Abstract
To investigate and describe the influence of intra-articular effusion on knee joint kinematics and electromyographic (EMG) profiles during jogging. Thirteen individuals underwent a 20 cc 0.9% saline insufflation of the knee joint capsule and completed 8 jogging trials. Stance phase, sagittal plane knee joint kinematics and thigh muscular EMG profiles were compared pre- and post-insufflation utilizing a paired t-test ( = 0.05). Mild knee effusion caused a reduction in vastus medialis (p = 0.005) and lateralis (p = 0.006) EMG activity. The rectus femoris, biceps femoris and medial hamstring muscles did not exhibit changes due to this protocol. There were no changes in the sagittal plane knee joint kinematic pattern. Twenty cc effusion can cause quadriceps inhibition in the vastus medialis and the vastus lateralis in otherwise healthy individuals during jogging. This study provides baseline data for the effects of mild knee joint effusion on thigh musculature during jogging.
Key Points.
20 cc of knee effusion can cause vastus medialis and lateralis inhibition as noted by decreases in EMG amplitude.
This effusion does not appear to alter sagittal plane knee joint kinematics during jogging.
This finding if different from previous work investigating knee joint kinematic changes during a less dynamic activity (gait) with 20 cc of effusion.
Key words: Electromyography (EMG), kinematics, jogging, muscle inhibition, knee
Introduction
The neuromuscular system acts to regulate intra-articular knee joint loading by acting as a shock absorber, producing and controlling movement and by providing functional stability via sensory and proprioceptive valuation (Andriacchi and Alexander, 2000; Hurwitz et al., 1997; O’Connor, 1993). Quadriceps inhibition has been suggested to be a causative factor in quadriceps strength deficits observed in many knee pathologies (Hurley, 1998; Hurley and Scott, 1998; Itoh et al., 1998; O’Reilly et al., 1998). Muscular weakness in association with quadriceps inhibition has also been implicated in the development of functional and structural changes of the knee joint in some knee pathologies such as patellofemoral pain (Sakai et al., 2000; Thomee et al., 1996; Thomee et al., 1995) and tibio-femoral osteoarthritis (OA) (Brandt, 2000; Brandt et al., 1999; Hurley, 1998; Hurley and Scott, 1998; Hurley et al., 1997; O’Reilly et al., 1998).
Studies that have investigated the neuromuscular performance after knee injury have shown that quadriceps electromyographic (EMG) amplitude is reduced compared to healthy controls, implying muscle inhibition is a consequence of the disease process (Hurley and Newham, 1993; Hurley and Scott, 1998; Hurley et al., 1997; Thomee et al., 1996; Thomee et al., 1995). Hurley et al. (1994) investigated the role of muscle inhibition and isometric and isokinetic muscle strength in 10 patients with unilateral osteoarthritic (OA) knees. The quadriceps of all OA legs demonstrated muscular inhibition and were significantly weaker than the non- diseased legs. The authors suggested that muscular inhibition may be partially responsible for the unilateral muscle weakness and thus may be associated with the cause or progression of OA.
Thomee et al. (1995) assessed muscle function in patients with patellofemoral pain syndrome (PFP) and healthy controls. Patients with PFP exhibited lower knee extensor strength in the most symptomatic knee compared to the least symptomatic knee and less vertical jumping ability compared to the controls. These findings correlated with lower EMG activity in the vastus medialis and the rectus femoris muscles in the PFP group. Other reports support the notion of quadriceps muscular imbalances as a function of PFP (Cerny, 1995; Souza and Gross, 1991).
A major limitation of all these reports, however, is that they only assess the current functional capacity of these individuals under the influences of the current state of the disease process. Thus, these studies cannot address the question of whether the observed muscular inhibition is or was a consequence of the knee pathology or contributed to its etiology.
Quadriceps inhibition, particularly of the vastus medialis, has been demonstrated by simulated knee joint effusion in humans without prior knee injuries (Kennedy et al., 1982; Spencer et al., 1984; Torry et al., 2000). This inhibition has been reported to alter quadriceps EMG (Stratford, 1981; Torry et al., 2000) patterns and decrease quadriceps strength (Fahrer et al., 1988; Jensen and Graf, 1993; McNair et al., 1996) in various isometric and isokinetic exercises. Intra-articular knee joint effusion has also been shown to cause altered EMG, kinematic and kinetic characteristics in the stance phase of gait (Torry et al., 2000) that are similar to those reported in knee injured groups (Boucher et al., 1992; Messier et al., 1992).
While the influences of knee joint effusion have been reported for slower motions such as gait (Torry et al., 2000), a specific goal of this research was to investigate and describe the influence of intra-articular effusion on knee joint kinematics and EMG profiles during a more dynamic activity such as running. Despite the plausible mechanical association of the neuromuscular system to the development of many knee joint pathologies in the active individual, few studies have investigated the role of muscular weakness, dysfunction and imbalance on the pathogenesis of these knee injuries. Because individuals employed in this study did not have confounding pathology (OA or PFP), the results of this protocol may help explain performance differences that have been reported in these populations in previous studies. Furthermore, the results of this study may help researchers and clinicians begin to understand the relationship muscular inhibition may possess in the pathogenesis of knee injuries in active individuals and add to our growing understanding of why strengthening exercises are effective in safely treating these injuries.
Methods
Subjects
Thirteen healthy subjects (8 male; 5 female) with no history of lower extremity pathology (mean age = 28.5, SD 5.1 years; mean mass = 76.50, SD 3.7 kg; mean height = 181.2, SD 5.1 cm) volunteered for participation in this study. Prior to testing, all participants provided their written informed consent according to a protocol approved by an Institutional Review Board retained by Local Ethics Committee.
Jogging protocol
The subjects were allowed to familiarize themselves with the runway and testing apparatus prior to testing. Infra-red timing lights evenly positioned before and after the force plate 1.5 m apart measured jogging speed. Each participant practiced jogging on the 16 m runway at a self-selected speed until they could provide a consistent jogging speed and full foot-strike on the force platform. Upon satisfying these requirements, the average speed of 5 consecutive, practice trials was used as the self-selected speed during the testing protocol. Only the trials within ± 2.5% of the self-selected speed were considered acceptable for analysis in both the pre and post-test conditions.
Knee effusion and test protocol
After 8 pre-effusion jogging trials were collected, a sub-cutaneous injection of 1.5 cc of 25% Marcaine and 1.5 cc of 1% Lidocaine was administered at the supra-patellar portal. After this injection had taken affect (~ 5 minutes), 20 cc of 0.9% saline were injected into the joint capsule to simulate knee joint effusion. A physician (PM) administered all injections using an aseptic sterile technique identical to the methods described previously (Torry et al., 2000). To ensure saline was administered into the joint space, an intra-articular (weight-bearing) pressure reading (mmHg) was recorded via a pressure transducer aligned in parallel with the syringe (Kennedy et al., 1982; Torry et al., 2000). The needle was withdrawn a sterile dressing applied and the individuals performed 8 post-effusion jogging trials. All jogging tests were completed within 10 min to avoid the stretch-relax cycle of the human knee joint capsule (Levick, 1983). In accordance with Internal Review Board recommendations, all participants were instructed to refrain from weight bearing exercises for two-weeks after testing. This was to promote the return of Donnan’s osmotic pressure gradient within the hyaline cartilage, as increased or decreased water content has been experimentally shown to have a strong influence on the mechanical properties of articular cartilage (Mow and Ateshian, 1997; Mow and Ratcliff, 1997).
Instrumentation and data processing
Lower extremity kinematic performance during level ground jogging was recorded using a three-dimensional motion analysis system (Motion Analysis Corporation, Santa Rosa, CA, USA). A four segment, rigid-link model of the lower limb was defined by 13 retro-reflective, spherical markers (diameter = 25 mm) (Kadaba et al., 1990). Five synchronized cameras captured the gait motion at a frequency of 120 Hz. The cameras were calibrated with mean residual errors in the range of 1.55 - 2.95 mm over a volumetric space of 1.50 x 1.10 x 1.50 m centered over the force platform.
The coordinate data for each marker trajectory were smoothed using a fourth-order Butterworth filter with a 9 Hz cut-off frequency (Wood, 1982). The smoothed coordinates were used to calculate joint coordinate system angles for the knee as described previously (Kadaba et al., 1990; Kadaba et al., 1989; Torry et al., 2000). The force plate was used to determine the period of the stance phase defined as heel strike to toe-off. An average, stance phase, knee joint angle was calculated for each individual trial, by summing the values from heel strike to toe-off and dividing by the total number of values in the series. These values were then averaged for all 8 trials to yield average knee flexion angles pre- and post-effusion. For graphical purposes only, custom software utilizing a cubic spline function was used to time normalize the kinematic data, expressed as 0 to 100% of the stance phase (Torry et al., 2000).
The EMG patterns were recorded with pre-gelled, silver-silver/chloride bipolar surface electrodes (Medicotest A/S, Rugmaken, Denmark) for the vastus medialis, vastus lateralis, biceps femoris, and the medial hamstrings (semitendinosus and semimembranosus) according to Basmajiian and Deluca (1985) and Delagi et al. (1981). After the skin was shaved and cleansed with alcohol, the electrodes were placed over each muscle belly in line with the direction of the fibers with a center to center distance of approximately 2.5 cm. Electrode placement was confirmed for each muscle with manual muscle testing and visual biofeedback monitoring (Torry et al., 2000). A single ground electrode was placed over the anterior tibial spine.
EMG data were collected (1200 Hz) with the TeleMyo telemetric hardware system (Noraxon, USA, Inc., Scottsdale, AZ) on-line with a 16-bit A/D board (National Instruments, Austin, TX) of the motion capture system. Each EMG signal had a bandwidth of 3 dB at 16-500 Hz. The lower cutoff filter is a first order high-pass design and the upper cut-off filter is a sixth order Butterworth low-pass design. The differential amplifier has a fixed gain of 1700, an input impedance of >10 MΩ , and a common mode rejection ratio of 130 dB. Although the transmitter automatically removes the low frequency noise component from the EMG signals, a resting trial was collected and used to remove any additional noise. After removing signal offset, the raw dynamic EMG, and maximum voluntary contractions (MVCs) for each muscle, were processed with 15 and 50 ms root mean square (RMS) smoothing window algorithms, respectively (Deluca, 1997; Lange et al., 1996; Torry et al., 2000).
Five trials of pre-test MVCs were collected using methods previously described by Lange et al. (Lange et al., 1996). EMG reference values were calculated for each muscle using the average of the five peak EMG signals and represented 100% MVC. The mean peak EMG amplitude derived from the MVC protocol was used to scale the raw dynamic EMG recorded during each jogging trial (%MVC). The scaled data were then averaged over the stance phase.
Statistical analysis
Differences in the average, knee joint flexion angle and the average EMG (%MVC) of the five muscles were compared pre- and post-insufflation with a paired t-test with an a priori alpha level set at 0.05.
Results
Individual intra-articular pressures, jogging speeds and average knee angles for pre- and post-insufflation are reported in Table 1. The effusion did not cause a significant change (t=2.00, df=12, p=0.068, 1-ß=.440) in the average knee angle over the stance phase (Table 1 and Figure 1).
Table 1.
Individual scores for pre- and post-insufflation average knee angles through the stance phase, jogging speeds and intra-articular knee pressures.
| Subject # | Speed (m·sec-1) | Pressure (mmHg) | *Knee Angle (degrees) | |
|---|---|---|---|---|
| Pre | Post | |||
| 1 | 4.00 | 45.00 | -49.58 | -50.25 |
| 2 | 3.25 | 32.00 | -41.02 | -43.58 |
| 3 | 3.50 | 27.00 | -35.63 | -36.36 |
| 4 | 2.55 | 35.00 | -37.58 | -35.09 |
| 5 | 3.00 | 40.00 | -36.59 | -37.00 |
| 6 | 3.25 | 15.00 | -50.21 | -51.23 |
| 7 | 3.50 | 30.00 | -45.65 | -45.69 |
| 8 | 2.75 | 22.00 | -45.85 | -46.78 |
| 9 | 2.85 | 24.00 | -44.23 | -45.21 |
| 10 | 3.10 | 32.00 | -48.75 | -49.52 |
| 11 | 2.65 | 45.00 | -38.42 | -38.56 |
| 12 | 2.80 | 50.00 | -38.56 | -39.65 |
| 13 | 3.00 | 38.00 | -42.02 | -43.25 |
| Mean | 3.09 | 33.46 | -42.62 | -43.24 |
| SD | 0.41 | 10.10 | 5.11 | 5.50 |
| ES | -0.12 | |||
*No statistical difference (p > 0.05). SD = Standard deviations; ES = Effect size.
Figure 1.
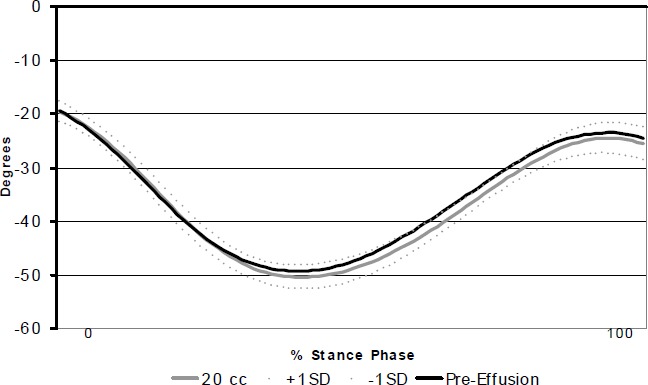
Time series knee joint angle from heel strike to toe-off of a representative subject. Negative values represent increasing knee flexion angle.
Table 2 presents EMG values and standard deviations pre- and post-insufflation for all subjects and each muscle tested. Eleven of the 13 subjects exhibited EMG inhibition in the vastus medialis while 10 of the 13 subjects exhibited inhibition of the vastus lateralis after insufflation. Specifically, vastus medialis and lateralis activity decreased on average 8.5% (t = 3.42, df = 12, p = 0.005) and 5.0% (t = 3.33, df = 12, p = 0.006), compared to the respective pre-effusion values. Although seven of 13 subjects showed an increase in EMG, rectus femoris activity did not demonstrate significant changes between conditions (t = -2.16, df = 12, p = 0.052, 1-ß = .500). Neither the medial hamstrings (t = -1.74, df = 12, p = 0.107, 1-ß = .340) nor the biceps femoris (t = -1.89, df = 12, p = 0.083, 1-ß = .400) muscles exhibited a significant change in EMG activity after knee effusion.
Table 2.
Subject means for average EMG (%MVC) amplitude pre- and post-insufflation.
| Subject# | *Vastus Medialis | *Vastus Lateralis | Rectus Femoris | Biceps Femoris | Medial Hamstrings | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| pre | post | pre | post | pre | post | pre | post | pre | post | |
| 1 | 96.1 | 74.6 | 50.4 | 49.4 | 51.1 | 50.3 | 176.6 | 261.9 | 62.4 | 68.0 |
| 2 | 98.7 | 96.6 | 72.5 | 70.3 | 64.0 | 63.4 | 111.3 | 116.1 | 70.3 | 75.7 |
| 3 | 66.8 | 65.5 | 63.8 | 61.6 | 34.4 | 33.5 | 64.4 | 75.6 | 30.8 | 34.5 |
| 4 | 9.3 | 10.5 | 42.6 | 42.1 | 75.3 | 75.0 | 22.2 | 26.9 | 18.6 | 22.4 |
| 5 | 48.9 | 44.9 | 21.8 | 19.9 | 23.8 | 23.6 | 33.3 | 38.6 | 34.0 | 36.3 |
| 6 | 72.3 | 64.2 | 60.1 | 57.1 | 91.4 | 91.4 | 34.9 | 48.9 | 20.4 | 18.2 |
| 7 | 84.9 | 75.3 | 94.9 | 86.3 | 47.0 | 49.6 | 70.8 | 87.0 | 64.0 | 54.3 |
| 8 | 50.5 | 46.0 | 43.1 | 39.5 | 42.1 | 46.8 | 22.7 | 29.9 | 52.8 | 56.7 |
| 9 | 45.6 | 43.4 | 41.4 | 41.6 | 28.6 | 30.0 | 11.3 | 12.1 | 24.5 | 24.3 |
| 10 | 69.4 | 64.1 | 65.2 | 59.0 | 57.6 | 59.6 | 94.3 | 98.6 | 70.4 | 75.9 |
| 11 | 50.5 | 46.0 | 43.1 | 43.1 | 28.6 | 30.0 | 22.7 | 22.9 | 52.8 | 56.7 |
| 12 | 45.6 | 43.4 | 21.4 | 18.6 | 57.6 | 59.6 | 11.3 | 11.6 | 24.5 | 24.3 |
| 13 | 69.4 | 64.1 | 25.2 | 25.6 | 28.6 | 30.3 | 94.3 | 94.6 | 70.4 | 75.9 |
| Mean | 62.1 | 56.8 | 49.6 | 47.2 | 48.5 | 49.5 | 59.2 | 71.1 | 45.8 | 47.9 |
| SD | 24.2 | 21.1 | 21.4 | 19.7 | 20.4 | 20.1 | 49.2 | 67.4 | 20.8 | 22.2 |
| Effect Size | 0.0 | 0.2 | 0.1 | |||||||
*p < 0.05
Discussion
The neuromuscular system is integral in controlling and maintaining the mechanical environment of the internal knee joint. It is plausible that alterations in thigh muscular activity patterns may reflect muscular force adaptations that could have a profound affect on the internal loading of the joint and its tissues. In the present study, vastus medialis and vastus lateralis inhibition occurred with mild knee effusion during jogging without a significant change in sagittal plane knee joint kinematics. These results are similar to those reported for walking (Torry et al., 2000), where 20 cc of effusion caused vastus medialis and lateralis inhibition. In contrast to a previous study (Torry et al., 2000), the present investigation did not observe significant changes in the sagittal knee joint kinematic pattern. This was surprising given the notable changes in the EMG. One possible explanation for this is that the inertial forces experienced by the lower limb during jogging are significantly higher compared to walking and may be of sufficient magnitude to overcome the muscular deficits of the medialis and lateralis in order to passively extend the leg. This further implicates the important functional and adaptive role the bi-articulate rectus femoris muscle may play in knee joint pathology, as this muscle would beprimarily responsible for generating the increased inertia for the lower extremity by acting at the hip.
It is believed that increased fluid in the knee distends the joint capsule and produces quadriceps inhibition that leads to weakness and atrophy (Spencer et al., 1984; Stratford, 1981; Suter and Herzog, 2000). Suter and Herzog (2000) hypothesized that muscle inhibition via knee injuries may lead to joint degeneration. However, in that study, it was not known whether the injury itself may have initiated the joint degeneration process as all participants were injured prior to the start of the study. The results of this study supports the concept that an otherwise healthy knee may experience reduced EMG drive to the vastus medialis and lateralis due to effusion and/or capsular distension as the individuals in this study were healthy and the observed effects can not be attributed to a pre-existing injury. Other studies have also reported reduced EMG drive to the medialis in PFP patients performing activities of daily life (Souza and Gross, 1991; Thomee et al., 1996; Thomee et al., 1995). These authors suggest that the reduced EMG to the quadriceps may be due to pain inhibition. The present study also offers an alternative hypothesis, suggesting that many of the observed changes in EMG in the pathological knee may also be, in part, due to knee joint effusion that often accompanies many of these clinical pathologies.
While reduced EMG activity does not necessarily translate into muscle force reduction during a dynamic contraction, several investigators have reported reduced EMG in the vastus medialis for knee injured groups (Boucher et al., 1992; Cerny, 1995; Souza and Gross, 1991; Thomee et al., 1995) where simultaneous knee extensor strength deficits were also noted. In support, a study conducted by Boucher et al. (1992) determined that EMG activity of the vastus medialis was reduced in patients with PFP syndrome compared to a non-pathological group. Thus, in addition to pain inhibition, it is plausible that knee joint effusions may also contribute to those findings.
In vivo function influences the mechanical environment of articular cartilage. Thus, in vivo function is coupled to the health of a joint. Muscle weakness has been associated with degenerative joint disease of the knee (Hurley, 1998; 1999) and others have suggested its importance in the initiation and progression of knee OA (Suter and Herzog, 2000). Recently, Andriacchi et al. (2004) suggested that the initiation of knee OA is associated with the kinematic change in tibiofemoral load bearing to areas where cartilage is not accustom to such loads and breaks down. Based on this data, it is not unreasonable to infer that similar cartilage breakdown may occur on the retro surface of the patella when the normal activation patterns of the quadriceps are interrupted as in the current study; and this altered quadriceps function will most likely produce some aberrant patellar tracking. Unfortunately, measuring patellar motion is not feasible with non-invasive means and thus we cannot determine if indeed patellar motion was altered in these subjects post-effusion.
The capsular tissue of the knee joint is visco-elastic (Levick, 1983). Previous researchers have demonstrated that saline is absorbed or the stretch-relaxation of the capsular tissues can accommodate to the increase in joint volume (Levick, 1983; Wood et al., 1988). In a case study, Spencer et al. (1984) examined the prolonged effect of 60 cc of saline injected into the knee joint on H-reflexes and pressures by leaving the fluid in the joint for 20 min. After 20 min, Spencer et al. (1984) noted a 3.0 mm Hg drop in intra-articular pressure but a continued decrease in the magnitude of the H-reflex. In the present study, all trials were conducted in a short time period (under 10 min from the time of insufflation) to help negate the confounding effects absorption and the capsular stretch-relaxation cycle may have had in this study design (Levick, 1983). Likewise, this study investigated the immediate effects of effusion and did not address the effects prolonged exposure may have on quadriceps or hamstring EMG values during jogging. We chose a volume of 20 cc to represent a mild effusion as it constitutes a clinical representation of knee effusions (see Table 3), and has been reported as having an inhibitory effect in previous studies and did not cause pain (Shakespeare et al., 1985; Spencer et al., 1984; Torry et al., 2000).
Table 3.
Demographics, recreational activity and amount of effusion removed for subjects with chronic patellofemoral pain and degenerative joint disease of the knee.
| Subject # | Diagnosis | Age | Gender | Recreational Activity | cc Removed |
|---|---|---|---|---|---|
| 1 | PFP | 34 | Female | Tele-Ski | 20 |
| 2 | PFP | 43 | Female | Alpine-Ski | 9 |
| 3 | PFP | 55 | Female | Bike | 17 |
| 4 | PFP | 32 | Female | Run | 15 |
| 5 | PFP | 39 | Male | Run/Hike | 22 |
| 6 | PFP | 34 | Female | Hike/Bike | 7 |
| 7 | PFP | 43 | Male | Run/Hike | 11 |
| 8 | DJD | 70 | Female | Alpine-Ski | 9 |
| 9 | DJD | 41 | Male | Hike/Bike | 38 |
| 10 | DJD | 64 | Male | Bike | 10 |
| 11 | DJD | 68 | Male | Golf | 7 |
| 12 | DJD | 55 | Male | Golf | 12 |
| 13 | DJD | 60 | Female | Tennis | 23 |
| 14 | DJD | 57 | Female | Cross Country Ski | 24 |
| 15 | DJD | 48 | Female | Alpine-Ski | 17 |
| 16 | DJD | 53 | Male | Tennis | 13 |
Abbreviations: PFP = Patello-femoral pain; DJD = Tibiofemoral degenerative joint disease.
This study demonstrated the effects of a simulated knee effusion on the quadriceps and hamstring musculature during jogging. Vastus medialis and lateralis inhibition were observed in EMG data during jogging. It is speculated that this inhibition was caused by joint capsular distension as reported by previous authors (Kennedy et al., 1982; Spencer et al., 1984; Torry et al., 2000). Because the subjects employed in this study were healthy, these results can be considered the isolated effects knee joint effusion may have on knee joint function during jogging as no other factor (injury, surgery or rehabilitation) could have caused these adaptations. Thus, these data suggest that knee effusion may be one factor that causes vastus medialis and lateralis EMG deficits that are often associated with knee joint pathologies such as OA or PFP. The results of this study may used as a baseline when comparing the functional capabilities of pathological groups who exhibit knee joint effusions.
Conclusions
Previous research conducted on knee injured individuals is limited as these reports can only assess the current functional capacity of these individuals under the influences of the current state of their disease process. Thus, these studies cannot address the question of whether the observed muscular inhibition is or was a consequence of the knee pathology or contributed to its etiology. This study has shown that knee effusion can cause reductions in the vastus medialis and lateralis EMG output potentially altering patello-femoral kinematics. These effects may, in part, help to explain results obtained in injured or diseased knees.
Biographies
Michael R. TORRY
Employment
Director of Research in the Biomechanics Research Lab of the Steadman Hawkins Research Foundation. Dr. Torry holds Adjunct Faculty positions in the Depart. of Bioengineering at the Univ. of Pittsburgh, at Colorado State Univ. in the Depart. of Veterinary Medicine and Biomedical Engineering and in the Depart. of Integrative Physiology at the Univ. of Colorado at Boulder.
Degree
PhD
Research interests
Knee and shoulder biomechanics.
E-mail: mike.torry@shsmf.org
Michael J. DECKER
Employment
PhD student at the University of Texas-Austin.
Degree
MS
Research interests
Neuromuscular mechanics.
Peter J. MILLETT
Employment
The Depart. of Orthopedics, Brigham and Women’s Hospital, Harvard Univ. Boston, MA.
Degree
MD
Research interests
Knee and shoulder surgery and function.
J. Richard STEADMAN
Employment
Executive Director, Steadman Hawkins Research Foundation in Vail, Colorado.
Degree
MD
Research interests
Knee surgery and mechanics.
William I. STERETT
Employment
The Head Team Physician for the United States Women’s Alpine Ski Team and a Partner at the Steadman Hawkins Clinic in Vail, Colorado. He is also the United States Medical Consultant to the International Federation of Skiing (FIS).
Degree
MD
Research interests
Knee surgery and mechanics.
References
- Andriacchi T.P., Alexander E.J. (2000) Studies of human locomotion: past, present and future. Journal Biomechanics 33, 1217-1224 [DOI] [PubMed] [Google Scholar]
- Andriacchi T.P., Mundermann A., Smith R.L., Alexander E.J., Dyrby C.O., Koo S. (2004) A framework for the in vivo pathomechanics of osteoarthritis at the knee. Annals Biomedical Engineering 32, 447-457 [DOI] [PubMed] [Google Scholar]
- Basmajian J., DeLuca C.J. (1985) Lower limb. Muscles alive, their functions revealed by electromyography. Butler J.Baltimore: Williams and Wilkins; 310-353 [Google Scholar]
- Boucher J.P., King M.A., Lefebvre R., Pepin A. (1992) Quadriceps femoris muscle activity in patellofemoral pain syndrome. American Journal Sports Medicine 20, 527-532 [DOI] [PubMed] [Google Scholar]
- Brandt K.D., Heilman D.K., Slemenda C., Katz B.P., Mazzuca S.A., Braunstein E.M., Byrd D. (1999) Quadriceps strength in women with radiographically progressive osteoarthritis of the knee and those with stable radiographic changes. Journal Rheumatology 26, 2431-2437 [PubMed] [Google Scholar]
- Brandt K.D. (2000) Putting muscle into osteoarthritis. Annals of Internal Medicine 127, 154-155 [DOI] [PubMed] [Google Scholar]
- Cerny K. (1995) Vastus medialis oblique/vastus lateralis muscle activity ratios for selected exercises in persons with and without patellofemoral pain syndrome. Physical Therapy 75, 672-683 [DOI] [PubMed] [Google Scholar]
- Delagi E., Perotto A., Lazzetti J., Morrison D. (1981) Section VII. Anatomic guide for electromyographer. Delagi E., Perotto A.Springfield, IL: Charles C. Thomas; 136-194 [Google Scholar]
- Deluca C.J. (1997) The use of surface electromyography in biomechanics. Journal of Applied Biomechanics 13, 135-163 [Google Scholar]
- Fahrer H., Rentsch H.U., Gerber N.J., Beyeler C., Hess C.W., Grunig B. (1988) Knee effusion and reflex inhibition of the quadriceps. A bar to effective retraining. Journal Bone Joint Surgery(British) 70, 635-638 [DOI] [PubMed] [Google Scholar]
- Hurley M.V., Newham D.J. (1993) The influence of arthrogenous muscle inhibition on quadriceps rehabilitation of patients with early, unilateral osteoarthritic knees. British Journal Rheumatology 32, 127-131 [DOI] [PubMed] [Google Scholar]
- Hurley M.V., Jones D.W., Newham D.J. (1994) Arthrogenic quadriceps inhibition and rehabilitation of patients with extensive traumatic knee injuries. Clinical Science (Colch) 86, 305-310 [DOI] [PubMed] [Google Scholar]
- Hurley M.V., Scott D.L., Rees J., Newham D.J. (1997) Sensorimotor changes and functional performance in patients with knee osteoarthritis. Annals Rheumatic Disease 56, 641-648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurley M.V. (1998) Quadriceps weakness in osteoarthritis. Current Opinion Rheumaologyl 10, 246-250 [DOI] [PubMed] [Google Scholar]
- Hurley M.V., Scott D.L. (1998) Improvements in quadriceps sensorimotor function and disability of patients with knee osteoarthritis following a clinically practicable exercise regime. British Journal Rheumatology 37, 1181-1187 [DOI] [PubMed] [Google Scholar]
- Hurley M.V. (1999) The role of muscle weakness in the pathogenesis of osteoarthritis. Rheumatic Disease Clinics North America 25, 283-298, vi. [DOI] [PubMed] [Google Scholar]
- Hurwitz D.E., Andriacchi T.P., Bush-Joseph C.A., Bach B.R., Jr. (1997) Functional adaptations in patients with ACL-deficient knees. Exercise Sport Science Reviews 25, 1-20 [PubMed] [Google Scholar]
- Itoh H., Kurosaka M., Yoshiya S., Ichihashi N., Mizuno K. (1998) Evaluation of functional deficits determined by four different hop tests in patients with anterior cruciate ligament deficiency. Knee Surgery Sports Traumatology Arthroscopy 6, 241-245 [DOI] [PubMed] [Google Scholar]
- Jensen K., Graf B.K. (1993) The effects of knee effusion on quadriceps strength and knee intraarticular pressure. Arthroscopy 9, 52-56 [DOI] [PubMed] [Google Scholar]
- Kadaba M.P., Ramakrishnan H.K., Wootten M.E., Gainey J., Gorton G., Cochran G.V. (1989) Repeatability of kinematic, kinetic, and electromyographic data in normal adult gait. Journal Orthopedic Research 7, 849-860 [DOI] [PubMed] [Google Scholar]
- Kadaba M.P., Ramakrishnan H.K., Wootten M.E. (1990) Measurement of lower extremity kinematics during level walking. Journal of Orthopedic Research 8, 383-392 [DOI] [PubMed] [Google Scholar]
- Kennedy J.C., Alexander I.J., Hayes K.C. (1982) Nerve supply of the human knee and its functional importance. American Journal Sports Medicine 10, 329-335 [DOI] [PubMed] [Google Scholar]
- Lange G.W., Hintermeister R.A., Schlegel T., Dillman C.J., Steadman J.R. (1996) Electromyographic and kinematic analysis of graded treadmill walking and the implications for knee rehabilitation. Journal Orthopedic Sports Physical Therapy 23, 294-301 [DOI] [PubMed] [Google Scholar]
- Levick J.R. (1983) Joint pressure-volume studies: their importance, design and interpretation. Journal Rheumatology 10, 353-357 [PubMed] [Google Scholar]
- McNair P.J., Marshall R.N., Maguire K. (1996) Swelling of the knee joint: effects of exercise on quadriceps muscle strength. Archives Physical Medicine Rehabilitation 77, 896-899 [DOI] [PubMed] [Google Scholar]
- Messier S.P., Loeser R.F., Hoover J.L., Semble E.L., Wise C.M. (1992) Osteoarthritis of the knee: effects on gait, strength, and flexibility. Archives Physical Medicine Rehabilitation 73, 29-36 [PubMed] [Google Scholar]
- Mow V.C., Ateshian G.A. (1997) Lubrication and wear of diarthrodial joints. Basic orthopedic biomechanics. Mow V.C., Hayes W.C.Philadelphia, PA: Lippencott-Raven; 275-316 [Google Scholar]
- Mow V.C., Ratcliff A. (1997) Structure and function of articular cartilage and meniscus. Basic orthopedic biomechanics. Mow V.C., Hayes W.C.Philadelphia, PA: Lippencott-Raven; 113-178 [Google Scholar]
- O’Connor J.J. (1993) Can muscle co-contraction protect knee ligaments after injury or repair? Journal Bone Joint Surgery (British) 75, 41-48 [DOI] [PubMed] [Google Scholar]
- O’Reilly S.C., Jones A., Muir K.R., Doherty M. (1998) Quadriceps weakness in knee osteoarthritis: the effect on pain and disability. Annals Rheumatic Disease. 57, 588-594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai N., Luo Z.P., Rand J.A., An K.N. (2000) The influence of weakness in the vastus medialis oblique muscle on the patellofemoral joint: an in vitro biomechanical study. Clinical Biomechanics (Bristol, Avon) 15, 335-339 [DOI] [PubMed] [Google Scholar]
- Shakespeare D.T., Stokes M., Sherman K.P., Young A. (1985) Reflex inhibition of the quadriceps after meniscectomy: lack of association with pain. Clinical Physiology 5, 137-144 [DOI] [PubMed] [Google Scholar]
- Souza D.R., Gross M.T. (1991) Comparison of vastus medialis obliquus: vastus lateralis muscle integrated electromyographic ratios between healthy subjects and patients with patellofemoral pain. Physical Therapy 71, 310-316; discussion 317-320. [DOI] [PubMed] [Google Scholar]
- Spencer J.D., Hayes K.C., Alexander I.J. (1984) Knee joint effusion and quadriceps reflex inhibition in man. Archives Physical Medicine Rehabilitation 65, 171-177 [PubMed] [Google Scholar]
- Stratford P. (1981) Electromyography of the quadriceps femoris muscles in subjects with normal knees and acutely effused knees. Physical Therapy 62, 279-283 [DOI] [PubMed] [Google Scholar]
- Suter E., Herzog W. (2000) Does muscle inhibition after knee injury increase the risk of osteoarthritis? Exercise Sports Science Reviews. 28, 15-18 [PubMed] [Google Scholar]
- Thomee R., Renstrom P., Karlsson J., Grimby G. (1995) Patellofemoral pain syndrome in young women. II. Muscle function in patients and healthy controls. Scandinavian Journal of Medicine and Science in Sports 5, 245-251 [PubMed] [Google Scholar]
- Thomee R., Grimby G., Svantesson U., Osterberg U. (1996) Quadriceps muscle performance in sitting and standing in young women with patellofemoral pain syndrome and young healthy women. Scandinavian Journal of Medicine and Science in Sports 6, 233-241 [DOI] [PubMed] [Google Scholar]
- Torry M.R., Decker M.J., Viola R.W., O’Connor D.D., Steadman J.R. (2000) Intra-articular knee joint effusion induces quadriceps avoidance gait patterns. Clinical Biomechanics (Bristol, Avon) 15, 147-159 [DOI] [PubMed] [Google Scholar]
- Wood G.A. (1982) Data smoothing and differentialization procedures in biomechanics. Exercise Sports Science Reviews 10, 308-362 [PubMed] [Google Scholar]
- Wood L., Ferrell W.R., Baxendale R.H. (1988) Pressures in normal and acutely distended human knee joints and effects on quadriceps maximal voluntary contractions. Quarterly Journal Experimental Physiology 73, 305-314 [DOI] [PubMed] [Google Scholar]


