Abstract
Obesity has been considered a public health issue in many countries and is of increasing concern for authorities over the past 6 years. The Zucker rat is a good experimental model for obesity and diabetes studies due to its metabolic characteristics that are similar to those developed by humans. A total of 12 obese Zucker rats and their lean littermates were killed in pubertal and young adult phases for assessing organ weights (testis and epididymis), testicular histomorphometric and stereological analyses, daily sperm production, and transit time in the epididymis. Sperm integrity was also investigated in the adult animals using the Comet assay. Alterations in organ weights, seminiferous epithelium architecture, sperm production, and transit time were noticed in the pubertal fatty rats. The volume density of the lymphatic space was decreased in both the ages. Adult animals had a significant increase in the extent of damage found in sperm DNA. Our results show for the first time that leptin receptor deficiency compromises sperm production during puberty and that genetic obese Zucker rats have increased sperm DNA fragmentation.
Keywords: Zucker rat, obesity, spermatogenesis, sperm production, DNA fragmentation
Introduction
Obesity has been considered a public health issue in many countries and is of increasing concern for authorities over the past 6 years. In both genders, the side effect caused by body fat accumulation compromises not only the well-being of the obese individuals but also their fertility.1–4 Several studies have been pointing out that men showing the body mass index (BMI) higher than 25 kg/m2 present alterations in seminal parameters like oligospermia and reduced motility.1,5
Although the alteration in the sexual hormones production and in the metabolism of obese man were attributed to be the main causes for sperm quality reduction,2,6 the effects of obesity on male fertility may be triggered by different mechanisms,4 including genetic defects.7 In humans, the accumulation of the abdominal adipose tissue can be linked to the seminal quality decrease due to an increase in testicular temperature.8 As a matter of fact, the increase in the production of reactive oxygen species can also be related to the BMI, thus damaging the germ cell line.9 Indeed, a study developed by Fariello and colleagues10 showed a reduction in the mitochondrial activity and an increase in sperm DNA fragmentation in overweight and obese individuals.
The leptin-receptor-deficient Zucker rat has been used as an experimental model for obesity and diabetes studies due to its metabolic characteristics that are similar to those developed by humans.1,11 Animals from this lineage develop hyperphagia and hyperinsulinemia as a result of a recessive gene.12 Such condition is related to a reduction in the leptin-receptor gene expression, which produces, as a consequence, symptoms of diabetes in the recessive homozygote rats.1 Besides the metabolic syndrome, another known effect is the complete sterility of the female rats,13 as the Zucker male rats reproductive capacity is reduced in an age-dependent manner.14,15 Obese rat infertility has been considered as caused by an inappropriate behavior during copulation and not as a consequence of fat accumulation.14,15 So far, no difference was found either in the testicular morphometric parameters or in sperm motility between the obese rats and their littermates.15 However, descriptive studies concerning the effects of the lack of leptin on spermatogenesis, sperm maturation, and quality are still to be addressed.
Leptin is a newly identified hormone produced in white adipose tissue that has been shown to have direct implications on fertility in both males and females.16,17 In the rat testis, leptin-receptor is expressed only in Leydig cells and has a very complex function in male reproduction. It seems that the Ob gene regulation and the ob gene receptor (ob-R) system in the testis may serve to negatively regulate testosterone production by Leydig cells during adulthood but not in prepubertal life; however, it could play a role during testicular embryogenesis and Leydig cell maturation in prenatal life.18
Yet, only few studies are available showing the effects of the lack of leptin signaling on the spermatogenesis19; moreover, there are no reports covering the sperm transportation through the epididymis and sperm DNA quality. Thus, in the present study we aimed to investigate these reproductive targets in 2 different moments of male sexual maturity in the Zucker rat model, the pubertal and the young adult phases.
Materials and Methods
Animals
A total of 12 obese Zucker rats aging 50 days and their lean littermates were purchased from the local animal facility (“Centro de Desenvolvimento de Modelos Experimentais para Medicina e Biologia—CEDEME”, from Federal University of Sao Paulo, Brazil). Throughout the experiment time, all the rats were maintained under 12/12-hour light/dark cycles, at 21°C to 23°C room temperature, fed with standardized laboratory chow (Labina; Purina, Brazil) and water provided ad libitum. This study was approved by the Ethics Committee for Animal Research of the Federal University of Sao Paulo, Brazil (protocol number 1030-10).
Organ Weights and Histological Procedures
Obese (n = 12) and lean (n = 12) rats were subjected to euthanasia by asphyxiation in the pubertal phase (6 obese/6 lean), when they were 64-day-old20 and in the sexually mature phase (6 obese/6 lean), when they were 100-day-old.21 Testes and epididymides were collected and weighted. The volume of the testes was obtained according to Scherle's method.22 The testicular axes (the major and the minor axes) were also measured using a micrometer caliper; thus, the left testis was immersed in Bouin fixative for 48 hours; the right testis and the right epididymis were frozen (−20°C) for sperm counting. The left epididymis cauda was used for sperm collection according to Vendramini et al.23 Samples containing spermatozoa were stored at −80°C in aliquots containing 1 mL of 4 × 106 spermatozoa/mL. Testicular fragments were processed and embedded in paraffin after fixation. Cross-sections of 5 μm thickness were stained with hematoxylin and eosin method. For histopathological analysis, the alterations in spermatogenesis were randomly analyzed in 100 tubular sections per animal and recorded using the Leica QWin V3 (Cambridge, United Kingdom) image analysis system. Testicular section images were captured by a digital camera connected to a light microscope.
Testes Histomorphometry
The histomorphometric parameters analyzed were the seminiferous tubule diameter and seminiferous epithelium height. Seminiferous tubule diameter and epithelium height measurements were performed under light microscope (Olympus BX-50;Olympus, Sao Paulo, Brazil) connected to a computer equipped with Leica QWin V3 (Cambridge, United Kingdom) image analysis system, under 10× and 20× objective lenses, respectively. When the sections were oblique, only the minor axis was considered.24
Stereological Analysis
The estimation of volume density (Vv) of the testicular compartments, which are sensitive to hormonal changes,25 was assessed using an integrating lens with 25 equidistant points at ×100 magnification,26 and 750 points were randomly counted per testis; thus, the percentage of interstitial tissue and lymphatic space compartments occupying the testis was achieved; then, the volume (V) of each compartment was calculated according to the referred percentages in the total volume of the organ. For all the analyses, 30 fields were randomly scrutinized in each testicular cross-section.
Daily Sperm Production per Testis, Sperm Number, and Transit Time in the Epididymis
The formerly frozen organs were thawed at room temperature for 30 minutes before the homogenization procedure. Homogenization-resistant spermatids (stage 19 of spermiogenesis) in the testis were counted according to Robb et al21; first, the testis was decapsulated and weighed soon after collection, homogenized in 5 mL of 0.9% NaCl containing 0.5% Triton X-100, followed by sonication for 30 seconds. After a 10-fold dilution, 1 sample was transferred to Neubauer chambers (4 samples per animal), and late spermatids were counted. To calculate the daily sperm production (DSP), the number of homogenization-resistant spermatids (per testis and per gram of testis) was divided by 6.1, which refers to the number of days these spermatids are present in the seminiferous epithelium. Likewise, caput/corpus and cauda epididymis portions were cut with scissors and homogenized, and the sperm was counted as described earlier. The sperm transit time through the epididymis was determined by dividing the number of sperm in each portion by the DSP.
Single-Cell Gel Electrophoresis
To assess single- and double-strand DNA breaks, spermatozoa were prepared as described previously for single-cell gel eletrophoresis analysis, known as the Comet assay,23,27–29 with the following minor changes: frozen sperm samples were thawed at 37°C for 2 minutes in a water bath and diluted in prewarmed (37°C) 0.5% low-melting point (LMP) agarose (LGC Laboratories, Sao Paulo, Brazil) at a dilution of 1:10. The solution (200 μL) was placed on the slides precoated with 1% normal melting point agarose (LGC Laboratories); cover slips were used to flatten the LMP agarose containing the spermatozoa, and the slides were stored at 4°C for 10 minutes. To avoid further damage to the sperm DNA, the following steps were done in the dark: first, each slide was covered with 1.5 mL of prechilled lysis buffer (2.5 mol/L NaCl, 100 mmol/L EDTA, and 10 mmol/L Tris-HCl; final pH 10) containing 10% dimethylsulfoxide, 1% Triton X-100, and 40 mmol/L dithiothreitol under 4°C for 1 hour; after washing the slides with dH2O, a second lysis buffer, prewarmed (37°C) and this time containing proteinase K (100 mg/mL), was used to cover the slides for 3 hours in a 37°C incubator. The slides were then gently washed in chilled dH2O and covered with freshly prepared alkaline solution (1 mmol/L EDTA and 0.05 mol/L NaOH, pH 12.1) for 45 minutes. The slides were washed twice with Tris-borate-EDTA (TBE) buffer (0.89 mol/L Tris, 0.89 mol/L boric acid, and 0.5 mol/L EDTA, pH 8) and then placed inside a horizontal electrophoresis box filled with TBE buffer, where they were submitted to electrophoresis at 30 V for 20 minutes. Finally, the slides were fixed in prechilled 70% alcohol, air dried, and stored in the dark until analysis.30 On the day of the analysis, the slides were stained with ethidium bromide (20 μg/mL in dH2O; 1.5 mL/slide) under an epifluorescence microscope Olympus BX-51 (Olympus), and 50 spermatozoa per slide (2 slides/animal) were analyzed using Komet 6.0 software (Andor Technology, Sao Paulo, Brazil). The following parameters were checked for statistical differences: tail DNA (%), tail length (µm), tail extent moment (tail length × tail DNA %/100), and olive tail moment (tail DNA % × a fraction of the tail length).
Statistical Analysis
Data were submitted to parametric Student t test using SPSS version 20.0 (SPSS Inc, Chicago, Illinois). Statistical significance was considered when P < .05 and expressed as letters and asterisks in the tables and figures.
Results
Organ Weights and Histopathology of the Seminiferous Epithelium
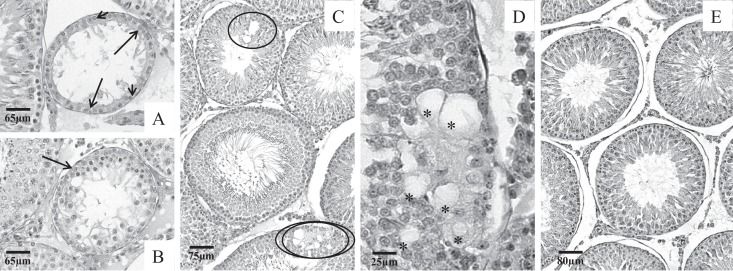
Body weight differences between lean and obese Zucker male rats were of 32.3% in the 64-day-old animals and 37.7% in the 100-day-old animals. However, a significant reduction in the testis and the epididymis weights was noticed only in the pubertal phase; on the other hand, as a consequence of the increased body weights seen in both pubertal and adult phases, the relative testicular and epididymides weights were decreased in the fatty rats in both the ages (Table 1). Under light microscopy, the testicular histopathology revealed damage only in the fatty rat testes analyzed here; germ cell loss in the intermediate and adluminal layers and intraepithelial vacuolization were the most common alterations observed in both ages (Figures 1A-C and 2A-D); interestingly, such alterations occurred more frequently in tubules characterized as being in the X to XII phase of the seminiferous cycle. In the pubertal rats, picnotic nuclei were also noticed in many tubular sections, generally located in the intermediate layers of the seminiferous epithelium (Figures 1B and C). Lean Zucker rats presented tubular sections containing concentric germ cell layers independent of the age analyzed (Figures 1D and 2E).
Table 1.
Biometric and Morphometric Parameters Obtained for the Lean (L) and Fatty (F) Zucker Rats Aging 64 and 100 Days.a
| Parameter | Groups | |||
|---|---|---|---|---|
| L64 (n = 6) | F64 (n = 6) | L100 (n = 6) | F100 (n = 6) | |
| Body weight, g | 233.01 ± 7.31 | 344.00 ± 13.64b | 305.31 ± 0.04 | 489.86 ± 0.06b |
| Testis weight, g | 1.17 ± 0.04 | 0.87 ± 0.05b | 1.31 ± 0.04 | 1.40 ± 0.06 |
| Testis relative weight, g | 0.50 ± 0.09 | 0.30 ± 0.08b | 0.45 ± 0.04 | 0.28 ± 0.06b |
| Volume testicular, cm3 | 1.06 ± 0.09 | 0.96 ± 0.22 | 1.28 ± 0.04 | 1.31 ± 0.06 |
| Testis major axis, mm | 18.30 ± 0.50 | 17.06 ± 1.05 | 19.19 ± 0.35 | 19.36 ± 3.15 |
| Testis minor axis, mm | 10.44 ± 0.12 | 9.96 ± 0.35 | 11.21 ± 0.18 | 12.25 ± 0.17b |
| Epididymides weight, g | 0.25 ± 0.02 | 0.17 ± 0.03b | 0.45 ± 0.04 | 0.40 ± 0.06 |
| Epididymides relative weight, g | 0.11 ± 0.03 | 0.05 ± 0.01b | 0.13 ± 0.01 | 0.08 ± 0.01b |
Abbreviation: SEM, standard error of the mean.
aData are reported as mean ± SEM. Student t test was used in this analysis.
bSignificant compared to the lean rats (P < .05).
Figure 1.
Photomicrographs of testicular cross-sections of lean and fatty littermates in the pubertal phase (64 days old). In the fatty animals, many damaged tubules were seen across the testicular section, as shown in (A–C). Disorganized and discontinuous epithelium (ellipses), vacuolization (asterisks), and cells containing picnotic nuclei (thick arrow) were the commonly found alterations in this phase, mainly in tubules in X to XII stage of the seminiferous epithelium cycle (A and B). Lean rat testes showed normal testicular histology composed of germ cells in many different stages of maturation and disposed in concentric and organized layers (D).
Figure 2.
Photomicrographs of testicular cross-sections of lean and fatty littermates in the adult phase (100 days old). Damaged tubules were still seen in the adult phase, although most part of the testes analyzed was composed by apparently normal seminiferous tubules (A–D). As shown in (A) and (B), some tubules presented a serious cell depletion; in some fatty rats, tubules contained only the first layer of germ cells (short arrows) surrounded by Sertoli cells (long arrows). In (C) and (D), note the discontinuity and vacuolization in the epithelium (ellipses); inside the doubled ellipsis is the region detailed in (D). Lean rat testes showed normal testicular histology (E). Vacuoles (asterisks).
Testes Histomorphometry
No statistical significance was found between lean and fatty rats, neither for the tubular diameter nor for the epithelium height analyzed in the pubertal and adult phases (Figure 3).
Figure 3.
Testicular histomorphometric analyses of pubertal and adult Zucker rats. The parametric Student t test did not detect significant differences neither for tubular diameter nor for the epithelium height in any of the ages investigated. Mean ± standard error of the mean (SEM); Student t test; not significant (P < .05).
Stereological Analysis
The volume densities were expressed by the percentage of the interstitial tissue and lymphatic space observed in the testicular cross-sections of the lean and fatty rats, and the obtained results are shown in Figure 4. A significant decrease was noticed only in the lymphatic space of fatty rat testes in both ages.
Figure 4.
Testicular volume densities (Vv) of the interstitial tissue and the lymphatic space observed in the pubertal and adult testes. The Vv of the lymphatic space was decreased in the fatty rats in both the phases of sexual maturity. Mean ± standard error of the mean (SEM); Student t test; *significant (P < .05).
Daily Sperm Production per Testis, Sperm Number, and Transit Time in the Epididymis
Table 2 shows the results obtained in the testicular and epididymal sperm analyses carried out in the present study. The results showed that the number of mature spermatids counted in the pubertal Zucker rat testes was reduced when compared to the means obtained in the lean littermates. Nevertheless, the ratio of spermatids in stage 19 per gram of testis did not follow the same pattern in the aforementioned age. The sperm transit time, estimated for sperm transportation time in the 2 different regions of the epididymis, was significantly decreased only in the cauda; conversely, the number of sperm found per organ (corpus/caput and cauda) was significantly decreased in the pubertal phase.
Table 2.
Testis and Epididymal Sperm Parameters in the Lean (L) and Fatty (F) Zucker Rats Aging 64 and 100 Days.a
| Parameter | Groups | |||
|---|---|---|---|---|
| L64 (n = 6) | F64 (n = 6) | L100 (n = 6) | F100 (n = 6) | |
| Testis | ||||
| Testis weight, g | 1.17 ± 0.03 | 0.87 ± 0.05b | 1.31 ± 0.04 | 1.40 ± 0.06 |
| Spermatid number, ×106/testis | 111.67 ± 7.53 | 81.75 ± 13.25b | 186.41 ± 13.30 | 223.50 ± 29.24 |
| Spermatid number, ×106/g/testis | 107.85 ± 8.15 | 128.25 ± 47.66 | 165.50 ± 7.96 | 184.56 ± 18.99 |
| Daily sperm production, ×106/testis/d | 18.33 ± 1.15 | 13.40 ± 2.2b | 30.53 ± 2.17 | 36.65 ± 5.12 |
| Daily sperm production, ×106/g testis/d | 15.82 ± 1.58 | 15.64 ± 3.64 | 25.67 ± 1.83 | 30.25 ± 3.11 |
| Epididymal caput/corpus | ||||
| Caput/corpus epididymal weight, g | 0.17 ± 0.02 | 0.10 ± 0.04b | 0.25 ± 0.02 | 0.23 ± 0.12 |
| Sperm number, ×106/organ | 50.03 ± 3.48 | 19.06 ± 10.09b | 77.94 ± 3.73 | 81.06 ± 7.53 |
| Sperm number, ×106/g/organ | 193.72 ± 69.96 | 244.70 ± 94.56 | 313.31 ± 9.80 | 312.93 ± 24.62 |
| Transit time, day | 2.86 ± 0.29 | 2.04 ± 0.51 | 2.59 ± 0.17 | 2.38 ± 0.32 |
| Epididymal cauda | ||||
| Epididymal cauda weight, g | 0.07 ± 0.01 | 0.03 ± 0.01b | 0.17 ± 0.03 | 0.09 ± 0.06 |
| Sperm number, ×106/organ | 37.64 ± 3.52 | 2.02 ± 0.51b | 119.95 ± 14.60 | 101.40 ± 11.98 |
| Sperm number, ×106/g/organ | 504.86 ± 39.55 | 124.00 ± 77.75b | 690.82 ± 69.46 | 703.53 ± 52.18 |
| Transit time, day | 1.22 ± 0.16 | 0.07 ± 0.01b | 3.60 ± 0.32 | 2.84 ± 0.29 |
Abbreviation: SEM, standard error of the mean.
aData are reported as mean ± SEM. Student t test was used in this analysis.
bSignificant compared to the lean rats (P < .05).
Single-Cell Gel Electrophoresis
Alcali Comet assay analysis (Figure 5) provided evidence that sperm produced by the fatty animals have an increase in DNA damage when compared to the lean animals as revealed by the parameters “tail length” and “tail extent moment.”
Figure 5.
Sperm DNA fragmentation obtained in obese and lean Zucker rats. Obese rats had more extended chromatin fragmentation than the lean rats. Mean ± standard error of the mean (SEM); Student t test; * significant (P < .05).
Discussion
In the present study, we demonstrate for the first time that pubertal obese Zucker rats present alterations in the spermatogenesis, as observed in the histological level, which persists up to the adult phase; in the quantitative analysis, sperm production in the fatty animals was reduced as well, but only in the pubertal rats; on the other hand, the increased sperm DNA fragmentation found in the adult rats points out to side genetic damage generated in the fatty rat gamete, which can be a lead for understanding the obese Zucker rat infertility.
The obese Zucker rat has been considered a promising model for the metabolic syndrome and diabetes11,17 The characteristic hyperlipidemia found in this model occurs due to a monogenetic deficiency that lacks the ob-R; as a consequence, the obese rat’s leptin levels are increased, testosterone is slightly decreased, and gonadotrophin-releasing hormone is not altered.12 Over the past decade, leptin has gained more attention, while its role in the regulation of the reproductive hormones started to be unfolded. In the pubertal onset, serum leptin concentration rises and is considered to be an important signal for further hormonal changes.31,32 However, the onset of puberty as judged by the peripubertal testosterone rise, secondary sexual organ growth, appearance of elongating spermatids, and/or the peripubertal decline in serum follicle-stimulating hormone (FSH) precedes the increase in serum leptin. In male monkeys, nocturnal levels of leptin are elevated just prior to the onset of the increase in luteinizing hormone (LH) pulse amplitude, thus indicating hypothalamic puberty in those animals.33 Although none of these studies showed that leptin induces puberty onset in male mammals, a rise in serum leptin concentrations may be a significant component of the pubertal process.32–34 In the fatty Zucker rat, a delay in puberty onset is believed to occur due to the interruption of leptin pathway, but it has not yet been extensively studied.35
Besides the hormonal profile, the lack of information about the morphofunctional analysis of the obese Zucker rat testis has propelled us toward this investigation. Along with its primary actions at the hypothalamic–pituitary level, leptin has direct effects on the proliferation, differentiation of germ cells, and modulation of testicular steroidogenesis, using both autocrine and paracrine mechanisms36 that can utterly affect the reproductive function. Some reports have already showed that the obese Zucker rats present alterations in some reproductive organ weights,14,15,35 which could be caused by the lack of leptin stimulus32; however, the study presented here is the first to evaluate the testicular histological characteristics and sperm production in the pubertal phase of the Zucker rat as well as its effects on DNA integrity in its sexual maturity. Nevertheless, obesity is also linked to the increase in testicular lipid peroxidation and therefore the increase in intratesticular oxidative stress37 that could also be contributing to the increase in the DNA damage seen in the fatty rats observed in the present report.
The analysis of the 64-day-old rats, when these rodents are considered pubertal,21 brings to light the need of a deeper investigation including different time points of sexual development in the leptin-receptor-deficient model. At this age, rodent sperm can be found in the epididymis cauda, and the seminiferous epithelium is composed of many concentric layers of germ cells as observed in the lean rats studied here.38,39 In the obese Zucker rat, we observed the presence of intraepithelial vacuoles, which is a clear sign of germ cell loss40; this was confirmed by the reduction in sperm production in the pubertal phase, albeit the mean of the epithelium height found was not different between lean and obese animals. We tend to believe that the germ cell loss can explain the reduced organ weights observed in the fatty animals at puberty, since the testicular axis mean was not different between lean and obese rats; besides that, a great reduction was also found in the lymphatic space in those rats, which could have contributed to the weight decrease as well. The volume density reduction in the lymphatic space in both the phases indicates that testicular hormonal environment is altered in both puberty and adulthood.25
The quantitative analysis showed that sperm counts per organ, observed in the 2 different portions of the epididymis and in the testis, was lower in the obese rats indicating that the decrease in organ weights is related to the reduction in sperm output. The decrease in testicular and epididymal weights may be a consequence of a delayed reproductive development of the obese Zucker rat; however, this condition was transient, since the organ weights gain achieved normal levels and were no longer significant in adulthood. In this matter, it is important to highlight that DSP efficiency was not compromised in any of the ages studied here, according to the mean obtained per gram of testis.41
Suh et al35 have observed that Zucker obese rats aging 14 weeks (98 days) have a statistically significant reduction in epididymis weight but not in testicular weight; similarly, the testicular weight mean of the adult obese Zucker rats obtained in the present study was comparable to those of the lean rats. We believe that the main difference between ours and the aforementioned study was the epididymis weight of the lean rats, which in our study was reduced when compared to the mean reported by Suh et al35 probably due to colony differences.42 On the other hand, our results corroborate the idea of the developmental delay in sexual organs of the obese Zucker rats proposed by Suh et al.35
We confirmed that Ob-R-deficient rat can produce sperm in sexual mature life as reported previously,14,15 although spermatogenesis is truncated in some seminiferous tubules; besides, it was shown here that the gametes recovered in young adult rats carry extended DNA fragmentation. In humans, obesity was already associated to sperm with low motility, decreased mitochondrial activity, and increased DNA fragmentation.10 Sperm containing highly fragmented DNA do not necessarily lose the fertility ability, but it can contribute to embryo developmental disruption.23,43 So far, no increase in preimplantation loss was observed after neither natural nor artificial insemination using obese Zucker rats and lean females.14 Conversely, a recent report showed that embryos derived from the sperm of obese mice submitted to high calorie diet for 10 weeks had a reduced ability to implant in the uterine wall of a recipient female following blastocyst transfer.44 This same report also showed that paternal diet-induced obesity caused early mouse embryo development delay from as early as syngamy; unfortunately, they did not assess the sperm DNA integrity.
Sperm reserves found in the epididymis cauda were dramatically decreased in the pubertal obese rats, which is likely to have reflected in the acceleration of the sperm transit time observed in this portion. We hypothesize that obese Zucker rats also have low testicular sperm production when they are around 50 days old; therefore, providing a lower amount of sperm reserves in the epididymis cauda at puberty (64 days old), since sperm usually takes between 10 to 14 days to be transported into the epididymis cauda.20 Considering that sperm storage is the main function of the epididymis cauda,45 in this case, we tend to understand such decrease in sperm transit time as a harmless event, but another consequence of the epididymis developmental delay.35
Currently, the fertility impairment condition reported in the Zucker rat model is far to be completely clear and is mainly attributed to the altered hormonal balance and behavioral dysfunction.13,14, 17 Allied to the histological alterations reported here, the reduction in the genome quality might be another lead for important damage that may be occurring in the gametogenesis process as a consequence of hormone-related dysfunction.46,47 It is likely that the alterations showed in pubertal Zucker rats were caused by the lack of leptin stimulus in the signaling cascade for sexual hormones production and androgens delivering,48 and this correlation should be investigated soon.
Substantial abnormalities in testicular morphology and impaired spermatogenesis associated with leptin deficiency were already reported in the murine model.49,50 Both the leptin-deficient (a.k.a. ob/ob) and the leptin-receptor-deficient (a.k.a. db/db or diabetic) mice develop morbid obesity due to hyperphagia and decreased energy expenditure, along with innumerous endocrine abnormalities.51–53 Low levels of FSH and testosterone and a lack of LH critical peaks are considered the main causes for the hypogonadism that is the characteristic of the ob/ob mouse.54 Immature hypothalamic–pituitary axis was also referred to occur in these 2 models of obesity.53,55 Indeed, in humans, both obesity and high levels of leptin are associated with early puberty56; however, the consequences of leptin deregulation to the spermatogenesis are not yet fully elucidated.
Spermatogenesis is a complex synchronized process of cell division and maturation to accomplish the male gametes production, followed by its release and storage in the epididymis.57,58 Apoptosis is the regulation process which the germ cells commonly undergo in case of injury.59–61 In the seminiferous tubule, increased number of intraepithelial vacuolization is often associated with apoptosis.60 Leptin deficiency in mice is related to the upregulation of proapoptotic genes within the testes, increased germ cell apoptosis, and impairment of spermatogenesis.50 In the present work, we have documented many occurrences of cell depletion characterized by intraepithelial vacuolization probably due to an increase in germ cell apoptosis triggered in the fatty rats. Interestingly, the seminiferous epithelium cycles that were noticed to contain germ cells displaying picnotic nuclei were mostly from X to XII.62 Indeed, these cell cycles are usually described as when susceptible germ cells are more likely to undergo apoptosis following a variety of injuries.63 Last but not least, the increased sperm DNA fragmentation found in obese Zucker rat might also be a result of affected germ cells that escaped apoptosis and managed to pursue further on the final steps of spermatogenesis, thus producing gametes carrying damaged chromatin.23
In conclusion, this report highlights that spermatogenesis is susceptible to the indirect deregulation in leptin signaling in the Zucker obese rat, producing cell depletion and, as a consequence, the reduction in sperm production in the pubertal phase. Extended DNA damage in sperm in sexual maturity certainly contributes to decreased fertility in the Zucker obese rat; however, the relationship between fertility competence and sperm DNA damage remains obscure in the leptin-receptor-deficient rat. Further investigations must be carried out to address the mechanistic events that are involved in the alterations described herein.
Footnotes
Authors’ Note: V. Vendramini performed all the experimental work and the confection of this manuscript. S.M. Miraglia, A.P. Cedenho, and D.M. Spaine have intellectually contributed to the experimental design, the analyses of the results, and the revision of the manuscript.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This project was financially supported by the National Council for the Improvement of Higher Education (CAPES). VV received grants for 1 year from the Brazilian Agency for Improvement of Higher Education Personnel (CAPES) and all the expenses with the study were funded by the Division of Urology and the Discipline of Developmental Biology laboratory at UNIFESP.
References
- 1. van der Steeg JW, Steures P, Eijkemans MJ, et al. Obesity affects spontaneous pregnancy chances in subfertile, ovulatory women. Hum Reprod. 2008;23(2):324–328. [DOI] [PubMed] [Google Scholar]
- 2. Jensen T, Andersson A, Jorgensen N, et al. Body mass index in relation to semen quality and reproductive hormones among 1,558 Danish men. Fertil Steril. 2004;82(4):863–870. [DOI] [PubMed] [Google Scholar]
- 3. Aggerholm AS, Thulstrup AM, Toft G, Ramlau-Hansen CH, Bonde JP. Is being overweight a risk factor for reduced semen quality and altered serum sex hormone profile? Fertil Steril. 2008;90(3):619–626. [DOI] [PubMed] [Google Scholar]
- 4. Kasturi SS, Tannir J, Brannigan ER. The metabolic syndrome and male infertility. J Androl. 2008;29(3):251–259. [DOI] [PubMed] [Google Scholar]
- 5. Hammoud AO, Wilde N, Gibson M, Parks A, Carrell DT, Meikle AW. Male obesity and alteration in sperm parameters. Fertil Steril. 2008;90(6):2222–2225. [DOI] [PubMed] [Google Scholar]
- 6. MacDonald GP, Herbison M, Showell Farquhar CM. The impact of body mass index on semen parameters and reproductive hormones in human males: a systematic review with meta-analysis. Hum Reprod Update. 2010;16(3):293–311. [DOI] [PubMed] [Google Scholar]
- 7. Farooqi IS, Wangensteen T, Collins S, et al. Clinical and molecular genetic spectrum of congenital deficiency of the leptin receptor. N Engl J Med. 2007;356(3):237–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Shafik A, Olfatt S. Lipectomy in the treatment of scrotal lipomatosis. Br J Urol. 1981;53(1):55–61. [DOI] [PubMed] [Google Scholar]
- 9. Kort H, Massey J, Elsner C, et al. Impact of body mass index values on sperm quantity and quality. J Androl. 2006;27(3):450–452. [DOI] [PubMed] [Google Scholar]
- 10. Fariello RM, Pariz JR, Spaine DM. Association between obesity and alteration of sperm DNA integrity and mitochondrial activity. Fertil Steril. 2009;92(6):28–29. [DOI] [PubMed] [Google Scholar]
- 11. Clark JB, Palmer CJ, Shaw WN. The diabetic Zucker fatty rat. Proc Soc Exp Bio Med. 1983;173(1):68–75. [DOI] [PubMed] [Google Scholar]
- 12. Bray GA. The Zucker-fatty rat: a review. Fed Proc. 1977;36(2):148–153. [PubMed] [Google Scholar]
- 13. Chelich AM, Edmonds ES. Copulatory behavior and reproductive capacity of the genetically obese female Zucker rat. Physiol Behav. 1980;27(2):331–335. [DOI] [PubMed] [Google Scholar]
- 14. Edmonds ES, Dallie SK, Withyachumnarnkul B. Reproductive system of the obese male Zucker rat. Reproductive capacity, artificial insemination and plasma testosterone levels. Biol Reprod. 1982;27(4):891–897. [DOI] [PubMed] [Google Scholar]
- 15. Piser JA, Edmonds ES, Hoftiezer V. Sperm motility and histomorphometry of the testis of the genetically obese Zucker rat. J Androl. 1981;2(4):200–204. [Google Scholar]
- 16. El-Hefnawy T, Loffe S, Dym M. Expression of the leptin receptor during germ cell development in the mouse testis. Endocrinology. 2000;141(7):2624–2630. [DOI] [PubMed] [Google Scholar]
- 17. Teerds KJ, de Rooij DG, Keijer J. Functional relationship between obesity and male reproduction: from humans to animal models. Hum Reprod Update. 2011;17(5):667–683. [DOI] [PubMed] [Google Scholar]
- 18. Caprio M, Fabbrini E, Ricci G, et al. Ontogenesis of leptin receptor in rat Leydig cells. Biol Reprod. 2003;68(4):1199–1207. [DOI] [PubMed] [Google Scholar]
- 19. Young RA, Frink R, Longcope C. Serum testosterone and gonadotropins in the genetically obese male Zucker rat. Endocrinology. 1982;111(3):977–981. [DOI] [PubMed] [Google Scholar]
- 20. Clegg EJ. The age at which male rats become fertile. J Reprod Fert. 1960;1:119–120. [Google Scholar]
- 21. Robb GW, Amainn PR, Killian GJ. Daily sperm production and epididymal sperm reserves of pubertal and adult rats. J Reprod Fert. 1978;54(1):103–107. [DOI] [PubMed] [Google Scholar]
- 22. Scherle W. A simple method for volumetry of organs in quantitative sterology. Mikroskopie. 1970;26(1):57–63. [PubMed] [Google Scholar]
- 23. Vendramini V, Robaire B, Miraglia SM. Amifostine–doxorubicin association causes long-term prepubertal spermatogonia DNA damage and early developmental arrest. Hum Reprod. 2012;27(8):2457–2466. [DOI] [PubMed] [Google Scholar]
- 24. Miraglia SM, Hayashi H. Histomorphometry of immature rat testis after heating. J Morphol. 1993;217(1):65–74. [DOI] [PubMed] [Google Scholar]
- 25. Sharpe RM, Cooper I. Testicular interstitial fluid as a monitor for changes in the intratesticular environment in the rat. J Reprod Fert. 1983;69(1):125–135. [DOI] [PubMed] [Google Scholar]
- 26. Gundersen HJ, Bendtsen TF, Korbo L, et al. Some new, simple and efficient stereological methods and their use in pathological research and diagnosis. APMIS. 1988;96(5):379–394. [DOI] [PubMed] [Google Scholar]
- 27. Tice RR, Agurell E, Anerson D, et al. Single cell gel/Comet assay: guidelines for in vitro and in vivo genetic toxicology testing. Environ Mol Mutagen. 2000;35(3):206–221. [DOI] [PubMed] [Google Scholar]
- 28. Codrington AM, Hales BF, Robaire B. Spermiogenic germ cell phase-specific DNA damage following cyclophosphamide exposure. J Androl. 2004;25(3):354–362. [DOI] [PubMed] [Google Scholar]
- 29. Delbès G, Hales BF, Robaire B. Effects of the chemotherapy cocktail used to treat testicular cancer on sperm chromatin integrity. J Androl. 2007;28(2):241–249. [DOI] [PubMed] [Google Scholar]
- 30. Haines GA, Hendry JH, Daniel CP, Morris ID. Germ cell and dose-dependent DNA damage measured by the comet assay in murine spermatozoa after testicular x-irradiation. Biol Reprod. 2002;67(3):854–861. [DOI] [PubMed] [Google Scholar]
- 31. Harris GC, Levine JE. Pubertal acceleration of pulsatile gonadotropin-releasing hormone release in male rats as revealed by microdialysis. Endocrinology. 2003;144(1):163–171. [DOI] [PubMed] [Google Scholar]
- 32. Nazian SJ, Cameron DF. Temporal relation between leptin and various indices of sexual maturation in the male rat. J Androl. 1999;20(4):487–491. [PubMed] [Google Scholar]
- 33. Suter KJ, Pohl CR, Wilson ME. Circulating concentrations of nocturnal leptin, growth hormone, and insulin-like growth factor-I increase before the onset of puberty in agonadal male monkeys: potential signals for the initiation of puberty. J Clin Endocrinol Metab. 2000;85(2):808–814. [DOI] [PubMed] [Google Scholar]
- 34. Urbanski HF, Pau KY. A biphasic developmental pattern of circulating leptin in the male rhesus macaque (Macaca mulatta). Endocrinology. 1998;139(5):2284–2286. [DOI] [PubMed] [Google Scholar]
- 35. Suh M, Krystal JM, Dick A, Taylor CG. Testes of obese rats are highly responsive to n-3 long-chain fat acids. Brit J Nutr. 2011;106(7):1005–1012. [DOI] [PubMed] [Google Scholar]
- 36. Herrid M, O’Shea T, McFarlane JR. Ontogeny of leptin and its receptor expressed in mouse testis during the postnatal period. Mol Reprod Dev. 2008;75(5):874–880. [DOI] [PubMed] [Google Scholar]
- 37. Vigueras-Villaseñor RM, Rojas-Castañeda JC, Chávez-Saldaña M, et al. Alterations in the spermatic function generated by obesity in rats. Acta Histochem. 2011;113(2):214–220. [DOI] [PubMed] [Google Scholar]
- 38. Leblond CP, Clermont Y. Spermiogenesis of rat, mouse, hamster and guinea pig as revealed by the periodic acid-fuchsin sulfurous acid technique. Am J Anat. 1952;90(2):167–215. [DOI] [PubMed] [Google Scholar]
- 39. Clermont Y, Morgentaler H. Quantitative study of spermatogenesis in the hypophysectomized rat. Endocrinology. 1955;57(3):369–382. [DOI] [PubMed] [Google Scholar]
- 40. Vendramini V, Sasso-Cerri E, Miraglia SM. Amifostine reduces the seminiferous epithelium damage in doxorubicin-treated prepubertal rats without improving the fertility status. Reprod Biol Endocrinol. 2010;8:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Bellentani FF, Fernandes GS, Perobelli JE, et al. Acceleration of sperm transit time and reduction of sperm reserves in the epididymis of rats exposed to sibutramine. J Androl. 2011;32(6):718–724. [DOI] [PubMed] [Google Scholar]
- 42. Peterson RG, Shaw WN, Neel MA, et al. Zucker diabetic fatty rat as a model for non-insulin-dependent diabetes mellitus. ILAR News. 1990;32(3):16–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Barton TS, Robaire B, Hales BF. Epigenetic programming in the preimplantation rat embryo is disrupted by chronic paternal cyclophosphamide exposure. Proc Natl Acad Sci USA. 2005;102(22):7865–7870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Binder NK, Hannan NJ, Gardner DK. Paternal diet-induced obesity retards early mouse embryo development, mitochondrial activity and pregnancy health. PLoS One. 2012;7(12):e52304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Robaire B, Hinton BT, Orgebin-Crist MC. The epididymis. In: Neill JD, ed. Knobil and Neill’s Physiology of Reproduction. 3rd ed New York, NY: Elsevier; 2006:1071–1148. [Google Scholar]
- 46. Franca LR, Parreira GG, Gates RJ, Russell LD. Hormonal regulation of spermatogenesis in the hypophysectomized rat: quantitation of germ-cell population and effect of elimination of residual testosterone after long-term hypophysectomy. J Androl. 1998;19(3):335–342. [PubMed] [Google Scholar]
- 47. Tena-Sempere M, Manna PR, Zhang FP, et al. Molecular mechanisms of leptin action in adult rat testis: potential targets for leptin-induced inhibition of steroidogenesis and pattern of leptin receptor messenger ribonucleic acid expression. J Endocrinol. 2001;170(21):413–442. [DOI] [PubMed] [Google Scholar]
- 48. Landry D, Cloutier F, Martin LJ. Implications of leptin in neuroendocrine regulation of male reproduction. Reprod. Biol. 2013;13(1):1–14. [DOI] [PubMed] [Google Scholar]
- 49. Bhat GK, Hamm ML, Igietseme JU, Mann DR. Does leptin mediate the effect of photoperiod on immune function in mice? Biol Reprod. 2003;69(1):30–36. [DOI] [PubMed] [Google Scholar]
- 50. Bhat GK, Sea TL, Olatinwo MO, et al. Influence of a leptin deficiency on testicular morphology, germ cell apoptosis, and expression levels of apoptosis-related genes in the mouse. J Androl. 2006;27(2):302–310. [DOI] [PubMed] [Google Scholar]
- 51. Coleman DL. Obese and diabetes: two mutant genes causing diabetes-obesity syndromes in mice. Diabetologia. 1978;14(3):141–148. [DOI] [PubMed] [Google Scholar]
- 52. Swerdloff RS, Batt RA, Bray GA. Reproductive hormonal function in the genetically obese (ob/ob) mouse. Endocrinology. 1976;98(6):1359–1364. [DOI] [PubMed] [Google Scholar]
- 53. Leibel RL, Chung WK, Chua SC., Jr The molecular genetics of rodent single gene obesities. J Biol Chem. 1997;272(51):31937–31940. [DOI] [PubMed] [Google Scholar]
- 54. Batt RA, Everard DM, Gillies G, Wilkinson M, Wilson CA, Yeo TA. Investigation into the hypogonadism of the obese mouse (genotype ob/ob). J Reprod Fertil. 1982;64(2):363–371. [DOI] [PubMed] [Google Scholar]
- 55. Israel DD, Sheffer-Babila S, de Luca C, et al. Effects of leptin and melanocortin signaling interactions on pubertal development and reproduction. Endocrinology. 2012;153(5):2408–2419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Shalitin S, Phillip M. Role of obesity and leptin in the pubertal process and pubertal growth—a review. Int J Obes. 2003;27(8):869–874. [DOI] [PubMed] [Google Scholar]
- 57. Sharpe RM. Testosterone and spermatogenesis. J Endocrinol. 1987;113(1):1–2. [DOI] [PubMed] [Google Scholar]
- 58. Russell LD, Ettlin RA, Sinha Hikim AP, Clegg ED. Histological and Histopathological Evaluation of the Testis. Clearwater, FL: Cache River Press; 1990. [Google Scholar]
- 59. Hasegawa M, Wilson G, Russell LD, Meistrich ML. Radiation-induced cell death in the mouse testis: relationship to apoptosis. Radiat Res. 1997;147(4):457–467. [PubMed] [Google Scholar]
- 60. Stumpp T, Sasso-Cerri E, Freymüller E, Miraglia SM. Apoptosis and testicular alterations in albino rats treated with etoposide during the prepubertal phase. Anat Rec [A]. 2004;279(1):611–622. [DOI] [PubMed] [Google Scholar]
- 61. Lirdi LC, Stumpp T, Sasso-Cerri E, Miraglia SM. Amifostine protective effect on cisplatin-treated rat testis. Anat Rec. 2008;291(7):797–808. [DOI] [PubMed] [Google Scholar]
- 62. Hess RA. Quantitative and qualitative characteristics of the stages and transitions in the cycle of the rat seminiferous epithelium: light microscopic observations of perfusion-fixed and plastic-embedded testes. Biol Reprod. 1990;43(3):525–542. [DOI] [PubMed] [Google Scholar]
- 63. Blanco-Rodríguez J, Martínez-García C. Apoptosis pattern elicited by several apoptogenic agents on the seminiferous epithelium of the adult rat testis. J Androl. 1998;19(4):487–497. [PubMed] [Google Scholar]