Abstract
Biomarkers are important for accurate diagnosis of complex disorders such as traumatic brain injury (TBI). For a complex and multifaceted condition such as TBI, it is likely that a single biomarker will not reflect the full spectrum of the response of brain tissue to injury. Ubiquitin C-terminal hydrolase L1 (UCH-L1) and glial fibrillary acidic protein (GFAP) are among of the most widely studied biomarkers for TBI. Because UCH-L1 and GFAP measure distinct molecular events, we hypothesized that analysis of both biomarkers would be superior to analysis of each alone for the diagnosis and prognosis of TBI. Serum levels of UCH-L1 and GFAP were measured in a cohort of 206 patients with TBI enrolled in a multicenter observational study (Transforming Research and Clinical Knowledge in Traumatic Brain Injury [TRACK-TBI]). Levels of the two biomarkers were weakly correlated to each other (r=0.364). Each biomarker in isolation had good sensitivity and sensitivity for discriminating between TBI patients and healthy controls (area under the curve [AUC] 0.87 and 0.91 for UCH-L1 and GFAP, respectively). When biomarkers were combined, superior sensitivity and specificity for diagnosing TBI was obtained (AUC 0.94). Both biomarkers discriminated between TBI patients with intracranial lesions on CT scan and those without such lesions, but GFAP measures were significantly more sensitive and specific (AUC 0.88 vs. 0.71 for UCH-L1). For association with outcome 3 months after injury, neither biomarker had adequate sensitivity and specificity (AUC 0.65–0.74, for GFAP, and 0.59–0.80 for UCH-L1, depending upon Glasgow Outcome Scale Extended [GOS-E] threshold used). Our results support a role for multiple biomarker measurements in TBI research. (ClinicalTrials.gov Identifier NCT01565551)
Key words: : biomarker, common data elements, human studies, TBI
Introduction
Traumatic brain injury (TBI) is often a diagnostic and therapeutic challenge, particularly at the mild end of the injury spectrum. TBI is traditionally classified as mild, moderate, or severe, based on the initial Glasgow Coma Scale (GCS) score recorded in the Emergency Department (ED).1 Although it is recognized that this classification scheme is one dimensional and leaves much to be desired,2 it has been universally utilized as a major inclusion criterion by all prior and ongoing TBI clinical trials. Severe TBI has been the primary focus of clinical investigation over the past 30 years,3 but mild TBI (mTBI0 is at least 10-fold more prevalent.4,5 Whereas the likelihood of favorable recovery is higher in mTBI than in moderate or severe TBI, many patients with mTBI are left with disabilities that impair their ability to fulfill their work, school, or family responsibilities. It is likely that the societal burden resulting from mTBI is at least equivalent to that resulting from severe TBI, given its much higher prevalence.4–6
mTBI has been relatively understudied in clinical investigations for several reasons. First, mTBI can often be difficult to diagnose, as symptoms are primarily subjective and nonspecific and overlap with psychological disorders that frequently confound the clinical picture. Second, many mTBI patients make a complete recovery, and early identification of mTBI patients likely to have persistent symptoms and develop cognitive and neuropsychological deficits is not currently possible. Third, as mortality and severe disability are relatively rare in mTBI, the outcome measures that are traditionally used in clinical research are insufficiently sensitive for the type of cognitive and behavioral disabilities that most commonly result from mTBI.3
One approach to this problem is to identify biomarkers that can be measured in the acute period, and that may be useful for selecting patients at risk of long-term disabilities and for guiding pharmacological interventions. Biomarkers are objectively measured indicators of biological processes, which can be assessed by biochemical, physiological, radiological, or other quantitative techniques,7 and are often very useful in clinical practice and research. Despite much effort over the past decade to develop diagnostic and prognostic biomarkers useful for TBI, as of yet, none meet the criteria needed for qualification for drug or biotechnology product development as identified by the United States Food and Drug Administration (FDA).7 Additionally, the focus on biomarker development in TBI has been on the moderate and severe end of the severity spectrum,8–10 whereas the potential for biomarkers changing clinical practice is likely greatest for mTBI, given the particular diagnostic and prognostic challenges presented by mild injuries.
Ubiquitin C-terminal hydrolase -L1 (UCH-L1) is a promising candidate biomarker for TBI, which is currently under investigation.9–12 UCH-L1 is highly and specifically expressed in neurons, and has been useful as a histological marker. It is involved in the process of ubiquitination of proteins destined for degradation via the proteosomal pathway, thus playing an important role in the removal of oxidized or misfolded proteins in both normal and pathological conditions.13 UCH-L1 levels in both cerebrospinal fluid (CSF) and serum are elevated for several days after severe TBI,11 and levels are higher in patients who died than in those who survived.9,11 Serum UCH-L1 levels in mTBI have been analyzed in two studies. Berger et al14 found that UCH-L1 levels were increased after moderate and severe pediatric TBI, but not after mTBI. A more recent study of 86 mTBI patients15 found modest elevations in serum UCH-L1 levels in mTBI compared with uninjured or orthopedically injured controls, which was primarily noted in patients with lesions visible on cranial computed tomography (CT) scanning. However, this study did not attempt to determine whether UCH-L1 elevations in serum were associated with incomplete recovery after mTBI.
More recently, glial fibrillary acidic protein (GFAP) and GFAP breakdown products (GFAP-BDP) have been studied as acute biomarkers for TBI.9,16–18 GFAP is an intermediate filament protein expressed almost exclusively in astrocytes, where it is induced by neural injury and released upon disintegration of the astrocyte cytoskeleton. In moderate and severe TBI, GFAP8,12 and GFAP-BDP9,10 levels are elevated in CSF and serum, particularly in patients who experienced an unfavorable outcome. In a study of predominantly mTBI,17 GFAP measurements were able to distinguish patients with TBI from uninjured controls with a high level of sensitivity and specificity (area under the curve [AUC] of receiver-operator characteristics [ROC] of 0.90, 95% CI 0.86–0.94). Further, GFAP was also able to discriminate between those with intracranial lesions on CT scanning from those without CT lesions (AUC 0.79, 95% CI 0.69–0.89). Metting et al.18 reported similar findings. In their study of 94 patients with mTBI, GFAP was elevated in those with abnormal CT, as well as in those with axonal injury on magnetic resonance imaging (MRI).
In principle, measurements of GFAP are complementary to UCH-L1 measures, as they are produced by different cell types and may reflect distinct injury mechanisms. A study of severe TBI9,10 suggests that GFAP elevations are primarily a reflection of focal mass lesions, whereas diffuse injuries primarily result in UCHL-1 elevations. As patients with diffuse injuries may require different therapies from those with focal lesions, such findings, if confirmed, hold promise for the ability to select patients for targeted therapies.19 In severe TBI, UCH-L1 concentrations are moderately correlated to GFAP (R=0.53).9 The relationship between these two biomarkers has not been directly studied in mTBI.
The Transforming Research and Clinical Knowledge in Traumatic Brain Injury (TRACK-TBI) study is a multicenter effort to improve TBI research and clinical practice through large-scale prospective collection of highly granular clinical, neuroimaging, biomarker, and outcome data throughout the continuum of TBI, including mTBI. In the pilot phase of TRACK-TBI, serum UCH-L1and GFAP levels were collected at three level 1 trauma centers in acute TBI patients, 85% of whom had mTBI. Outcome was assessed at 3 and 6 months after injury. An initial analysis of TRACK-TBI data20 confirms the findings of Papa et al.,17 and further indicates that elevated GFAP selects for patients who have persistent disabilities 3 months after injury.
This article presents the TRACK-TBI UCH-L1 results, and compares them with the GFAP findings. We hypothesized that 1) serum UCH-L1 levels are higher in moderate and severe TBI than in mTBI, 2) serum UCH-L1 levels are higher in mTBI patients with abnormal CT scans than in patients with normal CT scans, 3) higher serum UCH-L1 levels are predictive of outcome 3 months after injury, and 4) the combination of UCH-L1 and GFAP measurements are more informative than either biomarker alone.
Methods
Study population
Subjects were identified and recruited upon arrival at one of three level 1 trauma centers as part of the multicenter prospective TRACK-TBI study.21 Study protocols were approved by the institutional review boards of participating centers (San Francisco General Hospital; University of Pittsburgh Medical Center [UPMC]; University Medical Center Brackenridge [UMCB], Austin, Texas). All participants or their legal authorized representatives gave written informed consent. At follow-up outcome assessments, participants previously consented by legal authorized representative were consented for continuation in the study, if sufficient improvement in neurological status had occurred.
To be eligible for the TRACK-TBI study, patients had to present within 24 h of injury with a history of trauma to the head sufficient to triage to noncontrast head CT using the American College of Emergency Physicians/Centers for Disease Control (ACEP/CDC) evidence-based joint practice guideline.22 All levels of GCS scores were eligible. Details of loss of consciousness, amnesia, and source of trauma were recorded upon screening, and informed consent was obtained. GCS score was assessed by a neurosurgeon at admission, and was reconfirmed by study personnel at the time of biomarker collection.
Controls were 175 healthy, uninjured volunteers recruited through advertisements in a local periodical in Orlando and Gainesville, Florida. They were matched by age and gender to the TBI patients.
Sample collection and measurement of UCHL-1 and GFAP-BDP
Blood samples were collected from subjects who consented to genetic and proteomic analysis within 24 h of injury. All samples were dated and time stamped to compare with time of injury. The TBI-Common Data Elements (CDE) Biospecimens and Biomarkers Working Group Guidelines for plasma preparation were followed.23 Samples were centrifuged and plasma aliquots stored at −80°C for future batch processing. UPMC and UMCB batch shipped samples, overnight on dry ice, to University of California at San Francisco (UCSF). All samples were stored in a de-identified manner, with a unique study number specific to site and subject. A central database was maintained by the coordinating center (UCSF) with each site entering site-specific data for final statistical reporting. Blinded sample analysis occurred in a single laboratory (Banyan Biomarkers, Alachua, FL) using a sandwich enzyme linked immunosorbent assay (ELISA) to UCHL-1 and GFAP.
UCH-L1 was assayed using a sandwich ELISA as previously described.15 Both mouse monoclonal antibody (capture antibody) and rabbit polyclonal antibody (detection antibody) were made in-house (Banyan Biomarkers) against recombinant UCH-L1 full-length and partial protein, respectively. Both were affinity purified, and specificity was confirmed by immunoblotting. The lower limit of detection was 0.03 ng/mL. The GFAP ELISA utilized a proprietary mouse monoclonal antibody for solid phase immobilization and a proprietary polyclonal rabbit antibody for detection. The antibodies detect both whole GFAP molecules as well as GFAP-BDPs, potentially resulting in a more complete measure of GFAP levels in circulation.20 The estimated limit of detection (LOD) for GFAP is 0.1 ng/mL. The test sample was allowed to react sequentially with the capture and detection antibodies. Quantitative determination of the biomarker concentration was achieved by comparing the unknown sample results to a standard curve obtained from the same assay. All samples were analyzed in duplicate concomitantly with calibrators prepared in compatible matrix. Specifically, a serial dilution of the calibrator protein was prepared, and aliquots were assayed in the same volume and under the same conditions as the samples. The calibrator signal intensities were used to generate a dose-response curve and to calculate the sample concentrations using a four parameter logistic function (Mars Software for OPTIMA reader). The same amount of sample, quality controls, and calibrators were used for each assay (dilution factor of 1).
Evaluation of CT scans according to TBI-CDE
All patients underwent CT imaging of the brain at the time of initial presentation to the ED. Each patient's head CT was characterized using the recommendations of the TBI-CDE Neuroimaging Working Group.24 The Neuroimaging TBI-CDEs are consensus-based recommendations for data collection regarding specific radiological features, data definitions needed to characterize injuries, and best practices needed to optimize and harmonize imaging data acquisition for TBI research. Each CT was de-identified, electronically uploaded to a central imaging database, and reviewed by a blinded central reader who was a board-certified neuroradiologist. Imaging features were extracted and entered into the TRACK-TBI database.
Outcome evaluation
Patient outcomes included mortality and neurological assessment at 6 months after injury. The primary outcome measure was the 6 month Glasgow Outcome Scale-Extended score (GOS-E).25 The GOS-E provides eight categories of outcome: Dead, Vegetative State, Lower Severe Disability, Upper Severe Disability, Lower Moderate Disability, Upper Moderate Disability, Lower Good Recovery, and Upper Good Recovery. Ratings are based on patient consciousness, independence, ability to work, social and leisure activities, social relationships, and other sequalae of TBI. Upper Good Recovery (GOS-E score of 8) indicates return to pre-injury baseline with no residual effects of the TBI.
Statistical analysis
Descriptive statistics with means and proportions were used to describe categorical variables. Biomarker levels were treated as continuous data. Data were assessed for equality of variance and distribution. Intracranial lesions shown on initial CT were scored and analyzed as categorical variable (present or absent), with biomarker levels as the dependent variables, and the Student's t test was used to compare means across lesion groups. We assessed the ability of biomarker levels to separate patients with different injury patterns and outcomes; this is quantified as the area under the ROC curve. In line with current statistical consensus, AUC of 0.8–0.9 is considered very good, 0.7–0.8 is considered adequate, and <0.7 is considered poor. Data were analyzed using GraphPad Prism, version 5.0 for Windows (GraphPad Software, San Diego, CA) or with Statistical Package for the Social Sciences (SPSS, version 20, IBM Corporation).
Results
Baseline demographics and CT results
There were 206 TRACK-TBI participants with UCH-L1 data available, representing the full spectrum of TBI encountered in urban level I trauma centers. Plasma samples were obtained within 24 h of injury (mean 10.9 h, SD 6.4 h, min 0.5 h, max 23.4 h. Demographic details have been published previously.25 Briefly, the majority of subjects (83%) were classified as having had an mTBI (admission GCS 13–15), 4% as having had a moderate TBI (GCS 9–12), and 13% as having had a severe TBI (GCS 3–8). Mean age (±SD) was 42±18 years, and 73% were male. CT scans demonstrated intracranial pathology in 43% of those with mTBI, in 78% of those with moderate TBI, and in 96% of those with severe TBI. For the uninjured controls, mean age (±SD) was 37±14 years, and 53% of the uninjured controls were male.
GOS-E was obtained at 3 months in 168 participants and at 6 months in 145. At 3 months, one third (34%) had made a full functional recovery (GOSE=8), whereas a minority (15%) had an unfavorable outcome (GOSE≤4). At 6 months after injury, outcomes were similar; 32% had made a full recovery (GOSE=8), and 14% had an unfavorable outcome (GOSE≤4).
Relationship of UCH-L1 to injury severity and outcome
UCH-L1 levels were higher in moderate to severe TBI than in mTBI. Further, they were higher in mTBI with cranial CT abnormalities (complicated mTBI) than in mTBI without CT abnormalities (Fig. 1). Assessing outcome through the GOSE 3 months after injury, UCH-L1 levels were poorly predictive of complete recovery; they were much better at predicting poor outcome (Fig. 2). When only patients with mTBI were included in the analysis, UCH-L1 levels did not distinguish between those who recovered fully (GOSE=8) and those who did not (AUC 0.511, data not shown).
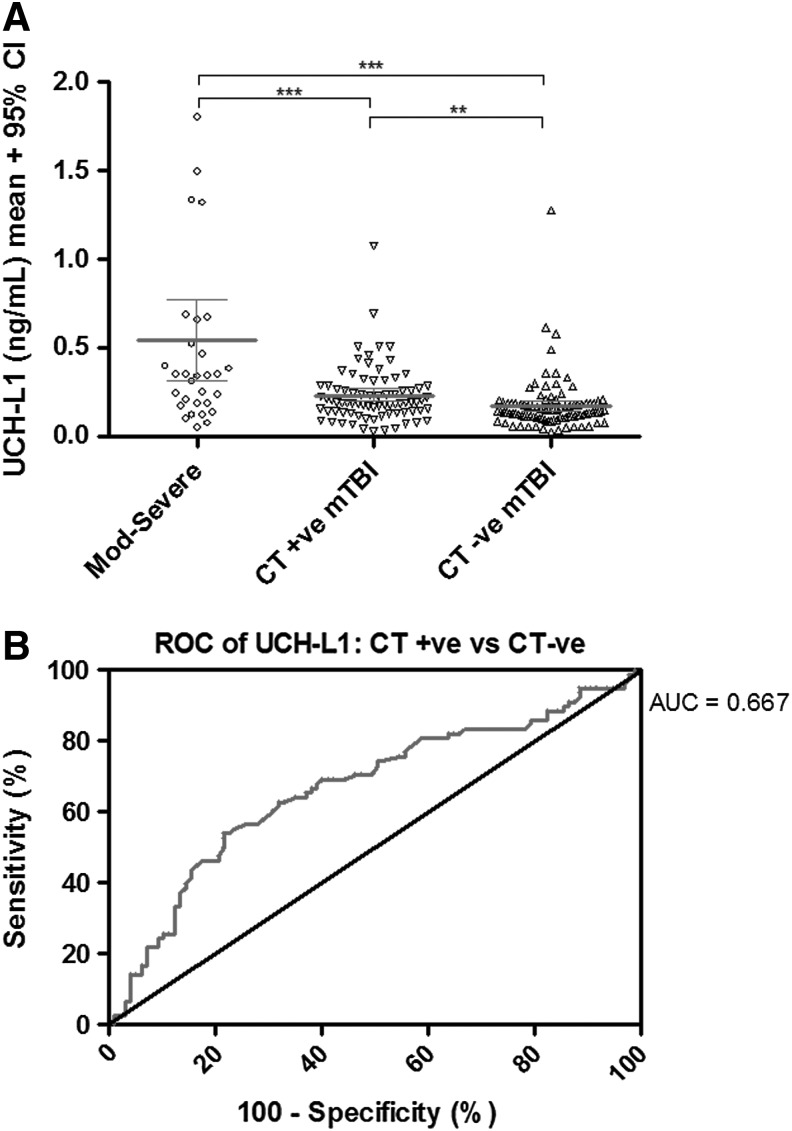
FIG. 1.
(A) Ubiquitin C-terminal hydrolase L1 (UCH-L1) levels as a function of injury severity. Mean UCH-L1 levels differ between moderate to severe TBI (GCS 3–12) and mild TBI (mTBI) (GCS 13–15) and also differ between complicated mTBI (GCS 13–15 with abnormal cranial CT) and uncomplicated mTBI. (***p<0.0001; **p=0.012) (B) Receiver-operator characteristics (ROC) curve for distinguishing CT positive from CT negative patients. UCH-L1 levels are modestly predictive of cranial CT abnormalities for mTBI patients (area under the curve [AUC] 0.713), data shown) or for all patients (AUC=0.667, data not shown).
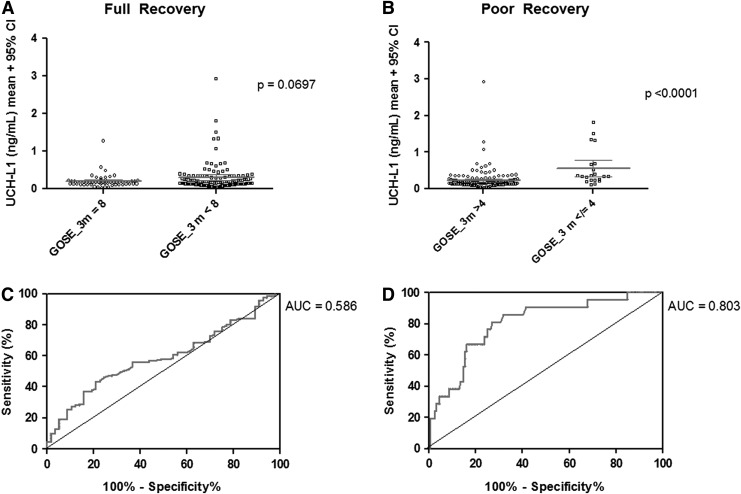
FIG. 2.
Relationship of ubiquitin C-terminal hydrolase (L1UCH-L1) levels with outcome. Outcome was assessed using the Glasgow Outcome Scale–Extended (GOS-E) at 3 months after injury. (A) Patients who recovered fully tended to have lower UCH-L1 levels that those who did not (p=0.07). (B) Patients who had poor outcomes (GOS-E≤4) had higher UCH-L1 levels. (C, D) UCH-L1 levels were poorly predictive of a complete recovery (area under the curve [AUC]=0.586), but reasonably predictive of poor outcome (GOS-E≤4) (AUC=0.803).
Relationship between UCH-L1 and GFAP
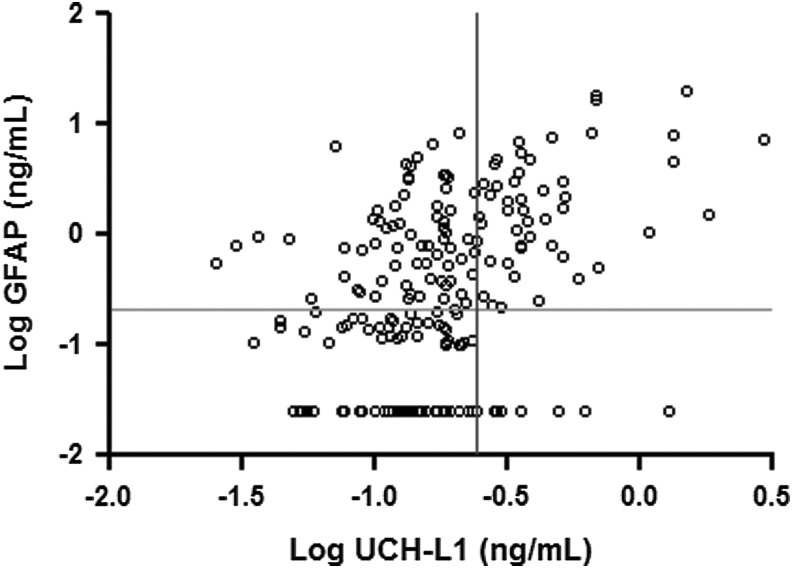
As UCH-L1 and GFAP appear to reflect different injury mechanisms, the combination of the two biomarkers may be more useful than either biomarker in isolation for predicting intracranial lesions on CT scanning or outcome 3 months after injury. First, we assessed the relationship between serum levels of the two biomarkers. There was a statistically significant but weak correlation (R=0.364, p<0.0001) between levels of UCH-L1 and GFAP. In order to visually represent the relationship between the two biomarkers, serum levels of each were plotted after log transformation (Fig. 3). Upper limits of normal were defined as mean+3 standard deviations, from Papa et al.15,17 For UCH-L1 mean (SD) was 0.073 (0.057) ng/mL, and for GFAP mean (SD) was 0.038 (0.059 ng/mL). Therefore, the upper limits of normal for UCH-L1 and GFAP were 0.244 and 0.215 ng/mL, respectively.
FIG. 3.
Relationship between serum ubiquitin C-terminal hydrolase (UCH-L1) and glial fibrillary acidic protein (GFAP) levels. Serum levels of the two biomarkers were weakly correlated (R=0.364, 95% CI 0.233–0.482, p<0.0001). Using log transformation to spread out data points at the low end of the distribution, it was apparent that most patients with elevated UCH-L1 levels also had elevated GFAP levels, whereas many patients with elevated GFAP levels had normal UCH-L1 levels.
UCHL-1 and GFAP distinguish between TBI and healthy controls
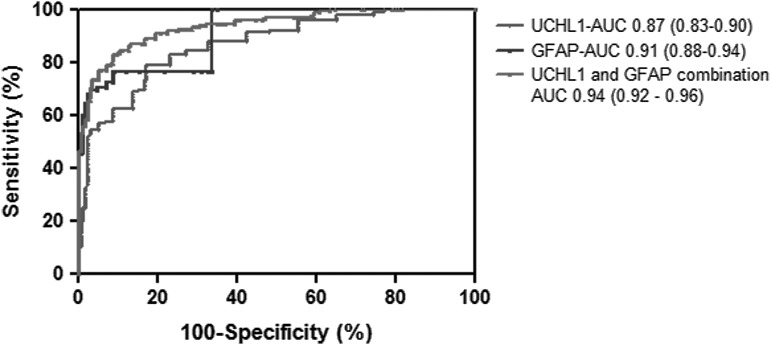
As has been demonstrated, UCH-L1 and GFAP levels readily discriminate between TBI patients and healthy controls. For the TRACK-TBI cohort, AUCs are 0.87 (95% CI 0.83–0.90) and 0.91 (95% CI 0.88–0.94), respectively. A novel finding of this study is that the combination of UCH-L1 levels and GFAP levels results in improved sensitivity and specificity compared with each biomarker considered in isolation (Fig. 4). The AUC for the combined biomarkers is 0.94 (0.92–0.96).
FIG. 4.
Receiver-operator characteristics (ROC) curves for ubiquitin C-terminal hydrolase (UCH-L1), glial fibrillary acidic protein (GFAP), and both biomarkers in combination for discriminating between traumatic brain injury (TBI) patients and healthy controls.
UCHL-1 levels do not improve prognosis compared with GFAP alone
Table 1 presents the ROC analysis for UCHL-1, GFAP, and both biomarkers together for four binary comparisons: 1) TBI patients versus uninjured controls, and within the TBI patients; 2) CT positive versus CT negative; 3) 3-month GOSE=8 versus GOSE<8; and 4) 3 month GOSE>4 versus GOSE≤4. For GFAP only, these data were included in a previously published manuscript,26 and are included here for purposes of comparison. Whereas consideration of both biomarkers together is better than either alone in distinguishing TBI patients from healthy volunteers, inclusion of UCH-L1 values does not improve upon GFAP alone in predicting intracranial lesions on CT or full recovery 3 months after injury. For predicting incomplete recovery (dichotomizing the 3-month GOSE at 7), the AUCs are all <0.65, considered a poor predictor, for GFAP alone, UCH-L1 alone, or both in combination. For predicting favorable versus unfavorable outcome (dichotomizing the 3 month GOSE at 4), UCH-L1 levels marginally outperform GFAP levels, and both biomarkers together are slightly better than either alone. Similar results were noted when the GOSE 6 months after injury was analyzed (Table 1).
Table 1.
Receiver-Operator Characteristic Analysis for UCH-L1, GFAP, and Both Biomarkers Considered Together for Four Binary Outcomes
| Biomarker | UCH-L1 | GFAP | UCH-L1+GFAP |
|---|---|---|---|
| TBI vs. healthy control |
0.87 (0.83–0.90) |
0.91 (0.88–0.94) |
0.94 (0.92–0.96) |
| CT positive vs. CT negative |
0.71 (0.64–0.78) |
0.88 (0.84–0.93) |
0.88 (0.83–0.93) |
| 3 month incomplete recovery (GOS-E<8 vs. GOS-E=8) |
0.58 (0.50–0.74) |
0.65 (0.55–0.74) |
0.64 (0.55–0.72) |
| 3 month poor outcome (GOS-E≤4 vs. GOS-E>4) |
0.80 (0.70–0.90) |
0.74 (0.61–0.87) |
0.83 (0.75–0.91) |
| 6 month incomplete recovery (GOS-E<8 vs. GOS-E=8) |
0.51 (0.39–0.63) |
0.60 (0.43–0.77) |
0.61 (0.48–0.75) |
| 6 month poor outcome (GOS-E≤4 vs. GOS-E>4) | 0.76 (0.60–0.91) | 0.74 (0.61–.87) | 0.81 (0.70–0.91) |
AUC with 95% CI are presented.
UCH-L1, ubiquitin C-terminal hydrolase; GFAP, glial fibrillary acidic protein, TBI, traumatic brain injury; GOS-E, Glasgow Outcome Scale–Extended; AUC, area under the curve.
Discussion
This analysis from the prospective, multicenter TRACK-TBI study examined the performance of a dual serum biomarker battery for early diagnosis of TBI. The combination of serum levels of UCH-L1 and GFAP produced superior sensitivity and specificity for distinguishing TBI patients from healthy controls. Both biomarkers discriminated between TBI patients with intracranial lesions on CT scan from those without such lesions; GFAP measures were significantly more sensitive and specific than UCH-L1. The addition of UCH-L1 values marginally improved upon GFAP alone in predicting dichotomized GOS-E at 3 months after injury.
The results of this analysis of patients enrolled in the TRACK-TBI cohort are largely consistent with those of Papa et al.15,17 Slight differences in the sensitivity and specificity for each biomarker for distinguishing between TBI patients and uninjured controls and for distinguishing between TBI patients with intracranial abnormalities on CT scans from those with normal CTs are likely the result of differences between the two cohorts. Whereas both the TRACK-TBI cohort and the one studied by Papa et al. were primarily mTBI (83% vs. 90%, respectively), and the majority had normal cranial CT scans (57% vs. 70%, respectively), in each case, injury severity in TRACK-TBI was slightly higher. Another potential reason for the difference is the timing of the samples. Samples from Papa et al. were obtained within 4 h of injury versus an average of 10.9 h in the TRACK-TBI study.
These results are also consistent with those of Metting et al.,18 who studied 94 mTBI patients recruited in The Netherlands. Although that study did not analyze healthy controls, GFAP was elevated in those with abnormal cranial CT scans, as well as in those with axonal injury on MRI. ROC analysis was not utilized in that study to determine the sensitivity and specificity of GFAP measurements for abnormal cranial CT results. As in TRACK-TBI, this study did not find that GFAP levels were predictive of incomplete recovery, measured either by GOS-E or by return to work status. Metting et al.18 studied a less severely injured cohort. Only 20% had abnormal CT scans, compared with 43% abnormal CTs found in the mTBI from TRACK-TBI. Additionally, 63% of the Dutch patients had indiscernible GFAP levels (<0.045 ng/mL). In TRACK-TBI, which used a different assay of comparable sensitivity, only 23% had GFAP levels below the lower limit of detection (0.1 ng/mL).
This study extends prior findings in two important ways. First, outcome information was obtained in TRACK-TBI using the GOS-E, which was not reported in the earlier studies by Papa et al. Whereas neither biomarker has good sensitivity and specificity for identifying those TBI patients who fail to make a complete recovery (GOS-E<8), GFAP modestly outperforms UCH-L1 in this regard (AUC 0.65 vs. 0.58). Although this level of sensitivity and specificity is too low for clinical use, it may be useful in clinical research by selecting patients with predominantly mTBI who are less likely to make a complete recovery. This is essential for clinical trials of neuroprotective or neuroregenerative therapies for mTBI, as without such enrichment the required sample sizes are very high, as most mTBI patients recover fully. For predicting patients who have a poor outcome (GOS-E≤4), UCH-L1 slightly outperforms GFAP-BDP, although only adequate AUCs are achieved (0.80 and 0.74, respectively). Although only a small number of mTBI patients have a poor outcome, the ability to identify those patients in the ED is important for clinical practice and research.
The second novel finding of this study is that the consideration of both biomarkers together improves the sensitivity and specificity for TBI diagnosis compared with each considered alone. As UCH-L1 and GFAP-BDP are produced by different cell types and theoretically measure different injury mechanisms, analytical strategies that consider levels of both biomarkers may, in principle, be superior to univariate considerations. Our findings indicate that that is the case for TBI diagnosis and for prediction of poor outcome, but not for prediction of intracranial CT abnormalities or for prediction of incomplete recovery. It is possible that analytical strategies including levels of other biomarkers may be better than consideration of only UCH-L1 and GFAP-BDP alone. It is likely that the ideal TBI biomarker panel may include a handful of biomarkers, and currently available multiplex immunoassay platforms make it feasible to measure up to 10 biomarkers with a high degree of sensitivity from small volumes of plasma.
The TRACK-TBI study obtained MRI scans in a subset of patients. The initial results of those studies have been published.27 MRI is significantly more sensitive than CT for identifying structural traumatic lesions, and it outperforms CT for identifying patients unlikely to fully recover. The levels of the two biomarkers analyzed in this study, alone or in combination, do not predict recovery with the level of sensitivity and specificity required for identification of patients who may need additional interventions. It is likely that, ultimately, a multidimensional prognostic model that incorporates CT and MRI data as well as results of multiple proteomic biomarker measurements, in addition to clinical and demographic variables, will be ultimately required to develop a prognostic model that would be useful in clinical practice and clinical research.
Another limitation of this study is that biomarker levels were measured only in the acute period after injury, during the patient's evaluation in the ED. The half-life of UCH-L1 is <12 h, and whereas GFAP has a longer half-life, it is still <2 days. Many patients with mTBI present for medical attention days (or later) after injury, and biomarkers for the subacute and chronic stages after TBI are desperately needed.
This study adds to the growing body of evidence regarding the clinical utility of blood biomarkers in TBI management. Although the ideal panel of biomarkers for TBI remains to be established, the findings that UCH-L1 and GFAP-BDP have reasonable sensitivity and specificity for predicting important clinical outcomes after predominantly mTBI provides encouragement that this research will ultimately be successful and should stimulate further research in larger, well-characterized cohorts.
Contributor Information
Collaborators: the TRACK-TBI Investigators, including
Acknowledgment
This study was funded by National Institutes of Health (NIH) Grant 1RC2 NS069409.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Stein S.C. (1996). Classification of head injury, in: Neurotrauma. Narayan R.K., Povlishock J.T., and Wilberger J.E. (eds.). McGraw Hill: New York, pps. 31–42 [Google Scholar]
- 2.Thurman D.J., Coronado V., and Selassie A. (2007). The epidemiology of TBI: implications for public health, in: Brain Injury Medicine: Principles and Practice. Zasler N.D., Katz D.I., and Zafonte R.D. (eds.). Demos: New York, NY, pps. 45–55 [Google Scholar]
- 3.Narayan R.K., Michel M.E., and The Clinical Trials in Head Injury Study Group (2002). Clinical trials in head injury. J. Neurotrauma 19, 503–557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.National Center for Injury Prevention and Control Report to Congress on Mild Traumatic Brain Injury in the United States: Steps to Prevent a Serious Public Health Problem. Atlanta, GH: Centers for Disease Control and Prevention, 2003 [Google Scholar]
- 5.Bazarian J.J., McClung J., Shah M.N., Cheng Y.T., Flesher W., and Kraus J. (2005). Mild traumatic brain injury in the United States, 1998–2000. Brain Inj. 19, 85–91 [DOI] [PubMed] [Google Scholar]
- 6.Max W., MacKenzie E.J., and Rice D.P. (1991). Head injuries: costs and consequences. J. Head Trauma Rehabil. 6, 76–91 [Google Scholar]
- 7.Food and Drug Administration, Center for Drug Evaluation and Research (2010). Qualification Process for Drug Development Tools. Food and Drug Administration: Washington, DC [Google Scholar]
- 8.Vos P.E., Jacobs B., Andriessen T.M., Lamers K.J., Borm G.F., Beems T., Edwards M., Rosmalen C.F., and Vissers J.L. (2010). GFAP and S100B are biomarkers of traumatic brain injury: an observational cohort study. Neurology 75, 1786–1793 [DOI] [PubMed] [Google Scholar]
- 9.Mondello S., Papa L., Buki A., Bullock M.R., Czeiter E., Tortella F.C., Wang K.K., and Hayes R.L. (2011). Neuronal and glial markers are differently associated with computed tomography findings and outcome in patients with severe traumatic brain injury: a case control study. Crit. Care 15, R156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mondello S., Jeromin A., Buki A., Bullock R., Czeiter E., Kovacs N., Barzo P., Schmid K., Tortella F., Wang K.K., and Hayes R.L. (2012). Glial neuronal ratio: a novel index for differentiating injury type in patients with severe traumatic brain injury. J. Neurotrauma 29, 1096–1104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brophy G.M., Mondello S., Papa L., Robicsek S.A., Gabrielli A., Tepas J., , III, Buki A., Robertson C., Tortella F.C., Hayes R.L., and Wang K.K. (2011). Biokinetic analysis of ubiquitin C-terminal hydrolase-L1 (UCH-L1) in severe traumatic brain injury patient biofluids. J. Neurotrauma 28, 861–870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Czeiter E., Mondello S., Kovacs N., Sandor J., Gabrielli A., Schmid K., Tortella F., Wang K.K., Hayes R.L., Barzo P., Ezer E., Doczi T., and Buki A. (2012). Brain injury biomarkers may improve the predictive power of the IMPACT outcome calculator. J. Neurotrauma 29, 1770–1778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gong B. and Leznik E. (2007). The role of ubiquitin C-terminal hydrolase L1 in neurodegenerative disorders. Drug News Perspect. 20, 365–370 [DOI] [PubMed] [Google Scholar]
- 14.Berger R.P., Hayes R.L., Richichi R., Beers S.R., and Wang K.K. (2012). Serum concentrations of ubiquitin C-terminal hydrolase-L1 and alphaII-spectrin breakdown product 145 kDa correlate with outcome after pediatric TBI. J. Neurotrauma 29, 162–167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Papa L., Lewis L.M., Silvestri S., Falk J.L., Giordano P., Brophy G.M., Demery J.A., Liu M.C., Mo J., Akinyi L., Mondello S., Schmid K., Robertson C.S., Tortella F.C., Hayes R.L., and Wang K.K. (2012). Serum levels of ubiquitin C-terminal hydrolase distinguish mild traumatic brain injury from trauma controls and are elevated in mild and moderate traumatic brain injury patients with intracranial lesions and neurosurgical intervention. J. Trauma Acute Care Surg. 72, 1335–1344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yasuhara T., Matsukawa N., Hara K., Maki M., Ali M.M., Yu S.J., Bae E., Yu G., Xu L., McGrogan M., Bankiewicz K., Case C., and Borlongan C.V. (2009). Notch-induced rat and human bone marrow stromal cell grafts reduce ischemic cell loss and ameliorate behavioral deficits in chronic stroke animals. Stem Cells Dev. 18, 1501–1514 [DOI] [PubMed] [Google Scholar]
- 17.Papa L., Lewis L.M., Falk J.L., Zhang Z., Silvestri S., Giordano P., Brophy G.M., Demery J.A., Dixit N.K., Ferguson I., Liu M.C., Mo J., Akinyi L., Schmid K., Mondello S., Robertson C.S., Tortella F.C., Hayes R.L., and Wang K.K. (2012). Elevated levels of serum glial fibrillary acidic protein breakdown products in mild and moderate traumatic brain injury are associated with intracranial lesions and neurosurgical intervention. Ann. Emerg. Med. 59, 471–483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Metting Z., Wilczak N., Rodiger L.A., Schaaf J.M., and van der Naalt J. (2012). GFAP and S100B in the acute phase of mild traumatic brain injury. Neurology 78, 1428–1433 [DOI] [PubMed] [Google Scholar]
- 19.Saatman K.E., Duhaime A.C., Bullock R., Maas A.I., Valadka A., and Manley G.T. (2008). Classification of traumatic brain injury for targeted therapies. J. Neurotrauma 25, 719–738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zoltewicz J.S., Scharf D., Yang B., Chawla A., Newsom K.J., and Fang L. (2012). Characterization of antibodies that detect human GFAP after traumatic brain injury. Biomark. Insights 7, 71–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yue J.K., Vassar M.J., Lingsma H.F., Cooper S.R., Okonkwo D.O., Valadka A.B., Gordon W.A., Maas A.I., Mukherjee P., Yuh E.L., Puccio A.M., Schnyer D.M., Manley G.T., Casey S.S., Cheong M., Dams-O'Connor K., Hricik A.J., Knight E.E., Kulubya E.S., Menon D.K., Morabito D.J., Pacheco J.L., Sinha T.K. (2013). Transforming research and clinical knowledge in traumatic brain injury pilot: multicenter implementation of the common data elements for traumatic brain injury. J. Neurotrauma [Epub ahead of print; PMID 23815563.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jagoda A.S., Bazarian J.J., Bruns J.J., , Jr., Cantrill S.V., Gean A.D., Howard P.K., Ghajar J., Riggio S., Wright D.W., Wears R.L., Bakshy A., Burgess P., Wald M.M., and Whitson R.R. (2008). Clinical policy: neuroimaging and decisionmaking in adult mild traumatic brain injury in the acute setting. Ann. Emerg. Med. 52, 714–748 [DOI] [PubMed] [Google Scholar]
- 23.Manley G.T., Diaz–Arrastia R., Brophy M., Engel D., Goodman C., Gwinn K., Veenstra T.D., Ling G., Ottens A.K., Tortella F., and Hayes R.L. (2010). Common data elements for traumatic brain injury: recommendations from the biospecimens and biomarkers working group. Arch. Phys. Med. Rehabil. 91, 1667–1672 [DOI] [PubMed] [Google Scholar]
- 24.Duhaime A.C., Gean A.D., Haacke E.M., Hicks R., Wintermark M., Mukherjee P., Brody D., Latour L., and Riedy G. (2010). Common data elements in radiologic imaging of traumatic brain injury. Arch. Phys. Med. Rehabil. 91, 1661–1666 [DOI] [PubMed] [Google Scholar]
- 25.Wilson J.T.L., Pettigrew L.E.L., and Teasdale G.M. (1998). Structured interviews for the Glasgow Outcome Scale and the Extended Glasgow Outcome Scale: guidelines for their use. J. Neurotrauma 15, 573–585 [DOI] [PubMed] [Google Scholar]
- 26.Okonkwo D.O., Yue J.K., Puccio A.M., Panczykowski D., Inoue T., McMahon P.J., Sorani M.D., Yuh E.L., Lingsma H., Maas A., Valadka A., et al. (2013). GFAP-BDP as an acute diagnostic marker in traumatic brain injury: results from the prospective TRACK-TBI Study. J. Neurotrauma 30, 1490–1497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yuh E.L., Mukherjee P., Lingsma H.F., Yue J.K., Ferguson A.R., Gordon W.A., Valadka A.B., Schnyer D.M., Okonkwo D.O., Maas A.I., and Manley G.T. (2012). Magnetic resonance imaging improves 3-month outcome prediction in mild traumatic brain injury. Ann. Neurol. 73, 224–235 [DOI] [PMC free article] [PubMed] [Google Scholar]