Abstract
Background
When challenged with information about the future, healthy participants show an optimistically biased updating pattern, taking desirable information more into account than undesirable information. However, it is unknown how patients suffering from major depressive disorder (MDD), who express pervasive pessimistic beliefs, update their beliefs when receiving information about their future. Here we tested whether an optimistically biased information processing pattern found in healthy individuals is absent in MDD patients.
Method
MDD patients (n = 18; 13 medicated; eight with co-morbid anxiety disorder) and healthy controls (n = 19) estimated their personal probability of experiencing 70 adverse life events. After each estimate participants were presented with the average probability of the event occurring to a person living in the same sociocultural environment. This information could be desirable (i.e. average probability better than expected) or undesirable (i.e. average probability worse than expected). To assess how desirable versus undesirable information influenced beliefs, participants estimated their personal probability of experiencing the 70 events a second time.
Results
Healthy controls showed an optimistic bias in updating, that is they changed their beliefs more toward desirable versus undesirable information. Overall, this optimistic bias was absent in MDD patients. Symptom severity correlated with biased updating: more severely depressed individuals showed a more pessimistic updating pattern. Furthermore, MDD patients estimated the probability of experiencing adverse life events as higher than healthy controls.
Conclusions
Our findings raise the intriguing possibility that optimistically biased updating of expectations about one's personal future is associated with mental health.
Key words: Belief updating, bias, depression, information processing, optimism
Introduction
Individuals suffering from major depressive disorder (MDD) process information about the self, the world and the future in a maladaptive fashion compared with healthy individuals (APA, 2000). According to prominent cognitive theories of depression, such as Beck's cognitive model (Beck et al. 1979; Haaga & Beck, 1995; Disner et al. 2011), Seligman's learned helplessness model (Seligman, 1972) and the more recent cognitive neuropsychological model (Clark et al. 2009; Roiser et al. 2012), maladaptive cognitive biases are central to the development and maintenance of MDD. Beck's cognitive model, for example, emphasizes the role of maladaptive cognitive schemas and has led to the development of cognitive therapy, an effective treatment focused on changing these maladaptive cognitive schemas (Beck, 2005; Beck & Dozois, 2011). Related neuropsychological models of depression emphasize the relationship between maladaptive cognition and vulnerability or resilience to MDD, highlighting that maladaptive cognition may be causal in the progression of depressive symptoms (Clark et al. 2009; Roiser et al. 2012).
Cognitive theories of MDD often highlight that maladaptive cognition manifests as negativity biases (e.g. Beck et al. 1979). However, in some instances the behavior of depressed individuals seems to be better characterized by realism relative to an objective standard or by an absence of a positivity bias relative to healthy individuals (Alloy & Ahrens, 1987; Moore & Fresco, 2012). The general reasoning that MDD may be related to a reduction, absence or reversal of positivity biases relative to mental health is motivated by considerable evidence showing that healthy individuals are characterized by a diverse array of positivity biases including illusions of superiority (Taylor & Brown, 1988; Taylor & Brown, 1994; Leary, 2007), illusions of control (Taylor & Brown, 1988; Thompson et al. 1998), positivity biases in memory (Walker et al. 2003) and also unrealistic optimism about the future (Weinstein, 1980; Taylor & Brown, 1988; Weinstein & Klein, 1995; Armor & Taylor, 2002; Puri & Robinson, 2007; Sharot, 2011). However, the precise relationship between mental health, MDD and cognitive information processing biases (or their absence) has remained surprisingly underexplored.
So far, information processing in MDD patients has been mostly investigated in tasks that are not directly related to positivity biases in healthy individuals (Mathews & MacLeod, 2005; Gotlib & Joorman, 2010). For example, depressed individuals show altered responses to performance feedback in cognitive tasks such as the Tower of London planning task (Elliot et al. 1997, 1998) or reversal learning tasks (Murphy et al. 2003; Robinson et al. 2012; see Eshel & Roiser, 2010 for review). Furthermore, depressed individuals show altered reward processing as demonstrated by signal-detection analyses (Pizzagalli et al. 2005, 2008), computational reinforcement learning approaches (Huys et al. 2009) and functional neuroimaging (Tremblay et al. 2005; Steele et al. 2007; Eshel & Roiser, 2010). These studies provide considerably evidence for altered learning and information processing in MDD and discuss whether MDD is better characterized by negative biases (i.e. increased responses to negative compared with positive stimuli) or by a blunting of responses (i.e. an insensitivity to both negative and positive stimuli) (Eshel & Roiser, 2010; Gotlib & Joorman, 2010). However, studies on information processing in MMD typically do not investigate domains in which healthy individuals tend to show positivity biases.
In the current study, we aimed at investigating cognitive biases in processing information about future life events. In contrast to healthy individuals (Sharot, 2011), MDD patients show a pervasive pessimism about their personal future (APA, 2000). For example, when estimating the likelihood of experiencing positive and negative everyday life events (e.g. being invited to a party or getting a parking ticket) within the next month, individuals with high depressive symptoms expected less positive and more negative events than they eventually experienced whereas healthy participants showed the opposite pattern (Strunk et al. 2006; Strunk & Adler, 2009). Thus, previous studies have highlighted that depressed individuals are pessimistic when predicting their personal future (Cropley & MacLeod, 2003; Strunk et al. 2006; Strunk & Adler, 2009) but they do not address how information that challenges these views is incorporated into existing beliefs.
We have recently shown that healthy individuals maintain optimistic expectations as a result of selective updating; that is they process information about their personal future in a positively biased way (Sharot et al. 2011, 2012a,b). Specifically, when healthy individuals estimated the probability of experiencing various adverse life events (e.g. robbery, Alzheimer's disease) and subsequently received information about how likely these events are to occur to persons living in the same sociocultural environment, they updated their beliefs more in response to desirable information that enforced optimism than to undesirable information that enforced pessimism. That is, participants changed their estimates more when the probabilities of the adverse events were lower than expected compared with when they were higher than expected, indicating a striking asymmetry in belief updating.
In the current study we tested the hypothesis that, unlike healthy individuals, depressed individuals are characterized by a breakdown of a selective updating bias in response to information about their future. To that end, we recruited healthy and MDD participants and quantified their belief changes in response to receipt of both desirable and undesirable information about future life events.
Method
Participants
Participants were recruited by flyers at the Freie Universität Berlin and from patients at the Charité–Universitätsmedizin Berlin and the Schlosspark-Klinik Berlin. Participants were assessed for psychiatric disorders using a structured clinical interview (SCID-I; Wittchen et al. 1997) by a cognitive neuroscientist (C.W.K.), who had been trained by a psychotherapist in conducting the SCID-I. For four in-patients an assessment provided by the referring clinical psychiatrist was used instead of the SCID-I. Participants completed the Beck Depression Inventory (BDI; Hautzinger et al. 1994). To relate task-related variables to participants' trait optimism, they also completed the Life Orientation Test – Revised (LOT-R; Scheier et al. 1994). To ensure that healthy controls and MDD patients were matched on IQ, all participants completed a test of verbal IQ (the Wortschatztest, WST, a vocabulary test implemented in the HAWIE-R, the German adaptation of the Wechsler Adult Intelligence Scale; Schmidt & Metzler, 1992). Participants with a diagnosis of MDD were assessed on the 21-item Hamilton Depression Rating Scale (HAMD-21; Hamilton, 1960) by a cognitive neuroscientist (C.W.K.), who had been trained by a psychotherapist in assessing the HAMD-21. As patients were only assessed by one person, inter-rater reliabilities could not be calculated. Additionally, to assess possible influences of participants' mood state on our task, they completed a multidimensional mood state questionnaire at the beginning and at the end of the study (Mehrdimensionaler Befindlichkeitsfragebogen, MDBF; Steyer et al. 1997). Participants gave informed consent and were paid. The study was approved by the Ethics Committee of the Charité – Universitätsmedizin Berlin.
We recruited two groups of participants: the healthy control group (n = 19) included participants with no psychiatric disorders according to SCID-I, a BDI score ≤ 10 and no history of MDD. The MDD patients group (n = 19) included participants with a diagnosis of MDD. One MDD patient was excluded because of a history of alcoholism (final n = 18). Co-morbid anxiety disorders in the MDD group were not excluded (n = 8). Of the 18 MDD patients, six received a single drug and seven two or more (eight took selective serotonin reuptake inhibitors, four selective serotonin and noradrenaline reuptake inhibitors, three bupropion, two tricyclic antidepressants, two pregabalin, one melperone, one lorazepam and one promethazine). Table 1 shows the demographics and clinical characteristics of the participants and their scores on the mood state questionnaire.
Table 1.
Demographic and clinical characteristics of the participants
| Characteristic | Healthy controls | MDD patients | Significant effects (p < 0.05) | ||
|---|---|---|---|---|---|
| n | 19 | 18 | |||
| Female sex | 15 | 12 | |||
| Age (years) | 26.5 (6.61) | 29.1 (7.06) | |||
| Education (years) | 17.1 (3.46) | 15.4 (3.25) | |||
| Verbal IQ (WST) | 109.84 (7.42) | 105.5 (11.8) | |||
| LOT-R | 16.6 (3.98) | 8.3 (4.89) | g | ||
| BDI | 4.3 (3.56) | 32.6 (7.96) | g | ||
| HAMD-21 | – | 24.7 (6.95) | |||
| Co-morbid anxiety disorder | 0 | 8 | |||
| Medication | 0 | 13 | |||
| Psychiatric hospitalization | 0 | 11 | |||
| MDBFa | Pre-task | Post-task | Pre-task | Post-task | |
| Good mood – bad mood | 32.8 (5.94) | 33.4 (5.51) | 19.5 (6.79) | 20.3 (6.49) | g, pre, post |
| Awake – tired | 28.8 (6.41) | 29.0 (7.05) | 28.8 (6.41) | 20.3 (6.68) | g, i, post |
| Calm – agitated | 32. 2 (5.76) | 32.7 (5.40) | 19.8 (6.27) | 19.9 (6.03) | g, pre, post |
MDD, Major depressive disorder; WST, Wortschatztest, a vocabulary test implemented in the HAWIE-R (German adaptation of the Wechsler Adult Intelligence Scale); LOT-R, Life Orientation Test – Revised; BDI, Beck Depression Inventory; HAMD-21, 21-item Hamilton Depression Rating Scale; MDBF, Multidimensional Mood State Questionnaire (Mehrdimensionaler Befindlichkeitsfragebogen); g, significant group difference or significant main effect: group (p < 0.05); i, significant interaction between time and group (p < 0.05); pre, significant difference between group in pre-task condition (p < 0.05); post, significant difference between group in post-task condition (p < 0.05).
Data are given as mean (standard deviation) or number of participants.
Data from two healthy controls were not collected post-task on the MDBF.
Stimuli
Stimuli and task were adapted from our previous studies (Sharot et al. 2011, 2012a,b). The original English task and stimuli were translated into German by a native German speaker with English as a second language (C.W.K.). Seventy short descriptions of negative life events were used (e.g. Alzheimer's disease, robbery; see Table 2 for a complete list of the original English and the translated German stimuli). For each adverse life event, the average probability or frequency of that event occurring at least once to a person living in the same sociocultural environment as the participants was determined based on online resources (e.g. Office for National Statistics, Eurostat, PubMed). As the probabilities of the events are roughly the same across Western Europe, we used the original event probabilities (from the UK). Participants were told that they would see the probability of the event happening to an average person of a similar background living in the same place. None of the participants reported doubts about this statistical information. Very rare and very common events were not included; that is, all event probabilities lay between 10% and 70%. To ensure that the range of possible overestimation was equal to the range of possible underestimation, participants were told that the range of probabilities lay between 3% and 77%. We excluded life events that are clearly related to depressive symptoms such as severe insomnia or anxiety disorder.
Table 2.
List of stimuli
| English original | German translation |
|---|---|
| Abnormal heart rhythm | Herzrhythmusstörungen |
| Age-related blindness | Altersblindheit |
| Alzheimer's disease | Alzheimer-Erkrankung |
| Appendicitis | Blinddarmentzündung |
| Arteries hardening (narrowing of blood vessels) | Arteriosklerose (Verkalkung der Blutgefäße) |
| Artificial joint | künstliches Gelenk |
| Asthma | Asthma |
| Autoimmune disease | Autoimmunerkrankung |
| Back pain | Rückenschmerzen |
| Being cheated by husband/wife | Ehemann/Ehefrau geht fremd |
| Being convicted of crime | für ein Verbrechen verurteilt werden |
| Being fired | gefeuert werden |
| Bicycle theft | Fahrraddiebstahl |
| Blood clot in vein | Thrombose |
| Bone fracture | Knochenbruch |
| Cancer (of digestive system/lung/prostate/breast/skin) | Krebserkrankung (Magen/Darm/Lunge/Prostata/Brust/Haut) |
| Car stolen | Autodiebstahl |
| Card fraud | Bank-/Kreditkartenbetrug |
| Chronic high blood pressure | chronischer Bluthochdruck |
| Chronic ringing sound in ear (tinnitus) | Tinnitus (Ohrgeräusche) |
| Death before age 60 | Tod vor dem 60. Lebensjahr |
| Death before age 70 | Tod vor dem 70. Lebensjahr |
| Death before age 80 | Tod vor dem 80. Lebensjahr |
| Death by infection | Tod durch Infektion |
| Dementia | Demenz |
| Diabetes (type 2) | Diabetes (Typ 2) |
| Disease of the spinal cord | Erkrankung der Wirbelsäule |
| Divorce | Scheidung |
| Domestic burglary | Einbruch in Haus/Wohnung |
| Drug abuse | Drogenabhängigkeit |
| Epilepsy | Epilepsie |
| Eye cataract (clouding of the lens of the eye) | Grauer Star (Linsentrübung) |
| Fraud when buying something on the internet | Betrug bei Internetkauf |
| Gallbladder stones | Gallensteine |
| Genital warts | Genitalwarzen |
| Gluten intolerance | Glutenunverträglichkeit |
| Having a stroke | Schlaganfall |
| Having fleas/lice | Flöhe/Läuse haben |
| Heart failure | Herzversagen |
| Hepatitis A or B | Hepatitis A oder B |
| Hernia (rupture of internal tissue wall) | Eingeweide- oder Leistenbruch |
| Herpes | Herpes |
| House vandalized | Haus/Wohnung wird mutwillig beschädigt |
| Household accident | Haushaltsunfall |
| Infertility | Unfruchtbarkeit |
| Irritable bowel syndrome (disorder of the gut) | Reizdarm |
| Kidney stones | Nierensteine |
| Knee osteoarthritis (causing knee pain and swelling) | Kniearthrose |
| Limb amputation | Amputation von Bein oder Arm |
| Liver disease | Lebererkrankung |
| Migraine | Migräne |
| More than £30000 of debts | Schulden über 50,000 Euro |
| Obesity | Fettleibigkeit |
| Osteoporosis (reduced bone density) | Osteoporose (Knochenschwund) |
| Parkinson's disease | Parkinson-Erkrankung |
| Restless legs syndrome | Syndrom der ruhelosen Beine |
| Serious hearing problems | schwere Hörprobleme |
| Severe injury due to accident (traffic or house) | schwere Verletzungen durch Unfall zu Hause oder im Straßenverkehr |
| Severe teeth problems when old | schwere Zahnprobleme im Alter |
| Skin burn | extremer Sonnenbrand |
| Sport-related accident | Sportunfall |
| Theft from person | Opfer von Taschendieben |
| Theft from vehicle | Diebstahl aus dem Fahrzeug |
| Ulcer | Magen-/Darmgeschwür |
| Victim of mugging | Opfer eines Überfalls auf der Straße |
| Victim of violence at home | Opfer von häuslicher Gewalt |
| Victim of violence by acquaintance | Opfer von Gewalt durch einen Bekannten |
| Victim of violence by stranger | Opfer von Gewalt durch einen Fremden |
| Victim of violence with need to go to A&E | Gewaltopfer mit Notaufnahmenaufenthalt |
| Witness a traumatizing accident | Zeuge eines traumatisierenden Unfalls |
| Events used during the training sessions | |
| Dying before age 90 | Tod vor dem 90. Lebensjahr |
| Glaucoma | Grüner Star |
Task
The task was programmed using the MATLAB toolbox Cogent 2000 (www.vislab.ucl.ac.uk/cogent.php). Participants completed four blocks of stimuli. The 70 adverse life events were split into two lists of 35 events each, which were matched for event probability. One list was used for blocks 1 and 2, the other for blocks 3 and 4. In blocks 1 and 3, participants first estimated their probability of encountering the events and then were presented with the average probability of the events for a demographically similar population (Fig. 1). To assess how participants used the information provided in block 1, they were asked to re-estimate their likelihood of encountering the events in block 2. Similarly, the likelihoods of events estimated in block 3 were re-estimated in block 4.
Fig. 1.
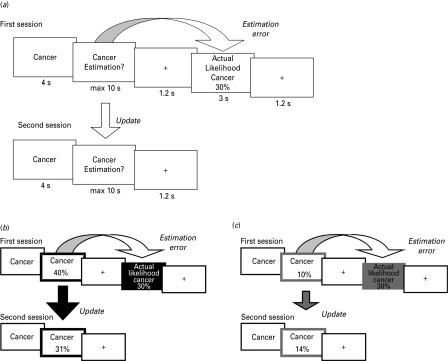
Paradigm. (a) On each trial participants were presented with a short description of one of 70 adverse life events and asked to estimate how likely this event was to occur to them in their lifetime. They were then presented with the average probability of that event occurring to a person living in the same sociocultural environment. The second session was the same as the first session, except that the average probability of the event occurring was not presented again. For each event an update term was calculated as the difference between the participants' first and second estimations. (b,c) Examples of trials for which the participant's estimate was (b) higher or (c) lower than the average probability. Here, for illustration purposes, the thick black and gray frames denote the participant's response (either an overestimation or an underestimation respectively). The black and gray filled boxes denote information that calls for an adjustment in (b) a desirable (optimistic) or (c) an undesirable (pessimistic) direction.
Estimates and average frequencies were framed as either ‘happening’ or ‘not happening’ so as to exclude the possibility that the results could be attributed to different processing strategies for high or low numbers. Specifically, in half of the blocks (either blocks 1 and 2 or blocks 3 and 4) participants had to estimate the probability of adverse life events happening to them (and were presented with the probability of the event happening for a demographically similar population) whereas in the other half they estimated the probability of the events not happening to them (and were presented with the probability of the event not happening for a demographically similar population). List assignment and order of the aforementioned framing were counter-balanced across participants. Participants completed two training trials before blocks 1 and 3 to get familiar with the task and the change in framing.
On each trial, participants were presented with one of the 70 adverse life events for 4 s (Fig. 1) and were instructed to imagine that event happening to them in the future. Then they provided their estimate of how likely the event was to happen (or not to happen) to them in the future. Participants had up to 10 s to respond using the keyboard. They then saw a fixation cross for 1.2 s. In the blocks where participants indicated their first estimate (blocks 1 and 3), they were then presented with the average probability of the event happening (or not happening) for a demographically similar population for 3 s followed by a fixation cross of 1.2 s. In the blocks in which participants re-estimated their likelihood of encountering the events (blocks 2 and 4), they were not presented with the average frequencies of the events. The order of life events was randomized within each block.
Memory and subjective scales
After completing the four blocks of the main task we tested participants' memory for the information presented. Specifically, we asked them to indicate the average probability of each event happening as presented previously in blocks 1 and 3 (self-paced). Participants then rated all stimuli on six subjective Likert scales (self-paced): vividness (How vividly could you imagine this event? 1 = not vivid at all, 6 = very vivid), familiarity (Regardless if this event has happened to you before, how familiar do you feel it is to you from TV, friends, movies and so on? 1 = not familiar at all, 6 very familiar), prior experience (Has this event happened to you before? 1 = never, 6 = very often), emotional arousal (When you imagine this event happening to you how emotionally arousing is the image in your mind? 1 = not arousing at all, 6 = very arousing), negativity (How negative would this event be for you? 1 = not negative at all, 6 = very negative) and controllability (How much control do you have over this event? 1 = not at all, 6 = very much).
Data analysis
Data were analyzed using MATLAB and SPSS. All estimates and average frequencies in the ‘not happen’ sessions were transformed into the corresponding numbers of the ‘happen’ sessions by subtracting the respective number from 100. For each event an estimation error term was calculated as the difference between participants' first estimate and the corresponding average probability presented:
| (1) |
By this definition, estimation errors were positive for overestimations and negative for underestimations. Note that all life events were negative events and that the desirability of the information arose out of whether participants over- or underestimated the probability of the events. When participants initially overestimated the probability of the adverse event relative to the average probability they received desirable information (i.e. the negative event is less likely to happen than estimated; Fig. 1b). By contrast, when participants underestimated the probability of the event relative to the average probability they received undesirable information (i.e. the negative event is more likely to happen than estimated; Fig. 1c). Therefore, for each participant, trials were classified according to whether the participant initially overestimated or underestimated the probability of the event (i.e. according to whether estimation errors were positive or negative).
To assess how much participants changed their ratings after receiving information, an update term was calculated as the difference between the first and second estimates:
| (2) |
We expected participants to change their estimates on average towards the information presented. That is, for desirable information (overestimations) first estimates should be larger than second estimates (i.e. mean updates for desirable information should be positive). For undesirable information (underestimations) first estimates should be smaller than second estimates (i.e. mean updates for undesirable information should be negative). The crucial test for biased updating was whether participants changed their estimates numerically more (or less) towards desirable information than towards undesirable information. Therefore, we compared absolute mean updates for desirable and for undesirable information across participants. To exclude the possibility that differences in mean estimation errors across participants and conditions could account for differences in updating, we calculated scaled absolute mean update scores (i.e. we divided absolute mean update scores for each participant and condition by the respective absolute mean estimation errors).
Trials were excluded if (1) participants failed to answer within the allotted time (maximal 10 s) in the first or second session (healthy controls: mean = 1.79, s.d. = 1.18; MDD patients: mean = 1.50, s.d. = 1.29) or (2) the estimation error was zero (i.e. participants gave a first estimate of their own likelihood that was the same as the presented probability; healthy controls: mean = 0.68, s.d. = 1.20; MDD patients: mean = 1.28, s.d. = 1.78). These trials were excluded because they could not be classified as desirable or undesirable. In both cases the number of excluded trials did not differ between healthy controls and MDD patients as assessed by Mann–Whitney U tests (all p > 0.1).
To test the strength of association between estimation errors and updates, Pearson correlation coefficients were calculated separately for desirable and undesirable trials within each participant.
Memory errors were calculated as the absolute differences between the frequencies previously presented and participants' recollection of these statistical numbers:
| (3) |
Unless specified otherwise, we conducted desirability (desirable/undesirable) by group (MDD/healthy) ANOVAs.
Results
Groups did not differ with regard to sex, age, education and verbal IQ, as confirmed by independent t tests, Mann–Whitney U tests or χ2 tests as appropriate (all p > 0.1; Table 1). MDD patients showed lower scores of trait optimism on the LOT-R compared with healthy controls (t35 = 5.6, p < 0.001; Table 1). MDD patients also reported having more negative mood, and being more tired and more agitated as assessed by significant main effects of group in group (MDD/healthy) by time (pre-task/post-task) ANOVAs of the MDBF scores (Table 1). Additionally, there was a significant interaction of group and time on the subscale ‘awake-tired’ of the MDBF: pre-task the groups did not differ but post-task MDD patients were more tired than healthy controls.
Comparison of updating behavior between MDD patients and healthy controls
Our main hypothesis was that belief updating behavior would differ between MDD patients and healthy controls. We predicted healthy controls would show optimistically biased updating (i.e. we expected them to update their beliefs more in response to desirable than in response to undesirable information regarding adverse life events) and that this optimistic bias would be reduced, absent or reversed in MDD patients. In line with our hypothesis, there was a significant desirability (desirable/undesirable) by group (MDD/healthy) interaction of absolute mean update scores (F1,35 = 6.9, p = 0.013, η2p = 0.17; Fig. 2a; Table 3). This interaction was characterized by an asymmetry in belief updating for healthy participants but not for MDD patients. Specifically, healthy participants updated their beliefs to a greater extent in response to desirable, compared with undesirable, information (t18 = 3.0, p = 0.008). No difference in updating was evident between desirable and undesirable trials in MDD patients (t17 = –0.11, p > 0.9). The significant interaction was further characterized by reduced updating in response to desirable information of MDD patients relative to healthy controls (t35 = –2.2, p = 0.033), with no significant difference for updating in response to undesirable information (t35 = 1.4, p > 0.1). The main effect of desirability was significant (F1,35 = 7.4, p = 0.010, ηp2 = 0.17). The main effect of group was not significant (F1,35 = 0.73, p > 0.3, ηp2 = 0.02).
Fig. 2.
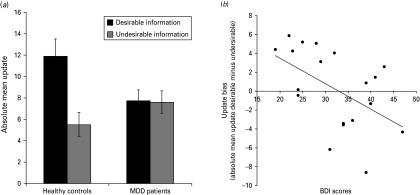
Updating behavior. (a) In the healthy group absolute mean updates were greater on trials where participants received desirable information than on trials where they received undesirable information. This bias was absent in the major depressive disorder (MDD) group. (b) Relationship between Beck Depression Inventory (BDI) scores and update bias (desirable minus undesirable) in MDD patients. Error bars indicate standard error of the mean.
Table 3.
Task-related variables, subjective scales, memory and reaction times
| Healthy controls | MDD patients | Significant effects (p < 0.05) | |||
|---|---|---|---|---|---|
| Desirable | Undesirable | Desirable | Undesirable | ||
| Task-related variables | |||||
| Number of trials | 32.9 (8.48) | 34.6 (8.23) | 43.0 (8.97) | 24.2 (8.54) | v, i, d, u |
| Number of trials excluded due to missed answers | 0.68 (1.20) | 1.28 (1.78) | |||
| Number of trials excluded due to estimation errors of zero | 1.79 (1.18) | 1.50 (1.29) | |||
| Updates | 11.96 (6.84) | 5.55 (4.81) | 7.76 (4.26) | 7.65 (4.37) | v, i, d |
| Estimation errors | 22.45 (5.65) | 17.92 (2.45) | 25.98 (6.66) | 17.86 (3.33) | v |
| Pearson correlation coefficients (Fisher transformation): updates and estimation errors | 0.71 (0.31) | 0.28 (0.35) | 0.44 (0.26) | 0.26 (0.32) | v, i, d |
| Memory errors | 15.05 (6.26) | 13.62 (4.01) | 14.68 (7.39) | 15.04 (5.93) | |
| Reaction time first estimate, ms | 2483 (561) | 2325 (529) | 2434 (831) | 2520 (739) | i |
| Reaction time second estimate, ms | 1993 (613) | 1917 (592) | 1944 (745) | 1874 (780) | |
| Subjective scales: (1) low – (6) high | |||||
| Vividness | 3.34 (0.73) | 2.92 (0.72) | 3.72 (0.57) | 3.10 (0.55) | v |
| Familiarity | 3.34 (0.721) | 2.90 (0.79) | 3.78 (0.54) | 3.42 (0.66) | v, g, d, u |
| Prior experience | 1.30 (0.19) | 1.16 (0.14) | 1.59 (0.29) | 1.37 (0.28) | v, g, d, u |
| Emotional arousal | 3.57 (1.00) | 3.40 (0.92) | 3.65 (0.85) | 3.53 (0.64) | v |
| Negativity | 4.26 (0.67) | 4.23 (0.64) | 4.06 (0.59) | 3.98 (0.67) | |
| Controllability | 2.86 (0.85) | 3.13 (0.77) | 2.62 (0.60) | 3.09 (0.63) | v |
MDD, Major depressive disorder; v, significant main effect: valence (p < 0.05); g, significant main effect: group (p < 0.05); i, significant interaction between valence and group (p < 0.05); d, significant difference between group in desirable condition (p < 0.05); u, significant difference between group in undesirable condition (p < 0.05).
Data are given as mean (standard deviation).
Additional analyses: updating behavior
To exclude the possibility that the observed difference in updating between MDD patients and healthy controls was driven by differences in estimation errors (first estimations minus probabilities presented), we performed an additional ANOVA on scaled absolute mean update scores (i.e. we accounted for differences in mean estimation errors by dividing absolute mean update scores for each participant and condition by the respective absolute mean estimation errors). The desirability by group interaction of scaled absolute mean update scores was significant (interaction desirability by group: F1,35 = 6.0, p = 0.020, ηp2 = 0.15; main effect desirability: F1,35 = 0.67, p > 0.4, ηp2 = 0.02; main effect group: F1,35 = 0.53, p > 0.4).
In our previous study on healthy participants, we analyzed the association between estimation errors and updates because formal learning models suggest that updates rely on error signals (i.e. the differences between expectations and outcomes) (Sharot et al. 2011). The strength of the association between estimation errors and updates is indicative of an optimistic bias in healthy individuals. Specifically, for desirable information estimation errors are more closely tied to updates than for undesirable information. Given these previous results in healthy individuals, we expected, as for updates, a desirability by group interaction of the strength of the association between estimation errors and updates. For each participant we calculated the correlation between estimation errors and updates separately for desirable and undesirable trials. There was a significant desirability by group interaction (mean Fisher-transformed Pearson correlation coefficients revealed an interaction of desirability by group: F1,35 = 4.19, p = 0.048, ηp2 = 0.11; main effect desirability: F1,35 = 26.9, p = 0.001, ηp2 = 0.43; main effect group: F1,35 = 2.96, p = 0.094, ηp2 = 0.08; Table 3). This further suggests that belief updating shows a less optimistic pattern in MDD patients compared with healthy controls.
Relationship between differential updating and MDD symptoms
Next, we sought to test whether the update bias (i.e. the difference between updates for desirable versus undesirable information) was related to depressive symptoms as measured by the BDI (Fig. 2b). BDI scores correlated negatively with the update bias across MDD patients (Pearson's r = –0.50, p = 0.036) but no significant correlation emerged for healthy controls (r = –0.001, p = 0.996), for which the range of BDI scores was limited. Thus, MDD patients with more severe symptoms showed less optimistic updating and more pessimistic updating (i.e. updating more in response to undesirable information compared with desirable information).
To elucidate whether group differences in update bias are potentially independent from depression severity, we tested whether group differences remained when partialling out BDI scores. We conducted a hierarchical regression analysis across all participants entering update bias as the dependent variable. As independent variables, we entered BDI scores on the first level and group membership on the second. BDI scores significantly predicted update bias (F1,35 = 8.41, p = 0.006) but group membership explained no additional variance beyond BDI scores [Fchange(1,34) = 0.00, p > 0.9], suggesting that differential updating is not independent of depression severity.
Additional analyses: relationship between differential updating and participants' characteristics and task-related variables
To test whether the update bias was related to demographic characteristics, mood states, task-related variables or subjective scales, we conducted a stepwise linear regression analysis entering update bias as the dependent variable. As independent variables we entered group membership, BDI, and their interaction, along with age, gender, education, presence of co-morbid anxiety disorder, medication status, verbal IQ, LOT-R scores, initial mood scores (Table 1) and differential measures (desirable minus undesirable) of memory errors, reaction times, estimation errors, and also differential scores on all subjective scales (vividness, familiarity, prior experience, arousal, negativity, controllability; Table 3). The model that best predicted differential updating only included the interaction of group membership with BDI score (F1,35 = 8.60, p = 0.006). Demographic characteristics, mood states, task-related variables or subjective scales were not retained in the stepwise regression. That is, the update bias was influenced by whether participants were healthy or depressed and the BDI score predicted the update bias in MDD patients.
In addition, we specifically analyzed the relationship between update bias and LOT-R scores. In MDD patients, LOT-R scores correlated with the update bias (r = –0.52, p = 0.027) but they did not explain additional variance beyond the BDI in a hierarchical regression [Fchange(1,15) = 1.107, p > 0.3]. In healthy controls, LOT-R scores did not correlate significantly with the update bias (r = –0.19, p > 0.4).
Additional analyses: comparison of initial estimates between MDD patients and healthy controls
In accord with the general pessimistic tendency of MDD patients, MDD patients initially estimated their overall probability of experiencing adverse life events as greater than healthy controls (MDD patients: first estimate = 39.9, s.d. = 8.20; healthy controls: first estimate = 31.5, s.d. = 7.47; t35 = 3.3, p = 0.002). MDD patients overestimated their probability of experiencing negative life events in relation to the average frequencies for a demographically similar population; that is mean estimation errors (first estimation minus probability presented) were positive and significantly different from zero (estimation error: mean = 10.4, s.d. = 8.18 ; t17 = 5.4, p < 0.001). This was not the case for healthy controls (estimation error: mean = 1.80, s.d. = 7.40; t18 = 1.1, p > 0.3). Mean estimation errors differed between groups (t35 = 3.3, p = 0.002).
As MDD patients were more pessimistic overall compared with healthy controls, the number of overestimations (desirable trials) and the number of underestimations (undesirable trials) differed between the two groups. Specifically, there was a significant desirability (desirable/undesirable) by group interaction of the number of trials (interaction desirability by group: F1,35 = 13.6, p < 0.001, ηp2 = 0.28; main effect desirability: F1,35 = 9.40, p = 0.004, ηp2 = 0.21; Table 3). For MDD patients the number of trials with desirable information was greater than the number of trials with undesirable information (t17 = 4.6, p < 0.001) because they overestimated the probabilities more often than they underestimated them. For healthy controls the number of trials with desirable and undesirable information did not differ (t18 = –0.46, p > 0.6). Furthermore, the interaction was characterized by MDD patients receiving desirable information on more trials and undesirable information on less trials than healthy controls (desirable: t35 = 3.54, p = 0.001; undesirable: t35 = –3.80, p < 0.001). There was no group difference in the overall number of trials (main effect group: F1,35 = 0.25, p > 0.6, ηp2 = 0.01).
Taken together, even though MDD patients received more desirable information than healthy individuals (and thus had more opportunities to change their beliefs in an optimistic direction), MDD patients showed no evidence for optimistically biased updating.
Additional analyses: memory, subjective rating scales, framing and reaction times
Differences in updating are not explained by differences in memory for the presented probability, by differences in subjective ratings of events, or by differences related to the framing of the presented probability. Specifically, we asked participants to recollect the presented probability of the event happening and computed memory errors for each event as the absolute differences between participants' recollection and the probabilities presented. There was no significant desirability by group interaction for absolute memory errors (interaction desirability by group: F1,35 = 1.46, p > 0.2, ηp2 = 0.04; main effect desirability: F1,35 = 0.52, p > 0.4, ηp2 = 0.02; main effect group: F1,35 = 0.08, p > 0.7, ηp2 = 0.00; Table 3). Additionally, we asked participants to rate all negative events on six scales for vividness, familiarity, prior experience, emotional arousal, negativity and controllability. There was no significant desirability by group interaction of any of these measures (all p > 0.1; see Table 3 for significant main effects).
To control for framing effects (effects due to information being presented in a positive or negative context) we asked participants to estimate how likely the events were to happen on half of the trials and how likely they were not to happen on the other half of the trials. In a frame (happen/not happen) by desirability by group ANOVA, only the desirability by group interaction reached significance (F1,34 = 5.74, p = 0.022, ηp2 = 0.14; all other main effects and interactions: p > 0.1; one healthy participant had to be excluded from this analysis because there were no trials in the desirable-happen condition). Thus, the framing of the estimates had no effect on updating behavior.
Reaction times for the first estimate did not show significant main effects of desirability or group (all p > 0.1) but a significant interaction (F1,35 = 5.98, p = 0.020, ηp2 = 0.15; Table 3). The interaction was characterized by healthy controls being slower for estimates for which they subsequently received desirable information compared with estimates for which they subsequently received undesirable information (t18 = –0.46, p = 0.006). This was not the case for MDD patients (p > 0.3). Reaction times for the second estimate showed no significant main effects or interaction (all p > 0.5; Table 3).
Nevertheless, differential measures (desirable minus undesirable) of memory errors, scores on all subjective scales and reaction times were included in the stepwise linear regression analysis described earlier, which revealed that only the interaction of group membership with the BDI score was retained in the model that best predicted differential updating.
Discussion
We show an absence of optimistic bias in belief updating in depressed individuals and this absence correlated with their symptom severity. Healthy individuals updated their beliefs more when presented with desirable information about the likelihood of experiencing adverse life events relative to undesirable information. By contrast, updating from desirable versus undesirable information correlated with symptom severity in MDD patients: less severely depressed individuals showed a positive bias but more severely depressed individuals showed a negative bias (i.e. they update more from undesirable compared with desirable information). Overall, this resulted in an absence of updating asymmetry across our sample of depressed individuals. Note that both groups were responsive to the information presented in the task and updated their estimates accordingly. The key difference between the groups was that, whereas controls showed a valence-dependent updating bias, the MDD group on average showed an absence of this bias. This lack of biased updating in MDD patients was due to reduced updating from desirable information about the future.
The observed pattern was selective for updating behavior (i.e. memory for the information presented and subjective ratings of the events did not show interactions between the two groups and the desirability of the information). The current results in healthy individuals replicate our previous findings (Sharot et al. 2011, 2012a,b) in a German sample and are in line with studies demonstrating that healthy individuals see emotionally laden future events through rose-colored spectacles (Sharot et al. 2007; Szpunar et al. 2012).
In accord with cognitive theories of depression (e.g. Beck et al. 1979), depressed individuals exhibited a pessimistic view of the future, evident in their inflated estimates of the probabilities of experiencing adverse events relative to controls and to the average probabilities of these events in the population. These results are also in line with previous studies (Strunk et al. 2006; Strunk & Adler, 2009) showing that depressed individuals expect more negative events and less positive events within the upcoming month than healthy controls. In our task, MDD patients received more desirable and less undesirable information because of their pessimistic views. Nevertheless, in contrast to controls, MDD patients did not take desirable information more into account than undesirable information but showed an absence of optimistically biased updating despite receiving more information that would warrant such an optimistic bias. It is possible that the more optimistic expectations of healthy compared with depressed individuals are a result of increased responsiveness to desirable information relative to negative information regarding the future; although cause and effect may also be reversed. Taken together, the current study showed that depressed individuals were characterized by pessimistic expectations and the absence of an optimistic updating bias.
Compared to many previous studies that have shown evidence for altered learning and feedback processing (Gotlib & Joorman, 2010; Eshel & Roiser, 2010), our study is more directly related to the research on positivity biases in healthy individuals. That is, participants in our study received explicit information about the personal probability of future life events whereas participants in previous studies typically received outcomes in the form of performance feedback, reward or punishment (e.g. Steele et al. 2007; Huys et al. 2009; Chase et al. 2010; Robinson et al. 2012). Using information about future life events, we show that the updating behavior of the MDD patients was less responsive to desirable information relative to controls, but similarly responsive to undesirable information. Therefore, the updating behavior of the MDD patients in our task seems to be better described by a lack of a positivity bias than by notions of general emotional blunting, as discussed in previous studies (see Eshel & Roiser, 2010).
Testing whether the updating bias shown by healthy individuals is adaptive is beyond the remit of the present study. However, the relative absence of optimistically biased updating in MDD needs to be considered in the context of previous research suggesting that positivity biases can be adaptive for mental and physical health and also economic success (Taylor & Brown, 1988, 1994; Scheier & Carver, 1992; Weinstein & Klein, 1995; Peterson, 2000; Armor & Taylor, 2002; Haselton & Nettle, 2006; Leary, 2007; Puri & Robinson, 2007; McKay & Dennett, 2009; Varki, 2009; Johnson & Fowler, 2011; Sharot, 2011). For example, all else being equal, it seems that optimists live longer, recover faster from diseases (see Rasmussen et al. 2009 for review), and earn more (Puri & Robinson, 2007). This needs to be weighted by evidence that extreme optimists do engage in unhealthy and risky behavior such as smoking and failing to save for retirement (Puri & Robinson, 2007; see Sharot, 2011 for review). Mild to moderate positivity biases may exert an adaptive effect in at least three ways. Positive beliefs can reduce stress and anxiety (Solberg Nes & Segerstrom, 2006). They can enhance a motivation to obtain desired goals; for example, optimists exercise more and work harder (Puri & Robinson, 2007; see Sharot, 2011 for review). Furthermore, positive beliefs enhance exploratory behavior that can enhance individual and group success (see Sharot, 2011 for review). In the context of MDD, recent research suggests that depressed individuals benefit from therapy approaches that focus on inducing positive biases, such as positive psychology interventions (Sin & Lyubomirsky, 2009) and cognitive bias modification (Hallion & Ruscio, 2011).
Future studies are needed to determine the generality of our findings in a larger sample that includes more male participants, and also to determine possible influences of medication and co-morbidity on the observed updating behavior. Importantly, we emphasize that the current study examines biased updating for negative but not for positive life events. Previous studies have shown that a similar updating bias exists in healthy individuals when they learn about positive stimuli (Eil & Rao, 2011; Möbius et al. 2011; Korn et al. 2012; Wiswall & Zafar, 2013), but whether such a lack of bias exists in MDD patients for positive events is an empirical question that needs to be tested.
The optimistic updating pattern described for healthy participants in our task might be associated with resilience to depression. Our study does not address whether altered information processing has a causal role in MDD. Longitudinal studies are needed to establish whether an absence of optimistically biased processing precedes the onset of depressive episodes and whether an increase in optimistic updating predicts treatment effects. Future studies will also be crucial in examining whether techniques that enhance updating from desirable information and/or techniques that reduce updating from undesirable information might be beneficial in the treatment of depression. Our results provide a starting point for such investigations by suggesting that an absence of optimistically biased belief updating of information regarding future life events may be relevant for mental health.
Acknowledgments
This study was supported by a Senior Investigator Award (098362/Z/12/Z) and a Max Planck Award to R.J.D. and a Wellcome Trust Career Development Fellowship to T.S. The Wellcome Trust Centre for Neuroimaging is supported by core funding from the Wellcome Trust 091593/Z/10/Z. We thank F. Bermpohl, M. Adli, P. Sterzer and R. Schneibel for help with recruiting participants.
Declaration of Interest
None.
References
- Alloy LB, Ahrens AH (1987). Depression and pessimism for the future: biased use of statistically relevant information in predictions for self versus others. Journal of Personality and Social Psychology 52, 366–378 [DOI] [PubMed] [Google Scholar]
- APA (2000). Diagnostic and Statistical Manual of Mental Health Disorders, 4thedn, text revision. American Psychiatric Association: Washington, DC [Google Scholar]
- Armor DA, Taylor SE (2002). When predictions fail: the dilemma of unrealistic optimism In Heuristics and Biases: The Psychology of Intuitive Judgment (ed. Gilovich T., Griffin D. W. and Kahneman D.), pp. 334–438 Cambridge University Press: New York [Google Scholar]
- Beck AT (2005). The current state of cognitive therapy: a 40-year retrospective. Archives of General Psychiatry 62, 953–959 [DOI] [PubMed] [Google Scholar]
- Beck AT, Dozois DJA (2011). Cognitive therapy: current status and future directions. Annual Review of Medicine 62, 397–409 [DOI] [PubMed] [Google Scholar]
- Beck AT, Rush AJ, Shaw B, Emery G (1979). Cognitive Therapy of Depression. Guilford Press: New York [Google Scholar]
- Chase HW, Frank MJ, Michael A, Bullmore ET, Sahakian BJ, Robbins TW (2010). Approach and avoidance learning in patients with major depression and healthy controls: relation to anhedonia. Psychological Medicine 40, 433–440 [DOI] [PubMed] [Google Scholar]
- Clark L, Chamberlain SR, Sahakian BJ (2009). Neurocognitive mechanisms in depression: implications for treatment. Annual Review of Neuroscience 32, 57–74 [DOI] [PubMed] [Google Scholar]
- Cropley M, MacLeod A (2003). Dysphoria, attributional reasoning and future event probability. Clinical Psychology and Psychotherapy 10, 220–227 [Google Scholar]
- Disner SG, Beevers CG, Haigh EAP, Beck AT (2011). Neural mechanisms of the cognitive model of depression. Nature Reviews Neuroscience 12, 467–477 [DOI] [PubMed] [Google Scholar]
- Eil D, Rao JM (2011). The good news-bad news effect: asymmetric processing of objective information about yourself. American Economic Journal: Microeconomics 3, 114–138 [Google Scholar]
- Elliott R, Baker SC, Rogers RD, O'Leary DA, Paykel ES, Frith CD, Dolan RJ, Sahakian BJ (1997). Prefrontal dysfunction in depressed patients performing a complex planning task: a study using positron emission tomography. Psychological Medicine 27, 931–942 [DOI] [PubMed] [Google Scholar]
- Elliott R, Sahakian BJ, Michael A, Paykel ES, Dolan RJ (1998). Abnormal neural response to feedback on planning and guessing tasks in patients with unipolar depression. Psychological Medicine 28, 559–571 [DOI] [PubMed] [Google Scholar]
- Eshel N, Roiser JP (2010). Reward and punishment processing in depression. Biological Psychiatry 68, 118–124 [DOI] [PubMed] [Google Scholar]
- Gotlib IH, Joormann J (2010). Cognition and depression: current status and future directions. Annual Review of Clinical Psychology 6, 285–312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haaga DAF, Beck AT (1995). Perspectives on depressive realism: implications for cognitive theory of depression. Behaviour Research and Therapy 33, 41–48 [DOI] [PubMed] [Google Scholar]
- Hallion LS, Ruscio AM (2011). A meta-analysis of the effect of cognitive bias modification on anxiety and depression. Psychological Bulletin 6, 940–958 [DOI] [PubMed] [Google Scholar]
- Hamilton M (1960). A rating scale for depression. Journal of Neurology, Neurosurgery, and Psychology 23, 56–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haselton MG, Nettle D (2006). The paranoid optimist: an integrative evolutionary model of cognitive biases. Personality and Social Psychology Review 10, 47–66 [DOI] [PubMed] [Google Scholar]
- Hautzinger M, Bailer M, Worall H (1994). The Beck Depression Inventory (BDI) [in German]. Huber: Bern [Google Scholar]
- Huys QJM, Vogelstein JT, Dayan P (2009). Psychiatry: insights into depression through normative decision-making models In Advances in Neural Information Processing Systems 21 (ed. Koller D, Schuurmans D, Bengio Y and Bottou L), pp. 729–736 MIT Press: Cambridge, MA [Google Scholar]
- Johnson DDP, Fowler HF (2011). The evolution of overconfidence. Nature 477, 317–320 [DOI] [PubMed] [Google Scholar]
- Korn CW, Prehn K, Park SQ, Walter H, Heekeren HR (2012). Positively biased processing of self-relevant social feedback. Journal of Neuroscience 32, 16832–16844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leary MR (2007). Motivational and emotional aspects of the self. Annual Review of Psychology 58, 317–344 [DOI] [PubMed] [Google Scholar]
- Mathews A, MacLeod C (2005). Cognitive vulnerability to emotional disorders. Annual Review of Clinical Psychology 1, 167–195 [DOI] [PubMed] [Google Scholar]
- McKay RT, Dennett DC (2009). The evolution of misbelief. Behavioral and Brain Sciences 32, 493–561 [DOI] [PubMed] [Google Scholar]
- Möbius MM, Niederle M, Niehaus P, Rosenblat TS (2011). Managing self-confidence: theory and experimental evidence. National Bureau of Economic Research (NBER) Working Paper No. 17014.
- Moore MT, Fresco DM (2012). Depressive realism: a meta-analytic review. Clinical Psychology Review 32, 496–509 [DOI] [PubMed] [Google Scholar]
- Murphy FC, Michael A, Robbins TW, Sahakian BJ (2003). Neuropsychological impairment in patients with major depressive disorder: the effects of feedback on task performance. Psychological Medicine 33, 455–467 [DOI] [PubMed] [Google Scholar]
- Peterson C (2000). The future of optimism. American Psychologist 55, 44–55 [DOI] [PubMed] [Google Scholar]
- Pizzagalli DA, Iosifescu D, Hallett LA, Ratner KG, Fava M (2008). Reduced hedonic capacity in major depressive disorder: evidence from a probabilistic reward task. Journal of Psychiatric Research 43, 76–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizzagalli DA, Jahn AL, O'Shea JP (2005). Toward an objective characterization of an anhedonic phenotype: a signal-detection approach. Biological Psychiatry 57, 319–327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puri M, Robinson DT (2007) Optimism and economic choice. Journal of Financial Economics 86, 71–99 [Google Scholar]
- Rasmussen HN, Scheier MF, Greenhouse JB (2009). Optimism and physical health: a meta-analytic review. Annals of Behavioral Medicine 37, 239–256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson OJ, Cools R, Carlisi CO, Sahakian BJ, Drevets WC (2012). Ventral striatum response during reward and punishment reversal learning in unmedicated major depressive disorder. American Journal of Psychiatry 196, 152–158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roiser JP, Elliott R, Sahakian BJ (2012). Cognitive mechanisms of treatment in depression. Neuropsychopharmacology 37, 117–136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheier MF, Carver CS (1992). Effects of optimism on psychological and physical well-being: theoretical overview and empirical update. Cognitive Therapy and Research 16, 201–228 [Google Scholar]
- Scheier MF, Carver CS, Bridges MW (1994). Distinguishing optimism from neuroticism (and trait anxiety, self-mastery, and self-esteem): a re-evaluation of the Life Orientation Test. Journal of Personality and Social Psychology 67, 1063–1078 [DOI] [PubMed] [Google Scholar]
- Schmidt K-H, Metzler P (1992). Wortschatztest (WST). Beltz Test GmbH: Weinheim, Germany [Google Scholar]
- Seligman ME (1972). Learned helplessness. Annual Review of Medicine 23, 407–412 [DOI] [PubMed] [Google Scholar]
- Sharot T (2011). The Optimism Bias. Pantheon Books: New York, NY [Google Scholar]
- Sharot T, Guitart-Masip M, Korn CW, Chowdhury R, Dolan RJ (2012a). How dopamine enhances an optimism bias in humans. Current Biology 21, 1477–1481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharot T, Kanai R, Marston D, Korn CW, Rees G, Dolan RJ (2012b). Selectively altering belief formation in the human brain. Proceedings of the National Academy of Sciences USA 109, 17058–17062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharot T, Korn CW, Dolan RJ (2011). How unrealistic optimism is maintained in the face of reality. Nature Neuroscience 4, 1475–1479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharot T, Riccardi AM, Raio CM, Phelps EA (2007). Neural mechanisms mediating optimism bias. Nature 450, 102–105 [DOI] [PubMed] [Google Scholar]
- Sin NL, Lyubomirsky S (2009). Enhancing well-being and alleviating depressive symptoms with positive psychology interventions: a practice-friendly meta-analysis. Journal of Clinical Psychology 65, 467–487 [DOI] [PubMed] [Google Scholar]
- Solberg Nes L, Segerstrom SC (2006). Dispositional optimism and coping: a meta-analytic review. Personality and Social Psychology Review 10, 235–251 [DOI] [PubMed] [Google Scholar]
- Steele JD, Kumar P, Ebmeier KP (2007). Blunted response to feedback information in depressive illness. Brain 130, 2367–2374 [DOI] [PubMed] [Google Scholar]
- Steyer R, Schwenkmezger P, Notz P, Eid M (1997). Multidimensional Mood State Questionnaire (MDBF) [in German]. Hogrefe: Göttingen, Germany [Google Scholar]
- Strunk DR, Adler AD (2009). Cognitive biases in three prediction tasks: a test of the cognitive model of depression. Behaviour Research and Therapy 47, 34–40 [DOI] [PubMed] [Google Scholar]
- Strunk DR, Lopez H, DeRubeis RJ (2006). Depressive symptoms are associated with unrealistic negative predictions of future life events. Behaviour Research and Therapy 44, 861–882 [DOI] [PubMed] [Google Scholar]
- Szpunar K, Addis DR, Schacter DL (2012). Memory for emotional simulations: remembering a rosy future. Psychological Science 23, 24–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor SE, Brown JD (1988). Illusion and well-being: a social psychological perspective on mental health. Psychological Bulletin 103, 193–210 [PubMed] [Google Scholar]
- Taylor SE, Brown JD (1994). Positive illusion and well-being revisited: separating fact from fiction. Psychological Bulletin 116, 21–27 [DOI] [PubMed] [Google Scholar]
- Thompson SC, Armstrong W, Thomas C (1998). Illusions of control, underestimations, and accuracy: a control heuristic explanation. Psychological Bulletin 123, 143–161 [DOI] [PubMed] [Google Scholar]
- Tremblay LK, Naranjo CA, Graham SJ, Herrmann N, Mayberg HS, Hevenor S, Busto UE (2005). Functional neuroanatomical substrates of altered reward processing in major depressive disorder revealed by a dopaminergic probe. Archives of General Psychiatry 62, 1228–1236 [DOI] [PubMed] [Google Scholar]
- Varki A (2009). Human uniqueness and the denial of death. Nature 460, 684. [DOI] [PubMed] [Google Scholar]
- Walker RW, Skowronski JJ, Thompson CP (2003). Life is pleasant – and memory helps keep it that way! Review of General Psychology 7, 203–210 [Google Scholar]
- Weinstein ND (1980). Unrealistic optimism about future life events. Journal of Personality and Social Psychology 39, 806–820 [Google Scholar]
- Weinstein ND, Klein WM (1995). Resistance of personal risk perceptions to debiasing interventions. Health Psychology 14, 132–140 [DOI] [PubMed] [Google Scholar]
- Wiswall M, Zafar B (2013). How do college students respond to public information about earnings? Federal Reserve Bank of New York Staff Report No. 516.
- Wittchen H-U, Zaudig M, Fydrich T (1997). Structured Clinical Interview for DSM-IV Axis I: Disorders (SCID-I) [in German]. Hogrefe: Göttingen, Germany [Google Scholar]


