Abstract
Background
Chronic lymphocytic leukemia (CLL) is the most prevalent leukemia in the western world. Recent advances in understanding the biology of B-cell malignancies resulted in the development of novel agents targeting key pro-survival pathways in the neoplastic B-cell.
Objective
The goal of this article was to summarize current literature on the emerging therapeutic approaches in CLL and B-cell malignancies.
Methods
A literature review was performed, identifying pathways and key clinical trials involving novel therapies in CLL with special emphasis on B-cell receptor targeting agents.
Results
Understanding the biology of B-cell receptor signaling pathway led to identification of novel molecular targets. Most notably, inhibitors of Bruton tyrosine kinase and phosphatidylinositide 3-kinase-δ have entered clinical trials and demonstrated high response rates in CLL, including high-risk disease. Cyclin-dependent kinase inhibitors may evolve into an alternative therapeutic approach in CLL. New drugs which target molecules within and outside of the B-cell receptor signaling pathway show promise in pre-clinical studies.
Conclusions
Both pre-clinical and early clinical trial results involving novel targeted therapies suggest that the standard treatment paradigm in CLL and B-cell malignancies will soon change. Particular attention should be paid to the BCR-targeting agents, whose favorable side effect profile may improve lives of the elderly patients with CLL.
Keywords: chronic lymphocytic leukemia, B-cell receptor, ibrutinib, NF-kappaB
Introduction
Chronic lymphocytic leukemia (CLL) is the most prevalent leukemia in the west with about 100,000 patients living with the disease in the United States. It is estimated that 15,680 men and women will be diagnosed with and 4,580 patients will die from CLL and its complications in 2013.1 The median age of a patient with CLL at diagnosis is 72 years, and 2/3 of cases are diagnosed among those aged 65 and older. Age adjusted incidence of CLL is estimated at 3.9/100,000 people, increasing to 22.3/100,000 among greater than 65 year olds and an estimated under-reporting of 10 to 30% of cases.2,3
Therapy of CLL has undergone significant evolution in the past two decades resulting in an improved survival of patients with this disease.4 Alkylating agents and glucocorticoids were first used in treatment of CLL in the 1950's.5,6 Purine analogues (cladribine, fludarabine and pentastatin) were introduced in the 1980's. Development of rituximab by IDEC pharmaceuticals and its subsequent FDA approval for treatment of non-Hodgkin lymphoma in 1997 introduced highly efficacious chemo-immunotherapy regimens which to this day remain the standard approach to initial therapy of younger fit patients with CLL.7 FCR (fludarabine, cyclophosphamide, rituximab) leads to an overall response rate (ORR) of ~90% and a complete response rate (CR) of 30–72% when administered to previously untreated patients with CLL.7,8
Despite this, both fludarabine- and pentastatin-based regimens are associated with high frequency of grade 3–4 neutropenia which occurs in up to 71% of younger patients , as well as prolonged suppression of T cell-mediated immunity.7,9 In an attempt to ameliorate toxicity in the elderly, recent studies with dose-reduced (50–60%) oral or intravenous FCR were undertaken but unfortunately still reported similar occurrence of severe neutropenia, high frequency of lethal infections, and suboptimal treatment completion rates.10,11 This presents a significant problem since most patients with CLL are older and present with a median of 2 comorbidities at diagnosis.12 The majority (89%) have a concurrent medical condition, while 46% carry one or more major comorbidity (eg, cerebrovascular disease, coronary artery disease, diabetes mellitus or another malignancy ).12 Key clinical trials in CLL, including trials investigating newer chemotherapy agents (such as bendamustine), have generally accrued non-representative populations: either younger or “fit” CLL patients selected to have zero or minor medical comorbidities.13–16 Therefore, because a) current chemo-immunotherapy regimens are commonly associated with unfavorable adverse events, particularly in the elderly and patients with comoribidities, who represent the majority of patients with CLL; b) clonal evolution in response to chemotherapy in CLL17 and eventual emergence of fludarabine-resistant disease are both now well recognized; and c) of a lack of a curative strategy, as well as high risks associated with stem cell therapy, there is an acute need in new treatment approaches in CLL.
Arguably, prednisone is historically the first agent which could serve as an example of targeted therapy in CLL. While steroid hormones modulate many intracellular pathways, at least two examples of its "targeted" action are of relevance to lymphoid malignancies. First, glucocorticoid receptors interfere with nuclear factor -κB (NF-κB) pathway activity: by inducing the inhibitory proteins inhibitor of κB (IκB), they promote sequestration of the NF-κB in the cytoplasm.18 This results in diminished transcription of NF-κB target genes and reduced cell proliferation and survival. Second, glucocorticoids bind to and inhibit activity of AP-1 transcription factor which is necessary for cell proliferation.19 In their study Shaw et al demonstrated that patients with CLL placed on prednisone at an initial dose of 1 mg/kg followed by slow taper exhibited reduction in lymphadenopathy and organomegaly and improvement in bone marrow function6. Alas, responses were short lived. Interestingly, the authors noted marked responses in a patient with hemolytic anemia and 2 patients with "severe" (probably immune) thrombocytopenia and recommended further use of steroids under those circumstances, a practice which continues to this day.
Targeted antibody therapy represents a major success in treatment of CLL and associated autoimmune conditions. Rituximab is a first generation chimeric murine/human monoclonal IgG1antibody directed against CD20, a glycosylated phosphoprotein expressed on the surface of all B-cells beginning at the pro-B phase. Rituximab induces direct apoptosis as well as complement-dependent cytotoxicity and antibody-dependent cell-mediated cytotoxicity in neoplastic B-cells. While efficacy of rituximab as a single agent in CLL/SLL was underwhelming in the initial studies (PR rate 12%)20, use of higher doses of the drug (such as three times weekly) achieved improved activity in CLL.21 In 1997 rituximab received FDA approval for treatment of non-Hodgkin lymphomas. A second generation (humanized) antibody, ofatumumab, targets an alternative CD20 epitope and was FDA approved for treatment of CLL refractory to fludarabine and alemtuzumab in 2009. Third generation anti-CD20 monoclonal antibodies, in addition to being completely humanized, carry an engineered Fc region to increase their avidity to the FcγRIIIa receptor. Obinutuzumab (GA101, Genentech) entered early phase clinical trials in CLL. For detailed information on the anti-CD20 monoclolal antibodies, the reader is referred to the recently published review articles on this subject.22,23
Recently, encouraging results were seen among agents which target the B-cell receptor (BCR) pathway, and hence a brief introduction of this pathway along with more detailed discussion of some of its components follows.
B-cell receptor signaling pathways
B-cell receptor (BCR) is a transmembrane receptor complex which incorporates a surface immunoglobulin associated with a signal transduction moiety (CD79A/B). BCR is indispensible during the maturation process of a normal B cell. BCR signaling results in distinct outcomes in a fate of a B cell depending on a stage of its development as it intertwines with a great variety of downstream pathways. Signaling through BCR modulates clone expansion in a pre-B-cell, induces apoptosis of an immature B cell and, by contrast, growth and proliferation of a mature B cell. Cell surface receptors such as CD19 and CD40, and signaling molecules located close to cell membrane are able to modulate BCR signaling, providing both inhibitory and activatory costimuli.24
Since CLL B-cells carry a mature B cell phenotype, i.e. CD19+CD20+CD27+, it has been proposed long ago that BCR signaling promotes clonal proliferation in CLL. A relative restriction of BCR immunoglobulin heavy chain variable genes (IGHV) repertoire provides evidence of antigenic selection in CLL.25,26 Yet questions remain, including why CLL cells, being reliant on the pro-survival BCR signaling, express BCR at a lower density compared with normal B cell counterparts; why some CLL samples demonstrate poor response to BCR stimulation (predominantly IGHV mutated, M-CLL) while others elicit strong response (predominantly IGHV unmutated, U-CLL); which putative antigens are relevant and able to lead to BCR activation in vivo.
In a normal B-cell, BCR signaling relies mainly on two pathways: that involving G-proteins such as Ras and the other leading to phosphorylation of Akt. Upon BCR crosslinking (i.e. antigen binding to the membrane immunoglobulin), approximation of src family protein kinases, namely Lyn, Fyn and Blk to the intracellular tyrosine activation motifs (ITAMs) on CD79A/B results in enhanced phosphorylation of the latter followed by recruitment of Syk, an SH2-domain containing kinase. Phosphorylation of spleen tyrosine kinase (Syk) is a key act in BCR signaling cascade, without which further signal transmission cannot occur. Syk recruits a plasma membrane-associated signaling complex with involvement of several tyrosine kinases (including Bruton tyrosine kinase [BTK]) and adaptor molecules (such as B cell linker protein [BLNK]). The complex signals to activate phospholipase C-γ2 and Ras. Ras binds to and activates Raf kinase which then propagates the signal down to extracellular regulated kinase (ERK). In its turn, phospholipase C-γ2 through a sequence of events leads to activation of mitogen-activated protein kinase (MAPK) kinases, including ERK, c-Jun NH2-terminal kinase (JNK) and p38 and transcription factors including Myc and NFκB. In parallel to that, association of CD19 with Lyn and phosphatidylinositide 3-kinase (PI3K) leads to activation of Akt. To allow efficient interaction between BCR components and intracellular kinases, their approximation is achieved in a process of lipid raft formation.24 Since BCR signaling is a cascade of phosphorylation events, receptor activation may be assessed in vitro using relatively unsophisticated techniques. Phosphorylation of Syk and downstream kinases that are activated in response to BCR engagement (PI3K, Akt, ERK and JNK) may be detected by immunoblotting or flow cytometry. Meanwhile, intracellular calcium flux may be measured using fluorescent indicators.
CLL cells characteristically express low levels of surface immunoglobulin.27 This led some to believe that BCR does not transmit signal in CLL and thus may be irrelevant to pathogenesis of CLL. It was then noted over a decade ago that while in some CLL cases BCR crosslinking with F(ab’)2 fragments of goat anti-human IgM does not elicit response, strong BCR activation is possible in a subset of CLL.28 A correlation was observed between response to BCR crosslinking and IGHV mutational status: BCR engagement led to phosphorylation of Syk and ERK, increased Ca influx and improved viability in U-CLL cells, but not in M-CLL.29,30 Expectedly, since overexpression of CD38- and ZAP-70 are surrogate marker for U-CLL, such CLL samples tend to respond to BCR stimulation.31 Interestingly, whereas U-CLL tend to express more sIgM than M-CLL, IGHV mutational status but not the receptor density per se predicted the intensity of BCR response indicating that alternative explanations pertaining to this phenomenon must exist.32 In normal B-cells, BCR undergoes lipid raft translocation upon ligand binding.24 BCR failed to translocate to lipid rafts in M-CLL, as evidenced by low content of IgM and lack of Lyn phosphorylation in lipid rafts isolated from such B-cells.30 While lipid raft formation was independent of src kinase activity U-CLL, inhibition of Src kinases in M-CLL resulted in spontaneous translocation of BCR into lipid rafts, indicating that Src kinases facilitate exclusion of the BCR from lipid rafts.30 Hence, spatial organization of the BCR signaling complex may be at least one of the critical determinants of the ability of the neoplastic B cell to respond to BCR stimulation.
Recent successes in targeting BCR signaling pathway
A number of BCR signaling inhibitors have entered clinical trials in the past few years. Indeed, targeting BCR shows great promise in both CLL and other types of B-cell neoplasia. Ibrutinib is now at the forefront of BCR-targeting agents in the clinic and is most extensively studied with reported response rates of up to 70% in both treatment-naive and relapsed/refractory CLL, relapsed/refractory follicular lymphoma, mantle cell lymphoma and lymphoplasmacytic lymphoma. Through translational research, novel BCR-targeting agents provide important new knowledge about the mechanisms of disease progression n B-cell malignancies. The discussion which follows will review the biology, preclinical and clinical activity of these agents. Meanwhile, it is important to consider certain common features among them, such as :
Efficacy across a variety B-cell malignancies, including CLL/SLL, follicular lymphoma, mantle cell lymphoma, lymphoplasmacytic lymphoma, and diffuse large B-cell lymphoma, indicating the importance of BCR pathway in sustenance of a neoplastic B cell;
Development of lymphocytosis patients who receive such agents, thought to be due to disruption of homing of neoplastic B cells to the microenvironment.33 Importantly, this appears to be an efficient way of thwarting apoptosis resistance and in and of itself lays foundation for design of novel drug combinations. The duration of lymphocytosis is variable and may be abrogated by co-administration of Rituximab or cytotoxic chemotherapy Persistent lymphocytosis complicated response assessment using the IWCLL 2008 criteria which are commonly used in clinical trials in CLL34;
Favorable side effect profile, although at least with ibrutinib, the frequency of neutropenia and infections is higher in patients with previously treated CLL compared to patients who received iburtinib as their first-line therapy. This makes promise that BCR-targeting agents may revolutionize treatment of CLL in the elderly;
Ibrutinib
(PCI-32765, Pharmacyclics) is a novel BTK inhibitor which recently entered Phase IIL trials in CLL. . Several studies involving ibrutinib generated considerable excitement at a 2012 American Society of Hematology Annual Meeting. Ibrutinib has demonstrated promising activity in studies involving relapsed follicular lymphoma, CLL/SLL, diffuse large B-cell lymphoma and other non-Hodgkin lymphoma subtypes as a single agent and in combination with other drugs.35–39 A Phase Ib/II study enrolled 31 patients with treatment naive CLL (all older than 65 year old), 61 patients with relapsed and refractory CLL and 24 patients with high-risk CLL (those who relapsed within 2 years following combination chemoimmunotherapy or were identified to carry deletion 17p).39 Ibrutinib was administered at a dose of 420–840 mg/day. After a median follow-up of 17 months, ORR was observed in 71%, 67% and 50% and CR - in 10%, 3% and 0% of patient among the three respective cohorts. Since ibrutinib induced lymphocytosis in a subset of patients, a novel category of response, PR with lymphocytosis, was reported at a frequency of 10, 20 and 29%, correspondingly, thus documenting response at close to 80% in each of the above cohorts. Such impressive activity was accompanied by rather mild side effects, which primarily included diarrhea (54%), fatigue (29%), upper respiratory tract infection (29%), rash (28%), nausea (26%) and arthralgias (25%).39 High activity of ibrutinib in CLL with deletion 17p is remarkable and exceeds the efficacy of high-dose methylprednisone combinations in this setting. Ibrutinib induced responses in 68% of such patients, more than >50% of whom remained on study for longer than 2 years; this is in contrast to a PFS of 10 months documented after treatment with high dose steroids in combination with alemtuzumab.39–41 A recent review by Dr. Jennifer Brown42 provides additional details on recent studies of ibrutinib in CLL, with some of these highlighted in a Table.
Table 1.
| Disease type | Study (N) | ORR | CR | PFS, months |
|---|---|---|---|---|
| Follicular lymphoma (relapsed/refractory) | Phase 1 (16) | 55%$ | 27% | 13.4–19.6 |
| Mantle cell lymphoma (relapsed/refractory) | Phase 2 (115) | 68% | 22% | 13.9 |
|
CLL, therapy-naïve 55%: U-CLL 7% – 17p |
Phase 1b/2 (31) | 96% | 10% (13%*) | 96% at 26 months |
|
CLL, relapsed/refractory 85%: U-CLL 35%: del17p 39%: del 11q |
Phase 2 (85) | 75% | 2% (18%*) | 75% at 26 months |
frequency of PR with lymphocytosis
at a dose level exceeding 2.5 mg/kg daily
Ongoing clinical trials with ibrutinib enroll patients with CLL include Phase III studies in treatment-naive and previously treated CLL (RESONATE, HELIOS, RESONATE-2); Phase II study in patients with deletion 17p (RESONATE -17); Phase II (SPARK) and III (SHINE and RAIN) studies in mantle cell lymphoma, follicular lymphoma (DAWN) and others.
Idelalisib
(GS-1101, formerly CAL-101, Gilead Sciences) is an orally bioavailable, small-molecule inhibitor of PI3-Kδ. The PI3K pathway is a key component of survival in many cancers, including CLL. There are three classes of PI3K isoforms. Class I isoforms are made up of two subsets: IA, which incorporates p110-α, p110-β and p110-δ (catalytic domains) bound by regulatory domains; and IB, made up only by p110-γ coupled with p101. Of those isoforms, PI3K-δ is abundantly expressed in CLL.43 Idelalisib has shown considerable monotherapy activity when given at dose levels of ≥100 mg twice daily in patients with heavily pretreated indolent non-Hodgkins lymphoma. In a Phase I study of idelalisib in patients with relapsed or refractory CLL, idelalisib was administered orally one or 2 times per day continuously for 28-day cycles for up to 12 cycles.44 Doses >150 mg twice daily resulted in minimal increases of plasma Cmax and AUC. Idelalisib reduced lymphadenopathy in all patients; 29/32 (91%) achieved a lymph node response. An initial increase in peripheral absolute lymphocyte counts of >50% from baseline was observed in 21/35 (60%) patients and those increases were maximal during the first 2 cycles and began to resolve thereafter.
Data on idelalisib combination Phase I studies was presented at a recent American Society of Hematology Annual Meeting. Patients (the majority of whom were 60 years old or older) were enrolled in three cohorts where idelalisib was combined with rituximab (N=30), bendamustine (N=33) or bendamustine and rituximab (BR; N=13) and given at a dose of 100–150 mg twice daily. The majority of patients in each group had relapsed follicular lymphoma (between 70 and 76% of patients in each of the treatment subgroups) and small lymphocytic lymphoma, as well as several patients with marginal zone lymphoma.45 Patients had a median of 3 prior therapies. Almost all patients had been exposed to rituximab and up to a third had received prior bendamustine. All but two patients experienced a decrease in lymphadenopathy with idelalisib combination therapy. CR/PR were reported in 13/64%, 16/69% and 30/47% of patients in the respective cohorts, with 1-year progression-free survival (PFS) of 78–90%. This treatment was fairly well tolerated with the most frequent adverse event being grade ≥3 neutropenia in 43–52% of patients. Febrile neutropenia was reported in 12% of patients who received idelalisib in combination with bendamustine, while infections were reported in 7%,15% and 8% of patients in the three cohorts. A dedicated Phase I study of idelalisib combination therapy in patients with CLL reported similar results.46 Again, most patients received prior rituximab, and a higher proportion than in the previous study (40–47%) had had bendamustine. In the three similarly designed patient cohorts, ORR ranged between 78 and 82% with 1-year PFS of 74–88%. Similarly, combination therapy was well tolerated with low rate of febrile neutropenia (7–12%) and infections (11–18%). Thus, idelalisib has both single agent activity and may be successfully combined with current standard therapies in CLL and lymphoma.
Several alternative selective inhibitors of PI3K are being tested in both pre-clinical and clinical setting and include AMG-319 (Amgen)47 and IPI-14548. A recent report however implicated that upregulation of alternative PI3K isoforms within neoplastic B cells, such as PI3K-α, may represent an emerging mechanism of eventual resistance to selective inhibitors of PI3K-δ in patients with B-cell malignancies.49
Finally, fostamatinib disodium (R788, Rigel Pharmaceuticals) is an ATP-competitive SYK kinase inhibitor that also inhibits a number of other kinases.50 Fostamatinib has been investigated in a Phase I/II study in patients with several types of recurrent non-hodgkin lymphoma.51 While compared to the above agents responses to fostamatinib were less prominent (ORR of 10% in follicular lymphoma, 55% in CLL, and 11% in mantle cell lymphoma), the drug was similarly well tolerated (grade ≥3 neutropenia - 18%, grade ≥3 anemia - 7%). Follow-up studies of fostamatinib in B-cell malignancies have not been initiated, although the drug has been intensely investigated in rheumatoid arthritis and other autoimmune disorders. On June 4, 2013, Astra Zeneca announced they would not proceed with regulatory filings for fostamatinib (www.nasdaq.com). Alternative SYK inhibitors demonstrate promising pre-clinical activity in CLL.52 A novel Syk inhibitor GS-9973 (Gilead Sciences) has entered clinical trials and is being investigated in combination with idelalisib in CLL.
Alternative and emerging targets: B-cell receptor and beyond
This section will discuss agents which target kinases other than BTK and PI-3K in the BCR signaling pathway (Lyn, Lck and ZAP-70) as well as other molecular targets which may cross-talk with, but are not directly linked to the BCR (cyclin-dependen kinases and NFκB).
Signaling through BCR results in B-cell survival and proliferation. As cells progress through cell cycle, they rely on a partnership between cyclin-dependent kinases (CDK's) and various cyclins to advance DNA duplication, cell mitosis and proliferation. CDK inhibitors is a novel class of agents whose clinical activity has been recently appreciated in CLL. Inhibition of transcription is arguably the best characterized mechanism of their action in CLL. This has been linked to inhibition of CDK-7 and -9 and a concomitant decrease in expression of short-lived proteins, among which the anti-apoptotic BCL2 family member proteins MCL1, BCLX are paramount.53,54 Just recently, Cosimo et al. provided convincing data where CR8, a roscovitine analog, decreases BCLX, MCL1 and XIAP expression in CLL cells. CR8 also disrupted NFκB pathway activity at the transcriptional level and led to loss of CD40L- mediated protection from apoptosis in CLL.55 Since many anti-apoptotic BCL2 family members are recognized as transcriptional targets of the NF-κB, it would be interesting to determine in a more direct set of experiments whether disruption of this pathway is the dominant mechanism leading to their decreased expression in presence of CDK inhibitors.
Dinaciclib (SCH727965, Merck) and flavopiridol (HMR-1275, Sanofi) are the CDK inhibitors which are currently at a most advanced stage in clinical trials. Those agents have non-selective activity in CLL with unfavorable features, including deletion 17p. Dinaciclib inhibits CDK's 1, 2, 5 and 9 and has poor selectivity between them in vitro.56 Dinaciclib shows prominent pre-clinical activity in CLL irrespective of the cytogenetics.57 While the Phase II results with dinaciclib in CLL have not been reported thus far, the drug showed efficacy in a Phase I study in patients with CLL. Dinaciclib was administered to five cohorts of patients with relapsed/refractory CLL (N=33) and an expansion cohort (N=16).58 Recommended Phase II dose was established at 14 mg/m2 on days 1, 8 and 15 of a 28-day cycle. PR was observed in 15/33 (45%) of all patients, including many who had prior fludarabine treatments and those with deletion 17p. Among the patients enrolled on the expansion cohort the PR rate was 62.5%, and several other patients with highly proliferative CLL achieved stable disease. Tumor lysis syndrome was observed in 5 patients, of whom two required dialysis. Otherwise the drug was well tolerated with diarrhea and hematologic toxicity being most prominent.58 Somewhat despite expectations, dinaciclib has not performed as well in a trial enrolling patients with indolent and large cell lymphoma, where only one partial response in a patient with diffuse large B-cell lymphoma was observed. Activity not meeting criteria for partial response was seen in 2 patients with follicular lymphoma and one patient with mantle cell lymphoma.59
Dasatinib is a non-specific Src/c-Abl kinase inhibitor. While it is used as a potent inhibitor of bcr-abl tyrosine kinase in Ph+ leukemias, dasatinib also has blocking activity against the proximal Src kinases within the BCR pathway such as Lyn.60 Lyn is vital to B-cell development as Lyn-deficient mice fail to maintain a mature B-cell population.61 Lyn functions to phosphorylate ITAM's on the Cd79a and CD79b following BCR stimulation and is consistently overexpressed in CLL.62 Dasatinib leads to decreased SYK phosphorylation and induces apoptosis of CLL cells in vitro.63 In a single institution phase II study dasatinib 140 mg once daily induced partial responses in 3/15 patients with high risk CLL, and additional 6 patients manifested nodal responses, albeit toxicity was not insignificant.64 Importantly, dasatinib at low concentrations also has inhibitory activity against other kinases within the BCR pathway including Lck and BTK60 which further explains its activity in CLL.
Lck is a Src family tyrosine kinase and a T-cell receptor signaling protein which was found to be expressed in B-CLL. ZAP-70 serves as a principal target for Lck in T cells.65 Lck is expressed in most B-cell malignancies, including mantle cell lymphoma, lymphomas of germinal center origin (such as follicular lymphoma) and CLL.66 Lck expression in CLL is variable and correlates with BCR responsiveness since Lck mediates phosphorylation of SYK and downstream BCR signaling events.67 Importantly, either siRNA-mediated knockdown or pharmacologic inhibition of Lck resulted in decreased signaling through BCR and abrogated the BCR crosslinking-mediated CLL cell survival67, thus implicating Lck as a potential therapeutic target in CLL.
ZAP-70
(Zeta-chain associated T cell receptor kinase 70 kDa), a key SH2-domain containing kinase in the T-cell receptor signaling cascade, is expressed at very low levels in normal B-cells, but was found to be overexpressed in U-CLL.68 ZAP-70 expression is now routinely measured in clinical laboratories in CLL where it is considered an unfavorable prognostic marker. ZAP-70 seems to be important in CLL cell survival, as destabilization of ZAP-70 via antagonizing hsp90 results in apoptosis.69 There is some evidence that ZAP-70 is involved in modulation of BCR signaling in CLL. For example, ZAP-70+ CLL B-cells show more robust BCR signaling70, while engineered expression of ZAP-70 in CLL cells and a Burkitt lymphoma cell line, BJAB, enhanced activation of Syk, Erk and Akt.71 Earlier work suggested that ZAP-70 is phosphorylated in the process of BCR signaling72, yet this has since been disputed.71 Moreover, contrary to Syk, ZAP-70 kinase activity is probably not required for the BCR signal to be successfully propagated, thus challenging the rationale of an ATP-competitive inhibitor.73 ZAP-70 associates with several key regulators of BCR signaling in CLL, including CD79b, and downstream molecules such as PI3K and Cbl72; however despite that knowledge, there is no definitive explanation as to how ZAP-70 is involved in BCR signal transduction. Since ZAP-70 associates with BCR components, it may be disrupting BCR internalization and/or promoting lipid raft formation thus facilitating recruitment of Src family kinases such as Lyn. Alternatively, ZAP-70 may compete for ubiquitination with other kinases in the BCR cascade.73 It is likely that the ability of ZAP-70 to serve as an adopter protein is critical to its function. ZAP-70 depends on the functionality of its SH2 domains to promote its effect on BCR signaling in CLL cells.73 While molecules targeting SH2 domains have been successfully generated, work in this area has not left pre-clinical realm.
NF-κB
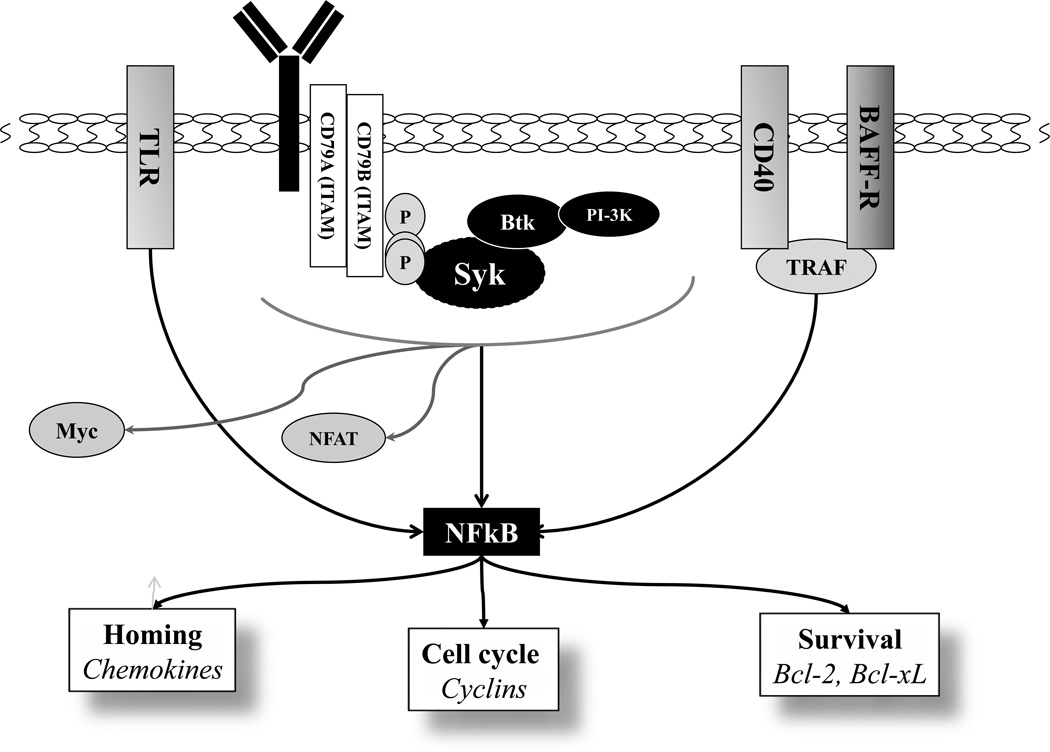
BCR signaling acts in synergy with the soluble factors expressed by the surrounding microenvironment in a CLL lymph node and bone marrow. A variety of soluble factors produced by T-cells, macrophages, dendritic cells and neutrophils drive B-cell homeostasis in a healthy setting and CLL B-cell perseverance in disease. Tumor necrosis factor receptor (TNFR) superfamily members B-cell activating factor receptor (BAFF-R) and CD40 play critical role in CLL. CD40L-mediated activation of the anti-apoptotic Bcl-2 family members (BCL2, BCLX, MCL1 and BFL-1) and downregulated expression of pro-apoptotic BH3-only proteins is a well-recognized phenomenon reproducible in vitro, that is accompanied by reduced spontaneous CLL cell apoptosis.74–77 Mechanistically, recruitment of the TNFR-associated factor (TRAF) proteins by TNFR family members leads to activation of the canonical and non-canonical NF-κB pathways (Figure). Importantly, NF-κB mediates cross-talk between BCR and TNFR signaling.75 As discussed earlier, activation of the proximal BCR signaling complex ultimately results in activation of IKK1/IKK2, phosphorylation and proteosomal degradation of IκBα and induction of the canonical NF-κB signaling. NF-κB transcriptional activity leads to upregulation of a number of chemokine molecules (CCL17, CCL22, CXCR5); cell cycle regulators (Cyclin D) and the anti-apoptotic proteins mentioned above. In a positive feedback mechanism, canonical NF-κB signaling enhances transcription of the non-canonical NF-κB pathway components (such as p100), as well as c-Rel and BAFF-R which are critical for BAFF-R signaling.75 Hence the NF-κB pathway represents an attractive target in B-cell malignancies. Moreover, CD40L- or BAFF-mediated NF-κB activation has strong clinical implications in that it may render malignant B cells less dependent on BCR signaling targeting agents ultimately leading to resistance to BCR-targeting agents.
Figure.
B-cell receptor- and other microenvironment-mediated signaling pathways converge on NF-κB.
In vitro inhibition of NF-κB has been shown to result in CLL cell apoptosis.78,79 However, targeting NF-κB in the clinic has thus far been less successful. For one, NFκB activity is proposed to be targeted by proteasome inhibitors bortezomib and carfilzomib due to disrupted degradation of IκBα. Bortezomib has been successfully used to augment the activity of R-CHOP regimen in activated B-cell diffuse large B-cell lymphoma, a subtype characterized by the constitutive activation of NF-κB.80 It received FDA approval as a second line treatment of mantle cell lymphoma, where it may have a similar mechanism of action.81 Despite initial enthusiasm due to significant activity of bortezomib in CLL in vitro82, clinical trials failed to elicit responses in this B-cell malignancy.83 This lack of activity has been attributed to dietary flavonoids, quercetin and myricetin, which were found in patients' plasma, and an ensuing chemical reaction resulting in a decreased activity of bortezomib in CLL cells but not in myeloma cells.84 A novel proteasome inhibitor carfilzomib recently received FDA approval in relapsed multiple myeloma. Carfilzomib was shown to induce growth inhibition in mantle cell lymphoma cell lines. Importantly, a preliminary report of an in vivo study comparing carfilzomib with bortezomib in a mantle cell lymphoma xenograft mouse model demonstrated superior efficacy of carfilzomib over bortezomib.85 Carfilzomib irreversibly blocked release of NF-κB to the nuclei thus abrogating pathway activity.85 Carfilzomib also demonstrated promising pre-clinical activity in CLL.86 Importantly, pro-apoptotic effect of carfilzomib persisted in the presence of human plasma, whereas bortezomib was rendered inactive. Interestingly, carfilzomib had little effect on baseline NF-κB activity in peripheral CLL cells in this study.86 However, NF-κB is significantly more active in the lymph node microenvironment.87 Therefore, it would be of interest to determine whether carfilzomib would reduce CLL cell survival in CD40L- or BAFF-expressing stromal co-cultures or in response to TLR9-mediated signaling in vitro. In a recently published phase I study, carfilzomib resulted in stable disease in four patients with follicular lymphoma and one patient with CLL/SLL.88 Other proteasome inhibitors, including MLN9708 (Millenium Pharmaceuticals), CEP-18770 (Teva/Cephalon) and orpozomib (Onyx Therapeutics) are currently being investigated in Phase I protocols in patients with relapsed and refractory B-cell lymphomas.
MLN4924 is an investigational small molecule inhibitor of Nedd8-activating enzyme (NAE) which has shown pre-clinical activity in hematologic malignancies.89,90 Nedd8-activating enzyme is necessary for activation of Cullin-RING ubiquitin ligases. In vitro MLN4294 leads to accumulation of Cullin-RING E3 ligase substrates - IκBα, p27 and Cdt1 and others.89,91 Disrupted ubiquitination of IκB in the presence of MLN4924 and subsequent de-activation of the NF-κB canonical pathway seems to be the underlying event which leads to apoptosis of the diffuse large B-cell lymphoma cell lines of the activated B-cell type.90 Those findings echo the bortezomib data in this lymphoma subtype. MLN4924 is currently being studied in Phase I/II clinical trials in B-cell lymphoma and multiple myeloma.
Conclusion
In summary, both pre-clinical and early clinical trial results involving novel targeted therapies strongly suggest that the standard treatment paradigm in CLL and B-cell malignancies will change in the next few years. Particular attention should be paid to the BCR-targeting agents, ibrutinib and idelalisib, which already demonstrate encouraging activity both as single agents and in combination with conventional chemotherapy across a variety of B-cell neoplastic conditions, including those with unfavorable genetic features. Meanwhile, the FDA granted breakthrough therapy designation to ibrutinib for patients with mantle cell lymphoma and Waldenström’s macroglobulinemia which will allow for its expedited development and review. Favorable side effect profile reported with those agents thus far may change lives of the elderly patients and patients with serious comorbidities. Novel therapies continue to emerge in the preclinical setting and will expand the armamentarium of drug combinations in hematologic oncology in the coming decades.
Acknowledgements
Support to AVD provided by a National Cancer Institute new faculty award (3P30CA023108-31S4) to the Norris Cotton Cancer Center.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest: the author has no conflicts of interest
References
- 1.Howlader NNA, Krapcho M, Garshell J, Neyman N, Altekruse SF, Kosary CL, Yu M, Ruhl J, Tatalovich Z, Cho H, Mariotto A, Lewis DR, Chen HS, Feuer EJ, Cronin KA, editors. SEER Cancer Statistics Review, 1975–2010. 2013 http://seer.cancer.gov/csr/1975_2010/
- 2.Zent CS, Kyasa MJ, Evans R, et al. Chronic lymphocytic leukemia incidence is substantially higher than estimated from tumor registry data. Cancer. 2001;92:1325–1330. doi: 10.1002/1097-0142(20010901)92:5<1325::aid-cncr1454>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 3.Kristinsson SY, Dickman PW, Wilson WH, et al. Improved survival in chronic lymphocytic leukemia in the past decade: a population-based study including 11,179 patients diagnosed between 1973–2003 in Sweden. Haematologica. 2009;94:1259–1265. doi: 10.3324/haematol.2009.007849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brenner H, Gondos A, Pulte D. Trends in long-term survival of patients with chronic lymphocytic leukemia from the 1980s to the early 21st century. Blood. 2008;111:4916–4921. doi: 10.1182/blood-2007-12-129379. [DOI] [PubMed] [Google Scholar]
- 5.Galton DA, Israels LG, Nabarro JD, et al. Clinical trials of p-(di-2-chloroethylamino)-phenylbutyric acid (CB 1348) in malignant lymphoma. Br Med J. 1955;2:1172–1176. doi: 10.1136/bmj.2.4949.1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shaw RKBDR, Silberman HR, Frei E., III A Study of prednisone in Chronic Lymphocytic Leukemia. Blood. 1961;17:182–195. [Google Scholar]
- 7.Keating MJ, O'Brien S, Albitar M, et al. Early results of a chemoimmunotherapy regimen of fludarabine, cyclophosphamide, and rituximab as initial therapy for chronic lymphocytic leukemia. J Clin Oncol. 2005;23:4079–4088. doi: 10.1200/JCO.2005.12.051. [DOI] [PubMed] [Google Scholar]
- 8.Byrd JC, Rai K, Peterson BL, et al. Addition of rituximab to fludarabine may prolong progression-free survival and overall survival in patients with previously untreated chronic lymphocytic leukemia: an updated retrospective comparative analysis of CALGB 9712 and CALGB 9011. Blood. 2005;105:49–53. doi: 10.1182/blood-2004-03-0796. [DOI] [PubMed] [Google Scholar]
- 9.Tam CS, O'Brien S, Wierda W, et al. Long-term results of the fludarabine, cyclophosphamide, and rituximab regimen as initial therapy of chronic lymphocytic leukemia. Blood. 2008;112:975–980. doi: 10.1182/blood-2008-02-140582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Smolej L, Brychtova Y, Specek M, Doubek M, Belada D, Motyckova M, Cmunt E, Prochazka V, Rohon P, Poul H, Klaskova K, Kozak T. Low-dose Fludarabine and Cyclophosphamide combined with Rituximab is a safe and effective treatment option for elderly and comorbid patients with chronic lymphocytic leukemia/small lymphocytic lymphoma: preliminary results of a Project Q-lite, by the Czech CLL Study Group. Clinical Lymphoma Myeloma & Leukemia; XIV International Workshop on CLL; Houston, TX. 2011. p. S261. [Google Scholar]
- 11.Mulligan SP, Gill D, Turner P, Renwick W, Harrup R, Latimer M, Berkahn L, Simpson D, Sulda M, Best G, Kuss B, Cull G. Safety and tolerability of oral Fludarabine, with or without oral Cyclophosphamide and intravenous Rituximab therapy, in previously untreated patients with chronic lymphocytic leukemia aged 65 years or older: second interim analysis from the Australian Leukemia and Lymphoma Group and CLL Australian Research Consortium CLL5 Study. Clinical Lymphoma, Myeloma & Leukemia; XIV International Workshop on CLL; Houston, TX. 2011. pp. S259–S260. [Google Scholar]
- 12.Thurmes P, Call T, Slager S, et al. Comorbid conditions and survival in unselected, newly diagnosed patients with chronic lymphocytic leukemia. Leuk Lymphoma. 2008;49:49–56. doi: 10.1080/10428190701724785. [DOI] [PubMed] [Google Scholar]
- 13.Rai KR, Peterson BL, Appelbaum FR, et al. Fludarabine compared with chlorambucil as primary therapy for chronic lymphocytic leukemia. N Engl J Med. 2000;343:1750–1757. doi: 10.1056/NEJM200012143432402. [DOI] [PubMed] [Google Scholar]
- 14.Eichhorst BF, Busch R, Hopfinger G, et al. Fludarabine plus cyclophosphamide versus fludarabine alone in first-line therapy of younger patients with chronic lymphocytic leukemia. Blood. 2006;107:885–891. doi: 10.1182/blood-2005-06-2395. [DOI] [PubMed] [Google Scholar]
- 15.Catovsky D, Richards S, Matutes E, et al. Assessment of fludarabine plus cyclophosphamide for patients with chronic lymphocytic leukaemia (the LRF CLL4 Trial): a randomised controlled trial. Lancet. 2007;370:230–239. doi: 10.1016/S0140-6736(07)61125-8. [DOI] [PubMed] [Google Scholar]
- 16.Flinn IW, Neuberg DS, Grever MR, et al. Phase III trial of fludarabine plus cyclophosphamide compared with fludarabine for patients with previously untreated chronic lymphocytic leukemia: US Intergroup Trial E2997. J Clin Oncol. 2007;25:793–798. doi: 10.1200/JCO.2006.08.0762. [DOI] [PubMed] [Google Scholar]
- 17.Landau DA, Carter SL, Stojanov P, et al. Evolution and impact of subclonal mutations in chronic lymphocytic leukemia. Cell. 2013;152:714–726. doi: 10.1016/j.cell.2013.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Auphan N, DiDonato JA, Rosette C, et al. Immunosuppression by glucocorticoids: inhibition of NF-kappa B activity through induction of I kappa B synthesis. Science. 1995;270:286–290. doi: 10.1126/science.270.5234.286. [DOI] [PubMed] [Google Scholar]
- 19.Bailey S, Hall AG, Pearson AD, et al. Glucocorticoid resistance and the AP-1 transcription factor in leukaemia. Adv Exp Med Biol. 1999;457:615–619. doi: 10.1007/978-1-4615-4811-9_68. [DOI] [PubMed] [Google Scholar]
- 20.McLaughlin P, Grillo-Lopez AJ, Link BK, et al. Rituximab chimeric anti-CD20 monoclonal antibody therapy for relapsed indolent lymphoma: half of patients respond to a four-dose treatment program. J Clin Oncol. 1998;16:2825–2833. doi: 10.1200/JCO.1998.16.8.2825. [DOI] [PubMed] [Google Scholar]
- 21.Byrd JC, Murphy T, Howard RS, et al. Rituximab using a thrice weekly dosing schedule in B-cell chronic lymphocytic leukemia and small lymphocytic lymphoma demonstrates clinical activity and acceptable toxicity. J Clin Oncol. 2001;19:2153–2164. doi: 10.1200/JCO.2001.19.8.2153. [DOI] [PubMed] [Google Scholar]
- 22.Stephens DM, Byrd JC. Improving the treatment outcome of patients with chronic lymphocytic leukemia through targeted antibody therapy. Hematol Oncol Clin North Am. 2013;27:303–327. doi: 10.1016/j.hoc.2012.12.003. [DOI] [PubMed] [Google Scholar]
- 23.Cang S, Mukhi N, Wang K, et al. Novel CD20 monoclonal antibodies for lymphoma therapy. J Hematol Oncol. 2012;5:64. doi: 10.1186/1756-8722-5-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kurosaki T. Regulation of B-cell signal transduction by adaptor proteins. Nat Rev Immunol. 2002;2:354–363. doi: 10.1038/nri801. [DOI] [PubMed] [Google Scholar]
- 25.Messmer BT, Albesiano E, Efremov DG, et al. Multiple distinct sets of stereotyped antigen receptors indicate a role for antigen in promoting chronic lymphocytic leukemia. J Exp Med. 2004;200:519–525. doi: 10.1084/jem.20040544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tobin G, Thunberg U, Karlsson K, et al. Subsets with restricted immunoglobulin gene rearrangement features indicate a role for antigen selection in the development of chronic lymphocytic leukemia. Blood. 2004;104:2879–2885. doi: 10.1182/blood-2004-01-0132. [DOI] [PubMed] [Google Scholar]
- 27.Stevenson FK, Caligaris-Cappio F. Chronic lymphocytic leukemia: revelations from the B-cell receptor. Blood. 2004;103:4389–4395. doi: 10.1182/blood-2003-12-4312. [DOI] [PubMed] [Google Scholar]
- 28.Lankester AC, Schijndel GM, Pakker NG, et al. Antigen receptor function in chronic lymphocytic leukemia B cells. Leuk Lymphoma. 1996;24:27–33. doi: 10.3109/10428199609045711. [DOI] [PubMed] [Google Scholar]
- 29.Lanham S, Hamblin T, Oscier D, et al. Differential signaling via surface IgM is associated with VH gene mutational status and CD38 expression in chronic lymphocytic leukemia. Blood. 2003;101:1087–1093. doi: 10.1182/blood-2002-06-1822. [DOI] [PubMed] [Google Scholar]
- 30.Allsup DJ, Kamiguti AS, Lin K, et al. B-cell receptor translocation to lipid rafts and associated signaling differ between prognostically important subgroups of chronic lymphocytic leukemia. Cancer Res. 2005;65:7328–7337. doi: 10.1158/0008-5472.CAN-03-1563. [DOI] [PubMed] [Google Scholar]
- 31.Cutrona G, Colombo M, Matis S, et al. Clonal heterogeneity in chronic lymphocytic leukemia cells: superior response to surface IgM cross-linking in CD38, ZAP-70-positive cells. Haematologica. 2008;93:413–422. doi: 10.3324/haematol.11646. [DOI] [PubMed] [Google Scholar]
- 32.Mockridge CI, Potter KN, Wheatley I, et al. Reversible anergy of sIgM-mediated signaling in the two subsets of CLL defined by VH-gene mutational status. Blood. 2007;109:4424–4431. doi: 10.1182/blood-2006-11-056648. [DOI] [PubMed] [Google Scholar]
- 33.Hoellenriegel J, Meadows SA, Sivina M, et al. The phosphoinositide 3'-kinase delta inhibitor, CAL-101, inhibits B-cell receptor signaling and chemokine networks in chronic lymphocytic leukemia. Blood. 2011;118:3603–3612. doi: 10.1182/blood-2011-05-352492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hallek M, Cheson BD, Catovsky D, et al. Guidelines for the diagnosis and treatment of chronic lymphocytic leukemia: a report from the International Workshop on Chronic Lymphocytic Leukemia updating the National Cancer Institute-Working Group 1996 guidelines. Blood. 2008;111:5446–5456. doi: 10.1182/blood-2007-06-093906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wilson JFG Wyndham H, Goy Andre, de Vos Sven, Kenkre Vaishalee P, Barr Paul M, Blum Kristie A, Shustov Andrei R, Advani Ranjana H, Lih Jason, Williams Mickey, Schmitz Roland, Yang Yandan, Pittaluga Stefania, Wright George, Kunkel Lori A, McGreivy Jesse, Balasubramanian Sriram, Cheng Mei, Moussa Davina, Buggy Joseph J, Staudt Louis M. The Bruton's Tyrosine Kinase (BTK) Inhibitor, Ibrutinib (PCI-32765), Has Preferential Activity in the ABC Subtype of Relapsed/Refractory De Novo Diffuse Large B-Cell Lymphoma (DLBCL): Interim Results of a Multicenter, Open-Label, Phase 2 Study. Blood. 2012:686. [Google Scholar]
- 36.Burger JA, Keating MJ, Wierda WG, et al. The Btk Inhibitor Ibrutinib (PCI-32765) in Combination with Rituximab Is Well Tolerated and Displays Profound Activity in High-Risk Chronic Lymphocytic Leukemia (CLL) Patients. Blood. 2012:187. [Google Scholar]
- 37.Fowler NH, Advani RH, Sharman J, et al. The Bruton's Tyrosine Kinase Inhibitor Ibrutinib (PCI-32765) Is Active and Tolerated in Relapsed Follicular Lymphoma. Blood. 2012:156. [Google Scholar]
- 38.Wang M, Rule SA, Martin P, et al. Interim Results of an International, Multicenter, Phase 2 Study of Bruton's Tyrosine Kinase (BTK) Inhibitor, Ibrutinib (PCI-32765), in Relapsed or Refractory Mantle Cell Lymphoma (MCL): Durable Efficacy and Tolerability with Longer Follow-up. Blood. 2012:904. [Google Scholar]
- 39.Byrd JC, Furman RR, Coutre S, et al. The Bruton's Tyrosine Kinase (BTK) Inhibitor Ibrutinib (PCI-32765) Promotes High Response Rate, Durable Remissions, and Is Tolerable in Treatment Naïve (TN) and Relapsed or Refractory (RR) Chronic Lymphocytic Leukemia (CLL) or Small Lymphocytic Lymphoma (SLL) Patients Including Patients with High-Risk (HR) Disease: New and Updated Results of 116 Patients in a Phase Ib/II Study. Blood. 2012:189. [Google Scholar]
- 40.Pettitt AR, Jackson R, Carruthers S, et al. Alemtuzumab in combination with methylprednisolone is a highly effective induction regimen for patients with chronic lymphocytic leukemia and deletion of TP53: final results of the national cancer research institute CLL206 trial. J Clin Oncol. 2012;30:1647–1655. doi: 10.1200/JCO.2011.35.9695. [DOI] [PubMed] [Google Scholar]
- 41.Castro JE, James DF, Sandoval-Sus JD, et al. Rituximab in combination with high-dose methylprednisolone for the treatment of chronic lymphocytic leukemia. Leukemia. 2009;23:1779–1789. doi: 10.1038/leu.2009.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Brown JR. Ibrutinib in CLL and B Cell Malignancies. Leuk Lymphoma. 2013 doi: 10.3109/10428194.2013.803226. [DOI] [PubMed] [Google Scholar]
- 43.Herman SE, Gordon AL, Wagner AJ, et al. Phosphatidylinositol 3-kinase-delta inhibitor CAL-101 shows promising preclinical activity in chronic lymphocytic leukemia by antagonizing intrinsic and extrinsic cellular survival signals. Blood. 2010;116:2078–2088. doi: 10.1182/blood-2010-02-271171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Furman RR, Byrd JC, Brown JR, Coutre SE, Benson DM, et al. CAL-101, An Isoform-Selective Inhibitor of Phosphatidylinositol 3-Kinase P110, Demonstrates Clinical Activity and Pharmacodynamic Effects In Patients with Relapsed or Refractory Chronic Lymphocytic Leukemia. Blood; ASH Annual Meeting; 2010. p. 55. [Google Scholar]
- 45.Fowler NHdVS, Schreder MT, Leonard JP, Flinn IW, et al. Combinations of the Phosphatidylinositol 3-Kinase-Delta (PI3Kδ) Inhibitor Gs-1101 (CAL-101) with Rituximab and/or Bendamustine Are Tolerable and Highly Active in Previously Treated, Indolent Non-Hodgkin Lymphoma: Results From a Phase I Study. Blood; ASH Annual Meeting; Atlanta, GA. 2012. p. 3645. [Google Scholar]
- 46.Coutre SE, Leonard JP, Furman RR, Barrientos JC, de Vos S, et al. Combinations of the Selective Phosphatidylinositol 3-Kinase-Delta (PI3Kdelta) Inhibitor GS–1101 (CAL-101) with Rituximab and/or Bendamustine Are Tolerable and Highly Active in Patients with Relapsed or Refractory Chronic Lymphocytic Leukemia (CLL): Results From a Phase I Study. Blood; ASH Annual Meeting; Atlanta, GA. 2012. p. 191. [Google Scholar]
- 47.Herko A, Mavis C, Czuczman MS, Hernandez F. AMG 319, a Novel Inhibitor of Phosphoinositide-3 Kinase Delta (PI3Kd), Demonstrates Activity in Lymphoma Pre-Clinical Models. Blood. 2012:3718. [Google Scholar]
- 48.Flinn IW, Horwitz SM, Patel M, Younes A, et al. Clinical Safety and Activity in a Phase 1 Trial of IPI-145, a Potent Inhibitor of Phosphoinositide-3-Kinase-gamma/delta in Patients with Advanced Hematologic Malignancies. Blood Blood. 2012:3663. [Google Scholar]
- 49.Iyengar S, Clear A, Bodor C, et al. P110alpha-mediated constitutive PI3K signaling limits the efficacy of p110delta-selective inhibition in mantle cell lymphoma, particularly with multiple relapse. Blood. 2013;121:2274–2284. doi: 10.1182/blood-2012-10-460832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Braselmann S, Taylor V, Zhao H, et al. R406, an orally available spleen tyrosine kinase inhibitor blocks fc receptor signaling and reduces immune complex-mediated inflammation. J Pharmacol Exp Ther. 2006;319:998–1008. doi: 10.1124/jpet.106.109058. [DOI] [PubMed] [Google Scholar]
- 51.Friedberg JW, Sharman J, Sweetenham J, et al. Inhibition of Syk with fostamatinib disodium has significant clinical activity in non-Hodgkin lymphoma and chronic lymphocytic leukemia. Blood. 2010;115:2578–2585. doi: 10.1182/blood-2009-08-236471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hoellenriegel J, Coffey GP, Sinha U, et al. Selective, novel spleen tyrosine kinase (Syk) inhibitors suppress chronic lymphocytic leukemia B-cell activation and migration. Leukemia. 2012;26:1576–1583. doi: 10.1038/leu.2012.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chen R, Wierda WG, Chubb S, et al. Mechanism of action of SNS-032, a novel cyclin-dependent kinase inhibitor, in chronic lymphocytic leukemia. Blood. 2009;113:4637–4645. doi: 10.1182/blood-2008-12-190256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chen R, Keating MJ, Gandhi V, et al. Transcription inhibition by flavopiridol: mechanism of chronic lymphocytic leukemia cell death. Blood. 2005;106:2513–2519. doi: 10.1182/blood-2005-04-1678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cosimo E, McCaig AM, Carter-Brzezinski LJ, et al. Inhibition of NF-kappaB-mediated signaling by the CDK inhibitor CR8 overcomes pro-survival stimuli to induce apoptosis in chronic lymphocytic leukemia cells. Clin Cancer Res. 2013 doi: 10.1158/1078-0432.CCR-12-2170. [DOI] [PubMed] [Google Scholar]
- 56.Parry D, Guzi T, Shanahan F, et al. Dinaciclib (SCH 727965), a novel and potent cyclin-dependent kinase inhibitor. Mol Cancer Ther. 2010;9:2344–2353. doi: 10.1158/1535-7163.MCT-10-0324. [DOI] [PubMed] [Google Scholar]
- 57.Johnson AJ, Yeh YY, Smith LL, et al. The novel cyclin-dependent kinase inhibitor dinaciclib (SCH727965) promotes apoptosis and abrogates microenvironmental cytokine protection in chronic lymphocytic leukemia cells. Leukemia. 2012;26:2554–2557. doi: 10.1038/leu.2012.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Flinn JMJJA, Andritsos L, et al. Phase I study of the CDK inhibitor dinaciclib (SCH727965) in patients with relapsed/refractory CLL. Journal of Clinical Oncology. 2011:6623. [Google Scholar]
- 59.Baiocchi RA, Flynn JM, Jones JA, et al. Early Evidence of Anti-Lymphoma Activity of the Cyclin Dependent Kinase Inhibitor Dinaciclib (SCH 727965) In Heavily Pre-Treated Low Grade Lymphoma and Diffuse Large Cell Lymphoma Patients. Blood. 2010:3966. [Google Scholar]
- 60.Karaman MW, Herrgard S, Treiber DK, et al. A quantitative analysis of kinase inhibitor selectivity. Nat Biotechnol. 2008;26:127–132. doi: 10.1038/nbt1358. [DOI] [PubMed] [Google Scholar]
- 61.Meade J, Fernandez C, Turner M. The tyrosine kinase Lyn is required for B cell development beyond the T1 stage in the spleen: rescue by over-expression of Bcl-2. Eur J Immunol. 2002;32:1029–1034. doi: 10.1002/1521-4141(200204)32:4<1029::AID-IMMU1029>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 62.Contri A, Brunati AM, Trentin L, et al. Chronic lymphocytic leukemia B cells contain anomalous Lyn tyrosine kinase, a putative contribution to defective apoptosis. J Clin Invest. 2005;115:369–378. doi: 10.1172/JCI22094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.McCaig AM, Cosimo E, Leach MT, et al. Dasatinib inhibits B cell receptor signalling in chronic lymphocytic leukaemia but novel combination approaches are required to overcome additional pro-survival microenvironmental signals. Br J Haematol. 2011;153:199–211. doi: 10.1111/j.1365-2141.2010.08507.x. [DOI] [PubMed] [Google Scholar]
- 64.Amrein PC, Attar EC, Takvorian T, et al. Phase II study of dasatinib in relapsed or refractory chronic lymphocytic leukemia. Clin Cancer Res. 2011;17:2977–2986. doi: 10.1158/1078-0432.CCR-10-2879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Weiss A, Littman DR. Signal transduction by lymphocyte antigen receptors. Cell. 1994;76:263–274. doi: 10.1016/0092-8674(94)90334-4. [DOI] [PubMed] [Google Scholar]
- 66.Paterson JC, Tedoldi S, Craxton A, et al. The differential expression of LCK and BAFF-receptor and their role in apoptosis in human lymphomas. Haematologica. 2006;91:772–780. [PubMed] [Google Scholar]
- 67.Talab F, Allen JC, Thompson V, et al. LCK is an important mediator of B cell receptor signaling in chronic lymphocytic leukaemia cells. Mol Cancer Res. 2013 doi: 10.1158/1541-7786.MCR-12-0415-T. [DOI] [PubMed] [Google Scholar]
- 68.Rosenwald A, Alizadeh AA, Widhopf G, et al. Relation of gene expression phenotype to immunoglobulin mutation genotype in B cell chronic lymphocytic leukemia. J Exp Med. 2001;194:1639–1647. doi: 10.1084/jem.194.11.1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Castro JE, Prada CE, Loria O, et al. ZAP-70 is a novel conditional heat shock protein 90 (Hsp90) client: inhibition of Hsp90 leads to ZAP-70 degradation, apoptosis, and impaired signaling in chronic lymphocytic leukemia. Blood. 2005;106:2506–2512. doi: 10.1182/blood-2005-03-1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Chen L, Apgar J, Huynh L, et al. ZAP-70 directly enhances IgM signaling in chronic lymphocytic leukemia. Blood. 2005;105:2036–2041. doi: 10.1182/blood-2004-05-1715. [DOI] [PubMed] [Google Scholar]
- 71.Gobessi S, Laurenti L, Longo PG, et al. ZAP-70 enhances B-cell-receptor signaling despite absent or inefficient tyrosine kinase activation in chronic lymphocytic leukemia and lymphoma B cells. Blood. 2007;109:2032–2039. doi: 10.1182/blood-2006-03-011759. [DOI] [PubMed] [Google Scholar]
- 72.Chen L, Widhopf G, Huynh L, et al. Expression of ZAP-70 is associated with increased B-cell receptor signaling in chronic lymphocytic leukemia. Blood. 2002;100:4609–4614. doi: 10.1182/blood-2002-06-1683. [DOI] [PubMed] [Google Scholar]
- 73.Chen L, Huynh L, Apgar J, et al. ZAP-70 enhances IgM signaling independent of its kinase activity in chronic lymphocytic leukemia. Blood. 2008;111:2685–2692. doi: 10.1182/blood-2006-12-062265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Soderquist R, Bates DJ, Danilov AV, et al. Gossypol overcomes stroma-mediated resistance to the BCL2 inhibitor ABT-737 in chronic lymphocytic leukemia cells ex vivo. Leukemia. 2013 doi: 10.1038/leu.2013.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Rickert RC, Jellusova J, Miletic AV. Signaling by the tumor necrosis factor receptor superfamily in B-cell biology and disease. Immunol Rev. 2011;244:115–133. doi: 10.1111/j.1600-065X.2011.01067.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Tromp JM, Tonino SH, Elias JA, et al. Dichotomy in NF-kappaB signaling and chemoresistance in immunoglobulin variable heavy-chain-mutated versus unmutated CLL cells upon CD40/TLR9 triggering. Oncogene. 2010;29:5071–5082. doi: 10.1038/onc.2010.248. [DOI] [PubMed] [Google Scholar]
- 77.Vogler M, Butterworth M, Majid A, et al. Concurrent up-regulation of BCL-XL and BCL2A1 induces approximately 1000-fold resistance to ABT-737 in chronic lymphocytic leukemia. Blood. 2009;113:4403–4413. doi: 10.1182/blood-2008-08-173310. [DOI] [PubMed] [Google Scholar]
- 78.Hewamana S, Alghazal S, Lin TT, et al. The NF-kappaB subunit Rel A is associated with in vitro survival and clinical disease progression in chronic lymphocytic leukemia and represents a promising therapeutic target. Blood. 2008;111:4681–4689. doi: 10.1182/blood-2007-11-125278. [DOI] [PubMed] [Google Scholar]
- 79.Pickering BM, de Mel S, Lee M, et al. Pharmacological inhibitors of NF-kappaB accelerate apoptosis in chronic lymphocytic leukaemia cells. Oncogene. 2007;26:1166–1177. doi: 10.1038/sj.onc.1209897. [DOI] [PubMed] [Google Scholar]
- 80.Dunleavy K, Pittaluga S, Czuczman MS, et al. Differential efficacy of bortezomib plus chemotherapy within molecular subtypes of diffuse large B-cell lymphoma. Blood. 2009;113:6069–6076. doi: 10.1182/blood-2009-01-199679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Pham LV, Tamayo AT, Yoshimura LC, et al. Inhibition of constitutive NF-kappa B activation in mantle cell lymphoma B cells leads to induction of cell cycle arrest and apoptosis. J Immunol. 2003;171:88–95. doi: 10.4049/jimmunol.171.1.88. [DOI] [PubMed] [Google Scholar]
- 82.Pahler JC, Ruiz S, Niemer I, et al. Effects of the proteasome inhibitor, bortezomib, on apoptosis in isolated lymphocytes obtained from patients with chronic lymphocytic leukemia. Clin Cancer Res. 2003;9:4570–4577. [PubMed] [Google Scholar]
- 83.Faderl S, Rai K, Gribben J, et al. Phase II study of single-agent bortezomib for the treatment of patients with fludarabine-refractory B-cell chronic lymphocytic leukemia. Cancer. 2006;107:916–924. doi: 10.1002/cncr.22097. [DOI] [PubMed] [Google Scholar]
- 84.Liu FT, Agrawal SG, Movasaghi Z, et al. Dietary flavonoids inhibit the anticancer effects of the proteasome inhibitor bortezomib. Blood. 2008;112:3835–3846. doi: 10.1182/blood-2008-04-150227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zhang L, Qian J, Ou Z, et al. Carfilzomib, An Irreversible Proteasome Inhibitor, Induces Long-Term Growth Inhibition of Mantle Cell Lymphoma In Vivo. Blood; ASH Annual Meeting; 2011. p. 3740. [Google Scholar]
- 86.Gupta SV, Hertlein E, Lu Y, et al. The Proteasome Inhibitor Carfilzomib Functions Independently of p53 To Induce Cytotoxicity and an Atypical NF-kappaB Response in Chronic Lymphocytic Leukemia Cells. Clin Cancer Res. 2013 doi: 10.1158/1078-0432.CCR-12-2754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Herishanu Y, Perez-Galan P, Liu D, et al. The lymph node microenvironment promotes B-cell receptor signaling, NF-kappaB activation, and tumor proliferation in chronic lymphocytic leukemia. Blood. 2011;117:563–574. doi: 10.1182/blood-2010-05-284984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Alsina M, Trudel S, Furman RR, et al. A phase I single-agent study of twice-weekly consecutive-day dosing of the proteasome inhibitor carfilzomib in patients with relapsed or refractory multiple myeloma or lymphoma. Clin Cancer Res. 2012;18:4830–4840. doi: 10.1158/1078-0432.CCR-11-3007. [DOI] [PubMed] [Google Scholar]
- 89.Swords RT, Kelly KR, Smith PG, et al. Inhibition of NEDD8-activating enzyme: a novel approach for the treatment of acute myeloid leukemia. Blood. 2010;115:3796–3800. doi: 10.1182/blood-2009-11-254862. [DOI] [PubMed] [Google Scholar]
- 90.Milhollen MA, Traore T, Adams-Duffy J, et al. MLN4924, a NEDD8-activating enzyme inhibitor, is active in diffuse large B-cell lymphoma models: rationale for treatment of NF-{kappa}B-dependent lymphoma. Blood. 2010;116:1515–1523. doi: 10.1182/blood-2010-03-272567. [DOI] [PubMed] [Google Scholar]
- 91.Soucy TA, Smith PG, Rolfe M. Targeting NEDD8-activated cullin-RING ligases for the treatment of cancer. Clin Cancer Res. 2009;15:3912–3916. doi: 10.1158/1078-0432.CCR-09-0343. [DOI] [PubMed] [Google Scholar]