Abstract
Significant follicle loss from frozen ovarian grafts is unavoidable. The authors evaluated the protective effects of the antiapoptotic agent sphingosine-1-phosphate (S1P) on vitrified ovarian grafts. Three-week-old sexually immature female FVB mice were divided into 4 groups, fresh, control without S1P, 0.5 mmol/L S1P, and 2 mmol/L S1P. The ovaries were pretreated with S1P for 1 hour and then cryopreserved by modified vitrification. The frozen–thawed ovaries were autotransplanted under the back muscles of mice for 10 days. Expression of apoptosis-related genes encoding caspase 3 and c-Myc was analyzed in the vitrified ovaries and 10 days after transplantation using real-time quantitative polymerase chain reaction. To quantify the ovarian reserve, anti-Müllerian hormone (AMH) levels and follicles were measured in the 10-day vitrified ovarian grafts. Caspase 3 and c-Myc messenger RNA did not differ significantly in the 4 groups after vitrification but was significantly upregulated in the control group after transplantation. The AMH levels and primordial follicle pool were significantly higher in the S1P-treated groups than in the control group but lower than that in the fresh group. The S1P protects vitrified ovarian grafts from ischemic reperfusion injury rather than from vitrification-associated process.
Keywords: sphingosine-1-phosphate, apoptosis, vitrification, anti-Müllerian hormone
Introduction
The cryopreservation of ovarian tissues before cancer treatment can restore fertility in women at risk of premature ovarian failure and is the only option that does not require ovarian stimulation in prepubertal girls.1–4 The transplantation of fresh and frozen ovarian tissues has resulted in around 28 healthy human babies,5,6 and all of these frozen ovarian tissues were prepared with a slow-freezing protocol. However, the slow freezing of human ovarian tissues remains problematic, because a diversity of cell types must be protected from ice crystallization damage, and the procedure is time consuming. Recent studies have suggested that vitrification is a more useful method, providing a rapid cooling rate that induces glass-like solidification inside the cells, protecting them from injury by ice crystals at all stages of cryopreservation.7 However, so far there have been no human live birth after ovarian vitrification. Vitrification is a procedure that precludes intracellular ice formation so that the embryos can be cooled and warmed at ultrarapid rates to minimize chilling injury, and viable embryos have been recovered following storage in liquid nitrogen (LN2).8 Vitrification was first reported in mouse embryos in the 1980s9 and has since been reported in oocytes,10 embryos,11 and ovarian tissues from various species, including the rat, sheep,12 and Chinese hamster.13 Vitrification simplifies the process of cooling to a great extent and reduces chilling injury. However, the highly concentrated cryoprotectants required may induce toxicity and is likely to penetrate ovarian tissues incompletely. Furthermore, an intrinsic program of apoptosis can be activated during tissue freezing and transplantation. However, cell death might be prevented if the apoptosis program of cells can be inactivated. Recent data have indicated that apoptosis is one of the major mechanisms responsible for developmental oocyte loss and the oocyte depletion induced by chemotherapy14,15 as well as the apoptosis induced by chemotherapy. Stress-induced apoptosis also makes tissues vulnerable to injury. Cryopreservation at very low temperatures and thawing are themselves stressors. Ovarian tissues are composed of various structures such as the stroma, fibrous tissue, vessels, nerves, somatic cells, and follicles. The antifreezing capacities of these tissues can differ from that of the germ cells. Ischemic reperfusion injury after transplantation may also exacerbate the damage to these tissues and can release oxidative agents that cause cell death. There is some evidence that during the process of apoptosis, 2 distinct sphingolipids, sphingosine-1-phosphate (S1P) and ceramide, play important roles in determining whether a cell will live or die (Figure 1).15–17 The S1P, which is synthesized from sphingosine by sphingosine kinase, regulates a variety of proliferative cellular processes, including cell growth and cell differentiation,18 and counterbalances the apoptosis that results from elevated levels of ceramide.16 Ceramide is formed from cell membrane sphingomyelin by sphingomyelinases and mediates antiproliferative responses such as cell growth arrest and apoptosis in many tissues and cell lines and is activated by a variety of stress factors.19 These sphingolipid-based signaling events have also been identified as key mediators of apoptosis, especially in the female gonads.14 Therefore, the protection afforded by S1P is very important in cell apoptosis. In this study, we evaluated whether S1P can protect ovarian follicles from vitrification-induced cell death and whether it can protect oocytes in frozen ovarian grafts to preserve the fertility of sexually immature females.15
Figure 1.

Schematic diagram of the interaction between S1P and ceramide. The intracellular levels of S1P and ceramide coordinately determine the cell fate. The S1P-mediated pathways are proliferative and antiapoptotic (acting through the inhibition of the proapoptotic protein, caspase 3). The expression of c-Myc protein c-Myc protein is modulated by ceramide-regulated pathways that lead to changes in growth arrest and apoptosis (Cuvillier et al,16 Ogretmen et al,17and Hancke et al15). S1P indicates sphingosine-1-phosphate.
Materials and Methods
Care and Use of Animals
All the FVB mice used were bred in the animal center of the National Defense Medical Center and housed under a 12-hour light/12-hour dark regime at 22°C to 24°C, with food and water supplied without restriction. All the procedures described here were reviewed and approved by the Animal Experimental Committee of the National Defense Medical Center in accordance with the Guiding Principles for the Care and Use of Laboratory Animals.
Study Design
The 20 3-week-old sexually immature female FVB mice were randomly assigned to 4 experimental groups. The ovaries in group 1 (n = 5) were used as the unvitrified fresh control. In keeping the optimal condition for the study design, we tested the dose course and time course to evaluate the most effective condition for S1P pretreatment of ovaries (Figure 2). In each group (n = 5 in each group), the whole ovaries were pricked from every direction under the light microscope and then immersed in 0, 0.5 mmol/L, or 2 mmol/L S1P solution (Biomol, Hamburg, Germany; prepared with PET as the vehicle: 5% polyethylene glycol, 2.5% ethanol, and 0.8% Tween-80), respectively, to facilitate S1P penetration and the consistence in the tissue concentration for 1 hour and then processed with vitrification. To simplify the procedure of solid surface vitrification (SSV) and to accommodate adequately the volume of the intact ovaries being frozen, we modified the vitrification procedure.20 The ovaries of groups 2 to 4 were exposed to 4% ethylene glycol in Dulbecco's phosphate-buffered saline (DPBS) + 10% fetal bovine serum (FBS) for 15 minutes. The ovaries were rinsed 3 times in a vitrification solution composed of 6 mol/L ethylene glycol and 0.4 mol/L trehalose in DPBS + 10% FBS and equilibrated in an ice bath for 5 minutes for vitrification. After their equilibration, the ovaries were dropped directly onto the top surfaces of cryostorage canes made from lightweight aluminum and firmly held in cryovials (designed for LN2 storage) containing LN2 until they had cooled to around −150°C during their partial immersion in LN2. Droplets (6 μL) of vitrification solution, each containing 1 ovary, were dropped onto the surface of aluminum foil that had been sprayed with LN2 to prevent the droplets taking a dome-shaped or a fried-egg-like form and to avoid the adherence of the vitrification solution to the surface of the foil. The droplets were moved with LN2-cooled forceps into 1-mL cryovials and then placed in LN2 for long-term storage. They were thawed by dropping them into a thaw solution composed of 0.3 mol/L trehalose in DPBS + 10% FBS at 37°C for 5 to 10 minutes and washing them 3 times with DPBS. The vitrified samples were thawed rapidly by immersing the ends of the tubes into a thawing solution composed of 1.0 mol/L sucrose in holding medium (TCM-199 + 20% FBS) for 10 minutes. The temperature of the medium used for warming was maintained at 37°C. The frozen–thawed ovarian tissues were autotransplanted under the dorsal back muscles of the animals, and the vitrified–thawed ovaries from 3-week-old mice were collected after 10 days.
Figure 2.
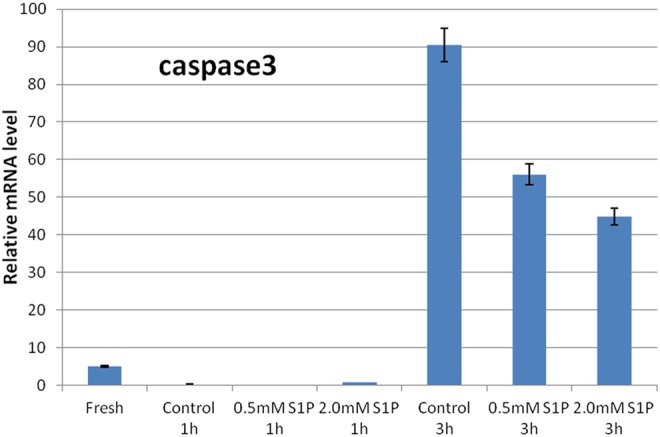
Expression of apoptosis-related marker caspase 3 by dose- and time-dependent effects of S1P-treated ovaries. A 1-hour S1P pretreatment yields the lowest expression of caspase 3 in the ovaries than that of 3-hour S1P pretreatment. S1P indicates sphingosine-1-phosphate.
Histological Analysis of Follicle Numbers
The ovaries were removed 10 days after transplantation, fixed in buffered formaldehyde, embedded in paraffin blocks, serially sectioned to a thickness of 6 μm, and stained with hematoxylin and eosin. The sections analyzed were taken at 36-μm intervals, and only follicles containing an oocyte were counted to avoid counting any follicles twice. Healthy nonatretic follicles were classified as primordial, intermediary, primary, secondary, or antral. The results of the 3 different groups were compared with those of the control group. To analyze the expression of apoptosis-related genes, including those encoding capase 3 and c-Myc, the status of the frozen–thawed ovaries was evaluated 10 days before and after transplantation. Anti-Müllerian hormone (AMH) expression and follicle counts were used to assess the ovarian reserve in the 10-day frozen–thawed grafts.
Reverse Transcription and Real-Time Quantitative Polymerase Chain Reaction
The ovaries were removed as quickly as possible after euthanasia and stored at −80°C until analysis. The total RNA was isolated from the individual treated ovaries using the Total RNA Mini Kit (Geneaid, Biotech Ltd, New Taipei City, Taiwan). The integrity of the RNA was checked by running a few microliters of each sample on an agarose gel, and its quantity and purity were analyzed spectrophotometrically.
Single-stranded complementary DNA (cDNA) was reverse transcribed from the total RNA (2 μg) using a High Capacity cDNA Reverse Transcription Kit for reverse transcriptase polymerase chain reaction (RT-PCR; Applied Biosystems Pty Ltd, UK) and random primers. The RT conditions consisted of 10 minutes of annealing at 25°C, 120 minutes of cDNA synthesis at 37°C, and 5 minutes of inactivation at 85°C.
The expression of the genes encoding c-Myc, caspase 3, AMH, and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was quantified by real-time PCR using a commercial kit (Power SYBR Green PCR Master Mix) and the StepOne Real-Time PCR System (Applied Biosystems Pty Ltd). The primers were designed using Primer3 (http://frodo.wi.mit.edu/cgi-bin/primer3/primer3_www.cgi) based on the sequences from the GenBank database. The amplification program consisted of initial activation at 95°C for 10 minutes, followed by 40 cycles of PCR (each cycle consisted of denaturation at 95°C for 15 seconds and annealing at 60°C for 60 seconds). The amount of template cDNA used in each reaction was normalized to the amount of GAPDH messenger RNA (mRNA). Amplification of the template cDNA was in the linear range for the number of PCR cycles used, and the GAPDH transcripts were RT-PCR amplified from the same amount of cDNA from each sample. The mRNA levels were normalized to those of GAPDH mRNA and were expressed as percentages of the mRNA levels measured in fresh ovaries transplanted for 1 day, which was considered to be 100%. Oligonucleotide PCR primers were selected with the assistance of a computer program (Primer3) and were designed to optimize their GC contents and melting temperatures and to minimize any hairpin and dimer formation. The PCR primers used were:
C-MYC, 5′-GCCACGTCTCCACACATCAG-3′;
C-MYC rev, 5′-TCTTGGCAGCAGGATAGTCCTT-3′;
Caspase 3, 5′-TGTCATCTCGCTCTGGTACG-3′;
Caspase 3 rev, 5′-AAATGACCCCTTCATCACCA-3′;
AMH, 5′-CTATTTGGTGCTAACCGTGGACTT-3′;
AMH rev, 5′-AAGGCTTGCAGCTGATCGAT-3′;
GAPDH, 5′-ACCCAGAAGACTGTGGATGG-3′; and
GAPDH rev, 5′-TGTGAGGGAGATGCTCAGTG-3′.
Statistical Analysis
All data are expressed as mean ± standard error of the mean and were analyzed by univariate analysis of variance. P < .05 was considered statistically significant.
Results
To investigate the effects of S1P on murine ovarian cryopreservation after SSV, we first quantified the mRNA expression of caspase 3 and c-Myc in the ovarian tissues after SSV and transplantation (Figure 3). There were no significant differences between the fresh group and the experimental groups after SSV. These results indicate that SSV had no significant effect on apoptosis. However, 10 days after transplantation, the caspase 3 and c-Myc mRNA levels of the control group (without S1P pretreatment) were significantly upregulated, indicating the occurrence of apoptosis, mainly during the process of transplantation. The S1P pretreatment reduced the amount of apoptosis-induced damage.
Figure 3.
Expression of caspase 3 and c-Myc mRNAs in the fresh, control, 0.5 mmol/L S1P, and 2 mmol/L S1P groups after vitrification (A and B) and after transplantation (C and D). There was no significant difference between the fresh group and the experimental groups after vitrification (A and B). After transplantation, the levels of caspase 3 and c-Myc mRNAs were significantly upregulated in the vitrified group without S1P pretreatment, whereas S1P pretreatment reduced apoptosis-induced damage (C and D). *P < .05, **P < .01, and ***P < .001. mRNA indicates messenger RNA; S1P, sphingosine-1-phosphate.
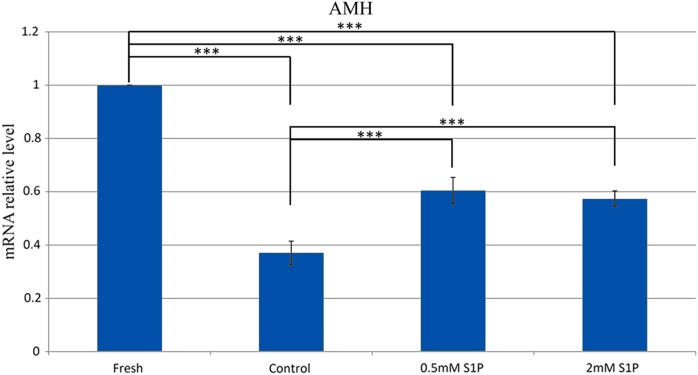
Next, we examined the levels of a granulosa cell marker (AMH) expressed in the ovaries. Cryopreservation caused a marked reduction in the level of AMH in the control group compared with that in the fresh group (P < .001), demonstrating that cryopreservation damaged the ovarian reserve without S1P pretreatment (Figure 4). In contrast, the AMH levels were significantly higher in the S1P-pretreated groups than in the control group (Figure 4), supporting the protective effect of S1P observed in the histological analysis (Figure 5). Reduced AMH levels were noted in the control and S1P groups compared with the levels in the fresh group, possibly indicating the vulnerability of the vitrified ovarian grafts, although there was no statistically significant difference between the groups pretreated with low and high concentrations of S1P.
Figure 4.
The expression of AMH mRNA in the fresh, control, 0.5 mmol/L S1P, and 2 mmol/L S1P groups 10 days after transplantation. The AMH levels were significantly higher in the S1P-pretreated groups than in the nonprotective vitrified group. Reduced AMH levels were noted in the S1P groups compared with the fresh group, but those of the low- and high-concentration S1P groups did not differ statistically significantly. The data are expressed as mean ± standard error of the mean. ***P < .001. AMH indicates anti-Müllerian hormone; mRNA, messenger RNA; S1P, sphingosine-1-phosphate.
Figure 5.
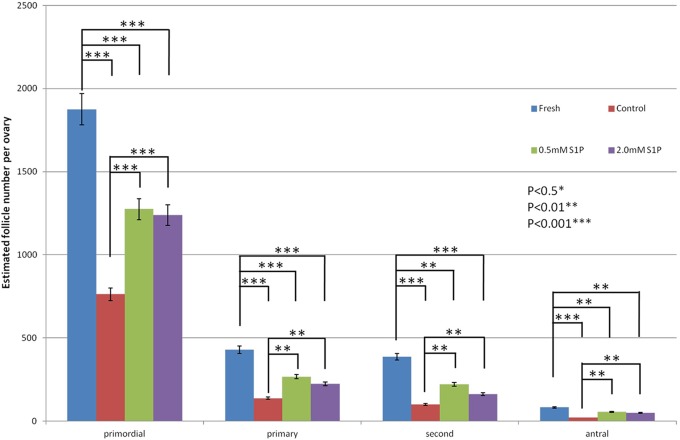
Estimated primordial, primary, secondary, and antral follicle counts were measured for the fresh, 0 mmol/L S1P, 0.5 mmol/L S1P, and 2 mmol/L S1P groups on day 10 after transplantation. A significant decline in the follicle pool was observed in the control group compared with that of the fresh group (P < .001). In the ovarian tissues pretreated with S1P, the follicle counts of all follicle types were reduced compared with those of the fresh group. The follicle pool was significantly higher in the S1P-pretreated groups than in the control group. The data are expressed as means ± standard error of the mean. *P < .05, **P < .01, ***P < .001. S1P indicates sphingosine-1-phosphate.
We also counted the number of primordial, primary, secondary, and antral follicles in the ovarian grafts 10 days after transplantation (Figures 5 and 6). A significant decline in the follicle pool was observed in the control group when compared with the fresh group (P < .001). In the S1P-treated groups, the follicle counts for all follicle types were reduced when compared with those of the fresh group (P < .01). These results indicate that although the S1P pretreatment was not completely effective, it reduced the depletion of primordial follicles and partially protected the ovaries from the damage caused by transplantation.
Figure 6.
Histological assessment of ovarian follicles for the fresh, control, 0.5 mmol/L S1P, and 2 mmol/L S1P groups on day 10 after transplantation. A, High density of a cluster of primordial follicles (arrow) and secondary follicles (arrowhead; magnification, ×200) in the fresh group. B, Medium density of primordial follicles in (arrow) and primary follicle (arrowhead, magnification ×200) in the 0.5 mmol/L S1P group. C, Medium density of primordial follicles in (arrow) and secondary follicle (arrowhead, magnification ×200) in the 2 mmol/L S1P group. D, Low density of primordial follicles (arrow) and secondary follicles (arrowhead; magnification, ×200) in control group. Bar = 50 μm. S1P indicates sphingosine-1-phosphate.
Discussion
The cryopreservation of ovarian tissues is one way to preserve the fertility of female patients with cancer. It has several advantages including its independence of the stage of sexual maturity and its suitability for prepubertal girls. The conventional strategy for ovarian tissue cryopreservation is based on a slow freezing method, but vitrification has been recently applied to ovarian tissues.21–24 Because the high concentrations of cryoprotectant required for vitrification are toxic, we assumed that these would affect the expression of some genes related to apoptosis. In this study, we examined the effects of S1P on murine ovarian cryopreservation by modified vitrification. The expression of caspase 3 and c-Myc mRNAs in the ovarian tissues did not differ significantly between the groups after SSV, indicating that ovarian injury by vitrification did not significantly affect apoptosis. When Salehnia et al evaluated the incidence of morphological changes and apoptosis in vitrified and rapidly cooled human ovarian tissues, they found that the cryopreservation protocols did not affect the incidence of apoptosis in human ovarian tissues.25 Ebrahimi et al also found that the cryotop vitrification of sheep cumulus–oocyte complexes did not affect the incidence of oocyte maturation or apoptotic gene expression.26
In this study, ovarian damage predominantly occurred during the process of transplantation and was unavoidable, even when the ovaries were pretreated with S1P. In the 4 groups tested, the apoptosis, the ovarian reserve, and the follicle counts in the ovaries after transplantation were worst in the nonprotective vitrified group, but there were no significant differences between the groups treated with 0.5 mmol/L S1P and 2 mmol/L S1P. In contrast to cryopreservation, which seems to have only a small deleterious effect on the primordial follicles, there is some evidence that the key factor responsible for follicle loss is postgrafting ischemia with hypoxia. This ischemia causes a proportion of cortical cells to undergo apoptosis after grafting. The ovary is a 3-dimensional structure composed of gametes and somatic cells. During the transplantation process, complete revascularization takes time, and the transplantation site selected is important. Therefore, reducing the damage caused by reperfusion–ischemia is an issue requiring urgent resolution. Yang et al demonstrated that the majority of apoptotic processes occur within the first week after transplantation, before well-organized blood vessels are established between the graft and the host. The survival of transplanted human ovarian tissue may be improved by treatment of both the host (with vitamin E and gonadotropins) and the graft (with vascular endothelial growth factor A [VEGF-A] and vitamins).27 Five young patients with cancer donated ovarian tissues that were transplanted into immunodeficient mice. Graft survival improved after the host was treated with melatonin, or the graft was incubated with hemagglutinin-rich biological glue, especially when combined with VEGF-A and vitamin E.28
The intracellular levels of S1P and ceramide coordinately determine the cell fate. Changes in S1P and ceramide have been implicated in a number of pathological conditions in which apoptosis plays an important role. Radiation-induced oocyte loss in adult female mice was completely prevented by an in vivo therapy with S1P or by the inhibition of ceramide-mediated apoptosis. Therefore, it has been suggested that the dynamic balance between the intracellular concentration of S1P and those of sphingosine and ceramide and the consequent activities of their respective and opposing signaling pathways are important factors determining the survival or death of mammalian cells. The protective effect of S1P during reperfusion–ischemia injury may also be useful in a cryopreservation–transplantation program. During ischemic injury, the ovarian tissues (gametes and somatic tissues) are likely to be exposed to cell death-related oxidative factors, which may be useful for S1P to lessen further cell apoptosis. Overall, our study seems to suggest that S1P is more protective against transplantation injury than against injury associated with cryopreservation. We can also use a strategy to improve the levels of oxidative stress, such as antioxidant supplementation and VEGF, to enhance the microvascular expression of S1P. Ammonium trichloro(dioxoethylene-0,0′) tellurate (AS101) is a nontoxic organotellurium compound with pleiotropic activities. The AS101 treatment reduced both apoptotic and inflammatory caspase activities and also inhibited protein tyrosine nitration in an ischemic stroke model in mice, suggesting that AS101 suppresses oxidative stress.29 In contrast to AS101, S1P has not yet been used in humans. The S1P can only be applied locally or topically (directly) but not systemically because of its tumor-inducing potential. The use of S1P requires more clinical trials in the future. The combined use of multiple supplements may be an important alternative strategy.
The S1P is a bioactive lysophospholipid present in plasma that is stored in and released from the circulating cells and that can be locally produced by vascular cells. Most of the biological effects of S1P are mediated by signaling through the cell surface receptors. It is established that the concentration of S1P in plasma is highly variable and much higher than the half-maximal concentration of S1P needed to stimulate its receptors.30
Despite unknown data about the bioavailability of S1P in mice, we use the same dosage of S1P used in the published study, which has demonstrated that S1P can protect ovarian follicles from chemotherapy-induced cell death.15
Concerning the safety issues and long-term effects, there is evidence demonstrating that injection of S1P in mouse ovarian bursa before radiotherapy and chemotherapy yields healthy litters without any significant phenotypic, behavioral, anatomic, histological, hematologic, or biochemical abnormality.15,31 Accordingly, it indicates the potential utility of S1P for fertility preservation. However, the long-term effects of S1P in the ovaries in vivo deserve to be investigated for a safeguard against the untoward side effects by S1P.
So far, no human baby has been born after the preservation of the ovary tissue by vitrification. Chen et al32 developed a modified vitrification method for murine ovarian grafts and successfully produced impregnated mice that delivered newborns. Suzuki et al33 studied vitrified ovarian grafts in monkeys (a primate model), but there were no pregnancies. Further research into the cryopreservation of the entire ovary is required in both humans and animal models.
In our study, S1P seemed to be effective in protecting vitrified ovarian grafts. The modified vitrification method we used in this rodent model was successful, and we will make every effort to apply it in humans in the future. Vitrification is still a promising technique for the cryopreservation of ovarian tissues.
The protective effects of S1P on ovarian tissue during cryopreservation by modified vitrification are demonstrable and promising. This is the first study of the use of S1P in cryopreservation and transplantation for the preservation of fertility. This use of S1P requires further study before it can be applied to humans.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported in part by grant CMNDMC9808 and in part by grant TMU100-AE1-B04, Taiwan, Republic of China.
References
- 1. Nisolle M, Casanas-Roux F, Qu J, Motta P, Donnez J. Histologic and ultrastructural evaluation of fresh and frozen-thawed human ovarian xenografts in nude mice. Fertil Steril. 2000;74(1):122–129. [DOI] [PubMed] [Google Scholar]
- 2. Chian RC, Gilbert L, Huang JY. et al. Live birth after vitrification of in vitro matured human oocytes. Fertil Steril. 2009;91(2):372–376. [DOI] [PubMed] [Google Scholar]
- 3. Radford JA, Lieberman BA, Brison DR, et al. Orthotopic reimplantation of cryopreserved ovarian cortical strips after high-dose chemotherapy for Hodgkin's lymphoma. Lancet. 2001;357(9263):1172–1175. [DOI] [PubMed] [Google Scholar]
- 4. Hancke K, Walker E, Strauch O, Göbel H, Hanjalic-Beck A, Denschlag D. Ovarian transplantation for fertility preservation in a sheep model: can follicle loss be prevented by antiapoptotic sphingosine-1-phosphate administration? Gynecol Endocrinol. 2009;25(12):839–843. [DOI] [PubMed] [Google Scholar]
- 5. Wallace WH, Anderson RA, Irvine DS. Fertility preservation for young patients with cancer: who is at risk and what can be offered? Lancet Oncol. 2005;6(4):209–218. [DOI] [PubMed] [Google Scholar]
- 6. Lobo RA. Potential options for preservation of fertility in women. N Engl J Med. 2005;353(1):64–73. [DOI] [PubMed] [Google Scholar]
- 7. Xu M, Pavone ME, Woodruff T. Fruitful progress to fertility: preserving oocytes from chemodestruction. Nat Med. 2011;17(12):1562–1563. [DOI] [PubMed] [Google Scholar]
- 8. Steponkus PL, Myers SP, Lynch DV, et al. Cryopreservation of Drosophila melanogaster embryos. Nature. 1990;345(6271):170–172. [DOI] [PubMed] [Google Scholar]
- 9. Gonfloni S, Di Tella L, Caldarola S, et al. Inhibition of the c-Abl-TAp63 pathway protects mouse oocytes from chemotherapy-induced death. Nat Med. 2009;15(10):1179–1185. [DOI] [PubMed] [Google Scholar]
- 10. Oktay K, Buyuk E, Libertella N, Akar M, Rosenwaks Z. Fertility preservation in breast cancer patients: a prospective controlled comparison of ovarian stimulation with tamoxifen and letrozole for embryo cryopreservation. J Clin Oncol. 2005;23(19):4347–4353. [DOI] [PubMed] [Google Scholar]
- 11. Silber SJ. Ovary cryopreservation and transplantation for fertility preservation. Mol Hum Reprod. 2012;18(2):59–67. [DOI] [PubMed] [Google Scholar]
- 12. Gosden RG, Baird DT, Wade JC, et al. Restoration of fertility to oophorectomized sheep by ovarian autografts stored at −196 degrees C. Hum Reprod. 1994;9(4):597–603. [DOI] [PubMed] [Google Scholar]
- 13. Baird DT, Webb R, Campbell BK, Harkness LM, Gosden RG. Long-term ovarian function in sheep after ovariectomy and transplantation of autografts stored at −196°C. Endocrinology. 1999;140(1):462–471. [DOI] [PubMed] [Google Scholar]
- 14. Morita Y, Perez GI, Paris F, et al. Oocyte apoptosis is suppressed by disruption of the acid sphingomyelinase gene or by sphingosine-1-phosphate therapy. Nat Med. 2000;6(10):1109–1114. [DOI] [PubMed] [Google Scholar]
- 15. Hancke K, Strauch O, Kissel C, Göbel H, Schäfer W, Denschlag D. Sphingosine 1-phosphate protects ovaries from chemotherapy-induced damage in vivo. Fertil Steril. 2007;87(1):172–177. [DOI] [PubMed] [Google Scholar]
- 16. Cuvillier O, Pirianov G, Kleuser B, et al. Suppression of ceramide-mediated programmed cell death by sphingosine-1-phosphate. Nature. 1996;381(6585):800–803. [DOI] [PubMed] [Google Scholar]
- 17. Ogretmen B, Hannun YA. Biologically active sphingolipids in cancer pathogenesis and treatment. Nat Rev Cancer. 2004;4(8):604–616. [DOI] [PubMed] [Google Scholar]
- 18. Spiegel S, Kolesnick R. Sphingosine 1-phosphate as a therapeutic agent. Leukemia. 2002;16(9):1596–1602. [DOI] [PubMed] [Google Scholar]
- 19. Perry DK. Ceramide and apoptosis. Biochem Soc Trans. 1999;27(4):399–404. [DOI] [PubMed] [Google Scholar]
- 20. Lane M, Gardner DK. Vitrification of mouse oocytes using a nylon loop. Mol Reprod Dev. 2001;58(3):342–347. [DOI] [PubMed] [Google Scholar]
- 21. Yeoman RR, Wolf DP, Lee DM. Coculture of monkey ovarian tissue increases survival after vitrification and slow-rate freezing. Fertil Steril. 2005;83 (suppl 1):1248–1254. [DOI] [PubMed] [Google Scholar]
- 22. Sugimoto M, Maeda S, Manabe N, Miyamoto H. Development of infantile rat ovaries autotransplanted after cryopreservation by vitrification. Theriogenology. 2000;53(5):1093–1103. [DOI] [PubMed] [Google Scholar]
- 23. Migishima F, Suzuki-Migishima R, Song SY, et al. Successful cryopreservation of mouse ovaries by vitrification. Biol Reprod. 2003;68(3):881–887. [DOI] [PubMed] [Google Scholar]
- 24. Salehnia M, Abbasian Moghadam E, Rezazadeh Velojerdi M. Ultrastructure of follicles after vitrification of mouse ovarian tissue. Fertil Steril. 2002;78(3):644–645. [DOI] [PubMed] [Google Scholar]
- 25. Salehnia M, Sheikhi M, Pourbeiranvand S, Lundqvist M. Apoptosis of human ovarian tissue is not increased by either vitrification or rapid cooling. Reprod Biomed Online. 2012;25(5):492–499. [DOI] [PubMed] [Google Scholar]
- 26. Ebrahimi B, Valojerdi MR, Eftekhari-Yazdi P, Baharvand H. In vitro maturation, apoptotic gene expression and incidence of numerical chromosomal abnormalities following cryotop vitrification of sheep cumulus-oocyte complexes. J Assist Reprod Genet. 2010;27(5):239–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Abir R, Fisch B, Jessel S, Felz C, Ben-Haroush A, Orvieto R. Improving posttransplantation survival of human ovarian tissue by treating the host and graft. Fertil Steril. 2011;95(4):1205–1210. [DOI] [PubMed] [Google Scholar]
- 28. Friedman O, Orvieto R, Fisch B, et al. Possible improvements in human ovarian grafting by various host and graft treatments. Hum Reprod. 2012;27(2):474–482. [DOI] [PubMed] [Google Scholar]
- 29. Okun E, Arumugam TV, Tang SC, et al. The organotellurium compound ammonium trichloro(dioxoethylene-0,0′) tellurate enhances neuronal survival and improves functional outcome in an ischemic stroke model in mice. J Neurochem. 2007;102(4):1232–1241. [DOI] [PubMed] [Google Scholar]
- 30. Hammad SM, Al Gadban MM, Semler AJ, Klein RL. Sphingosine 1-phosphate distribution in human plasma: associations with lipid profiles. J Lipids. 2012;2012:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Paris F, Perez GI, Fuks Z, et al. Sphingosine 1-phosphate preserves fertility in irradiated female mice without propagating genomic damage in offspring. Nat Med. 2002;8(9):901–902. [DOI] [PubMed] [Google Scholar]
- 32. Chen SU, Chien CL, Wu MY, et al. Novel direct cover vitrification for cryopreservation of ovarian tissues increases follicle viability and pregnancy capability in mice. Hum Reprod. 2006;21(111):2794–2800. [DOI] [PubMed] [Google Scholar]
- 33. Suzuki N, Hashimoto S, Igarashi S, et al. Assessment of long-term function of heterotopic transplants of vitrified ovarian tissue in cynomolgus monkeys. Hum Reprod. 2012;27(8):2420–2429. [DOI] [PubMed] [Google Scholar]