Abstract
This study investigates the influence of ovarian endometrioma on expression of steroid receptor RNA activator (SRA), estrogen receptors (ERs), vascular endothelial growth factor (VEGF), and thrombospondin 1 (TSP-1) in the surrounding ovarian tissues. Taken from the women with ovarian endometrioma and mature teratoma during laparoscopy, the biopsies were analyzed by real-time polymerase chain reaction and Western blot. Our results indicated that ovarian tissues surrounding endometrioma had lower SRA and ER-α levels but higher SRA protein (SRAP) and ER-β levels than ovarian endometrioma. With lower VEGF levels and higher TSP-1 levels, the surrounding ovarian tissues showed higher expression levels of SRA, SRAP, ER-α, and ER-β in the ovarian endometrioma group when compared to the controls. These data showed that ovarian endometrioma increases SRA, ERs, and TSP-1 but decreases VEGF levels in the surrounding ovarian tissues, suggesting that abnormal expression of these molecules may affect biological behaviors of ovarian endometrioma.
Keywords: ovarian endometrioma, steroid receptor RNA activator, estrogen receptor, vascular endothelial growth factor, thrombospondin 1
Introduction
Endometriosis is estimated to occur in 10% to 15% of women of reproductive age. Laparoscopy is an effective treatment for this condition and related symptoms, and most authors agree that it should be considered to be the gold standard.1–4 Despite its proven efficacy, surgical treatment of endometriosis is not without any perils. Recurrence after surgery remains a formidable challenge; about 40% to 45% of the patients, far higher than spontaneous incidence, have relapse of the disease 5 years after the primary surgery and would engender further surgery.5,6 Therefore, the trend is to opt for medical treatment. At present, no medical agents are more efficacious than other agents such as androgens, progestogens, oral contraceptive pills, and gona-dotropin-releasing hormone agonists. It is necessary to find a new medical agent with superior efficacy over other agents.
It has been reported that angiogenesis may play an important role in the pathogenesis of endometriosis. Similar to tumor metastases, endometriotic implants require neovascularization to proliferate, invade the extracellular matrix, and establish an endometriotic lesion.7,8 Vascular endothelial growth factor (VEGF) and thrombospondin 1 (TSP-1) as an angiogenic factor and angiogenic inhibitor, respectively, may be involved in the progression of endometriosis. An increase in VEGF and a decrease in TSP-1 may contribute to the neovascularization and development of endometriotic implants and even become capable of spreading to the surrounding host tissues. However, in some published reports, the authors found that ovarian endometriomas had lower levels of VEGF and higher levels of TSP-1 compared to eutopic endometrium or peritoneal lesions.9–11 If so, how does this condition initiate and develop with low capabilities of angiogenesis and metastasis?
The steroid receptor RNA activator (SRA) was first identified by Lanz et al in 1999 as a noncoding transcript able to increase the activity of steroid receptors.12 It is overexpressed in breast, uterine, and ovarian tumors13,14 and affects the growth of certain hormone-dependent breast and prostate cancer cell lines.15,16 Interestingly, the SRA precursor is subjected to an alternative splicing which generates an messenger RNA (mRNA) that codes for SRA protein (SRAP).17 In contrast with SRA, overexpression of SRAP represses estrogen receptor activity. The proportions of SRA and SRAP vary within breast cancer cell lines and might contribute to their phenotypes. However, some reports support the contrary view that SRAP may play an important role in enhancing steroid receptor activity in prostate cancer18,19 or breast cancer.20 Moreover, increased evidence suggests that target gene promoter, tissue, and cell line contexts can lead to functional inversion among coregulators.21 As such, coactivators can become corepressors and vice versa.
Ovarian endometrioma is an estrogen-dependent disease with the capabilities of metastasis and recurrence after resection. Overexpression of ER-β, favoring the suppression of ER-α level, is associated with accumulation of estradiol in endometriotic lesions.22 Estrogen is a potent stimulus of angiogenesis through a direct increase in VEGF expression.23,24 Furthermore, there are many pathways leading to the local accumulation of estrogen in endometriosis,25 many of which are also associated with angiogenesis. Conceivably, SRA/SRAP may regulate the expression of estrogen receptors, further affecting the development of ovarian endometrioma. So far, little is reported on the expression of noncoding and coding SRA in endometriosis. To our knowledge, the present study investigated for the first time the association of 2 molecules with this condition. This study aims to investigate the influence of ovarian endometrioma on expressions of SRA, ERs, VEGF, and TSP-1 in the surrounding ovarian tissues and their roles in the pathogenesis of ovarian endometrioma.
Materials and Methods
Endometrial Sample Collection
A group of women (n = 20) who underwent laparoscopic excision of ovarian endometrioma was included in this study. Their age ranged between 22 and 38 years, and the diameter of their ovarian cysts ranged between 30 and 82 mm by ultrasound; patients included were classified as stage III or IV endometriosis according to the American Society for Reproductive Medicine classification of endometriosis26; 85% (17 of 20) of the patients had chronic pelvic pain and 35% (7 of 20) had infertility. A group of women (n = 20) aged 23 to 37 years, with regular menstrual cycle, undergoing laparoscopic excision of mature teratoma, served as the controls. Mature ovarian teratomas are benign ovarian tumors, with absence of invasiveness and metastases, which usually present with a normal karyotype. There are very few reports describing chromosomal abnormalities in these tumors, none of which are recurrent.27 The study was approved by the review board of the Women's Hospital of Zhejiang University School of Medicine, and informed consent was obtained from all the patients before inclusion. A complete medical history was obtained, and physical examination was performed for each patient. Patients with endocrine disorders or with hormonal treatment in the last 3 months were excluded from the study. All specimens were collected during the proliferative phase of the menstrual cycle. The samples of endometriotic tissues and ovarian tissues surrounding endometrioma or mature teratoma, each 3 mm in diameter, were obtained by laparoscopy. The samples were immediately frozen in liquid nitrogen to analyze the expression at both the RNA and protein levels. These samples were confirmed histologically as endometriotic tissues by the histological criteria of Noyes et al28 or normal ovarian tissues.
Real-Time Polymerase Chain Reaction
Total RNA was isolated from ovarian endometriotic or ovarian tissues using Trizol reagent (Gibco, Carlsbad, California) according to the manufacturer’s instructions. After treatment for 20 minutes at 37°C with 1 unit of DNase I (Gibco, Carlsbad, California) to prevent genomic DNA contamination, 500 ng of total RNA was reverse transcribed using 1 µg of oligo (dT)18, 1 µL deoxyribonucleotide triphosphates (10 mmol/L), and 200 units of Superscript II RTase (Invitrogen) at 42°C for 1 hour in the appropriate buffer. The reaction was stopped by incubation at 70°C for 5 minutes. Then, quantitative real-time polymerase chain reaction (RT-PCR) analysis was performed using the iQ5 apparatus (Bio-Rad, Hercules, California). SYBR Premix Ex Taq (Perfect Real Time; Takara Bio Inc, Otsu, Japan) was used for real-time monitoring of amplification (1.0 µL of template complementary DNA, 45 cycles: 95°C/10 s, 62°C/25 s) with the following primers: SRA forward sequence 5′-TACCAGGCTTCCAGCAGGCTTCAT-3′ and SRA reverse sequence 5′-CCAAGTGACAGAAGGTCTCCAAGG-3′ as sense and antisense primers, respectively. Accurate amplification of the target amplicon was checked by performing a melting curve. Cycle time (Ct) was chosen to determine the amount of gene products in each sample. The average Ct value was calculated from triplicate wells for each sample. Duplicate samples varied by <0.5 Ct. Using human 18S ribosomal RNA (rRNA) sense (5′-GACTCAACACGGGAAACCTCAC-3′) and antisense (5′-CCAGACAAATCGCTCCACCAAC-3′) primers, a parallel amplification of human 18S rRNA transcript (assay identification No. NR_003286) was carried out to normalize the expression data of the SRA transcript (assay identification No. NM_001035235.3). The relative level of SRA expression is calculated using the following formula29: n = 2−ΔΔCt= 2−( ΔCt (patient)− ΔCt (min) (control)).
Western Blot Analysis
Total proteins were extracted from ovarian endometriotic or ovarian tissues and analyzed by Western blot as described previously.30,31 Four primary antibodies, a mouse monoclonal anti-SRA1 antibody (ab66378; Abcam), a rabbit anti-ER-α antibody (sc-102023; Santa Cruz, Dallas, Texas), a goat anti-ER-β antibody (sc-247989; Santa Cruz), a rabbit anti-VEGF antibody (ab46154; Abcam, Cambridge, Massachusetts), a goat anti-TSP-1 antibody (sc-12312; Santa Cruz), and a mouse anti-β-actin antibody (sc-69879; Santa Cruz), were used at dilutions of 1:500, 1:400, 1:500, 1:800, 1:600, and 1:1,000, respectively. Preincubation of the primary antibodies with its corresponding peptide, served as the controls, was performed as described previously.30 We detected immune complexes by SuperSignal West Dura Extended Duration Substrate detection kit (Pierce, Rockford, Illinois). Relative protein levels were quantified on band volume with respect to β-actin expression as assessed by Bandscan 5.0 software (Glyko Inc, Novato, California).
Statistical Analysis
All the data were assessed for normality of distribution using Shapiro-Wilk test. Continuous data are expressed as mean ± standard deviation, and categorical data are expressed as percentage. Continuous and categorical variables were compared using the student t test and Fisher exact test, respectively. P < .05 was considered statistically significant. The relationship between the 2 variables was assessed by Pearson correlation analysis. The data were analyzed using Statistical Package for the Social Sciences (SPSS) 16.0 for windows (SPSS Inc, Chicago, Illinois).
Results
Noncoding SRA Expression in Ovarian Tissues Surrounding Endometrioma Versus Mature Teratoma Detected by RT-PCR
There were no statistical differences in ages, parity, and gravidity between ovarian endometrioma and mature teratoma groups. The size of ovarian mass revealed no statistical differences (Table 1). The RNA expression of noncoding SRA was detected in the endometriotic and ovarian tissues by RT-PCR. Its expression in the endometriotic tissue was statistically higher than that in the ovarian tissue surrounding the endometrioma or mature teratoma (P < .05); meanwhile, its level in ovarian tissue surrounding the endometrioma was higher than that of mature teratoma (P < .05; Table 2; Figure 1).
Table 1.
Baseline Characteristics of Women With Ovarian Endometrioma Versus Control.a
| Parameters | Ovarian Endometrioma | Control | P Value |
|---|---|---|---|
| (n = 20) | (n = 20) | ||
| Age, years | 31.8 ± 5.3 | 31.4 ± 5.2 | .866 |
| Gravidity | 0.9 ± 1.0 | 1.7 ± 1.3 | .131 |
| Parity | 0.6 ± 0.5 | 0.8 ± 0.6 | .449 |
| Diameter of ovarian mass, cm | 5.2 ± 1.5 | 5.4 ± 1.6 | .778 |
| IUD, % | 15 (3/20) | 25 (5/20) | .695 |
| Smoking, % | 5 (1/20) | 10 (2/20) | 1.000 |
| Regular menstrual cycle, % | 100 (20/20) | 100 (20/20) | NS |
Abbreviations: IUD, intrauterus device; NS, not significant.
a Continuous and categorical variables were compared using the student t test and Fisher exact test, respectively.
Table 2.
The Expression Levels of Noncoding SRA in Ovarian Endometrioma and Surrounding Ovarian Tissue.a
| No. of Samples | Noncoding SRA | ||
|---|---|---|---|
| −ΔΔCt | Expression Level (2−ΔΔCt) | ||
| Ovarian endometrioma | 20 | −12.62 ± 0.15 | 2.67 ± 0.23 |
| Surrounding ovarian tissue | 20 | −13.15 ± 0.12 | 1.83 ± 0.11 |
| Control | 20 | −13.80 ± 0.15 | 1.20 ± 0.15 |
Abbreviation: steroid receptor RNA activator.
a Comparison of the relative expression level of noncoding SRA between the groups was evaluated using Student t test. Ovarian endometrioma versussurrounding ovarian tissue, P < .05; surrounding ovarian tissue versus control, P < .05.
Figure 1.
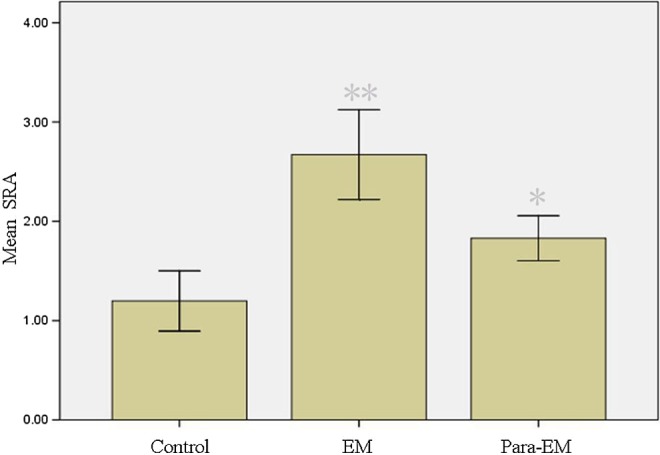
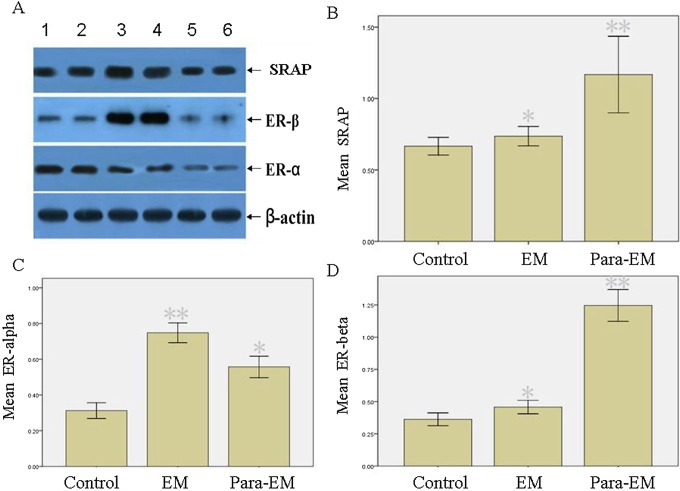
Real-time PCR analysis of SRA in different tissues. The relative level of expression is calculated by 2−ΔΔCt. Mean values indicated SRA expressions. Columns indicate data from separate experiments; bars indicate SD. *P < .05 versus control; **P < .05 versus control and versus *, respectively (Student t test); control, ovarian tissue surrounding mature teratoma; EM, ovarian endometrioma; Para-EM, ovarian tissue surrounding ovarian endometrioma; PCR, polymerase chain reaction; SD, standard deviation; SRA, steroid receptor RNA activator.
Quantification of the Proteins for SRAP, ER-α, ER-β, VEGF, and TSP-1 in Ovarian Tissues Surrounding Endometrioma Versus Mature Teratoma by Western Blot Analysis
The differences in levels of SRAP, ER-α, ER-β, VEGF, and TSP-1 in endometriotic and ovarian tissues were studied by Western blot analysis. The data showed ovarian endometrioma had higher ER-α levels (P < .05) than the surrounding ovarian tissues or ovarian tissues surrounding mature teratoma (Figure 2). Although ovarian endometrioma had higher VEGF levels and lower TSP-1 levels than surrounding ovarian tissues (P < .05, respectively; Figures 3 and 4), the former was lower in VEGF and higher in TSP-1 than ovarian tissues surrounding mature teratoma. Interestingly, ovarian tissues surrounding endometrioma, despite at a lower level of VEGF and higher level of TSP-1 (P<0.05, respectively), had higher levels of SRAP, ER-α, and ER-β than the controls (ovarian tissues surrounding mature teratoma; P < .05). In addition, higher SRAP and ER-β levels were detected in ovarian tissues surrounding endometrioma than in endometrioma (P < .05, respectively).
Figure 2.
Western blot analysis of SRAP, ER-α, and ER-β in different tissues. A, Lanes 1 and 2, ovarian endometrioma; lanes 3 and 4, surrounding ovarian tissues; lanes 5 and 6, ovarian tissues surrounding mature teratoma served as the controls. β-Actin expression is shown as the loading control. Normalized values indicated the ratios of 1 protein and β-actin protein expression. Mean values indicated SRAP (B), ER-α (C), and ER-β (D) expressions. Columns indicate data from separate experiments; bars indicate SD. *P < .05 versus control; **P < .05 versuscontrol and versus *, respectively (Student t test); control, ovarian tissue surrounding mature teratoma; EM, ovarian endometrioma; Para-EM, ovarian tissue surrounding ovarian endometrioma; ER, estrogen receptors; SRAP, steroid receptor RNA activator protein.
Figure 3.
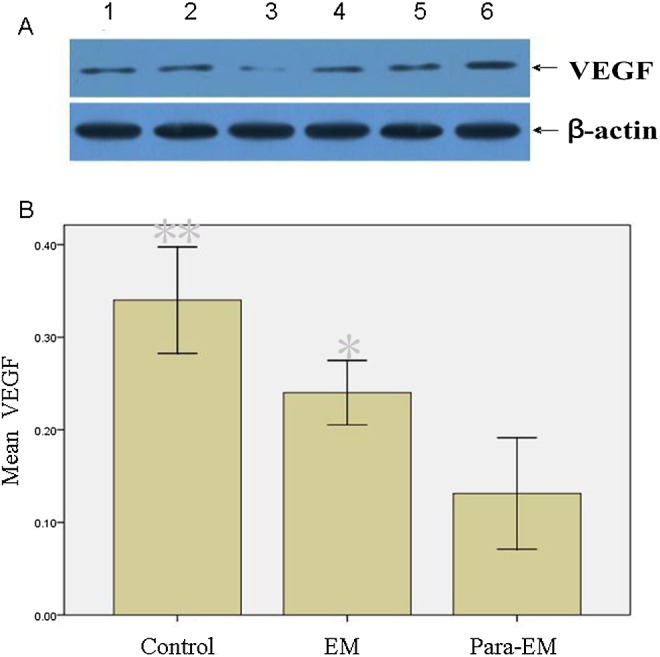
Western blot analysis of VEGF in different tissues. A, Lanes 1 and 2, ovarian endometrioma; lanes 3 and 4, surrounding ovarian tissues; lanes 5 and 6 ovarian tissues surrounding mature teratoma served as the controls. β-Actin expression is shown as the loading control. Normalized values indicated the ratios of 1 protein and β-actin protein expression. B, Mean values indicated VEGF expression. Columns indicate data from separate experiments; bars indicate SD. *P < .05 versus control; ** P < .05 versus control and versus *, respectively (Student t test); control, ovarian tissue surrounding mature teratoma; EM, ovarian endometrioma; Para-EM, ovarian tissue surrounding ovarian endometrioma; SD, standard deviation; VEGF, vascular endothelial growth factor.
Figure 4.
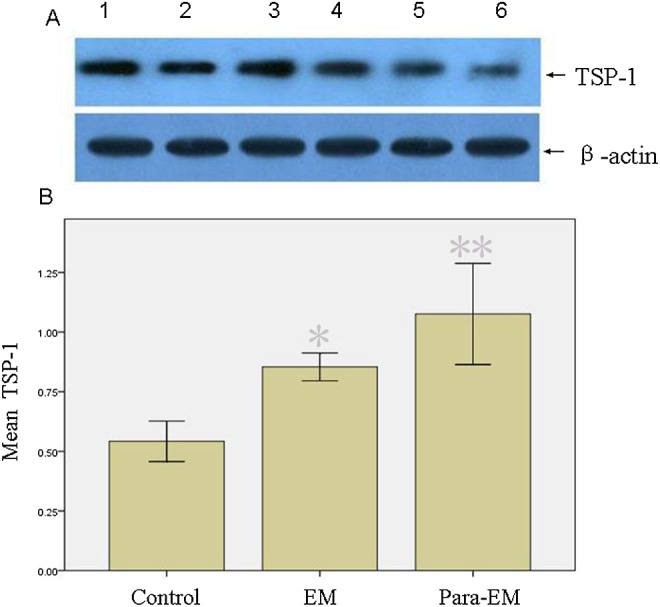
Western blot analysis of TSP-1 in different tissues. A, Lanes 1 and 2, ovarian endometrioma; (lanes 3 and 4) surrounding ovarian tissues; lanes 5 and 6, ovarian tissues surrounding mature teratoma served as the controls. β-Actin expression is shown as the loading control. Normalized values indicated the ratios of 1 protein and β-actin protein expression. B, Mean values indicated TSP-1 expression. Columns indicate data from separate experiments; bars indicate SD. *P < .05 versus control; ** P < .05 versus control and versus *, respectively (Student t test); control, ovarian tissue surrounding mature teratoma; EM, ovarian endometrioma; Para-EM, ovarian tissue surrounding ovarian endometrioma; SD, standard deviation; TSP-1, thrombospondin 1.
The Relationship Between SRA or SRAP and Estrogen Receptor was Analyzed by Pearson Correlations
Pearson correlation analysis shows the correlations between SRA and ER-α (r = .939, P = .000), between SRA and ER-β (r = .009, P = .971), between SRAP and ER-α (r = .191, P = .448), and between SRAP and ER-β (r = .928, P = .000). The result suggests that there is a relationship between these 2 types of genes in endometriotic tissues and ovarian tissues, especially SRA and ER-α and SRAP and ER-β with a tight coordinate expression.
Discussion
Endometriosis is a common, multifactorial disease in which angiogenesis may be involved in the growth of endometrium outside the uterus. Takehara et al32 have reported an increase in VEGF mRNA expression in endometriotic tissue (early stages) compared with the eutopic endometrium. However, in some previous reports, the authors found that ovarian endometriomas had lower VEGF levels and higher TSP-1 levels compared to eutopic endometrium or peritoneal lesions9–11 and thought that 3 clinically distinct lesions of endometriosis, including peritoneal lesion, ovarian endometrioma, and deep infiltrating endometriosis, may be variants of the same pathologic process, or they may be caused by different mechanisms.22,33 In the present study, we did not detect any increase in VEGF levels or any decrease in TSP-1 in ovarian endometrioma and even found a lower VEGF level and a higher TSP-1 level in comparison with ovarian tissues surrounding the mature teratoma that is a benign tumor without the capabilities of invasiveness and metastasis. Although ovarian endometrioma seems to be a progressive disease, this condition, with a reduction in the angiogenic activity, is a lesion with low capability of remodeling the surrounding tissue.
However, how does ovarian endometrioma initiate and develop? What results in multifocal foci? In the present study, ovarian endometrioma affects modulation of estrogen receptors in the surrounding ovarian tissues. The data showed that higher SRA and SRAP levels were detected in ovarian tissues surrounding endometrioma compared to the controls, associated with ER-α and ER-β levels, respectively. In the previous studies, overexpression of SRA and SRAP has been shown in some types of tumor cells, and compelling evidence suggests that these 2 molecules play critical roles in the development of tumor.13–15 Our results were in agreement with the previous studies that held that overexpression of SRA12–16 or SRAP18–20,34 enhances transcription transactivation of steroid receptors. Therefore, when reflux menstruation goes into the peritoneal cavity and its eutopic endometrium attaches to mesothelial cells, endothelial progenitor cells may be home to the lesions via ER-α and form blood vessels,35,36 enhancing the survival of the implants. Meanwhile, relatively high ER-β levels in the ovarian tissues may exhibit estrogen-like effects37 and enhancing cell survival.
Furthermore, previous studies showed that adult human endometrium contains rare epithelial progenitors and mesenchymal stem cells, likely responsible for its immense regenerative capacity, which may also have critical roles in the development of endometriosis.38,39 Clonal outgrowth is thought to be a fundamental feature of epithelial progenitor or stem cell. The clonality of a cellular expansion is taken to be a valuable tool capable of distinguishing the origin of tissues. In some previous reports on endometriosis,40–47 the investigators drew the following conclusions: (1) the majority of studies supported the monoclonal origin of endometriosis lesions; (2) in case of multifocal foci, different foci had independent origins; (3) although monoclonality was observed in samples for individual glands, different glands within the same lesion were from independent clones. If so, we speculate that the high SRA, SRAP, ER-α, and ER-β levels in ovarian tissues may benefit the successful implants of epithelial progenitors and mesenchymal stem cells of endometrium, and the multifocal endometriotic sites may be from different implants; thus, ovarian endometrioma shows a “metastasis” characteristic in clinic, although with low invasion capability.
The previous study showed that recurrence occurs in about half of the patients with endometriosis and needs further operation, with about 27% of them requiring 3 or more surgeries.48 The fact suggests that conservative surgical treatment does remove endometriotic tissues but not change predisposing factors and basal molecular defects for endometriosis. The present study supported this view that, after surgical removal of endometriotic tissue, the surrounding ovarian tissues with molecular defects, including upregulation of SRA and ERs, may demonstrate the predisposition for endometriosis, especially when abnormal eutopic endometrium49,50 which is similar to that of preoperation goes into the peritoneal cavity. The study of Exacoustos et al51 supported the predisposition for endometriosis that the majority (88.7%) of recurrent cases have recurrence involving the treated ovary.
In conclusion, ovarian endometrioma increases SRA, ERs, and TSP-1 but decreases VEGF levels in the surrounding ovarian tissues, which may affect biological behaviors of ovarian endometrioma. Therefore, our future work should investigate the SRA as a potential therapeutic target. However, endometriosis is a complex disease, and ovarian endometrioma is the only facet of the condition. Based on the characteristics of this condition and our collections of samples, it is plausible to postulate that the endometrioma shows just a small region of intense cellular activity; it is also plausible to postulate that most of the endometrioma carries a mass effect on adjacent ovarian tissue. Furthermore, it is common to observe a very flattened epithelial lining, and sometimes it is not easy to identify. These possibilities may affect the results of our study. These preliminary findings are based on a small number of patients, and further investigation is needed to determine the mechanism of SRA in this condition.
Acknowledgments
The authors thank Mr Jun Liu for his technical assistance.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was approved by the review board of the Women's Hospital of Zhejiang University School of Medicine, and informed consent was obtained from all patients before inclusion. Institutional review board (IRB) approval was obtained and the number is 20120037.
References
- 1. Daniell JF, Kurtz BR, Gurley LD. Laser laparoscopic management of large endometriomas. Fertil Steril. 1991;55(4):692–695. [PubMed] [Google Scholar]
- 2. Donnez J, Nisolle M, Gillet N, Smets M, Bassil S, Casanas-Roux F. Large ovarian endometriomas. Hum Reprod. 1996;11(3):641–646. [DOI] [PubMed] [Google Scholar]
- 3. Sutton CJ, Ewen SP, Jacobs SA, Whitelaw NL. Laser laparoscopic surgery in the treatment of ovarian endometriomas. J Am Assoc Gynecol Laparosc. 1997;4(3):319–323. [DOI] [PubMed] [Google Scholar]
- 4. Muzii L, Bellati F, Bianchi A, et al. Laparoscopic stripping of endometriomas: a randomized trial on different surgical techniques. Part II: Pathological results. Hum Reprod. 2005;20(7):1987–1992. [DOI] [PubMed] [Google Scholar]
- 5. Evers JL, Dunselman GA, Land JA, Bouckaert PX. Management of recurrent endometriosis. In: Couinho E, Spinola P, DeMoura LH, eds. Progress in the Management of Endometriosis. London, UK: Partheon, 1995:291–297. [Google Scholar]
- 6. Garry R. The effectiveness of laparoscopic excision of endometriosis. Curr Opin Obstet Gynecol. 2004;16(4):299–303. [DOI] [PubMed] [Google Scholar]
- 7. Laschke MW, Elitzsch A, Vollmar B, Vajkoczy P, Menger MD. Combined inhibition of vascular endothelial growth factor (VEGF), fibroblast growth factor and platelet-derived growth factor, but not inhibition of VEGF alone, effectively suppresses angiogenesis and vessel maturation in endometriotic lesions. Hum Reprod. 2006;21(1):262–268. [DOI] [PubMed] [Google Scholar]
- 8. Laschke MW, Menger MD. In vitro and in vivo approaches to study angiogenesis in the pathophysiology and therapy of endometriosis. Hum Reprod Update. 2007;13(4):331–342. [DOI] [PubMed] [Google Scholar]
- 9. Tan XJ, Lang JH, Liu DY, Shen K, Leng JH, Zhu L. Expression of vascular endothelial growth factor and thrombospondin-1 mRNA in patients with endometriosis. Fertil Steril. 2002;78(1):148–153. [DOI] [PubMed] [Google Scholar]
- 10. Gilabert-Estellés J, Ramón LA, España F, et al. Expression of angiogenic factors in endometriosis: its relation to fibrinolytic and metalloproteinase (MMP) systems. Hum Reprod. 2007;22(8):2120–2127. [DOI] [PubMed] [Google Scholar]
- 11. Ramón LA, Braza-Boïls A, Gilabert-Estellés J, et al. microRNAs expression in endometriosis and their relation to angiogenic factors. Hum Reprod. 2011;26(5):1082–1090. [DOI] [PubMed] [Google Scholar]
- 12. Lanz RB, McKenna NJ, Onate SA, et al. A steroid receptor coactivator, SRA, functions as an RNA and is present in an SRC-1 complex. Cell. 1999;97(1):17–27. [DOI] [PubMed] [Google Scholar]
- 13. Leygue E, Dotzlaw H, Watson PH, Murphy LC. Expression of the steroid receptor RNA activator in human breast tumors. Cancer Res. 1999;59(17):4190–4193. [PubMed] [Google Scholar]
- 14. Lanz RB, Chua SS, Barron N, Söder BM, DeMayo F, O’Malley BW. Steroid receptor RNA activator stimulates proliferation as well as apoptosis in vivo. Mol Cell Biol. 2003;23(10):7163–7176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cooper C, Guo J, Yan Y, et al. Increasing the relative expression of endogenous non-coding Steroid Receptor RNA Activator (SRA) in human breast cancer cells using modified oligonucleotides. Nucleic Acids Res. 2009;37(13):4518–4531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Agoulnik IU, Weigel NL. Coactivator selective regulation of androgen receptor activity. Steroids. 2009;74(8):669–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hube F, Guo J, Chooniedass-Kothari S, et al. Alternative splicing of the first intron of the steroid receptor RNA activator (SRA) participates in the generation of coding and noncoding RNA isoforms in breast cancer cell lines. DNA Cell Biol. 2006;25(7):418–428. [DOI] [PubMed] [Google Scholar]
- 18. Kawashima H, Takano H, Sugita S, Takahara Y, Sugimura K, Nakatani T. A novel steroid receptor co-activator protein (SRAP) as an alternative form of steroid receptor RNA-activator gene: expression in prostate cancer cells and enhancement of androgen receptor activity. Biochem J. 2003;369(pt 1):163–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kurisu T, Tanaka T, Ishii J, et al. Expression and function of human steroid receptor RNA activator in prostate cancer cells: role of endogenous hSRA protein in androgen receptor-mediated transcription. Prostate Cancer Prostatic Dis. 2006;9(2):173–178. [DOI] [PubMed] [Google Scholar]
- 20. Chooniedass-Kothari S, Vincett D, Yan Y, et al. The protein encoded by the functional steroid receptor RNA activator is a new modulator of ER alpha transcriptional activity. FEBS Lett. 2010;584(6):1174–1180. [DOI] [PubMed] [Google Scholar]
- 21. O’Malley BW, McKenna NJ. Coactivators and corepressors: what’s in a name? Mol Endocrinol. 2008;22(10):2213–2214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bulun SE. Endometriosis. N Engl J Med. 2009;360(3):268–279. [DOI] [PubMed] [Google Scholar]
- 23. Hyder SM, Nawaz Z, Chiappetta C, Stancel GM. Identification of functional estrogen response elements in the gene coding for the potent angiogenic factor vascular endothelial growth factor. Cancer Res. 2000;60(12):3183–3190. [PubMed] [Google Scholar]
- 24. Mueller MD, Vigne JL, Minchenko A, Lebovic DI, Leitman DC, Taylor RN. Regulation of vascular endothelial growth factor (VEGF) gene transcription by estrogen receptors alpha and beta. Proc Natl Acad Sci U S A. 2000;97(20):10972–10977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Attar E, Bulun SE. Aromatase and other steroidogenic genes in endometriosis: translational aspects. Hum Reprod Updat. 2006;12(1):49–56. [DOI] [PubMed] [Google Scholar]
- 26. American Society for Reproductive Medicine. Revised American Society for Reproductive Medicine classification of endometriosis 1996. Fertil Steril. 1997;67(5):817–821. [DOI] [PubMed] [Google Scholar]
- 27. Schmid-Braz AT, Cavalli LR, Cornélio DA, et al. Comprehensive cytogenetic evaluation of a mature ovarian teratoma case. Cancer Genet Cytogenet. 2002;132(2):165–168. [DOI] [PubMed] [Google Scholar]
- 28. Noyes RW, Hertig AT, Rock J. Dating the endometrial biopsy. Fertil Steril. 1950;1:3–5. [DOI] [PubMed] [Google Scholar]
- 29. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2 (-Delta Delta C (T)) method. Methods. 2001;25(4):402–408. [DOI] [PubMed] [Google Scholar]
- 30. Emberley E, Huang GJ, Hamedani MK, et al. Identification of new human coding steroid receptor RNA activator isoforms. Biochem Biophys Res Commun. 2003;301(2):509–15. [DOI] [PubMed] [Google Scholar]
- 31. Chooniedass-Kothari S, Emberley E, Hamedani MK, et al. The steroid receptor RNA activator is the first functional RNA encoding a protein. FEBS Lett. 2004;566(1-3):43–47. [DOI] [PubMed] [Google Scholar]
- 32. Takehara M, Ueda M, Yamashita Y, Terai Y, Hung YC, Ueki M. Vascular endothelial growth factor A and C gene expression in endometriosis. Hum Pathol. 2004;35(11):1369–1375. [DOI] [PubMed] [Google Scholar]
- 33. Nisolle M, Donnez J. Peritoneal endometriosis, ovarian endometriosis and adenomyotic nodules of the rectovaginal septum are three different entities. Fert Steril. 1997;68(4):585–596. [DOI] [PubMed] [Google Scholar]
- 34. Borth N, Massier J, Franke C, Sachse K, Saluz HP, Hänel F. Chlamydial protease CT441 interacts with SRAP1 co-activator of estrogen receptor alpha and partially alleviates its co-activation activity. J Steroid Biochem Mol Biol. 2010;119(1-2):89–95. [DOI] [PubMed] [Google Scholar]
- 35. Masuda H, Kalka C, Takahashi T, et al. Estrogen-mediated endothelial progenitor cell biology and kinetics for physiological postnatal vasculogenesis. Circ Res. 2007;101(6):598–606. [DOI] [PubMed] [Google Scholar]
- 36. Masuda H, Matsuzaki Y, Hiratsu E, et al. Stem cell-like properties of the endometrial side population: implication in endometrial regeneration. PLoS One. 2010;5(4):e10387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Hegele-Hartung C, Siebel P, Peters O, et al. Impact of isotype-selective estrogen receptor agonists on ovarian function. Proc Natl Acad Sci U S A. 2004;101(14):5129–5134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Gargett CE, Schwab KE, Zillwood RM, Nguyen HPT, Wu D. Isolation and culture of epithelial progenitors and mesenchymal stem cells from human endometrium. Biol Reprod. 2009;80(6):1136–1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Chan RWS, Ng EHY, Yeung WSB. Identification of cells with colony-forming activity, self-renewal capacity, and multipotency in ovarian endometriosis. Am J Pathol. 2011;178(6):2832–2844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Nilbert M, Pejovic T, Mandahl N, Iosif S, Willen H, Mitelman F. Monoclonal origin of endometriotic cysts. Int J Gynecol Cancer. 1995;5(1):61–63. [DOI] [PubMed] [Google Scholar]
- 41. Nabeshima H, Murakami T, Yoshinaga K, Sato K, Terada Y, Okamura K. Analysis of the clonality of ectopic glands in peritoneal endometriosis using laser microdissection. Fertil Steril. 2003;80(5):1144–1150. [DOI] [PubMed] [Google Scholar]
- 42. Jiang X, Hitchcock A, Bryan EJ, et al. Microsatellite analysis of endometriosis reveals loss of heterozygosity at candidate ovarian tumor suppressor gene loci. Cancer Res. 1996;56(15):3534–3539. [PubMed] [Google Scholar]
- 43. Jimbo H, Hitomi Y, Yoshikawa H, et al. Evidence for monoclonal expansion of epithelial cells in ovarian endometrial cysts. Am J Pathol. 1997;150(4):1173–1178. [PMC free article] [PubMed] [Google Scholar]
- 44. Jimbo H, Hitomi Y, Yoshikawa H, et al. Clonality analysis of bilateral ovarian endometrial cysts. Fertil Steril. 1999;72(6):1142–1143. [DOI] [PubMed] [Google Scholar]
- 45. Mayr D, Amann G, Siefert C, Diebold J, Anderegg B. Does endometriosis really have premalignant potential? A clonal analysis of laser-microdissected tissue. FASEB J. 2003;17(6):693–695. [DOI] [PubMed] [Google Scholar]
- 46. Tamura M, Fukaya T, Murakami T, Uehara S, Yajima A. Analysis of clonality in human endometriotic cysts based on evaluation of X chromosome inactivation in archival formalin-fixed, paraffin-embedded tissue. Lab Invest. 1998;78(2):213–218. [PubMed] [Google Scholar]
- 47. Wu Y, Basir Z, Kajdacsy-Balla A, et al. Resolution of clonal origins for endometriotic lesions using laser capture microdissection and the human androgen receptor (HUMARA) assay. Fertil Steril. 2003;79(suppl 1):710–717. [DOI] [PubMed] [Google Scholar]
- 48. Cheong Y, Tay P, Luk F, Gan HC, Li TC, Cooke I. Laparoscopic surgery for endometriosis: how often do we need to re-operate? J Obstet Gynaecol. 2008;28(1):82–85. [DOI] [PubMed] [Google Scholar]
- 49. Liu H, Lang J. Is abnormal eutopic endometrium the cause of endometriosis? The role of eutopic endometrium in pathogenesis of endometriosis. Med Sci Monit. 2011;17(4):RA92–RA99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Lucidi RS, Witz CA, Chrisco M, Binkley PA, Shain SA, Schenken RS. A novel in vitro model of the early endometriotic lesion demonstrates that attachment of endometrial cells to mesothelial cells is dependent on the source of endometrial cells. Fertil Steril. 2005;84(1):16–21. [DOI] [PubMed] [Google Scholar]
- 51. Exacoustos C, Zupi E, Amadio A, et al. Recurrence of endometriomas after laparoscopic removal: sonographic and clinical follow-up and indication for second surgery. J Minim Invasive Gynecol. 2006;13(4):281–288. [DOI] [PubMed] [Google Scholar]