Abstract
The objective of this study was to explore the evolution of apparent diffusion coefficient (ADC) values in magnetic resonance imaging (MRI) in normal-appearing tissue of the corpus callosum during the 1st year after traumatic brain injury (TBI), and relate findings to outcome. Fifty-seven patients (mean age 34 [range 11–63] years) with moderate to severe TBI were examined with diffusion weighted MRI at three time points (median 7 days, 3 and 12 months), and a sex- and age-matched control group of 47 healthy individuals, were examined once. The corpus callosum was subdivided and the mean ADC values computed blinded in 10 regions of interests without any visible lesions in the ADC map. Outcome measures were Glasgow Outcome Scale Extended (GOSE) and neuropsychological domain scores at 12 months. We found a gradual increase of the mean ADC values during the 12 month follow-up, most evident in the posterior truncus (r=0.19, p<0.001). Compared with the healthy control group, we found higher mean ADC values in posterior truncus both at 3 months (p=0.021) and 12 months (p=0.003) post-injury. Patients with fluid-attenuated inversion recovery (FLAIR) lesions in the corpus callosum in the early MRI, and patients with disability (GOSE score ≤6) showed evidence of increased mean ADC values in the genu and posterior truncus at 12 months. Mean ADC values in posterior parts of the corpus callosum at 3 months predicted the sensory-motor function domain score (p=0.010–0.028). During the 1st year after moderate and severe TBI, we demonstrated a slowly evolving disruption of the microstructure in normal appearing corpus callosum in the ADC map, most evident in the posterior truncus. The mean ADC values were associated with both outcome and ability to perform speeded, complex sensory-motor action.
Key words: : craniocerebral trauma, GOS, MRI, prospective studies, neuropsychological tests
Introduction
Traumatic axonal injury (TAI), or diffuse axonal injury, is considered an important pathoanatomical entity in all severities of traumatic brain injury (TBI).1,2 The corpus callosum is one of the predilection sites for TAI, as it is a less mobile structure connecting the more mobile hemispheres.3,4 The corpus callosum is the largest commissural structure with widespread interhemispheric communication,3 and TAI in this region has been associated with a worse outcome.5,6
The ability to detect TAI lesions depends upon the sequences included in the magnetic resonance imaging (MRI) scanning protocol. The application of diffusion weighted imaging (DWI) or diffusion tensor imaging (DTI) has yielded increased sensitivity in the detection of TAI.2,7 DWI is based on the random movements of water molecules in the tissue known as “Brownian motions.” The rate of net diffusion of molecules is referred to as the “apparent diffusion coefficient” (ADC) value.8 DTI is a technique in which a tensor is used to calculate three eigenvalues being used to estimate the fractional anisotropy (FA) indicating the direction of the diffusion. Mean diffusivity (MD) is also calculated based on these three eigenvalues, and is mathematically equivalent to the ADC values,9 which makes it possible to compare this DTI parameter directly with the DWI findings.
In human studies from the early phase after TBI, mostly decreased ADC values have been observed in visible DWI white matter lesions,7,10 whereas increased mean ADC values have been reported in normal-appearing tissue.11–13 A DWI study of chronic TBI patients demonstrated increased mean ADC values in both visible lesions and normal-appearing tissue in the corpus callosum.14 This finding corresponds to the increased MD observed in later DTI studies5,15,16 and such changes in indices of diffusion are considered to reflect pathology in microstructure and architectural organization.9
Some longitudinal studies have examined how changes in white matter diffusion properties evolve in the corpus callosum from the early to the chronic phase after TBI. Ljungqvist et al. studied 11 mild-severe TBIs without taking possible lesions in the corpus callosum into consideration, and found an increase in MD at 6 months.17 Also, Sidaros et al. observed increase in MD in the posterior corpus callosum from 2 to 12 months in normal- appearing white matter of 30 severe TBI patients.18
However, there exist no DWI studies in which the evolution of the ADC values in the corpus callosum is followed longitudinally from the early to the chronic phase, even though DWI is easily accessible and routinely implemented in the clinic. Hence, the aim of the present study was to explore the temporal changes of the ADC values in apparently normal- looking tissue of the corpus callosum. We studied different regions of the corpus callosum in the early phase, 3 and 12 months post-injury, and related the diffusion characteristics to global and cognitive outcome at 12 months.
Methods
Patients and controls
In the time period from October 2004 to July 2007, 125 individuals (11–65 years of age) were admitted to the Department of Neurosurgery, Trondheim University Hospital, Norway, with moderate to severe brain injury (Glasgow Coma Scale [GCS] scores 3–13). The prospective collection of the patients' clinical variables and the classification of the brain injury have been described in detail in a previous publication.19
Of the 125 patients admitted to the hospital during the study period 18 (14%) patients died and 9 (7%) refused to participate or were not able to cooperate. Fourteen (11%) patients with severe psychiatric conditions, cerebrovascular diseases, or ongoing/past substance abuse were excluded. Twenty-six (21%) patients could not complete all three repeated examinations for logistical, geographical, or other reasons. One patient was excluded from the study because of extensive corpus callosum lesions. Therefore, a total of 57 (46%) patients had all of the three MRI scans during the 1st year post-injury, and could be included in the study. The first scan was performed at a median of 7 days (range 0–26), whereas the following MRIs were performed at 3 and 12 months post-injury. For one patient, the ADC map was missing at 12 months.
A group of 47 healthy controls were matched with the TBI group with regard to age (mean age of 33.4; range 13–63 years) and sex (35 males; 74 %). The control group performed diffusion MRI at one time point, as it has been shown that MD values do not change over a 12 month period in healthy controls.20
Diffusion MRI and computation of ADC values
The MRI examinations were performed either at the study hospital or at one of the local hospitals in the region. In all cases, 1.5 Tesla (Siemens Symphony or Siemens Avanto; Siemens Medical, Erlangen Germany) scanners with a six-channel head coil were used. DWI was obtained with single-shot, spin-echo planar imaging sequences with 19 slices of 5 mm section thickness (TR 3300 msec, TE 110 msec, number of excitations 4, field of view [FOV] 23 cm, bandwidth 1240 Hz, acquisition time 1:44 min), obtaining baseline images (b=0 sec/mm2), and varying diffusion gradient strength along each of three orthogonal directions (b=500 and 1000 sec/mm2). Diffusion trace maps were computed from the isotropic diffusion image and were used to estimate the ADC map. The following sequences were also obtained 19 Sagittal turbo spin echo (TSE) T2 imaging (20 slices), sagittal, coronal, and transverse T2 fluid-attenuated inversion recovery (FLAIR) imaging (24 slices), transverse T2*-weighted gradient echo (T2*GRE) imaging (24 slices), and transverse SE T1 imaging (24 slices).
The first author (K.G.M.) performed the region of interest (ROI) analyses in the diffusion weighted images in PACS using the Sectra Workstation IDS5 11.4.1. All images were analyzed blinded for patient identification, clinical information, and time of examination. Circle shaped ROIs with a radius of 1.8 mm were positioned in apparently healthy-looking tissue in the ADC map. The standard deviations of the mean ADC values were by manual placement kept as low as possible within each ROI. Callosal sequelae following insertion of intraventricular drainage were not registered as a lesion. All other visible traumatic lesions detected in the corpus callosum on the ADC map were registered at all time points. The DWI scan was used clinically to screen for ADC map lesions. In addition, visible FLAIR lesions in the corpus callosum in the early MRI were registered, but not subclassified further with regard to callosal location. We also performed subgroup analyses of the patients with FLAIR lesions versus those without such lesions in the corpus callosum. The MRI scans were also evaluated for global atrophy (yes/no) using the T2 sequences, and we did subgroup analyses also for these groups.
If lesions in the ADC map were situated in the predefined ROIs, the ROIs were moved outside the visible lesion area and placed in the closest healthy-looking area within the specified callosal region. We also performed subanalyses where the moved ROIs were identically positioned for all the three investigations, after the blinding was removed.
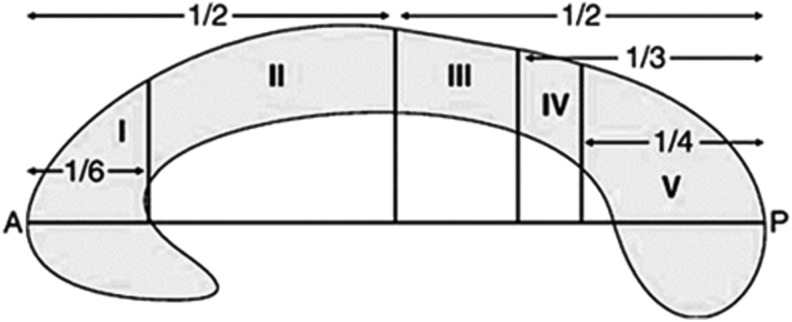
For all cases and controls, the ADC measurements were computed in 10 different predefined ROIs within the corpus callosum, based on the Hofer and Frahm scheme (Fig. 1).21 The fibers in region I project into the prefrontal region, whereas regions II, III and IV contain fibers projecting to premotor, motor, and sensory cortical areas, respectively.21 Posterior parietal, temporal, and occipital fibers cross the corpus callosum in region V. Mean ADC values were calculated in ROIs in each of these five regions on both sides of the corpus callosum (Fig. 2).
FIG. 1.
The Hofer and Frahm scheme illustrating the topography of the midsagittal corpus callosum. The regions of interest (ROIs) were computed in each region bilaterally. Region I corresponds to the genu and region V corresponds to the splenium, whereas regions II–IV correspond to the truncus. The fibers from the different regions project to the following regions of the brain: region I, prefrontal; region II, premotor and supplementary motor; region III, motor; region IV, sensory; region V, posterior parietal, temporal, and occipital. A, anterior. P, posterior. Redrawn from Hofer and Frahm21; reproduced with permission from the publisher and author.
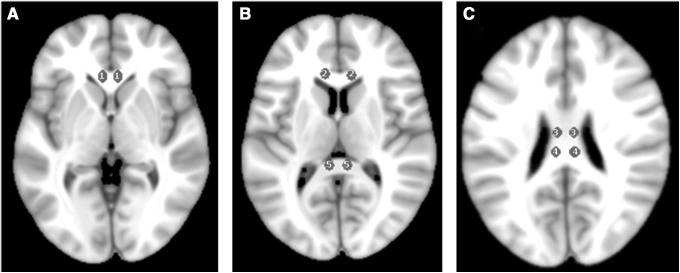
FIG. 2.
The 10 individual regions of interest (ROIs) are indicated in this axial T1 MRI template, with a similar cross-section as used in the apparent diffusion coefficient (ADC) maps. The two individual ROIs of the Hofer and Frahm region I of the corpus callosum are indicated in (A) (genu). The two most anterior ROIs in (B) indicate the second region, whereas the two posterior ROIs indicate the fifth region (splenium) of the corpus callosum. In (C), the two anterior ROIs and the two posterior ROIs indicate the third and fourth regions, respectively.
Throughout this article, the mean ADC value denotes the mean of the measurements performed on both sides in each specified region, whereas the total mean ADC value denotes the mean of the measurements performed at both sides in all the regions.
Assessment of outcome and neuropsychological function
Global outcome was assessed with Glasgow Outcome Scale Extended (GOSE) 12 months post-injury by telephone or personal contact with the patient and the relatives or caregivers.22 GOSE score of 7 and 8 was defined as a good recovery, whereas a GOSE score <7 was defined as disability.
Neuropsychological assessment was performed at 12 months post-injury. In some cases, one or more tests were not administered for various reasons. Raw scores were converted to T-scores by use of normative data provided by the manufacturers of the tests, except for the Symbol Digit Modality Test, in which a normative sample quoted by Lezak was used.23 T-scores on the individual neuropsychological tests were collapsed into composite scores reflecting the overall function on each cognitive domain, and in this study we used these different domains in the analyses.
1. Sensory-motor function: Grooved Pegboard using dominant and nondominant hand.24
2. Information processing speed: Trail Making Test (TMT); condition 2 (number sequencing), and condition 3 (letter sequencing), Color-Word Interference Test (CWIT); condition 1 (color naming) and 2 (word reading) from the Delis Kaplan Executive Function System (D-KEFS),25 and Symbol Digit Modality Test (SDMT), oral and written versions.26
3. Executive function: Category Test computer version,27 Verbal Fluency Test; condition 1 (letter fluency), and condition 3 (category change) from D-KEFS, TMT; condition 4 (Number-Letter Sequencing) from D-KEFS, CWIT; conditions 3 and 4 (Inhibition and Inhibition/Switching) from D-KEFS; Tower Test from D-KEFS.
The control group was not assessed on neuropsychological tests.
Statistical analyses
The statistical analyses were conducted using the IBM© Statistical Package for the Social Sciences (SPSS)© Statistics version 19 and STATA/SE version 11.2.
The ADC values were analysed with nonparametric tests because of lack of normal distribution. The Kruskal–Wallis Test and the Mann–Whitney U test were used, and in the multiple comparisons of mean ADC values in patients versus controls, we applied Bonferroni correction for multiple comparisons. Chi Square test was used for comparison of proportions, and for the nonparametric related samples, we used Wilcoxon signed rank test for pairwise comparisons. Statistical evaluation of the linear trend of the mean ADC values was evaluated using Jonckheere–Terpstra Test together with the correlation coefficient Kendall's tau-b to get a measure of the effect size.
The association between mean ADC values at 3 and 12 months and GOSE score or neuropsychological test scores at 12 months was assessed by Spearman Rank correlation, ordinal logistic regression models, or linear regression models. Only the regional mean ADC values that reached a p level<0.1 in the simple analyses were further examined in multiple regression models with adjustment for age.
The precision of the estimates was assessed either with respective standard deviations or 95% confidence intervals (CI). All tests were considered statistically significant at a probability value<0.05.
Ethics
The Regional Committee for Medical Research Ethics approved this study. Written consent was obtained either from the surviving patients or their next of kin if the patient was incapacitated or <16 years of age.
Results
Demographics and visible corpus callosum lesions
Demographic and injury related variables are presented in Table 1. A total of 47 patients (82%) were diagnosed with TAI on the early MRI. In the early phase, 37% of the patients had visible lesions in the corpus callosum on the ADC map, whereas 51% had FLAIR lesions. Two patients with visible lesions in the ADC map had no FLAIR lesions. The visible lesions on the ADC map were detected in the splenium (n=4), truncus (n=5), or both (n=9). In addition, three patients had isolated ADC map lesions in the genu. Only 9 (15%) patients had visible lesions in the ADC map located to one or more of the predefined ROIs, making it necessary to move 12 of the total 570 ROIs in the early MRI. No visible lesions were detected in the ADC maps at 3 or 12 months.
Table 1.
Patient Characteristics
| Variable | n | No. | Percent |
|---|---|---|---|
| Age (years)a |
57 |
33.5 |
(11.4 – 63.4) |
| Sex (male/female) |
57 |
42/15 |
(74%/26%) |
| Injury mechanism |
57 |
|
|
| Vehicle accident |
|
26 |
(46%) |
| Fall |
|
25 |
(44%) |
| Other |
|
6 |
(10%) |
| Severe TBI according to HISS |
57 |
26 |
(46%) |
| GCS scoreb |
57 |
9 |
(3 – 13) |
| Unilateral pupillary dilation |
57 |
5 |
(9%) |
| ADC map lesions in corpus callosum |
57 |
21 |
(37%) |
| FLAIR lesions in corpus callosum |
57 |
29 |
(51%) |
| Traumatic axonal injury (TAI) grade |
57 |
|
|
| No TAI |
|
10 |
(18%) |
| TAI grade 1 |
|
16 |
(28%) |
| TAI grade 2 |
|
14 |
(25%) |
| TAI grade 3 |
|
17 |
(30%) |
| Neurosurgery performed | |||
| Evacuation hematoma |
|
12 |
(21%) |
| External ventricular drainage |
|
4 |
(7%) |
| Intraparencymal ICP sensor |
|
20 |
(35%) |
| GOSE score at 12 months |
57 |
|
|
| 1–2 |
|
1 |
(2%) |
| 3–4 |
|
6 |
(10%) |
| 5–6 |
|
19 |
(33%) |
| 7–8 |
|
31 |
(54%) |
| Motor function at 12 monthsc |
42 |
43.9 |
10.3 |
| Processing speed at 12 monthsc |
42 |
46.5 |
11.7 |
| Executive functions at 12 monthsc | 35 | 47.7 | 7.1 |
Age listed as mean and range.
GCS score is listed with median and ranges.
All neuropsychological test domains are listed as mean of T-scores with standard deviations. More details for what these tests consist of, see the Methods section.
TBI, traumatic brain injury; HISS, Head Injury Severity Scale;
GCS, Glasgow coma scale; ADC, apparent diffusion coefficient. FLAIR; fluid-attenuated inversion recovery;
ICP, intracranial pressure; GOSE, Glasgow Outcome Scale Extended.
The age of the patients (mean 33.5 [SD 16.8]) did not differ from the control subjects (mean 33.3 [SD13.3], p=0.96), and the proportion of men in the patient group (n=42, 74%) did not differ from the control group (n=35, 74%, p=1.0).
Evolution of the mean ADC values during the 1st year after TBI
We found a gradual significant increasing mean ADC value in region IV of corpus callosum during the 1 year follow-up, and a similar trend was found in regions I and III (Table 2). In the early MRI, the mean ADC values in the different callosal regions did not differ from the those in the control group (p=0.51–1.0). However, at 3 months, the mean ADC value in region IV was significantly higher than in the control group (p=0.021). The same was found at 12 months, but with a stronger p value (p=0.003) and at this time point also the total mean ADC value was significantly higher than in the control group (p=0.042).
Table 2.
Mean ADC Values in the Corpus Callosum (mADC±95% CI×10−5mm2/sec) in TBI Patients during the First Year Post-Injury
| |
Controls (n=47) |
Early MRI (n=56) |
3 months MRI (n=57) |
12 months MRI (n=56) |
|
|
||||
|---|---|---|---|---|---|---|---|---|---|---|
| Corpus callosum region | mADC | 95% CI | mADC | (95% CI) | mADC | (95% CI) | mADC | (95% CI) | ra | p value |
| Region I |
70.2 |
(68.4; 72.0) |
71.0 |
(68.4; 73.5) |
72.1 |
(69.1; 75.0) |
73.0 |
(70.0; 76.0) |
0.10 |
0.060 |
| Region II |
68.8 |
(67.1; 70.5) |
69.2 |
(66.3; 72.1) |
67.8 |
(64.9; 70.6) |
71.0 |
(67.8; 74.2) |
0.03 |
0.56 |
| Region III |
72.9 |
(71.1; 74.6) |
74.4 |
(71.8; 77.1) |
73.6 |
(71.1; 76.1) |
75.6 |
(72.9; 78.4) |
0.10 |
0.058 |
| Region IV |
72.6 |
(70.5; 74.7) |
73.3 |
(69.9; 76.7) |
75.2 |
(72.6; 77.8) |
77.2 |
(74.5; 80.0) |
0.19 |
<0.001 |
| Region V |
71.2 |
(69.7; 72.8) |
69.6 |
(67.2; 72.0) |
70.8 |
(68.4; 73.2) |
71.8 |
(69.2; 74.4) |
0.01 |
0.88 |
| Total | 71.0 | (70.0; 71.9) | 71.0 | (68.9; 73.1) | 71.1 | (68.8; 73.5) | 73.0 | (70.5; 75.5) | 0.10 | 0.042 |
Kendalls tau b correlation coefficient.
Bold type indicates that the p value is<0.05 (i.e. statistically significant), and p value indicates linear trend across the groups.
ADC, apparent diffusion coefficient; mADC, mean apparent diffusion coefficient; MRI, magnetic resonance imaging;
95% CI, 95% confidence interval; TBI, traumatic brain injury.
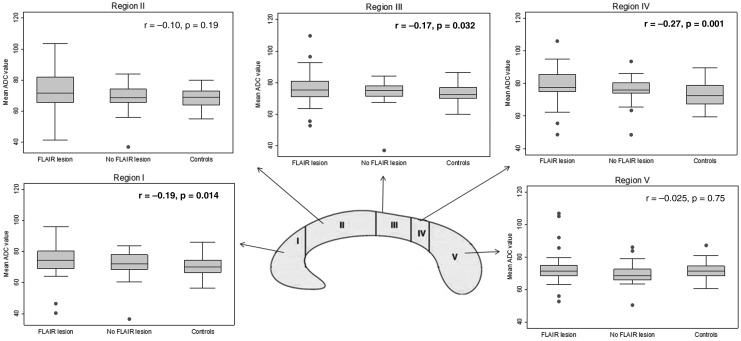
As some of the patients without visible lesions in the ADC map had FLAIR lesions in the corpus callosum, we analyzed ADC values in subgroups of patients with and without FLAIR lesions and compared these with controls. We found a gradual increase of the mean ADC values in regions I, III, and IV, with the highest values in patients with FLAIR lesions in the corpus callosum in the early MRI, followed by patients with no FLAIR lesions (Fig. 3). We also did similar subgroup analyses where we detected the highest ADC values in patients with global atrophy compared with those without atrophy and healthy controls. These findings were similarly for regions I (p=0.023), III (p=0.020), and IV (p=0.013).
FIG. 3.
Comparisons of the mean apparent diffusion coefficient (ADC) values in patients with fluid-attenuated inversion recovery (FLAIR) lesions in the corpus callosum with those in patients without FLAIR lesions in the corpus callosum, and healthy controls. In regions I, III, and IV Kendall's tau b (marked in bold) showed a significant decreasing trend, with highest mean ADC values among the patients with FLAIR lesions.
We repeated the analyses of the mean ADC values in the nine patients with moved ROIs after the blinding was removed, and no differences in the overall results of the evolution of the ADC values were detected.
Three months mean ADC values related to neuropsychological function at 12 months
We demonstrated that the mean ADC values at 3 months in regions IV and V predicted the sensory-motor domain score at 12 months using multiple linear regression analyses with adjustment for age (Table 3). We also found a tendency for prediction of sensory-motor function by mean ADC values in region III (p=0.06). No significant prediction with sensory-motor function was observed for regions I (p=0.77) or II (p=0.64). We found no association between mean ADC values in any of the regions and the executive function (p=0.14–0.85) or information-processing speed score (p=0.47–0.98).
Table 3.
Linear Regressions between Mean ADC Values at 3 Months Post-TBI and 12 Months Neuropsychological Test Scores
| |
|
|
Simple linear regressions |
|
Multiple linear regressionsa |
||||
|---|---|---|---|---|---|---|---|---|---|
| Dependent variable | Explanatory variable | n | β-coeff. | 95% CI | p value | n | β-coeff. | 95% CI | p value |
| Sensory-motor function |
3 months mADC total |
42 |
−0.81 |
(−1.58, −0.03) |
0.041 |
42 |
−0.80 |
(−1.67, −0.06) |
0.067 |
| Sensory-motor function |
3 months mADC region III |
42 |
−0.52 |
(−1.01, −0.03) |
0.037 |
42 |
−0.53 |
(−1.08, −0.02) |
0.060 |
| Sensory-motor function |
3 months mADC region IV |
42 |
−0.75 |
(−1.26, −0.23) |
0.006 |
42 |
−0.76 |
(−1.32, −0.20) |
0.010 |
| Sensory-motor function | 3 months mADC region V | 42 | −0.55 | (−1.07, −0.03) | 0.001 | 42 | −0.62 | (−1.18, −0.07) | 0.028 |
Bold type indicates that the p value is<0.05 (i.e., statistically significant).
In this table only the explanatory variables with a p value<0.1 were included, as they were analyzed further in the multiple model.
In the multiple linear regressions we have adjusted for age.
TBI, traumatic brain injury; ADC, apparent diffusion coefficient; mADC, mean apparent diffusion coefficient; β-coeff, β-coefficient; 95% CI, 95% confidence interval.
Twelve months mean ADC values related to outcome and neuropsychological function at 12 months
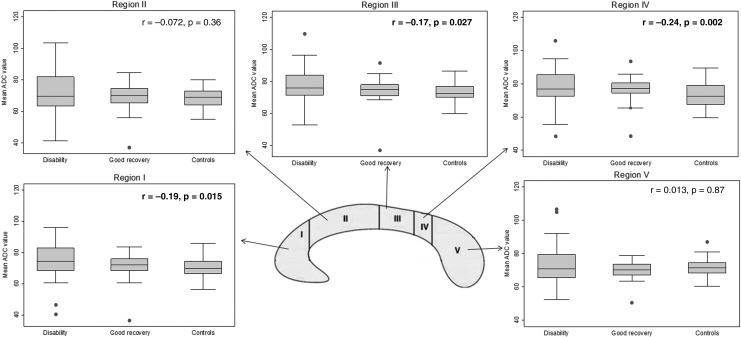
We found a significant gradual increment of the mean ADC values in regions I, III, and IV when we compared the healthy control group via the good recovery group to the disability group; the latter having the highest mean ADC values (Fig. 4).
FIG. 4.
Comparisons of the mean apparent diffusion coefficient (ADC) values in patients with disability (Glasgow Outcome Scale Extended [GOSE] score ≤6), good recovery (GOSE score 7 and 8), and the healthy control subjects. In regions I, III, and IV, Kendall's tau b (marked in bold) showed a significant decreasing trend, with highest mean ADC values among the patients in the disability group.
No significant predictions were found between the 12 month regional ADC values and motor function score (p=0.24–0.96), information-processing speed score (p=0.11–0.89), or executive function score (p=0.38–0.92).
Discussion
In this prospectve longitudinal DWI study of the corpus callosum, we have demonstrated that the mean ADC values in normal-appearing tissue increased during the 1st year after TBI. This increase in diffusivity of several regions of the corpus callosum unfolded over time with significantly increased ADC values through the subacute to the chronic phase in the posterior truncus. The same tendency was also found in the genu. We found a relationship between the ADC values and both global outcome and complex, speeded sensory-motor function at 12 months.
Evolution of mean ADC values during the 1st year after TBI
We observed a linear trend with gradual increasing mean ADC values from the early phase to the chronic phase in region IV of corpus callosum, and also a tendency in regions I and III. The increasing diffusion in posterior parts of corpus callosum is in accordance with findings in earlier longitudinal DTI studies in adults with TBI. Bendlin et al. used a voxel- based morphometric approach, and showed that most parts of the corpus callosum of moderate and severe TBI had increased MD at 12 months compared with MD at 2 months.20 In another DTI study of 16 TBI patients, the FA was significantly reduced from 6 to 24 months in normal-appearing truncus, but no reduction was found from 2 weeks to 6 months.5 Therefore, the evolution of diffusion characteristics in previous studies mostly demonstrates a progressively increasing MD or decreasing FA in the corpus callsoum into the chronic phase.18,20 Our larger study of mean ADC values obtained with DWI supports those findings. However, our study also revealed a tendency of gradual increase of the mean ADC values over time in genu, a finding that was not shown in the previous studies.
Mean ADC values and comparison with controls
In the early stage, we could not demonstrate any differences between the mean ADC values in the TBI patients and those in the healthy control group. Our findings are contradictory to those of some other smaller studies of normal-appearing white matter,11,13 and could be explained by the differing examination time points of the early MRI or the different regions studied. However, both at 3 and 12 months post-injury, we demonstrated a higher mean ADC value in region IV than in the control group. This was the most important finding in our study, and, therefore, region IV seems to be particularly prone to develop altered microstructure following TBI. Region IV had increased ADC values both in the comparison with the control group and also when the evolution of the ADC values was studied longitudinally during the 1st year after TBI.
The finding of higher mean ADC values in the corpus callosum following moderate and severe TBI has also been found in earlier studies, when comparing with controls. Even though no large-scale DWI studies have been performed, a DWI study of 10 patients in the chronic phase showed increased mean ADC values in ROIs in the corpus callosum both in lesion and non-lesion areas.14 The existing DTI studies addressing diffusivity in the corpus callosum in the chronic phase following TBI also support our findings. In a study of 30 TBI patients, higher MD was observed in the posterior corpus callosum at 12 months compared with healthy controls.18 Another study of mild TBI patients with ROIs in normal-appearing splenium showed higher MD in the chronic phase up to 5.7 years post-injury.2
Neuropathological origin of increased ADC values in different callosal regions
Although all regions of the corpus callosum may be affected by TAI,15 neuropathological and clinical studies show that visible lesions are mainly present in the truncus and splenium.4,7,28 Light microscopic studies have shown that the density of thin diameter fibers reach a minimum and large myelinated fibers reach a maximum in the posterior truncus.29,30 Increased ADC values could result from loss of myelin sheaths, a phenomenon that neuropathological review examinations have found to continue for 1–2 years post-injury.3 Vargas et al. found that myelin degradation in the central nervous system (CNS) is slow, probably because oligodendrocytes cannot phagocytose myelin debris, and there is no influx of peripheral macrophages in the CNS to speed up the degradation process.31 This supports our finding of increasing ADC values evolving into the chronic phase. As the posterior truncus is especially enriched in large myelinated fibers, TBI-induced demyelination may have larger impact in this region, and hence lead to the faster and more notable increase in ADC values in region IV.
A study by Tomasch et al. has shown that the density of fibers is highest in the genu and posterior parts of the corpus callosum,32 which were both areas with significant increase of the mean ADC values in the present study. The significantly more pronounced increase in ADC values in patients with atrophy gives further support to the notion that these fiber-dense callosal regions suffer from an overall axonal loss. Kraus et al. concluded that the findings of increased radial and axial diffusivity in ROIs obtained in chronic moderate and severe TBI patients resulted from a combination of both myelin and fiber loss.33 Based on the evolution of the ADC values in our study, it may be speculated that the effect of myelin loss on ADC values appears before the loss of fibers, as the myelinated fibers are most numerous in the posterior truncus.
Mean ADC values in patients with FLAIR lesions in the corpus callosum
In this study, we have focused on normal-appearing tissue in the ADC map, thus not taking into account callosal lesions in other MRI sequences. We know that the FLAIR sequence is important in demonstrating non-hemorrhagic lesions after TBI.34 A trend test showed gradual increment in the mean ADC values in regions I, III, and IV from controls to patients without FLAIR lesions to patients with FLAIR lesions. This supports not only that patients with any FLAIR lesions in the corpus callosum have greater injuries to the microstructure in the chronic phase, but also that patients without FLAIR lesions have such injuries.
Mean ADC values and relation to global outcome
In our study, we detected a gradual increment of mean ADC values in the genu and posterior truncus from controls to patients in the good recovery group to the patients in the disability group. This is in accordance with the study of Sidaros et al., who also found gradually increased MD values in these same groups in the analyses of the posterior part of the corpus callosum (regions III and IV).18 Another study showed no association between the ADC values in the posterior corpus callosum and 1 year outcome in TBI, whereas low FA was predictive of worse outcome.35 But in that study, the 30 patients had TBIs ranging from mild to severe. Newcombe et al. also observed higher ADC values and lower FA in ROIs in normal-appearing white matter with worse outcome, and this was also most pronounced in the patients with poor outcome.16
Therefore, our study demonstrates that not only DTI metrics, but also the ADC values, derived from DWI, may reveal clinically significant changes in diffusivity, and importantly, this applies to tissue that looks normal on conventional MRI.
Mean ADC values and relation to neuropsychological function
We observed that the mean ADC values at 3 months could predict the sensory-motor domain score at 12 months. Corpus callosum mediates inter-hemispheric communication, and the sagittal area of the corpus callosum has been positively associated with skilled hand movements.36 The grooved Pegboard Test used here is a test of higher order motor function and depends on visuomotor coordination, manipulative dexterity, and speed. It is a sensitive test in patients with TBI.37 The finding that ADC values in the posterior corpus callosum predicted grooved Pegboard performance at 12 months, is in line with the current knowledge of the topography of the corpus callosum, as these regions contain fibers converging to motor, sensory, and occipital areas, all involved in the execution of this kind of task.
Interestingly, this association was found only for the ADC values obtained at 3 months post-injury. This was a consistent, but confusing finding, as the ADC values were more clearly increased to pathological values by 12 months. We may speculate whether underlying subgroup differences in ADC values could be more pronounced by 3 months, and that these differences are predictive of outcome. We should also bear in mind that other factors than the mean ADC values in the corpus callosum probably are important for the sensory-motor function post-injury, that is, lesions in other brain structures.
Strengths and limitations of the study
The prospective study design, the high number of participants all with three repeated MRI examinations, the standardized time point of the second and third MRI at 3 and 12 months post-injury, and the blinding of the MRI analyses together with a large control group matched for age and sex, are all important strengths of the study.
However, the time span between injury and the first DWI imaging could preferably be narrower. The placement of the ROIs may differ to some degree, as they were placed manually, and we cannot exclude partial volume effects with the cerebrospinal fluid, as some of the ROIs were placed near the ventricles. As some of the patients in the chronic phase had developed a slim corpus callosum caused by atrophy, partial volume effects and difficulties with positioning of the ROIs may have become more pronounced. Neuropsychological test results from the entire TBI group and from the controls would have been preferable.
Conclusions
We have conducted a prospective, longitudinal study of diffusion properties in ROIs in normal-appearing tissue in the corpus callosum. The ADC values were obtained with DWI, a sequence included in standard MRI protocols. To our knowledge, no similar study exists. We observed increasing diffusion in the posterior truncus (region IV) during the 1 year follow-up, with a tendency also in regions I (genu) and III (posterior truncus). This indicates an increasing disruption of callosal microstructure also in normal-appearing tissue in conventional MRI.
In patients with disability, increased ADC values in several regions of the corpus callosum were present, and the mean ADC values at 3 months predicted complex, speeded sensory-motor function at 1 year post-injury. Importantly, this study shows that the DWI sequence can reveal injury also in the normal-appearing corpus callosum, and that these subtle changes are associated with both global and neuropsychological function in the chronic phase.
Contributorship Statement
Drs. Moen, Skandsen, Finnanger and Vik were responsible for data collection. Drs. Moen, Håberg, and Vik were responsible for study design. Drs. Moen and Vik were responsible for data analysis. All authors were responsible for data interpretation and for the writing of this article.
Acknowledgments
We thank Stine Borgen Lund and Beate Holmqvist Karlsen for participating in the management of the database and the GOSE interviews. Drs. Moen, Skandsen, and Finnanger received a research grant from the Liaison Committee between the Central Norway Regional Health Authority (RHA) and the Norwegian University of Science and Technology (NTNU) during the study period. This work was also supported by the Norwegian ExtraFoundation for Health and Rehabilitation (Dr. Finnanger, grant number 2010/2/0105).
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Gentry L.R., Godersky J.C., and Thompson B. (1988). MR imaging of head trauma: review of the distribution and radiopathologic features of traumatic lesions. AJR Am. J. Roentgenol. 150, 663–672 [DOI] [PubMed] [Google Scholar]
- 2.Inglese M., Makani S., Johnson G., Cohen B.A., Silver J.A., Gonen O. and Grossman R.I. (2005). Diffuse axonal injury in mild traumatic brain injury: a diffusion tensor imaging study. J Neurosurg. 103, 298–303 [DOI] [PubMed] [Google Scholar]
- 3.Meythaler J.M., Peduzzi J.D., Eleftheriou E., and Novack T.A. (2001). Current concepts: diffuse axonal injury-associated traumatic brain injury. Arch. Phys. Med. Rehabil. 82, 1461–1471 [DOI] [PubMed] [Google Scholar]
- 4.Gasparetto E.L., Rueda Lopes F.C., and Domingues R.C. (2011). Diffusion imaging in traumatic brain injury. Neuroimaging Clin. N. Am. 21, 115–125, viii. [DOI] [PubMed] [Google Scholar]
- 5.Kumar R., Saksena S., Husain M., Srivastava A., Rathore R.K., Agarwal S., and Gupta R.K. (2010). Serial changes in diffusion tensor imaging metrics of corpus callosum in moderate traumatic brain injury patients and their correlation with neuropsychometric tests: a 2-year follow-up study. J. Head Trauma Rehabil. 25, 31–42 [DOI] [PubMed] [Google Scholar]
- 6.Caeyenberghs K., Leemans A., Geurts M., Linden C.V., Smits–Engelsman B.C., Sunaert S., and Swinnen S.P. (2011). Correlations between white matter integrity and motor function in traumatic brain injury patients. Neurorehabil. Neural Repair 25, 492–502 [DOI] [PubMed] [Google Scholar]
- 7.Huisman T.A., Sorensen A.G., Hergan K., Gonzalez R.G., and Schaefer P.W. (2003). Diffusion-weighted imaging for the evaluation of diffuse axonal injury in closed head injury. J. Comput. Assist. Tomogr. 27, 5–11 [DOI] [PubMed] [Google Scholar]
- 8.Le Bihan D., Breton E., Lallemand D., Grenier P., Cabanis E., and Laval–Jeantet M. (1986). MR imaging of intravoxel incoherent motions: application to diffusion and perfusion in neurologic disorders. Radiology 161, 401–407 [DOI] [PubMed] [Google Scholar]
- 9.Basser P.J. and Jones D.K. (2002). Diffusion-tensor MRI: theory, experimental design and data analysis – a technical review. NMR Biomed. 15, 456–467 [DOI] [PubMed] [Google Scholar]
- 10.Liu A.Y., Maldjian J.A., Bagley L.J., Sinson G.P., and Grossman R.I. (1999). Traumatic brain injury: diffusion-weighted MR imaging findings. AJNR Am. J. Neuroradiol. 20, 1636–1641 [PMC free article] [PubMed] [Google Scholar]
- 11.Hou D.J., Tong K.A., Ashwal S., Oyoyo U., Joo E., Shutter L., and Obenaus A. (2007). Diffusion-weighted magnetic resonance imaging improves outcome prediction in adult traumatic brain injury. J. Neurotrauma 24, 1558–1569 [DOI] [PubMed] [Google Scholar]
- 12.Goetz P., Blamire A., Rajagopalan B., Cadoux–Hudson T., Young D., and Styles P. (2004). Increase in apparent diffusion coefficient in normal appearing white matter following human traumatic brain injury correlates with injury severity. J. Neurotrauma 21, 645–654 [DOI] [PubMed] [Google Scholar]
- 13.Brandstack N., Kurki T., Hiekkanen H., and Tenovuo O. (2011). Diffusivity of normal-appearing tissue in acute traumatic brain injury. Clin. Neuroradiol. 21, 75–82 [DOI] [PubMed] [Google Scholar]
- 14.Chan J.H., Tsui E.Y., Peh W.C., Fong D., Fok K.F., Leung K.M., Yuen M.K., and Fung K.K. (2003). Diffuse axonal injury: detection of changes in anisotropy of water diffusion by diffusion-weighted imaging. Neuroradiology 45, 34–38 [DOI] [PubMed] [Google Scholar]
- 15.Chang M.C., and Jang S.H. (2010). Corpus callosum injury in patients with diffuse axonal injury: a diffusion tensor imaging study. NeuroRehabilitation 26, 339–345 [DOI] [PubMed] [Google Scholar]
- 16.Newcombe V., Chatfield D., Outtrim J., Vowler S., Manktelow A., Cross J., Scoffings D., Coleman M., Hutchinson P., Coles J., Carpenter T.A., Pickard J., Williams G., and Menon D. (2011). Mapping traumatic axonal injury using diffusion tensor imaging: correlations with functional outcome. PLoS One 6, e19214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ljungqvist J., Nilsson D., Ljungberg M., Sorbo A., Esbjornsson E., Eriksson–Ritzen C., and Skoglund T. (2011). Longitudinal study of the diffusion tensor imaging properties of the corpus callosum in acute and chronic diffuse axonal injury. Brain Inj. 25, 370–378 [DOI] [PubMed] [Google Scholar]
- 18.Sidaros A., Engberg A.W., Sidaros K., Liptrot M.G., Herning M., Petersen P., Paulson O.B., Jernigan T.L., and Rostrup E. (2008). Diffusion tensor imaging during recovery from severe traumatic brain injury and relation to clinical outcome: a longitudinal study. Brain 131, 559–572 [DOI] [PubMed] [Google Scholar]
- 19.Skandsen T., Kvistad K.A., Solheim O., Strand I.H., Folvik M., and Vik A. (2009). Prevalence and impact of diffuse axonal injury in patients with moderate and severe head injury: a cohort study of early magnetic resonance imaging findings and 1–year outcome. J. Neurosurg. 113, 556–563 [DOI] [PubMed] [Google Scholar]
- 20.Bendlin B.B., Ries M.L., Lazar M., Alexander A.L., Dempsey R.J., Rowley H.A., Sherman J.E., and Johnson S.C. (2008). Longitudinal changes in patients with traumatic brain injury assessed with diffusion-tensor and volumetric imaging. Neuroimage 42, 503–514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hofer S., and Frahm J. (2006). Topography of the human corpus callosum revisited—comprehensive fiber tractography using diffusion tensor magnetic resonance imaging. Neuroimage 32, 989–994 [DOI] [PubMed] [Google Scholar]
- 22.Jennett B., Snoek J., Bond M.R., and Brooks N. (1981). Disability after severe head injury: observations on the use of the Glasgow Outcome Scale. J. Neurol. Neurosurg. Psychiatry 44, 285–293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lezak M.D., Howieson D.B., and Loring D.W. (2004). Neuropsychological Assessment, 4th ed. Oxford University Press: New York [Google Scholar]
- 24.Matthews C.G., and Klove K. (1964). Instruction Manual for the Adult Neuropsychology Test Battery. University of Wisconsin Medical School: Madison [Google Scholar]
- 25.Delis D.C., Kaplan E., and Kramer J. (2001). Delis Kaplan Executive Function System. The Psychological Corporation: San Antonio [Google Scholar]
- 26.Smith A. (2002). Symbol Digit Modality Test Manual, (SDMT), 9th ed. Western Psyhological Services: Los Angeles [Google Scholar]
- 27.Heaton R.K. (1993). Wisconsin Card Sorting Test, Computer Version −2 Research Edition. Psychological Assessment Resources, Inc.: Odessa, FL [Google Scholar]
- 28.Leclercq P.D., McKenzie J.E., Graham D.I., and Gentleman S.M. (2001). Axonal injury is accentuated in the caudal corpus callosum of head-injured patients. J. Neurotrauma 18, 1–9 [DOI] [PubMed] [Google Scholar]
- 29.Aboitiz F., Scheibel A.B., Fisher R.S., and Zaidel E. (1992). Fiber composition of the human corpus callosum. Brain Res. 598, 143–153 [DOI] [PubMed] [Google Scholar]
- 30.Aboitiz F. (1992). Brain connections: interhemispheric fiber systems and anatomical brain asymmetries in humans. Biol. Res. 25, 51–61 [PubMed] [Google Scholar]
- 31.Vargas M.E., and Barres B.A. (2007). Why is Wallerian degeneration in the CNS so slow? Annu. Rev. Neurosci. 30, 153–179 [DOI] [PubMed] [Google Scholar]
- 32.Tomasch J. (1954). Size, distribution, and number of fibres in the human corpus callosum. Anat. Rec. 119, 119–135 [DOI] [PubMed] [Google Scholar]
- 33.Kraus M.F., Susmaras T., Caughlin B.P., Walker C.J., Sweeney J.A., and Little D.M. (2007). White matter integrity and cognition in chronic traumatic brain injury: a diffusion tensor imaging study Brain 130, 2508–2519 [DOI] [PubMed] [Google Scholar]
- 34.Moen K.G., Skandsen T., Folvik M., Brezova V., Kvistad K.A., Rydland J., Manley G.T., and Vik A. (2012). A longitudinal MRI study of traumatic axonal injury in patients with moderate and severe traumatic brain injury. J. Neurol. Neurosurg. Psychiatry 93, 1193–1200 [DOI] [PubMed] [Google Scholar]
- 35.Perlbarg V., Puybasset L., Tollard E., Lehericy S., Benali H., and Galanaud D. (2009). Relation between brain lesion location and clinical outcome in patients with severe traumatic brain injury: a diffusion tensor imaging study using voxel-based approaches. Hum. Brain Mapp. 30, 3924–3933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kurth F., Mayer E.A., Toga A.W., Thompson P.M., and Luders E. (2013). The right inhibition? callosal correlates of hand performance in healthy children and adolescents callosal correlates of hand performance. Hum. Brain Mapp. 34, 2259–2265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Skandsen T., Finnanger T.G., Andersson S., Lydersen S., Brunner J.F., and Vik A. (2010). Cognitive impairment 3 months after moderate and severe traumatic brain injury: a prospective follow-up study. Arch. Phys. Med. Rehabil. 91, 1904–1913 [DOI] [PubMed] [Google Scholar]