Abstract
The aim of the present study was to examine the effects of endurance training on heart rate (HR) recovery after exercise and cardiac autonomic nervous system (ANS) modulation in female marathon runners by comparing with untrained controls. Six female marathon runners (M group) aged 32-40 years and eight age-matched untrained females (C group) performed a maximum-effort treadmill running exercise. Maximal oxygen uptake (VO2max) was measured during the exercise with a gas analyzer connected to subjects through a face mask. Heart rate, blood pressure and blood lactate were measured before and after the exercise. Rating of perceived exertion (RPE) to the exercise was obtained immediately after the exercise. Holter ECG was recorded and analyzed with power spectral analysis of heart rate variability (HRV) to investigate the cardiac ANS modulation. The M group had significantly higher VO2max, faster HR recovery after exercise, higher Mean RR, SDRR, HF power and lower LF/HF ratio at rest compared with the C group. The M group also presented greater percent decrease of blood pressure after exercise, although their blood pressure after exercise was higher than the C group. It is suggested that endurance training induced significant alterations in cardiac ANS modulation at rest and significant acceleration of HR recovery after exercise in female marathon runners. Faster HR recovery after exercise in the female marathon runners should result from their higher levels of HRV, higher aerobic capacity and exaggerated blood pressure response to exercise compared with untrained controls.
Key Points.
The effects of endurance training on HR recovery after exercise and cardiac ANS modulation were investigated in female marathon runners by comparing with untrained controls.
Time and frequency domain analysis of HRV was used to investigate cardiac ANS modulation.
As compared with untrained controls, the female marathon runners showed faster HR recovery after exercise, which should result from their higher levels of HRV, higher aerobic capacity and exaggerated blood pressure response to exercise.
Key words: Heart rate recovery, heart rate variability, female marathon runner
Introduction
During exercise, heart rate (HR) and myocardial contractility will be increased to satisfy energy demands of working muscles. Its nervous modulation is considered to be due to the vagal withdrawal at low-intensity exercise and the combination of vagal withdrawal and sympathetic activation at moderate or high-intensity exercise (Kluess et al., 2000). With the cessation of exercise, the decrease in HR immediately after exercise is mainly thought to be a function of a reactivation of the parasympathetic nervous system (Arai et al., 1989). Later, the further decrease in HR to the pre-exercise value also depends on the gradual withdrawal of the sympathetic system (Perini et al., 1989). Because increased vagal activity has been associated with a reduction in the risk of sudden cardiac death (Pardo et al., 2000), recently, some studies have demonstrated that a delayed decrease in HR after exercise would be a powerful and independent predictor of all cause mortality in patients or in general population (Cole et al., 1999; Cole et al., 2000; Nishime et al., 2000).
Physical training was shown to increase cardiac vagal tone (Levy et al., 1998) and to accelerate HR recovery after exercise (Darr et al., 1988), which may contribute to the reduction in mortality. Which kind of exercise could more efficiently accelerate HR recovery or increase cardiac autonomic nervous system (ANS) modulation remains unknown. Marathon running exercise is an endurance-promotion exercise practiced by a lot of athletes over the world. However, little is known about the effects of marathon training habit on HR recovery after exercise and cardiac ANS modulation. Time and frequency domain analysis of heart rate variability (HRV) has been proven to be a noninvasive technique capable of providing information on autonomic modulation of the sinus node. Therefore, employing this method, the present study investigated the changes over time in HR and ANS modulation both at rest and during post-exercise recovery periods of female marathon runners by comparing with their age-matched untrained controls.
Methods
Subjects
Six female marathon runners aged 32-40 years (M group) and eight untrained female controls aged 29- 42 years (C group) participated in this study. M group has been doing endurance running exercise for more than 15 years and participated in international marathon race. C group had not been involved in regular long-term exercise training for years, but participated in occasional recreational activity (bicycle, table-tennis or badminton). According to the results of questionnaire and electrocardiogram, all subjects were free of hypertension, hyperlipemia, cardiovascular disease and diabetes mellitus. In addition, they were nonsmokers, and none of them were taking any medicine known to effect cardiovascular function. Characteristics of the two groups are depicted in Table 1. This study was approved by the Ethics Committee of the Gifu University School of Medicine, and all subjects provided written informed consent for their participation in the experimental procedures.
Table 1.
Subjects’ characteristics. Data are means (±SE).
| Marathon Runners (n = 6) | Untrained controls (n = 8) | |
|---|---|---|
| Age (yr) | 36.5 (1.1) | 35.3 (1.4) |
| Height (m) | 1.59 (.02) | 1.62 (.02) |
| Weight (kg) | 46.7 (.8) | 52.3 (2.6) |
| BMI (kg·m-2) | 18.5 (.4) | 20.0 (.8) |
| Body fat (%) | 18.5 (.7)* | 22.8 (1.2) |
| VO2max (ml·min-1·kg-1) | 58.6 (2.0)** | 41.1 (2.1) |
| Running distanc(km·day-1) | 10.7 (.2) | - |
| Best marathon time (min) | 174.5 (5.8) | - |
| Treadmill gradientmax (%) | 13.3(.8)** | 6.8 (.7) |
| Treadmill speed (km·h-1) | 10.5 (.0)** | 8.1 (.1) |
| RPE to exercise | 17.6 (.3) | 17.7 (.3) |
*p < 0.05
** p < 0.01 (Significantly different from untrained controls).
Procedures
All measurements were performed in a quiet and air-conditioned (24°C) room, and all subjects did not consume any beverages containing alcoholic or coffee before the measurements. Body fat percentage was measured by an analyzer (Body Fat Analyzer TBF-31O, Tanita Company, Limited, Japan). After 10-min quiet rest in a supine position, the subjects were asked to perform maximal running exercise on an electrically treadmill. The running speed was chosen by the subjects according to their individual pace. With 3-min warm up period at 0% treadmill inclination, the gradient was increased by 1% per minute according to Balke method till exhaustion was reached. After the maximal exercise, the subjects rested quietly for 30 min in the supine position for recovery. During the exercise the subject wore a face mask connected to a gas analyzer (Cardiopulmonary Function Measuring System, Oxycon Alpha, Fukuda Electronic Company, Limited, Japan), by which inspired and expired gases were measured in breath by breath mode and analyzed in 5 sec intervals. The subject breathed spontaneously throughout the experimental procedure without the attempt to control the depth or frequency of the respiratory pattern in order to avoid the discomfort as well as the changes of metabolism and blood gas. The criteria for the establishment of VO2max included a plateau in oxygen consumption with increasing work rate, a respiratory exchange ratio > 1.1, near achievement of age-predicted maximal HR (±10) and failure to maintain the running speed despite encouragement. The electrocardiogram (ECG) was monitored by Holter recorder (Ambulatory ECG Recorder SM-50, Fukuda Electronic Company, Limited, Japan) at a rate of 1000 samples per second during the experiment for heart rate variability (HRV) analysis. Meanwhile, blood pressure and blood lactate were measured before exercise, immediately after exercise, 3 min, 5 min, 10 min, 20 min and 30 min after exercise. The rating of perceived exertion (RPE) (Borg, 1982) to the exercise was also obtained according to an oral questionnaire for the subjects immediately after the exercise.
Estimation of HRV
Holter ECG was analyzed by Holter Analyzer SCM-6000 System (SCM-6000 Dual Holter Workstation System, Fukuda Electronic Company, Limited, Japan). Spectral analysis was repeatedly performed within the selected segments of Holter ECG R-R interval data using 256-point fast Fourier transformation. Holter ECG R-R interval data at rest phase were divided into two segments (rest1: 0~256s, rest2: 300~556s) and recovery phase were divided into five segments (rec1: 300~556s, rec2: 600~856s, rec3: 900~1156s, rec4: 1200~1456s, rec5: 1500~1756s). The results of HRV at rest were shown by the mean values of rest1~rest2. Spectral analysis of fast Fourier transformation requires stationarity of the R-R interval time series and thus analysis of HRV during exercise is often skewed and may lead to inconsistent results (Casadei et al., 1995). In this study, after exercise the subjects need slow down the running to stop and then lie on a bed to rest. It was still not stationarity during the initial recovery after exercise, and the results of HRV analysis during this phase could not be confirmed to be true. Therefore, HRV at the recovery of 0~300s was not quantified. Power spectra obtained from spectral analysis were defined as two components: 0.04~0.15Hz (low frequency: LF) and 0.15~0.4Hz (high frequency: HF). HF power was shown to be almost entirely mediated by the vagal activity (Berger et al., 1989), whereas LF power reflects the mixed modulation of vagal and sympathetic activities (Bernardi et al., 1994). The ratio of LF power to HF power (LF/HF) was considered to reflect the sympathovagal balance, and high values suggested a sympathetic predominance (Pagani et al., 1986).
Data analysis
All corresponding data between the two groups were compared by student’s t-test. All data are expressed as means (±SE). Statistical significance was set at p < 0.05.
Results
Characteristics of the subjects
Body fat percentage was significantly lower (p < 0.05) in M group than in C group (Table 1). VO2max was significantly higher (p < 0.01) in M group than in C group. Treadmill gradient at maximal exercise was significantly higher (p < 0.01) in M group than in C group. Treadmill running speed chosen by M group was also significantly faster (p < 0.01) than that chosen by C group. The RPE to the exercise was almost the same for the two groups.
Changes of the systolic blood pressure (SBP) and diastolic blood pressure (DBP)
Resting blood pressure was almost the same for the two groups (Figure 1). SBP was significantly higher (p < 0.01) immediately after exercise, 3 min after exercise and 5 min after exercise in M group than in C group. DBP also showed a higher value (p < 0.01) immediately after exercise in M group than in C group.
Figure 1.
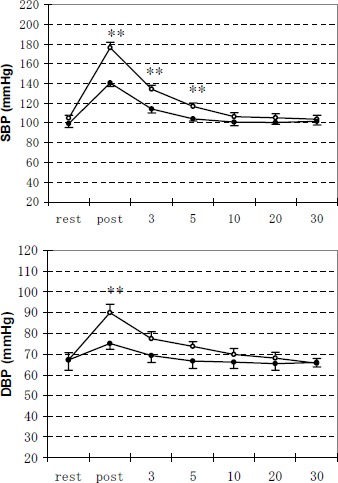
Changes of systolic blood pressure (SBP) and diastolic blood pressure (DBP) during the experiments of rest, immediately after exercise, 3 min, 5 min, 10 min, 20 min and 30 min after exercise. Data are means (±SE). Open and solid circles represent the values for M group and C group, respectively. ** Significantly different between M group and C group (p < 0.01).
Percent decrease of the systolic blood pressure (SBP) and diastolic blood pressure (DBP) after exercise
At 3 min, 5 min, 10 min, 20 min and 30 min after exercise, percent decrease of SBP or DBP from the peak exercise BP was significantly higher (p < 0.01 or p < 0.05) in M group than in C group (Figure 2).
Figure 2.
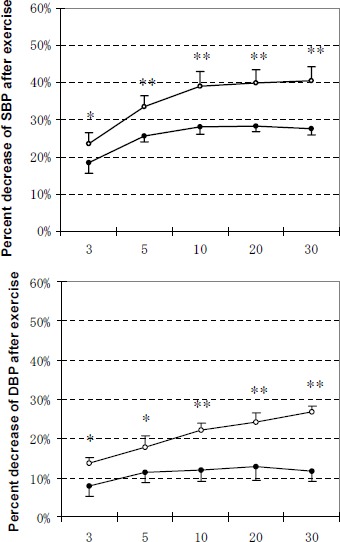
Decrease in SBP and DBP at 3 min, 5 min, 10 min, 20 min and 30 min after exercise as a percentage of peak BP. Data are means (±SE). Relative decrease of BP at time T after exercise as a percentage of peak BP: (peak BP - BPT) / peak BP x 100, where T = 3 min, 5 min, 10 min, 20 min or 30 min. Open and solid circles represent the values for M group and C group, respectively. *, ** Significantly different between M group and C group (* p < 0.05, ** p < 0.01).
Changes of blood lactate
Blood lactate was almost the same for the two groups at rest or at recovery phases(Figure 3). Within 30 min after exercise, blood lactate did not return to the pre-exercise values for both groups.
Figure 3.
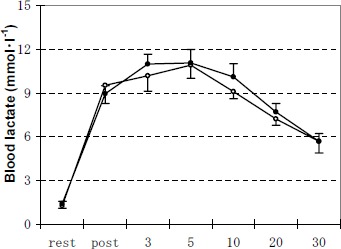
Changes of blood lactate concentration during the experiments of rest, immediately after exercise, 3 min, 5 min, 10 min, 20 min and 30 min after exercise. Data are means (±SE). Open and solid circles represent the values for M group and C group, respectively.
Changes of HR
Resting HR was significantly lower (p < 0.05) in M group than in C group(Figure 4). Maximal exercise HR was almost the same for the two groups. HR recovery after exercise indicated the initial fast phase and the followed slow phase in both groups. However, post-exercise HR was significantly lower (p < 0.01 or p < 0.05) in M group than in C group. Within 30 min after exercise, HR gradually decreased but did not return to the pre-exercise values for both groups.
Figure 4.
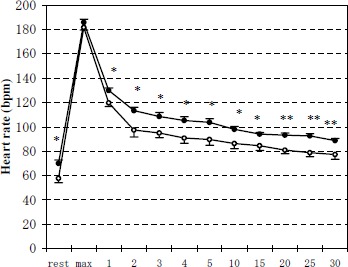
Changes of heart rate during the experiments of rest, maximal exercise and from 1 min to 30 min after exercise. Data are means (±SE). Open and solid circles represent the values for M group and C group, respectively. *, ** Significantly different between M group and C group (* p < 0.05, ** p < 0.01).
Percent decrease of HR after exercise
At 2 min to 30 min after exercise, percent decrease of HR from the peak exercise HR was significantly higher (p < 0.01 or p < 0.05) in M group than in C group (Figure 5).
Figure 5.
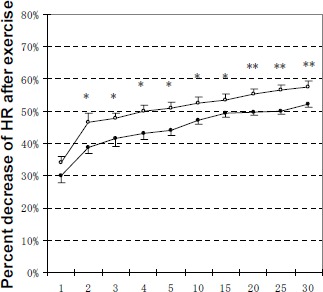
Decrease in HR from 1 min to 30 min after exercise as a percentage of peak HR. Data are means (±SE). Relative decrease of HR at time T after exercise as a percentage of peak HR: (peak HR - HRT) / peak HR x 100, where T = 1 min to 30 min. Open and solid circles represent the values for M group and C group, respectively. *, ** Significantly different between M group and C group (* p < 0.05, ** p < 0.01).
Parameters of HRV in time domains
Mean RR intervals were higher (p <0 .05) at rest or at recovery phases in M group than in C group. SDRR at rest phase was higher (p < 0.05) in M group than in C group. In addition, within 30 min after exercise, Mean RR and SDRR did not return to the pre-exercise values for both groups (Table 2).
Table 2.
Time domain or frequency domain heart rate variability indices. Data are means (±SE).
| rest | rec1 | rec2 | rec3 | rec4 | rec5 | ||
|---|---|---|---|---|---|---|---|
| Mean RR | M | 1098 (46)* | 688 (38)* | 717 (38)* | 737 (36)* | 763 (37)* | 794 (38)* |
| C | 833 (33) | 604 (14) | 624 (13) | 641 (12) | 654 (14) | 659 (12) | |
| SDRR | M | 108.2 (10.7)* | 13.5 (1.8) | 13.2 (1.3) | 16.7 (4.0) | 18.5 (3.6) | 19.7 (2.5) |
| C | 56.3 (8.8) | 15.5 (1.6) | 17.1 (2.8) | 16.0 (1.8) | 19.4 (2.9) | 20.6 (3.1) | |
| LF | M | 812.6 (12.0) | 30.8 (8.6) | 44.4 (12.7) | 80.6 (44.7) | 90.6 (47.9) | 156.4 (62.2) |
| C | 517.8 (140.5) | 42.7(10.0) | 55.4 (11.1) | 98.5 (20.0) | 96.1 (39.6) | 126.1 (27.6) | |
| HF | M | 654.3 (136.6)* | 9.9 (3.8) | 16.2 (5.1) | 25.7 (10.1) | 36.1 (15.1) | 45.5 (14.7) |
| C | 218.3 (64.2) | 9.5 (1.7) | 13.6 (2.1) | 17.9 (2.4) | 16.5 (3.5) | 26.7 (4.5) | |
| LF/HF | M | .9 (.3)* | 4.5 (1.1) | 4.2 (1.0) | 3.6 (1.1) | 3.5 (1.0) | 2.9 (.8) |
| C | 2.5 (.3) | 5.4 (1.6) | 5.4 (1.2) | 5.5 (1.1) | 5.2 (1.3) | 4.9 (1.5) |
Abbreviations: M = marathon runners; C = untrained controls.
*Significantly different from C group (p < 0.05). Rest, rec1, rec2, rec3, rec4, rec5: for a more detailed description, see Methods.
Parameters of HRV in frequency domains
At rest, LF spectral power was slightly higher, whereas HF spectral power was significantly higher (p < 0.05) and LF/HF ratio was significantly lower (p < 0.05) in M group than in C group(Table 2). At recovery phases, HF power was higher and LF/HF ratio was lower in M group than in C group, but there was no statistical significance. LF power, HF power and LF/HF ratio did not return to the pre-exercise values within 30 min after exercise for both groups.
Discussion
This study showed that as compared with untrained controls, female marathon runners had 1) a higher aerobic capacity; 2) a higher blood pressure after exercise; 3) a higher percent decrease of blood pressure after exercise; 4) a faster HR recovery after exercise; 5) higher HRV parameters and lower LF/HF ratio at rest. These results suggested that endurance training increased cardiorespiratory function and accelerated HR recovery after exercise.
HR recovery after exercise depends on several factors: the intensity of exercise, the cardiorespiratory fitness, cardiac ANS modulation, hormones changes and baroreflex sensitivity. Different degrees of intensity of exercise would result in diverse types of HR recovery. After light exercise, HR follows an exponential decline to resting level. After moderate or heavy exercise, however, the recovery pattern is characterized by two distinct phases, an initial exponential drop followed by a slower decline to resting level (Darr et al., 1988). In the present study, the initial fast phase and the followed slow phase of HR recovery after the maximum-effort exercise were also presented in both groups. But the female marathon runners showed a significantly faster decrease in HR to pre-exercise value than the untrained controls. Since the two age-matched groups performed the maximal exercise until exhaustion was reached, furthermore, the RPE to the exercise and the changes of blood lactate after exercise were almost the same for both groups, the relative intensity of exercise for the two groups may be expected to be identical. Obviously, other factors such as influences from long-term endurance training are suggested to be responsible for the faster HR recovery after exercise in the female marathon runners. It should be noted that the RPE to maximal exercise was only 17.6 for M group and 17.7 for C group, which were less than the expected maximal RPE values of 19-20. Since the local feeling in breathing or leg earlier appear exhaustion during the exercise, which made them have to stop running, while the overall feeling of the RPE to exercise still did not reach 19 or 20.
High aerobic capacity is associated with fast HR recovery after exercise. For males, HR recovery was shown to be faster in athletes, who had a higher aerobic capacity than nonathletes (Darr et al., 1988). The present study based on the females indicated that the marathon runners remarkably had a faster HR recovery after exercise and an anticipated higher aerobic capacity than untrained controls. This could imply that for females it is consistent with the previous studies based on males that the faster HR recovery after exercise depends on the higher aerobic capacity.
A number of studies have shown that physically active men or women demonstrated higher levels of HRV compared with sedentary controls (Jensen-Urstad et al., 1997; Davy et al., 1998; Rossy and Thayer, 1998). The present study also showed that the female marathon runners had significantly higher SDRR, HF power at rest and slightly higher HF power at recovery after exercise than untrained controls. High levels of HRV are associated with rapid HR recovery after exercise. Ohuchi et al. (2000) demonstrated that the greater cardiac parasympathetic activity at rest should be in part responsible for the faster HR recovery after exercise. Dixon et al. (1992) found that athletes, who had higher vagal activity and lower sympathetic activity, also had faster HR recovery after exercise than nonathletes. Javorka et al. (2002) showed that the percent decrease of HR during the first minute of recovery was positively correlated with HRV indices at post-exercise recovery. However, in the above-mentioned studies, most subjects are based on males. There were almost no studies based on females that have presented the relationship between HRV variables and HR recovery after exercise. Unfortunately, we could not find the significant correlations between HRV variables and HR recovery in our present study based on the female subjects. It is confused and it may be possible that the limited number of subjects investigated in this study was insufficient to reach statistical significance.
The change of blood pressure brings about reflex HR change by the arterial baroreceptors, which are important in attenuating the rapid change of blood pressure during daily perturbation (Lanfranchi and Somers, 2002). Studies have shown baroreflex sensitivity to be augmented in high-fit male subjects (Barney et al., 1988; Shin et al., 1995). Likewise, physically active women indicate higher baroreflex sensitivity than sedentary women (Davy et al., 1996; 1998), although baroreflex responsiveness is lower in women compared with men (Huikuri et al., 1996). The baroreflex-mediated response of HR to change in arterial blood pressure indicates the capacity of reflex cardiac autonomic modulation (Huikuri et al., 1996). Significant correlations between baroreflex sensitivity and HRV have been observed in a previous study (Pellizzer et al., 1996). In the present study, the marathon runners showed significantly higher cardiac vagal tone at rest and slightly higher cardiac vagal tone at post-exercise recovery, which suggested a greater baroreflex-mediated cardiac vagal outflow than untrained controls. From the results of HRV analysis and the literatures mentioned above, we could suggest that the faster HR recovery after exercise in the female marathon runners may be related to their augmented baroreflex function.
It is noteworthy that blood pressure at rest was almost the same for the two groups, but after exercise it was significantly higher in the female marathon runners. This exhibited an exaggerated blood pressure response to maximal exercise in the female marathon runners. Physical activity was shown to increase left ventricular (LV) mass (Maron, 1986; Levy et al., 1993; Whyte et al., 2004), which was related to maximal blood pressure during exercise (Michelsen et al., 1990; Molina et al., 1999; Kamarck et al., 2000; Sung et al., 2003). The large increment of blood pressure during the maximum-effort running exercise may be necessary for the marathon runners to achieve great work capacity of fast treadmill running speed and high treadmill gradient. Whether exaggerated exercise blood pressure is responsible for rapid HR recovery after exercise is unclear and further studies are need to clarify. Although blood pressure after exercise was higher in the female marathon runners, their percent decrease of blood pressure after exercise was significantly greater than untrained controls’. This also reflects that the female marathon runners have a greater capacity of reflex cardiovascular modulation after exercise than untrained controls (Laukkanen et al., 2004).
It is unlikely that hormonal changes contribute to the faster HR recovery in the marathon runners because according to some studies, endurance training could enhance plasma catecholamine concentration in response to moderate or strenuous exercise (Kjaer et al., 1986; Silverman and Mazzeo, 1996; Greiwe et al., 1999; Jacob et al., 2004) and the clearance rate of post-exercise plasma catecholamine was shown not to be significantly changed by training (Hagberg et al., 1979).
Conclusions
In summary, female marathon runners indicated faster HR recovery after exercise and altered cardiac ANS modulation at rest than untrained controls. The higher levels of HRV, higher aerobic capacity and exaggerated blood pressure response to exercise in the female marathon runners are suggested to be responsible for their faster HR recovery after exercise compared with untrained controls.
Biographies
Na DU
Employment
Depart. of Sports Medicine and Sports Science, Gifu Univ. Graduate School of Medicine
Degree
MD
Research interests
Cardiology, adaptation to altitude
E-mail: g2103004@guedu.cc.gifu-u.ac.jp
Siqin BAI
Employment
Depart. of Sports Medicine and Sports Science, Gifu Univ. Graduate School of Medicine
Degree
MS
Research interests
Plasticity, balance
E-mail: i2111302@guedu.cc.gifu-u.ac.jp
Kazuo OGURI
Employment
Faculty of Management, Shizuoka Sangyo University
Degree
PhD
Research interests
Muscle metabolism, body composition assessment.
E-mail: oguri@iwata.ssu.ac.jp
Yoshihiro KATO
Employment
Depart. of Sports Medicine and Sports Science, Gifu Univ. Graduate School of Medicine
Degree
MD, PhD
Research interests
Cardiology, pediatrics
E-mail: yoshi-kt@cc.gifu-u.ac.jp
Ichie MATSUMOTO
Employment
Depart. of Sports Medicine and Sports Science, Gifu Univ. Graduate School of Medicine.
Degree
MS
Research interests
Body composition.
E-mail: michie@cc.gifu-u.ac.jp
Harumi KAWASE
Employment
Section of Clinical Laboratory, Gifu Univ. Hospital.
Degree
MS
Research interests
Cardiology.
E-mail: kkawase@cc.gifu-u.ac.jp
Toshio MATSUOKA
Employment
Professor, Depart. of Sports Medicine and Sports Science, Gifu Univ. Graduate School of Medicine.
Degree
PhD
Research interests
Anaerobic threshold, lactate threshold.
E-mail: matsuoka@cc.gifu-u.ac.jp
References
- Arai Y., Saul J.P., Albrecht P., Hartley L.H., Lilly L.S., Cohen R.J., Colucci W.S. (1989) Modulation of cardic autonomic activity during and immediately after exercise. American Journal of Physiology 256, H132-H141 [DOI] [PubMed] [Google Scholar]
- Barney J.A., Ebert T.J., Groban L., Farrell P.A., Hughes C.V., Smith J.J. (1988) Carotid baroreflex responsiveness in high-fit and sedentary young men. Journal of Applied Physiology 65, 2190-2194 [DOI] [PubMed] [Google Scholar]
- Berger R.D., Saul J.P., Cohen R.J. (1989) Transfer function analysis of autonomic regulation. I. Canine atrial rate response. American Journal of Physiology 256, H142-H152 [DOI] [PubMed] [Google Scholar]
- Bernardi L., Leuzzi S., Radaelli A., Passino C., Johnston J.A., Sleight P. (1994) Low-frequency spontaneous fluctuations of R-R interval and blood pressure in conscious humans: a baroreceptor or central phenomenon? Clinical Science 87, 649-654 [DOI] [PubMed] [Google Scholar]
- Borg G.A. (1982) Psychophysical bases of perceived exertion. Medicine and Science in Sports and Exercise 14, 377-381 [PubMed] [Google Scholar]
- Casadei B., Cochrane S., Johnston J., Conway J., Sleight P. (1995) Pitfalls in the interpretation of spectral analysis of the heart rate variability during exercise in humans. Acta Physiologica Scandinavica 153, 125-131 [DOI] [PubMed] [Google Scholar]
- Cole C.R., Blackstone E.H., Pashkow F.J., Snader C.E., Lauer M.S. (1999) Heart rate recovery immediately after exercise as a predictor of mortality. The New England Journal of Medicine 341, 1351-1357 [DOI] [PubMed] [Google Scholar]
- Cole C.R., Foody J.M., Blackstone E.H., Lauer M.S. (2000) Heart rate recovery after submaximal exercise testing as a predictor of mortality in a cardiovascularly healthy cohort. Annals of Internal Medicine 132, 552-555 [DOI] [PubMed] [Google Scholar]
- Darr K.C., Bassett D.R., Morgan B.J., Thomas D.P. (1988) Effects of age and training status on heart rate recovery after peak exercise. American Journal of Physiology 254, H340-H343 [DOI] [PubMed] [Google Scholar]
- Davy K.P., Miniclier N.L., Taylor J.A., Stevenson E.T., Seals D.R. (1996) Elevated heart rate variability in physically active postmenopausal women: a cardioprotective effect? American Journal of Physiology 271, H455-H460 [DOI] [PubMed] [Google Scholar]
- Davy K.P., DeSouza C.A., Jones P.P., Seals D.R. (1998) Elevated heart rate variability in physically active young and older adult women. Clinical Science 94, 579-584 [DOI] [PubMed] [Google Scholar]
- Dixon E.M., Kamath M.V., McCartney N., Fallen E.L. (1992) Neural regulation of heart rate variability in endurance athletes and sedentary controls. Cardiovascular Research 26, 713-719 [DOI] [PubMed] [Google Scholar]
- Greiwe J.S., Hickner R.C., Shah S.D., Cryer P.E., Holloszy J.O. (1999) Norepinephrine response to exercise at the same relative intensity before and after endurance exercise training. Journal of Applied Physiology 86, 531-535 [DOI] [PubMed] [Google Scholar]
- Hagberg J.M., Hickson R.C., Mvlane J.A., Ehsani A.A., Winder W.W. (1979) Disappearance of norepinephrine from the circulation following strenuous exercise. Journal of Applied Physiology 47, 1311-1314 [DOI] [PubMed] [Google Scholar]
- Huikuri H.V., Pikkujamsa S.M., Airaksinen K.E., Ikaheimo M.J., Rantala A.O., Kauma H., Lilja M., Kesaniemi Y.A. (1996) Sex-related differences in autonomic modulation of heart rate in middle-aged subjects. Circulation 94, 122-125 [DOI] [PubMed] [Google Scholar]
- Jacob C., Zouhal H., Prioux J., Gratas-Delamarche A., Bentue-Ferrer D., Delamarche P. (2004) Effect of the intensity of training on catecholamine responses to supramaximal exercise in endurance-trained men. European Journal of Applied Physiology, 91, 35-40 [DOI] [PubMed] [Google Scholar]
- Javorka M., Zila I., Balharek T., Javorka K. (2002) Heart rate recovery after exercise: relations to heart rate variability and complexity. Brazilian Journal of Medical and Biological Research 35, 991-1000 [DOI] [PubMed] [Google Scholar]
- Jensen-Urstad K., Saltin B., Ericson M., Storck N., Jensen-Urstad M. (1997) Pronounced resting bradycardia in male elite runners is associated with high heart rate variability. Scandinavian Journal of Medicine & Science in Sports, 7, 274-278 [DOI] [PubMed] [Google Scholar]
- Kamarck T.W., Eranen J., Jennings J.R., Manuck S.B., Everson S.A., Kaplan G.A., Salonen J.T. (2000) Anticipatory blood pressure responses to exercise are associated with left ventricular mass in Finnish men: Kuopio Ischemic Heart Disease Risk Factor Study. Circulation 102, 1394-1399 [DOI] [PubMed] [Google Scholar]
- Kjaer M., Farrell P.A., Christensen N.J., Galbo H. (1986) Increased epinephrine response and inaccurate glucoregulatior in exercising athletes. Journal of Applied Physiology 61, 1693-1700 [DOI] [PubMed] [Google Scholar]
- Kluess H.A., Wood R.H., Welsch M.A. (2000) Vagal modulation of the heart and central hemodynamics during handgrip exercise. American Journal of Physiology 279, H1648-H1652 [DOI] [PubMed] [Google Scholar]
- Lanfranchi P.A., Somers V.K. (2002) Arterial baroreflex function and cardiovascular variability: interactions and implications. American Journal of Physiology- Regulatory, Integrative and Comparative Physiology 283, R815-R826 [DOI] [PubMed] [Google Scholar]
- Laukkanen J.A., Kurl S., Salonen R., Lakka T.A., Rauramaa R., Salonen J.T. (2004) Systolic blood pressure during recovery from exercise and the risk of acute myocardial infarction in middle-aged men. Hypertension 44, 820-825 [DOI] [PubMed] [Google Scholar]
- Levy W.C., Cerqueira M.D., Abrass I.B., Schwartz R.S., Stratton J.R. (1993) Endurance exercise training augments diastolic filling at rest and during exercise in healthy young and older men. Circulation 88, 116-126 [DOI] [PubMed] [Google Scholar]
- Levy W.C., Cerqueira M.D., Harp G.D., Johannessen K.A., Abrass I.B., Schwartz R.S., Stratton J.R. (1998) Effect of endurance exercise training on heart rate variability at rest in healthy young and older men. The American Journal of cardiology 82, 1236-1241 [DOI] [PubMed] [Google Scholar]
- Maron B.J. (1986) Structural features of athlete heart as defined by echocardiography. Journal of the American College of Cardiology 7, 190-203 [DOI] [PubMed] [Google Scholar]
- Michelsen S., Knutsen K.M., Stugaard M., Otterstad J.E. (1990) Is left ventricular mass in apparently healthy, normotensive men correlated to maximal blood pressure during exercise? European Heart Journal 11, 241-248 [DOI] [PubMed] [Google Scholar]
- Molina L., Elosua R., Marrugat J., Pons S. (1999) Relation of maximum blood pressure during exercise and regular physical activity in normotensive men with left ventricular mass and hypertrophy. The American Journal of Cardiology 84, 890-893 [DOI] [PubMed] [Google Scholar]
- Nishime E.O., Cole C.R., Blackstone E.H., Pashkow F.J., Lauer M.S. (2000) Heart rate recovery and treadmill exercise score as predictors of mortality in patients referred for exercise ECG. The Journal of the American Medical Association 284, 1392-1398 [DOI] [PubMed] [Google Scholar]
- Ohuchi H., Suzuki H., Yasuda K., Arakaki Y., Echigo S., Kamiya T. (2000) Heart rate recovery after exercise and cardiac autonomic nervous activity in children. Pediatric Research 47, 329-335 [DOI] [PubMed] [Google Scholar]
- Pagani M., Lombardi F., Guzzetti S., Rimoldi O., Furlan R., Pizzinelli P., Sandrone G., Malfatto G., Dell’Orto S., Piccaluga E., Turiel M., Baselli G., Cerutti S., Malliani A. (1986) Power spectral analysis of heart rate and arterial pressure variabilities as a marker of sympatho-vagal interaction in man and conscious dog. Circulation Research 59, 178-193 [DOI] [PubMed] [Google Scholar]
- Pardo Y., Merz C.N., Velasquez I., Paul-Labrador M., Agarwala A., Peter C.T. (2000) Exercise conditioning and heart rate variability: evidence of a threshold effect. Clinical Cardiology 23, 615-620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellizzer A.M., Kamen P.W., Jackman G., Brazzale D., Krum H. (1996) Non-invasive assessment of baroreflex sensitivity and relation to measures of heart rate variability in man. Clinical and Experimental Pharmacology & Physiology, 23, 621-624 [DOI] [PubMed] [Google Scholar]
- Perini R., Orizio C., Comande A., Castellano M., Beschi M., Veicsteinas A. (1989) Plasma norepinephrine and heart rate dynamics during recovery from submaximal exercise in man. European Journal of Applied Physiology, 58, 879-883 [DOI] [PubMed] [Google Scholar]
- Rossy L.A., Thayer J.F. (1998) Fitness and gender-related differences in heart period variability. Psychosomatic Medicine 60, 773-781 [DOI] [PubMed] [Google Scholar]
- Shin K., Minamitani H., Onishi S., Yamazaki H., Lee M. (1995) Assessment of training-induced autonomic adaptations in athletes with spectral analysis of cardiovascular variability signals. Japanese Journal of Physiology 45, 1053-1069 [DOI] [PubMed] [Google Scholar]
- Silverman H.G., Mazzeo R.S. (1996) Hormonal responses to maximal and submaximal exercise in trained and untrained men of various ages. The Journals of Gerontology: Series A, Biological Sciences and Medical Sciences 51, B30-B37 [DOI] [PubMed] [Google Scholar]
- Sung J., Ouyang P., Silber H.A., Bacher A.C., Turner K.L., DeRegis J.R., Hees P.S., Shapiro E.P., Stewart K.J. (2003) Exercise blood pressure response is related to left ventricular mass. Journal of Human Hypertension 17, 333-338 [DOI] [PubMed] [Google Scholar]
- Whyte G.P., George K., Nevill A., Shave R., Sharma S., McKenna W.J. (2004) Left ventricular morphology and function athletes: a meta-analysis. International Journal of Sports Medicine 25, 380-383 [DOI] [PubMed] [Google Scholar]


