A. W. Structure of the replicating DNA from bacteriophage T4. Proc Natl Acad Sci U S A. 1971 Dec;68(12):3049–3053. doi: 10.1073/pnas.68.12.3049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denhardt D. T. A membrane-filter technique for the detection of complementary DNA. Biochem Biophys Res Commun. 1966 Jun 13;23(5):641–646. doi: 10.1016/0006-291x(66)90447-5. [DOI] [PubMed] [Google Scholar]
- Eberle H. Concerning the influence of integrated episomes on chromosomal replication in Escherichia coli. Proc Natl Acad Sci U S A. 1970 Feb;65(2):467–474. doi: 10.1073/pnas.65.2.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginsberg B., Hurwitz J. Unbiased synthesis of pulse-labeled DNA framents of bacteriophage lambda and T4. J Mol Biol. 1970 Sep 14;52(2):265–280. doi: 10.1016/0022-2836(70)90030-6. [DOI] [PubMed] [Google Scholar]
- Gottesman M. M., Hicks M. L., Gellert M. Genetics and function of DNA ligase in Escherichia coli. J Mol Biol. 1973 Jul 15;77(4):531–547. doi: 10.1016/0022-2836(73)90221-0. [DOI] [PubMed] [Google Scholar]
- Herrmann R., Huf J., Bonhoeffer F. Cross hybridization and rate of chain elongation of the two classes of DNA intermediates. Nat New Biol. 1972 Dec 20;240(103):235–237. doi: 10.1038/newbio240235a0. [DOI] [PubMed] [Google Scholar]
- Iyer V. N., Lark K. G. DNA replication in Escherichia coli: location of recently incorporated thymidine within molecules of high molecular weight DNA. Proc Natl Acad Sci U S A. 1970 Oct;67(2):629–636. doi: 10.1073/pnas.67.2.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson M. K., Lark K. G. DNA replication in Escherichia coli: evidence for two classes of small deoxyribonucleotide chains. J Mol Biol. 1973 Feb 5;73(4):371–396. doi: 10.1016/0022-2836(73)90088-0. [DOI] [PubMed] [Google Scholar]
- Klein A., Bonhoeffer F. DNA replication. Annu Rev Biochem. 1972;41(10):301–332. doi: 10.1146/annurev.bi.41.070172.001505. [DOI] [PubMed] [Google Scholar]
- Kuempel P. L., Veomett G. E. A possible function of DNA polymerase in chromosome replication. Biochem Biophys Res Commun. 1970 Nov 25;41(4):973–980. doi: 10.1016/0006-291x(70)90180-4. [DOI] [PubMed] [Google Scholar]
- LENNOX E. S. Transduction of linked genetic characters of the host by bacteriophage P1. Virology. 1955 Jul;1(2):190–206. doi: 10.1016/0042-6822(55)90016-7. [DOI] [PubMed] [Google Scholar]
- Lozeron H. A., Szybalski W. Congruent transcriptional controls and heterology of base sequences in coliphages lambda and phi-80. Virology. 1969 Nov;39(3):373–388. doi: 10.1016/0042-6822(69)90085-3. [DOI] [PubMed] [Google Scholar]
- Okazaki R., Arisawa M., Sugino A. Slow joining of newly replicated DNA chains in DNA polymerase I-deficient Escherichia coli mutants. Proc Natl Acad Sci U S A. 1971 Dec;68(12):2954–2957. doi: 10.1073/pnas.68.12.2954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olivera B. M., Bonhoeffer F. Discontinuous DNA replication in vitro. I. Two distinct size classes of intermediates. Nat New Biol. 1972 Dec 20;240(103):233–235. doi: 10.1038/newbio240233a0. [DOI] [PubMed] [Google Scholar]
- Polsinelli M., Milanesi G., Ganesan A. T. Short fragments from both complementary strands in the newly replicated DNA of bacteriophage SPP-1. Science. 1969 Oct 10;166(3902):243–245. doi: 10.1126/science.166.3902.243. [DOI] [PubMed] [Google Scholar]
- Schnös M., Inman R. B. Position of branch points in replicating lambda DNA. J Mol Biol. 1970 Jul 14;51(1):61–73. doi: 10.1016/0022-2836(70)90270-6. [DOI] [PubMed] [Google Scholar]
- Schnös M., Inman R. B. Starting point and direction of replication in P2 DNA. J Mol Biol. 1971 Jan 14;55(1):31–38. doi: 10.1016/0022-2836(71)90278-6. [DOI] [PubMed] [Google Scholar]
- Sugimoto K., Okazaki T., Imae Y., Okazaki R. Mechanism of DNA chain growth. 3. Equal annealing of T4 nascent short DNA chains with the separated complementary strands of the phage DNA. Proc Natl Acad Sci U S A. 1969 Aug;63(4):1343–1350. doi: 10.1073/pnas.63.4.1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugino A., Hirose S., Okazaki R. RNA-linked nascent DNA fragments in Escherichia coli. Proc Natl Acad Sci U S A. 1972 Jul;69(7):1863–1867. doi: 10.1073/pnas.69.7.1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugino A., Okazaki R. Mechanism of DNA chain growth. Vii. Direction and rate of growth of T4 nascent short DNA chains. J Mol Biol. 1972 Feb 28;64(1):61–85. doi: 10.1016/0022-2836(72)90321-x. [DOI] [PubMed] [Google Scholar]
- WEIL R., VINOGRAD J. THE CYCLIC HELIX AND CYCLIC COIL FORMS OF POLYOMA VIRAL DNA. Proc Natl Acad Sci U S A. 1963 Oct;50:730–738. doi: 10.1073/pnas.50.4.730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu R., Kaiser A. D. Mapping the 5'-terminal nucleotides of the DNA of bacteriophage lambda and related phages. Proc Natl Acad Sci U S A. 1967 Jan;57(1):170–177. doi: 10.1073/pnas.57.1.170. [DOI] [PMC free article] [PubMed] [Google Scholar]



