Abstract
Free radical–induced reperfusion injury is a recognized cause of brain damage in the newborn after birth asphyxia. The xanthine oxidase inhibitor allopurinol reduces free radical synthesis and crosses the placenta easily. Therefore, allopurinol is a promising therapeutic candidate. This study tested the hypothesis that maternal treatment with allopurinol during fetal asphyxia limits ischemia–reperfusion (I/R) damage to the fetal brain in ovine pregnancy. The I/R challenge was induced by 5 repeated measured compressions of the umbilical cord, each lasting 10 minutes, in chronically instrumented fetal sheep at 0.8 of gestation. Relative to control fetal brains, the I/R challenge induced significant neuronal damage in the fetal hippocampal cornu ammonis zones 3 and 4. Maternal treatment with allopurinol during the I/R challenge restored the fetal neuronal damage toward control scores. Maternal treatment with allopurinol offers potential neuroprotection to the fetal brain in the clinical management of perinatal asphyxia.
Keywords: sheep, fetus, asphyxia, allopurinol, neuroprotection
Introduction
During labor and delivery, the most common challenge to the fetus is repeated compression of the umbilical cord. If severe or prolonged enough, this may result in perinatal asphyxia, leading to marked fetal acidosis and cardiovascular compromise with subsequent hypoxic–ischemic encephalopathy (HIE), predictive of future cerebral palsy and cognitive disability.1 The incidence of perinatal asphyxia is estimated to be 2 to 4 per 1000 full-term neonates, of whom 15% to 20% will die and about 25% of the survivors will permanently have neuropsychological deficits.2
To date, the only established treatment for HIE is therapeutic hypothermia of the neonate.3,4 However, only mild to moderately asphyxiated infants seem to significantly benefit and only a small reduction of death or disability is seen.3,5,6 Therefore, there is a great need for additional treatment options to help prevent or diminish cerebral damage in the infant due to perinatal asphyxia.
Repeated compressions of the umbilical cord not only induce fetal asphyxia and acidosis but also induce episodes of ischemia–reperfusion (I/R), promoting the excessive generation of reactive oxygen species (ROS) such as the superoxide (O2 .−) and hydroxyl (OH−) anions.7–9 Several potential antioxidant pharmacologic options to intervene in the putative neurotoxic cascade have already been studied.6,10 Drugs like tetrahydrobiopterin, melatonin, neuronal nitric oxide synthase (nNOS) inhibitors, xenon, and vitamin C showed promising results in experimental studies but have not yet been translated to clinical use.6,11
One of the main prooxidant pathways stimulated by I/R is the xanthine oxidase pathway.12,13 Allopurinol, a xanthine oxidase inhibitor and Food and Drug Administration (FDA)-approved drug, inhibits the conversion of hypoxanthine into xanthine and uric acid, thereby limiting the toxic overproduction of ROS. In high doses, it also functions as a chelator of nonprotein bound iron (NPBI) and a direct scavenger of the hydroxyl radical.14 Furthermore, substantial knowledge on the use of allopurinol in terms of dose and in vivo safety profiles is already available.15–21 Collectively, these properties make allopurinol a strong potential candidate for cerebral neuroprotection arising from I/R damage.
A recent follow-up study of 2 prospective randomized controlled trials determining the effects of postnatally administered allopurinol in term asphyxiated human neonates showed a beneficial effect of treatment on mortality and severe disabilities at 4 to 8 years of age but only in the moderately asphyxiated group.20 It was further reported that treatment with allopurinol of the asphyxiated neonate improved neonatal outcome.15 However, if the time interval between I/R and treatment had been prolonged, or again when asphyxia had been too severe, no reduction in serious morbidity or mortality was observed.16 Therefore, there has been accumulating interest in establishing whether the perinatal cerebral outcome may be improved if the window of treatment with allopurinol is advanced, for instance via maternal treatment to cover the actual period of fetal asphyxia and of I/R in complicated labor. Maternal treatment with allopurinol crosses the placenta, it suppresses O2 .− production in the fetus22 and yields therapeutic levels in the neonatal circulation,23 justifying this route of administration for preventative therapy in obstetric practice.
Therefore, this study tested the hypothesis that maternal treatment with allopurinol during repeated episodes of fetal asphyxia would limit I/R damage to the fetal brain. The hypothesis was tested in chronically instrumented fetal sheep in late gestation subjected to an I/R challenge involving repeated, measured compression of the umbilical cord with or without maternal treatment with allopurinol.
Materials and Methods
Ethical Approval
The study was approved by the Cambridge University Ethical Review Committee. All procedures were performed under the UK Animals (Scientific Procedures) 1986 Act and conducted under the authority of the appropriate project and personal licenses.
Animals and Surgical Procedures
A total of 11 Welsh Mountain sheep were surgically instrumented for long-term recording at 124 days of gestation (term is ∼145 days) using strict aseptic conditions, as previously described in detail.19,24,25 Under general anesthesia (1.5%-2.0% halothane in 50:50 O2-N2O), midline abdominal and uterine incisions were made, the fetal hind limbs were exteriorized and, on one side, fetal arterial (inner diameter [id], 0.86 mm; outer diameter [od], 1.52 mm; Critchley Electrical Products, New South Wales, Australia) and venous (id, 0.56 mm; od, 0.96 mm) catheters were inserted. Another catheter was anchored onto the fetal hind limb for recording of the reference amniotic pressure. In addition, a transit time flow transducer was implanted around the left umbilical artery close to the common umbilical artery inside the fetal abdominal cavity (4SB; Transonic Systems Inc, Ithaca, New York).24,26 An inflatable occluder cuff (In Vivo Metrics, Healdsburg, California) was positioned around the proximal end of the umbilical cord, as described previously in detail.19,26 Ewes were instrumented with arterial and venous catheters placed in the left femoral artery and vein, respectively. All incisions were closed in layers. The catheters, occluder cable, and flow probe leads were then exteriorized via a keyhole incision in the maternal flank and kept inside a plastic pouch sewn onto the maternal skin.
Postoperative Care and Experimental Protocol
Antibiotics were administered daily to the ewe (0.20-0.25 mg/kg intramuscularly [im] Depocillin; Mycofarm, Cambridge, UK) and fetus intravenously (iv) and into the amniotic cavity (150 mg/kg Penbritin; SmithKline Beecham Animal Health, Welwyn Garden City, Hertfordshire, UK). Following at least 5 days of postoperative recovery, all fetuses were submitted to an I/R challenge produced by 5 × 10 minutes inflations of the cord occluder with sterile saline at 10-minute intervals. Each cord compression was designed to reduce umbilical blood flow by 80% to 90% from baseline and to lead to a progressive fall in fetal arterial pH to 6.9 by the end of the fifth compression. In 5 fetuses, the I/R challenge was induced during maternal iv treatment with allopurinol (Sigma Ltd, Watford, Hertfordshire, UK, 20 mg/kg maternal weight, dissolved in buffered saline and infused over a 20-minute period). In the remaining 6 fetuses, the I/R challenge was induced during maternal infusion with buffered saline at the same rate. Infusion of either allopurinol or vehicle started 10 minutes before the fourth umbilical cord compression and finished immediately after the end of it. The dosing regimen of allopurinol was adopted from the only study that used the drug in women undergoing uncomplicated labor.23
Blood Sampling Regimen
To determine arterial blood gas, acid base, and metabolic status, maternal and fetal arterial blood samples (0.3 mL) were drawn into sterile syringes 1 hour prior to the I/R challenge, at 10-minute intervals during and for 48 hours following the I/R challenge (ABL5 Blood Gas Analyzer; Radiometer, Copenhagen, Denmark; see Figure 1). Values for percentage saturation of hemoglobin with oxygen (SatHb) were determined using a hemoximeter (OSM3; Radiometer). Blood glucose and lactate concentrations were measured using an automated analyzer (Yellow Springs 2300 Stat Plus; YSI Ltd, Farnborough, UK). Additional paired maternal and fetal blood samples (1 mL) were taken in the allopurinol-treated pregnancies at varying set intervals, starting at the onset of the infusion period and up to 5 hours following the end of infusion, to compile a comprehensive serial profile of maternal and fetal plasma concentrations of allopurinol and oxypurinol without affecting maternofetal concentrations of hemoglobin. Reversed-phase high-performance liquid chromatography with UV detection at 254 nm was used for the quantification of allopurinol and oxypurinol in both the fetal and maternal plasma. The method was linear between 0.5 and 25 mg/L, with a lower limit of detection of 0.2 mg/L for both compounds.27
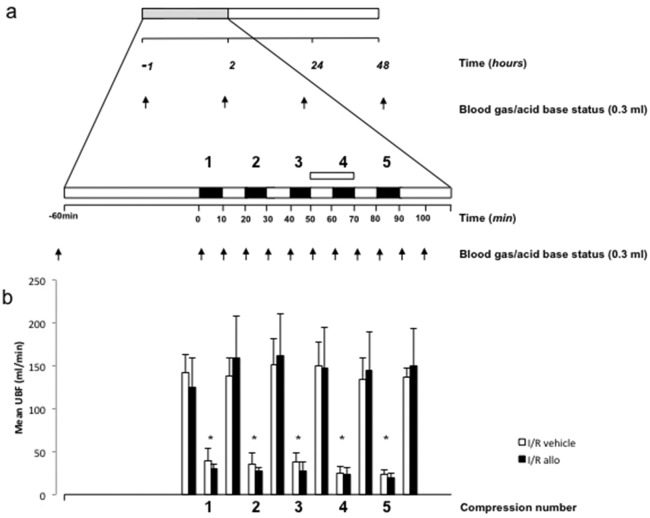
Figure 1.
A, Experimental protocol. At least 5 days after surgery, all fetuses were subjected to an ischemia–reperfusion (I/R) challenge (gray bar). After 1 hour of basal recording, the I/R challenge consisted of 5 compressions of the umbilical cord, each of 10 minutes’ duration (black bars) with a 10-minute interval. Maternal infusion with allopurinol or vehicle started 10 minutes before the fourth umbilical cord compression and finished immediately after the end of it (white bar). Fetal arterial blood samples (arrows) were taken for analysis of blood gas and metabolic status. B, Data are umbilical blood flow (UBF) mean ± standard error of the mean (SEM), before and after each umbilical cord compression in 6 I/R allopurinol pregnancies (black bars) and in 6 I/R vehicle pregnancies (white bars). Significant differences *P < .05 versus baseline (2-way repeated measures analysis of variance with t Newman-Keuls test).
Histological Evaluation
Forty-eight hours after the end of the experimental protocol, ewes and fetuses were subjected to humane euthanasia using a lethal dose of sodium pentobarbitone (200 mg/kg iv Pentoject; Animal Ltd, York, UK) for tissue collection. Tissues were also harvested from 6 uninstrumented fetal sheep at 0.8 gestation, which served as age-matched controls. Immediately after euthanasia, a cesarean section was performed and the fetal brains were perfusion fixed with formaldehyde delivered through the carotid arteries under constant pressure. Fixed brains were embedded in paraffin. Coronal sections of 4 µm were cut at the level of the dorsal hippocampus, caudate nucleus, and cerebellum and stained with hematoxylin and eosin (H&E) and a combined staining procedure using acid fuchsin–thionin (0.1% fuchsin, Gurr/BDH; 0.25% thionin, Chroma). The latter stain highlights (acidophilic) damaged neurons; these neurons have a bright pink cytoplasm on a blue Nissl background. Cells were considered to be necrotic if they had acidophilic cytoplasm, nuclear pyknosis, or loss of nuclear detail. The proportion of necrotic cells was determined in the hippocampus (cornu ammonis [CA] areas 1 + 2 and 3 + 4, dentate gyrus, and parahippocampal cortex), thalamus, and caudate nucleus by light microscopy (Zeiss standard 25, Germany) at ×100 and ×400 magnification. Examination was performed using a 6-point scale: 0 (0%), 1 (>0%-10%), 2 (>10%-50%), 3 (>50%-90%), 4 (>90%-99%), and 5 (100%).28–30 Sections were randomly numbered and scored simultaneously using a dual viewing attachment by 2 investigators (J.J.K. and M.G.H.) who were unaware of the experimental groups.
To assess apoptosis, another set of 4 µm coronal sections of the hippocampus, thalamus, basal nuclei, cortex, and white matter was stained with a cleaved caspase 3 stain. The degree of apoptosis was determined using a 3-point scoring system as previously described.31 Sections were randomly numbered and scored simultaneously using a dual viewing attachment by 2 investigators (H.L.T. and P.G.J.N.) who were unaware of the experimental groups.
Data and Statistical Analyses
Cardiovascular data were recorded continually at 1 second intervals using a computerized Data Acquisition System (Department of PDN, University of Cambridge, UK). Summary measures analysis was applied to the cardiovascular serial data to focus the number of comparisons, and the areas under the curve were calculated for statistical comparison, as previously described in detail.32 For all variables, values are expressed as mean ± standard error of the mean (SEM). Comparisons between groups were assessed using a 1- or 2-way analysis of variance (ANOVA) with an appropriate post hoc test. The relationships between indices of fetal brain damage and values for fetal arterial blood gases or acid base and metabolic status were assessed using the Pearson product moment correlation. For all comparisons, P < .05 was considered significant.
Results
Postoperative recovery time was 5 days in all groups before the start of the experiments.
Plasma Levels of Allopurinol
Administration of allopurinol to pregnant ewes led to elevations in fetal plasma levels of allopurinol between 4 and 7 mg/L within 90 minutes of the start of the infusion. These fetal plasma concentrations of allopurinol are within the human therapeutic range.23 Plasma levels of the active metabolite oxypurinol rose more gradually to levels between 1 and 1.5 mg/L at 2 hours after maternal administration, and these elevations lasted much longer, returning toward baseline 5 hours after treatment.
Maternal Variables
Both maternal heart rate (MHR) and mean arterial pressure (MAP) were not different between groups (allopurinol: MAP 99 ± 5 mm Hg, MHR 91 ± 5 beats per minute [bpm]; vehicle: MAP 104 ± 10 mm Hg, MHR 97 ± 6 bpm;). The MAP remained stable from baseline throughout the experimental protocol in both groups, but MHR transiently increased from baseline to a maximum of 156 ± 20 bpm at 52 minutes after the onset of infusion in ewes treated with allopurinol (P > .05). Basal maternal arterial blood gas and acid base status did not differ between the allopurinol- and vehicle-treated ewes (allopurinol group: pH 7.52 ± 0.01, PaCO2 37 ± 0.4 mm Hg, PaO2 100 ± 4 mm Hg, acid/base excess [ABE] 7.0 ± 0.9 mEq/L, SatHb 97% ± 1%; vehicle group: pH 7.52 ± 0.01, PaCO2 39 ± 1 mm Hg, PaO2 103 ± 3 mm Hg, ABE 7.9 ± 0.9 mEq/L, SatHb 97% ± 1%), and remained unaffected throughout the experimental protocol.
Fetal Arterial Blood Gas, Acid/Base, and Metabolic Status
Repeated compressions of the umbilical cord transiently decreased fetal PaO2, SatHb, pH, and ABE and increased PaCO2, glucose, and lactate concentrations (Table 1). Changes in the vehicle- and allopurinol-treated groups, respectively, before and at the end of the I/R challenge are summarized as follows: fetal pH was reduced from 7.36 ± 0 at baseline to 6.97 ± 0.03 versus 7.36 ± 0 to 6.97 ± 0.03 by the end of the fifth compression (both groups P < .05), ABE was reduced from 4.8 ± 0.5 to −15 ± 1.7 mEq/L versus 3.6 ± 0.8 to −15 ± 1.3 mEq/L (both groups P < .05), PaO2 was reduced from 21 ± 2 to 14 ± 1 mm Hg versus 21 ± 2 to 13 ± 2 mm Hg (both groups P < .05), SatHb was reduced from 55% ± 5% to 22% ± 2% versus 58% ± 7% to 23% ± 6% (both groups P < .05), PaCO2 was increased from 58 ± 1 to 91 ± 6 mm Hg versus 55 ± 2 to 91 ± 5 mm Hg (both groups P < .05), lactate levels rose from 1.1 ± 0.2 to 9.8 ± 1.7 mmol/L versus 1.1 ± 0.2 to 9.9 ± 1.2 mmol/L (both groups P < .05) and glucose levels rose from 1.0 ± 0.1 to 1.7 ± 0.1 mmol/L versus 0.8 ± 0.1 to 1.2 ± 0.3 mmol/L (both groups P < .05). There were no differences in the magnitude of any change in arterial blood gas, acid–base excess, or metabolic status between groups.
Table 1.
Fetal Arterial Blood Gases and Metabolic Status.a
| Experimental Group | Baseline | At Start Infusion | After End of Infusion | +48 hours Post | |
|---|---|---|---|---|---|
| pHa | I/R vehicle | 7.36 ± 0.01 | 7.11 ± 0.03b | 6.97 ± 0.03b | 7.34 ± 0.01 |
| I/R allopurinol | 7.36 ± 0.01 | 7.07 ± 0.02b | 6.97 ± 0.03b | 7.35 ± 0.01 | |
| ABE, mEq/L | I/R vehicle | 4.8 ± 0.5 | −6.7 ± 2.1b | −15.0 ± 1.7b | 3.0 ± 0.8 |
| I/R allopurinol | 3.6 ± 0.8 | −9.0 ± 0.98b | −15.0 ± 1.3b | 2.8 ± 0.5 | |
| PaO2, mm Hg | I/R vehicle | 21.3 ± 2.0 | 14.2 ± 1.9b | 14.0 ± 1.4b | 23.8 ± 2.0 |
| I/R allopurinol | 20.8 ± 1.9 | 13.6 ± 2.4b | 13.4 ± 3.7b | 21.4 ± 1.3 | |
| PaCO2, mm Hg | I/R vehicle | 58.0 ± 1.2 | 81.7 ± 3.4b | 90.8 ± 5.5b | 57.0 ± 0.91 |
| I/R allopurinol | 55.2 ± 2.0 | 84.4 ± 5.4b | 90.6 ± 5.4b | 54.8 ± 1.2 | |
| SatHb, % | I/R vehicle | 55.3 ± 5.0 | 24.0 ± 3.4b | 21.8 ± 2.1b | 58.8 ± 5.7 |
| I/R allopurinol | 57.9 ± 7.4 | 24.6 ± 5.0b | 22.8 ± 5.9b | 58.1 ± 5.5 | |
| Lactate, mmol/L | I/R vehicle | 1.13 ± 0.20 | 6.11 ± 1.17b | 9.79 ± 1.24b | 0.92 ± 0.14 |
| I/R allopurinol | 1.05 ± 0.20 | 6.09 ± 0.94b | 9.09 ± 0.99b | 1.19 ± 0.27 | |
| Glucose, mmol/L | I/R vehicle | 0.97 ± 0.09 | 1.66 ± 0.29b | 1.72 ± 0.10b | 1.04 ± 0.16 |
| I/R allopurinol | 0.80 ± 0.11 | 1.65 ± 0.38b | 1.18 ± 0.30b | 1.05 ± 0.23 |
Abbreviations: ABE, acid/base excess; I/R, ischemia–reperfusion; SatHb, saturation of hemoglobin with oxygen.
a Fetal physiologic parameters (arterial pH, blood gases, glucose, and lactate values) 60 minutes before umbilical cord compression (baseline), immediately before and immediately after the end of infusion of allopurinol or buffered saline, and at 48 hours after the I/R challenge (see Materials and Methods and Figure 1).
b Significant differences within groups are P < .05 versus baseline (2-way repeated measures analysis of variance with post hoc t Newman-Keuls test). There were no significant differences between the 2 treatment groups.
Assessment of Neuronal Necrosis
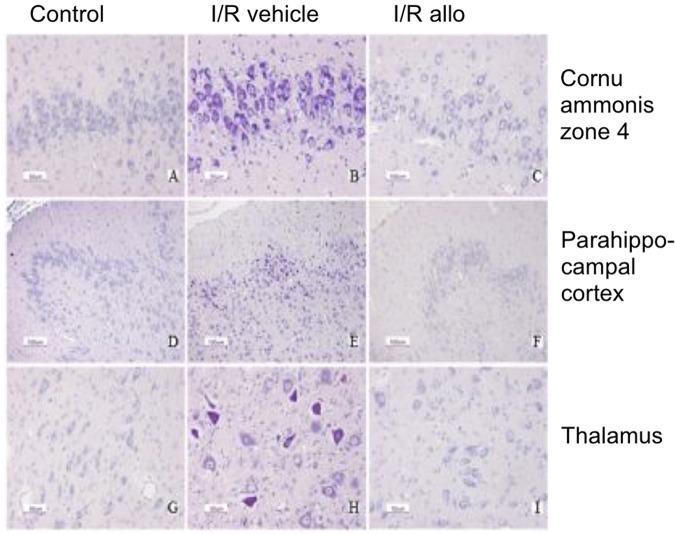
Relative to controls, the I/R challenge induced significantly greater neuronal damage in the hippocampal CA zones 3 and 4 of fetal sheep brains (Figures 2 and 3). Maternal treatment with allopurinol during the I/R challenge restored fetal cerebral neuronal damage in these areas toward control scores (Figures 2 and 3). Differences in fetal brain neuronal damage between control and I/R groups, and relative protection following I/R by maternal allopurinol treatment, were also prominent although outside statistical significance (P = .08), in the fetal dentate gyrus and thalamic regions (Figure 3).
Figure 2.
Histopathological images; acid fuchsin thionin staining. Representative images of the histopathological appearance of fetal sheep brains at 0.8 of gestation in controls (A, D, and G), the ischemia–reperfusion (I/R) vehicle group (B, E, and H), and the I/R allopurinol group (C, F, and I). Panels A to C represent zone 4 of the cornu ammonis; panels D to F are images of the parahippocampal cortex; and panels G, H, and I represent the thalamus. An acid fuchsin and thionin staining was used to detect neuronal loss (acidophilic positive neurons).
Figure 3.
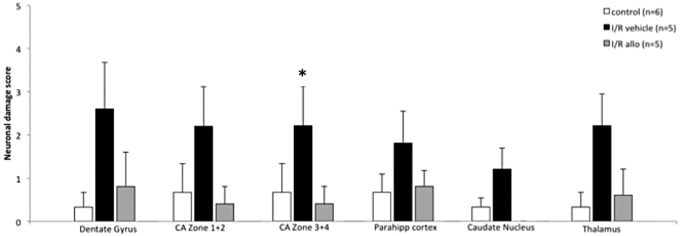
Neuronal damage scores in fetal sheep brain. Values are mean ± standard error of the mean (SEM). *P < .05 versus control (repeated measures analysis of variance + Bonferroni test).
Assessment of Neuronal Apoptosis
Relative to controls, significantly greater apoptosis in the basal nuclei and cortex was measured in fetuses subjected to the I/R challenge. However, the magnitude of apoptosis in these fetal brain regions was similar between vehicle- and allopurinol-treated groups (Figure 4). Differences in the extent of neuronal apoptosis between control and fetuses subjected to the I/R challenge, independent of allopurinol treatment, were also apparent in other fetal brain regions. However, these differences fell outside statistical significance (P = .08; Figure 4).
Figure 4.
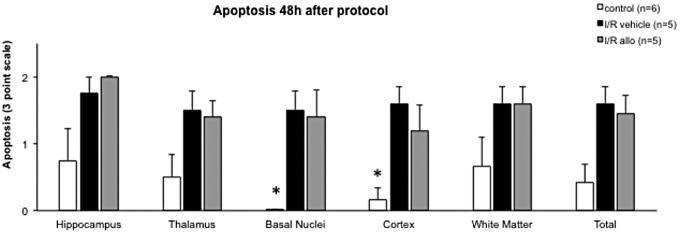
Apoptosis (assessed with cleaved caspase 3) in fetal sheep brain. Values are mean ± standard error of the mean (SEM). *P < .05 versus ischemia–reperfusion (I/R) vehicle and I/R allopurinol (1-way analysis of variance + Bonferroni).
When variables representing fetal arterial blood gases, acid/base, and metabolic status were correlated to indices of cerebral necrosis or apoptosis in all brain regions within individual animals for all groups, no significant relationships were found.
Fetal Cardiovascular Variables
Data concerning fetal cardiovascular variables are described in detail in a previous article by our group.19 These data show that maternal treatment with allopurinol helped maintain umbilical blood flow and reduced fetal cardiac oxidative stress after I/R.
Discussion
This study was designed to investigate the potential neuroprotective effects of the FDA-approved drug allopurinol, when administered to the mother during intrauterine asphyxia at term. To test the hypothesis, we developed an experimental model to simulate perinatal asphyxia by using 5 intermittent umbilical cord occlusions, leading to clinically relevant metabolic acidosis with subsequent brain damage in late gestation fetal sheep. At the start of maternal allopurinol infusion, mean pH was 7.07 ± 0.02 and 7.11 ± 0.03 in the I/R allopurinol and I/R vehicle groups, respectively, indicating severe fetal asphyxia. In the human clinical setting, this would be the time point to start preparing an emergency cesarean section or instrumented vaginal delivery, a window of time in which maternal infusion with allopurinol could be incorporated.
Data show that in the late gestation ovine fetus repeated, intermittent, measured compressions of the umbilical cord led to neuronal cell damage and apoptosis in most brain areas examined. Ischemia–reperfusion induced significantly greater neuronal damage in the hippocampal CA zones 3 and 4, as compared to nonischemic controls. Differences between groups were prominent, although outside statistical significance, in the dentate gyrus and thalamus regions. Maternal administration with allopurinol during I/R restored fetal neuronal damage toward control scores, confirming a possible role for xanthine oxidase in the pathway leading to neuronal damage and supporting a potential neuroprotective effect of antenatal antioxidant treatment.
Several experimental models to induce fetal asphyxia have previously been performed; partial occlusion of the umbilical cord for 30 minutes to several hours, repetitive infrequent occlusions lasting 5 minutes, occlusions of increasing length and/or frequency, and (nearly) total occlusion for 10 minutes, as in our model.28,29,33–40 Although a broad variation in distribution patterns of neuronal damage and apoptosis has been identified after using these different techniques, the observed (para)hippocampal vulnerability has frequently been described.28,34,35,41 In our study, the hippocampus and the parahippocampal cortex, areas involved in memory and spatial orientation and navigation, were also the most severely damaged brain areas, results in keeping with the characteristics of such studies.
Apparent differences in histological brain damage between the I/R allopurinol and I/R vehicle groups that did not reach statistical significance in the present study are most likely due to the small number of participants used. Alternatively, a lack of an effect of allopurinol treatment could reflect brain damage triggered via pro-oxidant pathways other than via the activation of xanthine oxidase. Several other pathways may promote oxidative stress, such as the glutamate-induced excitotoxicity, which proceeds via N-methyl-d-aspartate receptor activation, producing Ca2+ influx and thereby activation of Ca2+-dependent NOS, particularly nNOS. At high concentrations, NO− reacts with superoxide (O2 .−) to produce peroxynitrite (ONOO−), which in turn induces lipid peroxidation and mitochondrial nitrosylation. Consequently, mitochondrial dysfunction and membrane depolarization develop with further release of O2 .−. An increased influx of calcium also leads to the activation of cytosolic phospholipases, increasing eicosanoid release and inflammation with concomitant accumulation of neutrophils. A combined therapeutic approach using drugs which influence different parts of the neurotoxic cascade, like melatonin42 or tetrahydrobiopterin, might therefore further improve the outcome of antioxidant therapy.
In the present study, analysis of indices of apoptosis also revealed significant programmed cell death in most brain areas of fetal sheep that underwent I/R. However, maternal treatment with allopurinol during the I/R challenge did not have a significant effect on reducing the amount of apoptotic brain cells. This may be because histological assessment was performed at 48 hours after I/R , while the process of apoptosis normally reaches its maximum 72 hours after a hypoxic–ischemic event.30
Previous studies have reported on effects of allopurinol in postasphyxial reperfusion brain damage in both animals and humans. Although allopurinol has been administered both before and after hypoxic–ischemic insults in these studies, most of the experiments have been carried out postnatally. Only 5 other studies in the literature have reported on antenatally administered allopurinol, but none of them have described any effects on the histology of the brain.18,19,22,23,43
In the present study, therapeutic ranges of allopurinol and its metabolite oxypurinol have shown to be attainable after antenatal maternal treatment with allopurinol, while no adverse side effects were observed. These findings are in line with previous studies in both animals and humans.18,22,23,43 Masaoka et al were the first to investigate antenatally administered allopurinol during intermittent umbilical cord occlusion in fetal sheep and described a significantly reduced production of superoxide in the allopurinol-treated animals.22 This indicates a direct effect of allopurinol in preventing the production of free radicals, in scavenging free radicals once formed, or both. These results were confirmed in the ovine fetuses used in the present study, in which we found reduced indices of oxidative stress in the cardiovascular system after maternal treatment with allopurinol.19 A randomized controlled human clinical pilot study performed by our group has also shown a significant reduction in NPBI in the cord blood of allopurinol-treated infants, confirming the oxidative stress-reducing effect of allopurinol in humans. Furthermore, an inverse correlation between levels of allopurinol and levels of S-100B, a marker of brain damage, in umbilical cord blood was seen, further indicating a potential neuroprotective effect.18
In summary, this is the first report investigating the protective value of antenatal administration of allopurinol during birth asphyxia on brain damage in the late gestation sheep fetus. The data support the hypothesis tested that maternal treatment with allopurinol during repeated episodes of fetal asphyxia would limit I/R damage to the fetal brain. Maternal treatment with allopurinol therefore remains a promising therapeutic option to complement neonatal head cooling. A large prospective multicenter placebo-controlled trial in the Netherlands, investigating the effect of maternal administration of allopurinol on markers of brain damage and neonatal outcome in humans has recently completed recruitment.44
Acknowledgments
We thank Mr Scott Gentle and Mrs Sue Nicholls for their help with animal maintenance.
Footnotes
Authors’ Note: This study was performed at the Department of Physiology, University of Cambridge, UK. An abstract for this work received the President’s Presenter Award from The Society for Gynecologic Investigation at the 58th Annual Meeting, Miami, USA; 2011 (Joepe J. Kaandorp).
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Supported by funding from the Perinatal Center Wilhelmina Children’s Hospital Utrecht , the Royal Dutch Academy of Sciences (Ter Meulen Fonds), the Dutch Institute for Scientific Research (NWO), the Dutch Society of Obstetrics and Gynecology (NVOG), and The British Heart Foundation.
References
- 1. Cowan F, Rutherford M, Groenendaal F, et al. Origin and timing of brain lesions in term infants with neonatal encephalopathy. Lancet. 2003;361(9359):736–742. [DOI] [PubMed] [Google Scholar]
- 2. Vannucci RC, Perlman JM. Interventions for perinatal hypoxic-ischemic encephalopathy. Pediatrics. 1997;100(6):1004–1014. [DOI] [PubMed] [Google Scholar]
- 3. Gluckman PD, Wyatt JS, Azzopardi D, et al. Selective head cooling with mild systemic hypothermia after neonatal encephalopathy: multicentre randomised trial. Lancet. 2005;365(9460):663–670. [DOI] [PubMed] [Google Scholar]
- 4. Gunn AJ, Thoresen M. Hypothermic neuroprotection. NeuroRx. 2006;3(2):154–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Edwards AD, Brocklehurst P, Gunn AJ, et al. Neurological outcomes at 18 months of age after moderate hypothermia for perinatal hypoxic ischaemic encephalopathy: synthesis and meta-analysis of trial data. BMJ. 2010;340:c363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Robertson NJ, Tan S, Groenendaal F, et al. Which neuroprotective agents are ready for bench to bedside translation in the newborn infant? J Pediatr. 2012;160(4):544–552.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Clapp JF, Peress NS, Wesley M, Mann LI. Brain damage after intermittent partial cord occlusion in the chronically instrumented fetal lamb. Am J Obstet Gynecol. 1988;159(2):504–509. [DOI] [PubMed] [Google Scholar]
- 8. Masaoka N, Hayakawa Y, Ohgame S, Sakata H, Satoh K, Takahashi H. Changes in purine metabolism and production of oxygen free radicals by intermittent partial umbilical cord occlusion in chronically instrumented fetal lambs. J Obstet Gynaecol Res. 1998;24(1):63–71. [DOI] [PubMed] [Google Scholar]
- 9. Rogers MS, Wang CC, Lau TK, et al. Relationship between isoprostane concentrations, metabolic acidosis, and morbid neonatal outcome. Clin Chem. 2005;51(7):1271–1274. [DOI] [PubMed] [Google Scholar]
- 10. Fan X, van Bel F. Pharmacological neuroprotection after perinatal asphyxia. J Matern Fetal Neonatal Med. 2010;23(suppl 3):17–19. [DOI] [PubMed] [Google Scholar]
- 11. Miller SL, Wallace EM, Walker DW. Antioxidant therapies: a potential role in perinatal medicine. Neuroendocrinology. 2012;96(1):13–23. [DOI] [PubMed] [Google Scholar]
- 12. Saugstad OD. Role of xanthine oxidase and its inhibitor in hypoxia: reoxygenation injury. Pediatrics. 1996;98(1):103–107. [PubMed] [Google Scholar]
- 13. Ono T, Tsuruta R, Fujita M, et al. Xanthine oxidase is one of the major sources of superoxide anion radicals in blood after reperfusion in rats with forebrain ischemia/reperfusion. Brain Res. 2009;1305:158–167. [DOI] [PubMed] [Google Scholar]
- 14. Pacher P, Nivorozhkin A, Szabo C. Therapeutic effects of xanthine oxidase inhibitors: renaissance half a century after the discovery of allopurinol. Pharmacol Rev. 2006;58(1):87–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Van Bel F, Shadid M, Moison RM, et al. Effect of allopurinol on postasphyxial free radical formation, cerebral hemodynamics, and electrical brain activity. Pediatrics. 1998;101(2):185–193. [DOI] [PubMed] [Google Scholar]
- 16. Benders MJ, Bos AF, Rademaker CM, et al. Early postnatal allopurinol does not improve short term outcome after severe birth asphyxia. Arch Dis Child Fetal Neonatal Ed. 2006;91(3):F163–F165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gunes T, Ozturk MA, Koklu E, Kose K, Gunes I. Effect of allopurinol supplementation on nitric oxide levels in asphyxiated newborns. Pediatr Neurol. 2007;36(1):17–24. [DOI] [PubMed] [Google Scholar]
- 18. Torrance HL, Benders MJ, Derks JB, et al. Maternal allopurinol during fetal hypoxia lowers cord blood levels of the brain injury marker S-100B. Pediatrics. 2009;124(1):350–357. [DOI] [PubMed] [Google Scholar]
- 19. Derks JB, Oudijk MA, Torrance HL, et al. Allopurinol reduces oxidative stress in the ovine fetal cardiovascular system after repeated episodes of ischemia-reperfusion. Pediatr Res. 2010;68(5):374–380. [DOI] [PubMed] [Google Scholar]
- 20. Kaandorp JJ, van Bel F, Veen S, et al. Long-term neuroprotective effects of allopurinol after moderate perinatal asphyxia: follow-up of 2 randomised controlled trials. Arch Dis Child Fetal Neonatal Ed. 2012;97(3):F162–F166. [DOI] [PubMed] [Google Scholar]
- 21. Herrera EA, Kane AD, Hansell JA, et al. A role for xanthine oxidase in the control of fetal cardiovascular function in late gestation sheep. J Physiol. 2012;590(pt 8):1825–1837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Masaoka N, Nakajima Y, Hayakawa Y, et al. Transplacental effects of allopurinol on suppression of oxygen free radical production in chronically instrumented fetal lamb brains during intermittent umbilical cord occlusion. J Matern Fetal Neonatal Med. 2005;18(1):1–7. [DOI] [PubMed] [Google Scholar]
- 23. Boda D, Németh P, Kiss P, Orvos H. Treatment of mothers with allopurinol to produce therapeutic blood levels in newborns. Prenat Neonatal Med. 1999;4:130–134. [Google Scholar]
- 24. Thakor AS, Herrera EA, Seron-Ferre M, Giussani DA. Melatonin and vitamin C increase umbilical blood flow via nitric oxide-dependent mechanisms. J Pineal Res. 2010;49(4):399–406. [DOI] [PubMed] [Google Scholar]
- 25. Kane AD, Herrera EA, Hansell JA, Giussani DA. Statin treatment depresses the fetal defence to acute hypoxia via increasing nitric oxide bioavailability. J Physiol. 2012;590(pt 2):323–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Gardner DS, Fletcher AJ, Fowden AL, Giussani DA. A novel method for controlled and reversible long term compression of the umbilical cord in fetal sheep. J Physiol. 2001;535(pt 1):217–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. van Kesteren C, Benders MJ, Groenendaal F, van Bel F, Ververs FF, Rademaker CM. Population pharmacokinetics of allopurinol in full-term neonates with perinatal asphyxia. Ther Drug Monit. 2006;28(3):339–344. [DOI] [PubMed] [Google Scholar]
- 28. Gunn AJ, Parer JT, Mallard EC, Williams CE, Gluckman PD. Cerebral histologic and electrocorticographic changes after asphyxia in fetal sheep. Pediatr Res. 1992;31(5):486–491. [DOI] [PubMed] [Google Scholar]
- 29. De Haan HH, Gunn AJ, Williams CE, Gluckman PD. Brief repeated umbilical cord occlusions cause sustained cytotoxic cerebral edema and focal infarcts in near-term fetal lambs. Pediatr Res. 1997;41(1):96–104. [DOI] [PubMed] [Google Scholar]
- 30. Fraser M, Bennet L, Gunning M, et al. Cortical electroencephalogram suppression is associated with post-ischemic cortical injury in 0.65 gestation fetal sheep. Brain Res Dev Brain Res. 2005;154(1):45–55. [DOI] [PubMed] [Google Scholar]
- 31. Groenendaal F, Lammers H, Smit D, Nikkels PG. Nitrotyrosine in brain tissue of neonates after perinatal asphyxia. Arch Dis Child Fetal Neonatal Ed. 2006;91(6): F429–F433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Matthews JN, Altman DG, Campbell MJ, Royston P. Analysis of serial measurements in medical research. BMJ. 1990;300(6719):230–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Goni-de-Cerio F, Alvarez A, Caballero A, et al. Early cell death in the brain of fetal preterm lambs after hypoxic-ischemic injury. Brain Res. 2007;1151:161–171. [DOI] [PubMed] [Google Scholar]
- 34. Mallard EC, Gunn AJ, Williams CE, Johnston BM, Gluckman PD. Transient umbilical cord occlusion causes hippocampal damage in the fetal sheep. Am J Obstet Gynecol. 1992;167(5):1423–1430. [DOI] [PubMed] [Google Scholar]
- 35. Mallard EC, Williams CE, Johnston BM, Gluckman PD. Increased vulnerability to neuronal damage after umbilical cord occlusion in fetal sheep with advancing gestation. Am J Obstet Gynecol. 1994;170(1 pt 1):206–214. [DOI] [PubMed] [Google Scholar]
- 36. de Haan HH, Van Reempts JL, Vles JS, de Haan J, Hasaart TH. Effects of asphyxia on the fetal lamb brain. Am J Obstet Gynecol. 1993;169(6):1493–1501. [DOI] [PubMed] [Google Scholar]
- 37. Ikeda T, Choi BH, Yee S, Murata Y, Quilligan EJ. Oxidative stress, brain white matter damage and intrauterine asphyxia in fetal lambs. Int J Dev Neurosci. 1999;17(1):1–14. [DOI] [PubMed] [Google Scholar]
- 38. Falkowski A, Hammond R, Han V, Richardson B. Apoptosis in the preterm and near term ovine fetal brain and the effect of intermittent umbilical cord occlusion. Brain Res Dev Brain Res. 2002;136(2):165–173. [DOI] [PubMed] [Google Scholar]
- 39. Rocha E, Hammond R, Richardson B. Necrotic cell injury in the preterm and near-term ovine fetal brain after intermittent umbilical cord occlusion. Am J Obstet Gynecol. 2004;191(2):488–496. [DOI] [PubMed] [Google Scholar]
- 40. Berger R, Lehmann T, Karcher J, Schachenmayr W, Jensen A. Relation between cerebral oxygen delivery and neuronal cell damage in fetal sheep near term. Reprod Fertil Dev. 1996;8(3):317–321. [DOI] [PubMed] [Google Scholar]
- 41. Castillo-Melendez M, Chow JA, Walker DW. Lipid peroxidation, caspase-3 immunoreactivity, and pyknosis in late-gestation fetal sheep brain after umbilical cord occlusion. Pediatr Res. 2004;55(5):864–871. [DOI] [PubMed] [Google Scholar]
- 42. Miller SL, Yan EB, Castillo-Melendez M, Jenkin G, Walker DW. Melatonin provides neuroprotection in the late-gestation fetal sheep brain in response to umbilical cord occlusion. Dev Neurosci. 2005;27(2-4):200–210. [DOI] [PubMed] [Google Scholar]
- 43. van Dijk AJ, Parvizi N, Taverne MA, Fink-Gremmels J. Placental transfer and pharmacokinetics of allopurinol in late pregnant sows and their fetuses. J Vet Pharmacol Ther. 2008;31(6):489–495. [DOI] [PubMed] [Google Scholar]
- 44. Kaandorp JJ, Benders MJ, Rademaker CM, et al. Antenatal allopurinol for reduction of birth asphyxia induced brain damage (ALLO-trial); a randomized double blind placebo controlled multicenter study. BMC Pregnancy Childbirth. 2010;10:8. [DOI] [PMC free article] [PubMed] [Google Scholar]