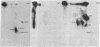Abstract
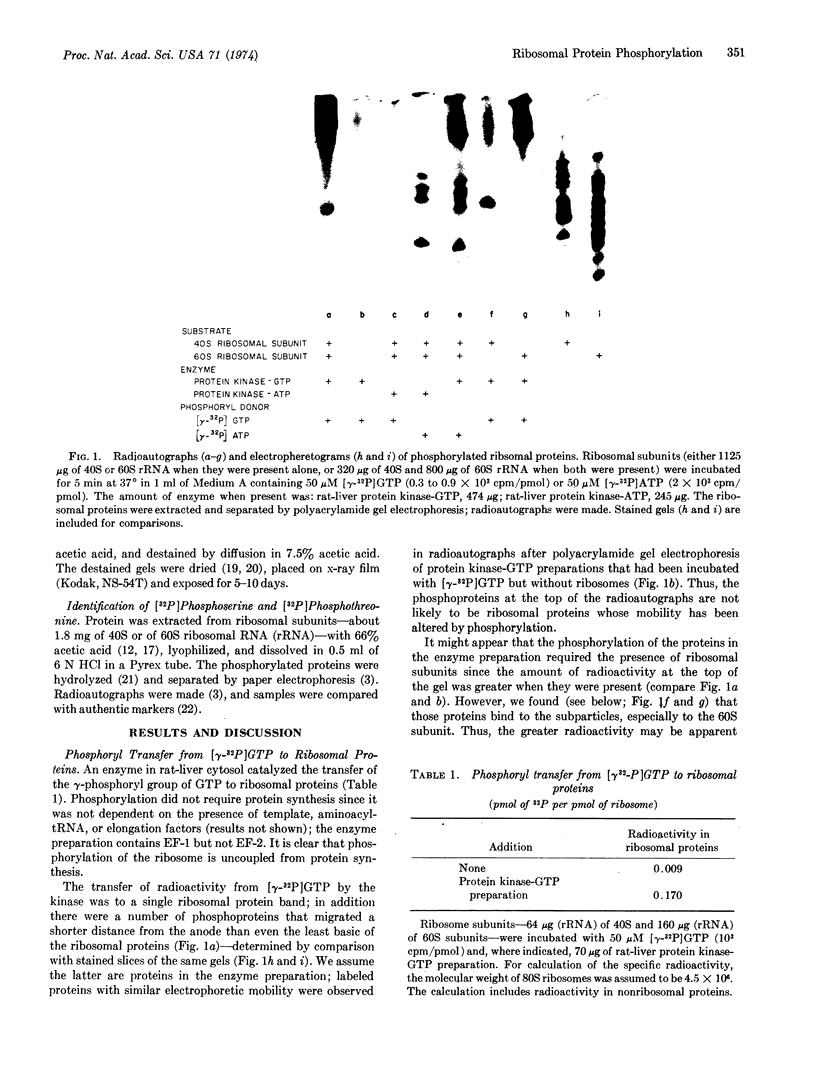
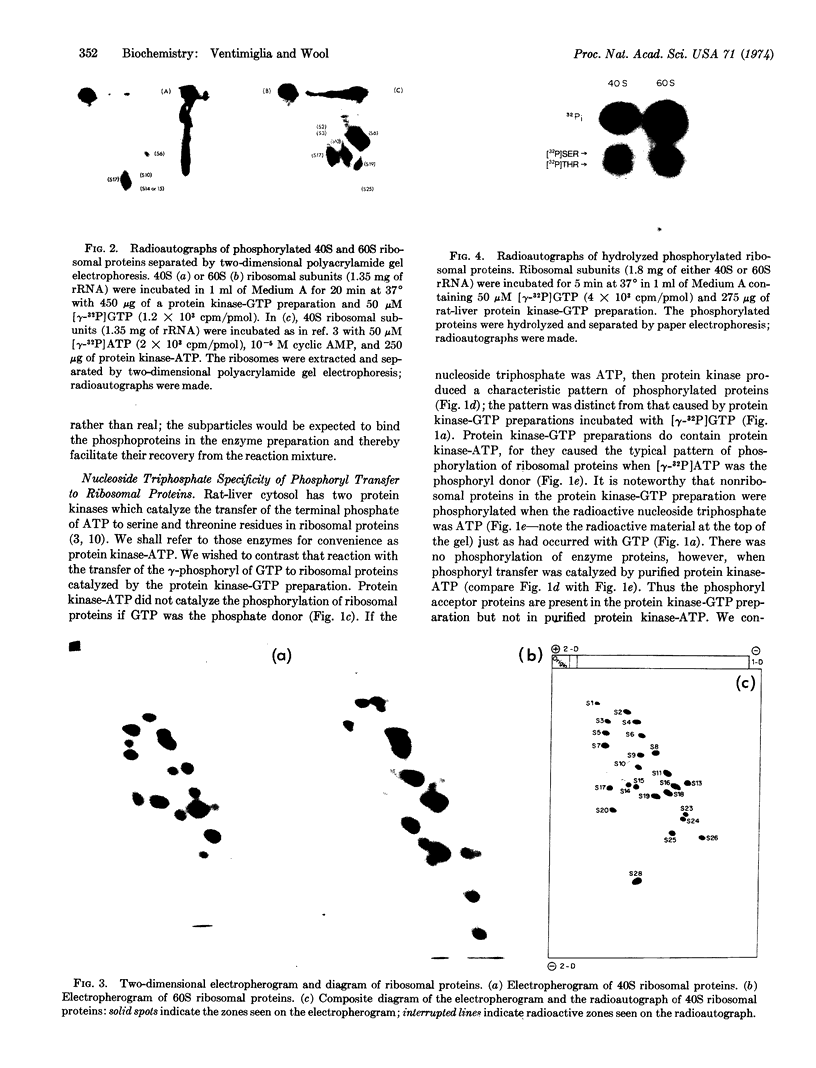
An enzyme in rat-liver cytosol transferred the γ-phosphoryl of GTP to serine and threonine residues of at least four proteins (S6, S10, S14 or S15, and S17) of the small (40S) subunit of rat-liver ribosomes. A number of nonribosomal proteins in the enzyme preparation were also phosphorylated; they were preferentially and tightly bound to the large subunit. The enzyme could be distinguished from protein kinase-ATP (which also phosphorylated ribosomal proteins) by a number of criteria: (1) GTP was the phosphoryl donor; (2) the pattern of phosphorylation of ribosomal proteins by the two enzymes was different; and (3) the protein kinase that used GTP as the phosphoryl donor was not stimulated by cyclic AMP (or by cyclic GMP).
Keywords: rat-liver cytosol
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Czernilofsky A. P., Collatz E. E., Stöffler G., Kuechler E. Proteins at the tRNA binding sites of Escherichia coli ribosomes. Proc Natl Acad Sci U S A. 1974 Jan;71(1):230–234. doi: 10.1073/pnas.71.1.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eil C., Wool I. G. Phosphorylation of rat liver ribosomal subunits: partial purification of two cyclic AMP activated protein kinases. Biochem Biophys Res Commun. 1971 Jun 4;43(5):1001–1009. doi: 10.1016/0006-291x(71)90561-4. [DOI] [PubMed] [Google Scholar]
- Fairbanks G., Jr, Levinthal C., Reeder R. H. Analysis of C14-labeled proteins by disc electrophoresis. Biochem Biophys Res Commun. 1965 Aug 16;20(4):393–399. doi: 10.1016/0006-291x(65)90589-9. [DOI] [PubMed] [Google Scholar]
- Glynn I. M., Chappell J. B. A simple method for the preparation of 32-P-labelled adenosine triphosphate of high specific activity. Biochem J. 1964 Jan;90(1):147–149. doi: 10.1042/bj0900147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy S. J., Kurland C. G., Voynow P., Mora G. The ribosomal proteins of Escherichia coli. I. Purification of the 30S ribosomal proteins. Biochemistry. 1969 Jul;8(7):2897–2905. doi: 10.1021/bi00835a031. [DOI] [PubMed] [Google Scholar]
- Kabat D. Phosphorylation of ribosomal proteins in rabbit reticulocytes. A cell-free system with ribosomal protein kinase activity. Biochemistry. 1971 Jan 19;10(2):197–203. doi: 10.1021/bi00778a001. [DOI] [PubMed] [Google Scholar]
- Kabat D. Phosphorylation of ribosomal proteins in rabbit reticulocytes. Characterization and regulatory aspects. Biochemistry. 1970 Oct 13;9(21):4160–4175. doi: 10.1021/bi00823a019. [DOI] [PubMed] [Google Scholar]
- Kaltschmidt E., Wittmann H. G. Ribosomal proteins. VII. Two-dimensional polyacrylamide gel electrophoresis for fingerprinting of ribosomal proteins. Anal Biochem. 1970 Aug;36(2):401–412. doi: 10.1016/0003-2697(70)90376-3. [DOI] [PubMed] [Google Scholar]
- Kaulenas M. S. Rapid isolation of insect ribosomal subunits by ethanol-magnesium precipitation. Anal Biochem. 1971 May;41(1):126–131. doi: 10.1016/0003-2697(71)90197-7. [DOI] [PubMed] [Google Scholar]
- LEBOY P. S., COX E. C., FLAKS J. G. THE CHROMOSOMAL SITE SPECIFYING A RIBOSOMAL PROTEIN IN ESCHERICHIA COLI. Proc Natl Acad Sci U S A. 1964 Dec;52:1367–1374. doi: 10.1073/pnas.52.6.1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Loeb J. E., Blat C. Phosphorylation of some rat liver ribosomal proteins and its activation by cyclic AMP. FEBS Lett. 1970 Sep 24;10(2):105–108. doi: 10.1016/0014-5793(70)80427-6. [DOI] [PubMed] [Google Scholar]
- Low R. B., Wool I. G. Mammalian ribosomal protein: analysis by electrophoresis on polyacrylamide gel. Science. 1967 Jan 20;155(3760):330–332. doi: 10.1126/science.155.3760.330. [DOI] [PubMed] [Google Scholar]
- Martin T. E., Rolleston F. S., Low R. B., Wool I. G. Dissociation and reassociation of skeletal muscle ribosomes. J Mol Biol. 1969 Jul 14;43(1):135–149. doi: 10.1016/0022-2836(69)90084-9. [DOI] [PubMed] [Google Scholar]
- Martin T. E., Wool I. G. Formation of active hybrids from subunits of muscle ribosomes from normal and diabetic rats. Proc Natl Acad Sci U S A. 1968 Jun;60(2):569–574. doi: 10.1073/pnas.60.2.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKeehan W. L., Hardesty B. Purification and partial characterization of the aminoacyl transfer ribonucleic acid binding enzyme from rabbit reticulocytes. J Biol Chem. 1969 Aug 25;244(16):4330–4339. [PubMed] [Google Scholar]
- Schneir M., Moldave K. The isolation and biological activity of multiple forms of aminoacyl transferase I of rat liver. Biochim Biophys Acta. 1968 Aug 23;166(1):58–67. doi: 10.1016/0005-2787(68)90490-5. [DOI] [PubMed] [Google Scholar]
- Sherton C. C., Wool I. G. Determination of the number of proteins in liver ribosomes and ribosomal subunits by two-dimensional polyacrylamide gel electrophoresis. J Biol Chem. 1972 Jul 25;247(14):4460–4467. [PubMed] [Google Scholar]
- Stirewalt W. S., Castles J. J., Wool I. G. Skeletal muscle ribosome subunits and peptidyl transfer ribonucleic acid. Biochemistry. 1971 Apr 27;10(9):1594–1598. doi: 10.1021/bi00785a014. [DOI] [PubMed] [Google Scholar]
- Terhorst C., Wittmann-Liebold B., Möller W. 50-S ribosomal proteins. Peptide studies on two acidic proteins, A 1 and A 2 , isolated from 50-S ribosomes of Escherichia coli. Eur J Biochem. 1972 Jan 31;25(1):13–19. doi: 10.1111/j.1432-1033.1972.tb01661.x. [DOI] [PubMed] [Google Scholar]
- Wool I. G., Cavicchi P. Insulin regulation of protein synthesis by muscle ribosomes: effect of the hormone on translation of messenger RNA for a regulatory protein. Proc Natl Acad Sci U S A. 1966 Sep;56(3):991–998. doi: 10.1073/pnas.56.3.991. [DOI] [PMC free article] [PubMed] [Google Scholar]