Abstract
Mild Traumatic Brain Injury (mTBI), or concussion, is a major public health concern. There is controversy in the literature regarding the true incidence of postconcussion syndrome (PCS), with the constellation of physical, cognitive, emotional, and sleep symptoms after mTBI. In the current study, we report on the incidence and evolution of PCS symptoms and patient outcomes after mTBI at 3, 6, and 12 months in a large, prospective cohort of mTBI patients. Participants were identified as part of the prospective, multi-center Transforming Research and Clinical Knowledge in Traumatic Brain Injury Study. The study population was mTBI patients (Glasgow Coma Scale score of 13–15) presenting to the emergency department, including patients with a negative head computed tomography discharged to home without admission to hospital; 375 mTBI subjects were included in the analysis. At both 6 and 12 months after mTBI, 82% (n=250 of 305 and n=163 of 199, respectively) of patients reported at least one PCS symptom. Further, 44.5 and 40.3% of patients had significantly reduced Satisfaction With Life scores at 6 and 12 months, respectively. At 3 months after injury, 33% of the mTBI subjects were functionally impaired (Glasgow Outcome Scale-Extended score ≤6); 22.4% of the mTBI subjects available for follow-up were still below full functional status at 1 year after injury. The term “mild” continues to be a misnomer for this patient population and underscores the critical need for evolving classification strategies for TBI for targeted therapy.
Key words: : clinical trial, health-related quality of life, postconcussion syndrome, outcome, traumatic brain injury
Introduction
Approximately 1.7 million people sustain a traumatic brain injury (TBI) each year in the United States. An estimated 75% of TBIs are mild TBI (mTBI), though the true incidence of mTBI is most likely higher, with the Centers for Disease Control and Prevention (CDC) estimating as many as 4 million actual mTBI cases per year.1,2
The sequelae of mTBI are most commonly grouped into a constellation of symptoms termed “post-concussion syndrome” (PCS).3,4 Currently, there is no clear understanding of the evolution, duration, or resolution of PCS symptoms after mTBI, with little agreement in the literature. Though some reports have found no significant impairment in any neuropsychological function past 3 months subsequent to mTBI,5 other studies, utilizing self-reported measures of symptoms and distress, report that patients continue to experience measurable impairment up to 1 year after injury.6–9 A major cause of the absence of consensus in the field is the lack of universally adopted mTBI outcomes and the use of different measures, making direct comparisons between studies difficult. Further, mTBI may be classified into complicated and uncomplicated, with the former indicating the presence of intracranial pathology on radiographic imaging.9
Recent workshops sponsored by the National Institutes of Health (NIH) and the Department of Defense highlighted this lack of standardization in data collection as a major impediment to improved understanding of TBI diagnosis and prognosis. With broad agency support, a multi-disciplinary group of thought leaders proposed standards for data collection, referred to as the TBI common data elements (TBI-CDEs), across TBI studies. In 2009, the NIH funded the TRACK-TBI (Transforming Research and Clinical Knowledge in Traumatic Brain Injury) Study, a multi-center, prospective collaboration among four U.S. centers to develop, test, and refine the TBI-CDEs in four domains: demographics; neuroimaging; biomarkers; and outcome measures. A unique feature of the target TBI population under investigation is that it spans the entire range of TBI from mild to severe (e.g., enrollment included a large cohort of mTBI patients with negative neuroimaging results, as well mTBI patients discharged from the emergency department [ED]).
The aim of this study was to use selected CDEs to characterize prospectively the incidence and evolution of PCS symptoms and patient outcomes after mTBI at 3, 6, and 12 months in a large, prospective cohort of mTBI patients presenting to an ED. Patients were assessed using a PCS symptoms checklist as well as the Glasgow Outcome Scale-Extended (GOSE) score, Brief Symptom Inventory-18 (BSI-18), Rivermead Post-Concussion Questionnaire (RPQ), and the Satisfaction With Life Scale (SWLS). The use of individual symptoms, in addition to multiple measures of outcome over multiple time points, provided a more comprehensive assessment of global patient status, as well as the ability to capture the more subtle sequelae of mTBI.
Methods
Study population
Subjects were identified and recruited upon arrival at one of three level 1 trauma centers as part of the multi-center, prospective TRACK-TBI Study.10 Study protocols were approved by the institutional review boards of participating centers (University of California San Francisco, University of Pittsburgh Medical Center, and University Medical Center Brackenridge). All participants or their legal authorized representatives gave written informed consent. At their follow-up outcome assessments, participants previously consented by their legal authorized representative were assessed for cognizance. If appropriate, participants personally consented for continued participation in the study.
To be eligible for the TRACK-TBI Study, patients had to present within 24 h of injury and have history of trauma to the head sufficient to triage to noncontrast head computed tomography (CT) using the American College of Emergency Physicians/CDC evidence-based joint practice guideline.11 For the purposes of this study, patients were considered complicated or uncomplicated mTBI based on the presence or absence of pathology on CT scan. Details of loss of consciousness, amnesia, and source of trauma were recorded upon screening, and informed consent was obtained. Medical history collected included psychiatric history, neurologic history, previous TBI, and history of drug use, assessed as a series of “yes/no” questions, with a “yes” to any item scored as a positive history. Psychiatric history included any report of anxiety, depression, sleep problems, schizophrenia, bipolar, post-traumatic stress disorder, and “other.” Neurologic history included transient ischemic attack, seizure, epilepsy, headaches, cerebrovascular accident, vascular abnormality, multiple sclerosis, degenerative disease, encephalopathy, brain tumor, and nerve sheath tumor. At presentation, Glasgow Coma Scale (GCS) score was assessed by an emergency physician or neurosurgeon. Patients with a negative head CT in the ED who were discharged to home were also recruited into the cohort.
Data were collected from each subject during the acute hospital stay and at 3, 6, and 12 months after injury. Patient interviews were conducted by phone or face to face by a trained neuropsychological technician. Interview duration was approximately 45 min per patient.
For the current study, data were extracted from the larger TRACK-TBI database. Patient inclusion criteria were age 18 and over, an admission GCS score of 13–15, and complete PCS symptom assessment data recorded for at least 1 of the follow-up time points (3, 6, and 12 months). Those patients with a GOSE of 8 at 6 months were initially not included for 12-month follow-up in the larger database because of resource constraints as well as the low likelihood of patients worsening from the best possible outcome score. However, the study soon expanded to include follow-up with all patients at 12 months, regardless of GOSE score. Patients who died at any point during the study period were excluded.
Outcome measures
Postconcussion syndrome symptoms
Postconcussive symptoms were assessed at 3, 6, and 12 months after injury using a symptom checklist, containing 22 subjective questions classified into four domains: physical; cognitive; emotional; and sleep. Patients were questioned regarding the presence or absence of each symptom. Ten questions assessed physical symptoms, and the remaining domains were assessed with four questions each. The total number of symptoms for each patient at each follow-up point was recorded. PCS symptoms were used as a symptoms checklist only, measuring the absolute number and type of symptoms each patient experienced at each follow-up time. PCS symptoms were not used in the context of the International Classification of Diseases, Tenth Revision diagnosis of PCS.
Glasgow Outcome Scale-Extended score
The GOSE is considered the “gold standard” for assessing patient outcomes after TBI.12 Based on a structured interview, patient outcome is assigned an ordinal score of 1–8, with a score of 1 indicating “deceased” and a score of 8 being “high good” outcome.13 A GOSE score of ≥7 (good recovery), indicating a return to full functional status at work and in daily activity, was used as a cut-off value in some of the analyses. The GOSE is a reliable measure, capable of being administered over the phone or by correspondence using a standardized interview structure, with kappa scores from 0.85 to 0.89.14,15 Validity is supported by the scale's association with initial injury severity, the Disability Rating Scale, and self-reported measures of health outcomes.16 The GOSE was administered at the 3-, 6-, and 12-month time periods after injury.
Brief Symptom Inventory-18
The BSI-18 is a screening tool to assess the level of psychological distress after TBI.17 Three dimensions covered by the scale are somatization, depression, and anxiety. Each contains six questions that rate the level of distress over the past 7 days using a 4-point “Likert-type” scale, ranging from “not at all” to “extremely often.”17 The Global Severity Index (GSI) T score, calculated from the summed responses in each domain and normalized across age and sex, was utilized for this study. A maximum T score of 72 is possible, and a T score at or above 63 is considered a clinically significant level of distress and was set as the cut-off value for some of the analyses.17 The overall reliability of the GSI is high, with a kappa of 0.89 and a retest reliability kappa of approximately 0.66.17 In tests of validity, the BSI correlates significantly with other validated psychosocial and functional tests in TBI patients.17 The BSI-18 was administered at 6 and 12 months.
Rivermead Post-Concussion Questionnaire
The RPQ is a measure of severity of 16 PCS symptoms, as compared to premorbid level. Symptoms are rated on a 4-point Likert scale, ranging from “not experienced at all” to “a severe problem.”18 Because a score of 1 for any item indicates “no worse than before the injury” and was included in the total score, a cut-off value could not be utilized for this measure. The test is divided into the RPQ-3 and the RPQ-13, with the RPQ-3 items assessing headaches, nausea and/or vomiting, and dizziness that are considered to be early concussion symptoms. The RPQ-13 items assess cognitive, mood, sleep, and other physical symptoms and are considered later symptoms of PCS.18 It is recommended that the scores remain separate for analysis, because, individually, they show good test-retest reliability and construct validity, which is potentially lost if they are combined.19 The RPQ was administered at 6 and 12 months after injury.
Satisfaction With Life Scale
The SWLS is a series of five statements that assess current patient life satisfaction. Patients rank each question from 1 (“strongly disagree”) to 7 (“strongly agree”).20 Responses are summed, with a maximum score of 35 indicating “extremely satisfied” and a score of 20 indicating neutral feelings. The average score for economically developed nations is within the range of 20–24, indicating general satisfaction, but with desire for improvement in some areas.20 A cut-off value of 19 or below was used to indicate significant dissatisfaction. The SWLS is considered to have good reliability and validity and is believed to be an accurate measure of general, overall well-being.21 The SWLS was administered at 6 and 12 months after injury.
Statistical analysis
Data analysis was performed using the STATA 12 software package (2011; StataCorp LP, College Station, TX). Parametric or nonparametric analysis was performed, where appropriate, with a significance level set at (p<0.05). Patient demographic data included age, sex, and initial GCS. The difference between total PCS symptoms at 3 and 6, 6 and 12, and 3 and 12 months was calculated. Similar calculations were performed for the BIS-18, the SWLS, and the RPQ at 6 and 12 months. Analysis of change between follow-ups only included patients with data for both time points. Interactions between outcome measures and demographics were assessed. For all measures except the GOSE score, an unpaired one-sample, two-tailed t-test was used to test for significant change in score between time points. Wilcoxon's signed-rank test was used to analyze the difference in GOSE score from 3 to 6, 6 to 12, and 3 to 12 months. McNemar's chi-square test was used for analysis of dichotomized scores, using the cut-off values mentioned previously. Additional analysis was performed comparing those patients with positive versus negative findings on CT scan as well as the subset of patients with complete PCS symptom data at all three follow-up time points using an unpaired one-sample, two-tailed t-test or Wilcoxon's signed-rank test, when appropriate.
Results
Postconcussion syndrome symptoms
A total of 375 patients met inclusion criteria. The population was 70.1% male, with a mean age of 44 years (range, 18–94). Approximately 38% of patients were between 20 and 40 years of age, 34% were between 40 and 60, and 19% were between 60 and 80. The majority of patients had a GCS of 15 (75.7%). Approximately 44% of the population (total, 165) was considered complicated mTBI, having positive findings on CT scan. Patients with positive CT scans were significantly older than patients with negative CT scans (48.7±19.2 and 40.4±16.5 years, respectively). GCS score was not significantly different between CT-positive and CT-negative patients. Mean Injury Severity Score (ISS), calculated only for those patients admitted to the hospital, was 9.37±9.72 (total, 279). ISS was significantly higher for CT-positive patients (16.5±8.39), compared to CT negative patients (3.8±6.6; Table 1). Patient medical history revealed approximately 30% of the population with a history of psychiatric problems (total, 114), 22% with a previous neurological condition (total, 81), 52% with a previous TBI (total, 195), and 20% with a history of drug use (total, 75). Patient histories were examined in the context of radiographic findings as well (Table 1). The number of patients with psychiatric history was not significantly different between CT-positive and CT-negative patients; however, there were significantly more patients with neurologic history (p<0.001), previous TBI (p<0.001), and history of drug use (p<0.001) among the CT-negative patients. Analysis of age, gender, and GCS revealed no significant interaction with PCS symptoms or any outcome scores. Age specifically did not significantly correlate with PCS symptoms or outcome, except for a negative correlation with the GOSE at 3 months. Complete PCS symptom data were recorded for 348 (92.8%) of patients at 3 months, 305 (81.3%) at 6 months, and 199 (53.1%) at 12 months.
Table 1.
Demographics of the TRACK-TBI Patient Population Included in the Study
| Demographics | CT negative | CT positive | Total |
|---|---|---|---|
|
N, % (n) |
56 (210) |
45.2 (165) |
375 |
| Age | |||
| Mean (SD) |
40.4 (16.5) |
48.7 (19.2)* |
44 (18.2) |
| Min-Max |
18–94 |
18–93 |
18–94 |
| Gender, % (n) | |||
| Male |
67.1 (141) |
73.9 (122) |
70.1 (263) |
| Female |
32.9 (69) |
26.1 (43) |
29.9 (112) |
| Admission GCS, % (n) | |||
| 13 |
2.4 (5) |
4.8 (8) |
3.47 (13) |
| 14 |
21.9 (46) |
19.4 (32) |
20.8 (78) |
| 15 |
75.7 (159) |
75.8 (125) |
75.7 (284) |
| ISS | |||
| Mean (SD) |
16.5 (8.4)* |
3.8 (6.6) |
9.4 (9.7) |
| Patient history, % (n) | |||
| Psychiatric |
59.6 (68) |
40.4 (46) |
30.4 (114) |
| Neurologic |
72.8 (59)* |
27.2 (22) |
21.6 (81) |
| Previous TBI |
68.7 (134)* |
31.3 (61) |
52.0 (195) |
| Drug use | 73.3 (55)* | 26.7 (20) | 20.0 (75) |
p<0.05.
TRACK-TBI, Transforming Research and Clinical Knowledge in Traumatic Brain Injury Study; SD, standard deviation; Min, minimum; Max, maximum; GCS, Glasgow Coma Scale; TBI, traumatic brain injury; CT, computed tomography.
Average total and by-domain PCS symptoms at each follow-up time point are shown in Table 2. At 3 months after mTBI, 77% (n=348) of patients reported at least one PCS symptom. At both 6 and 12 months postinjury, 82% of the sample was reporting at least one symptom (n=250 and n=163, respectively). Average reported PCS symptoms increased significantly from 3 to 6 months (n=278; p<0.001) and from 3 to 12 months (n=181; p<0.001), with approximately 67% of patients at either interval experiencing no change or an increase in the number of symptoms. Although average PCS symptoms decreased significantly from 6 to 12 months (n=188; p<0.001), approximately 50% of patients still experienced no change or an increase in symptoms across this same time (Table 2).
Table 2.
Mean Symptoms Reported and Percentage Reporting More Than One Symptom for All Patients at Each Follow-Up Time, by Total PCS Symptoms and Each PCS Functional Domain
| |
3 months (N=348) |
6 months (N=305) |
12 months (N=199) |
|||
|---|---|---|---|---|---|---|
| PCS symptom domain | Mean (SD) | %≥1 symptom | Mean (SD) | %≥1 symptom | Mean (SD) | %≥1 symptom |
| Total PCS symptoms |
5.8 (5.8) |
74.7 |
7.3 (6.1)* |
82.4 |
6.8 (6.0)*† |
81.2 |
| Physical symptoms |
2.4 (2.6) |
67.0 |
2.9 (2.8)* |
71.8 |
2.8 (2.8)*† |
69.4 |
| Cognitive symptoms |
1.4 (1.6) |
49.4 |
1.7 (1.7)* |
59.3 |
1.7 (1.7)† |
62.8 |
| Emotional symptoms |
1.1 (1.5) |
43.4 |
1.5 (1.5)* |
56.7 |
1.3 (1.5)* |
49.3 |
| Sleep symptoms | 1.0 (1.1) | 50.2 | 1.3 (1.2)* | 60.3 | 1.1 (1.2)* | 53.5 |
p<0.05, compared to immediately previous follow-up time; †p<0.05, compared to 3-month follow-up time.
PCS, postconcussion syndrome; SD, standard deviation.
Analysis of mean number of reported PCS symptoms by presence or absence of pathology on CT scan revealed no significant difference at 3 months. However, mean PCS symptoms reported were significantly higher for CT-negative patients, compared to CT-positive patients, at both 6 (8.0±6.1 and 6.36±5.9, respectively; p=0.019) and 12 months (7.6±6.1 and 5.85±5.76, respectively; p=0.042). Mean symptoms reported by those with positive CT scans was stable across all three follow-up times, whereas patients with negative CT scans had significantly increased symptoms at 6 and 12 months, compared to 3 months (Fig. 1). Upon removal of those patients with positive medical histories (psychiatric problems, neurologic problems, previous TBI, or drug use), there was no significant difference in mean reported symptoms between CT-positive and CT-negative patients at any time. Analysis of those patients with negative CT scans and negative medical histories revealed a nonsignificant trend of increasing mean reported symptoms from 3 to 6 months, though sample size was markedly reduced (<30 patients).
Fig. 1.
Mean number of reported PCS symptoms at 3-, 6-, and 12-month follow-up by CT-positive and CT-negative patients. Significantly more symptoms were reported by CT-negative patients, compared to CT-positive patients at both 6 (8.0±6.1 and 6.4±5.9, respectively; p=0.019) and at 12 months (7.6±6.1 and 5.9±5.8, respectively; p=0.04), as indicated by the black diamond. PCS, postconcussion syndrome; CT, computed tomography.
Of the 170 patients in this mTBI cohort with complete symptom data at all three follow-up times, 110 (64.7%) had documented PCS symptomatology at all three follow-up points. When mean PCS symptoms in this subgroup were compared between CT-positive patients (total, 93) and CT-negative patients (total, 77), no significant difference was observed for any follow-up time. Further analysis of those patients without any positive medical history revealed no significant difference in mean reported PCS symptoms at any follow-up time between CT-positive and CT-negative patients. These patients reported mean PCS symptoms of 3.15±4.3 at 3 months, 4.4±4.6 at 6 months, and 2.8±4.5 at 12 months.
Evolution of PCS symptoms by domain was also examined. Physical symptoms increased significantly from 3 to 6 (p<0.001) and from 3 to 12 months (p=0.009). Sleep and emotional symptoms significantly increased from 3 to 6 months (p<0.001) and significantly decreased from 6 to 12 months (p<0.001 and p=0.002, respectively), with the reported number of symptoms at 12 months no different from those at 3 months. Cognitive symptoms significantly increased from 3 to 6 months (p<0.002) and remained stable in the 6- to 12-month interval. Overall, cognitive symptoms remained significantly higher at 12 months than they were at 3 months (p=0.02; Table 2).
Glasgow Outcome Scale-Extended score
Global functional outcome in this prospective TRACK-TBI study cohort was assessed using the GOSE. One third of patients had a GOSE ≤6 (upper moderate recovery or worse) at both 3 and 6 months post-mTBI (Table 3). At 12 months postinjury, 22.4% of patients had still not returned to full preinjury functional status (GOSE ≤6; Fig. 2).
Table 3.
Mean Score and Percent of Patients with Clinically Significant Impairment, Distress or Dissatisfaction for the GOSE, BSI, SWLS, and RPQ-13, Respectively, (and Where Applicable) at Each Follow-Up Time Point
| Outcome and time | Mean (SD) | % with clinically poorer outcomes |
|---|---|---|
| GOSE at 3 months |
6.9 (1.1) |
33.2 |
| GOSE at 6 months |
6.9 (1.0) |
33.8 |
| GOSE at 12 months |
7.1 (1.0) |
22.4 |
| BSI-18 at 6 months |
55.6 (10.7) |
29.6 |
| BSI-18 at 12 months |
51.8 (11.1) |
21.1 |
| SWLS at 6 months |
20.3 (8.0) |
44.5 |
| SWLS at 12 months |
22.0 (8.2) |
40.3 |
| RPQ-13 at 6 months |
14.6 (12.2) |
n/a |
| RPQ-13 at 12 months | 11.7 (11.3) | n/a |
Mean (SD) scores reflect only those patients with data available for all applicable follow-up times. Percent scores include the total number of patients at each follow-up time.
GOSE, Glasgow Outcome Scale-Extended; BSI, Brief Symptom Inventory; SWLS, Satisfaction With Life Scale; RPQ, Rivermead Post-Concussion Questionnaire; SD, standard deviation; n/a, not available.
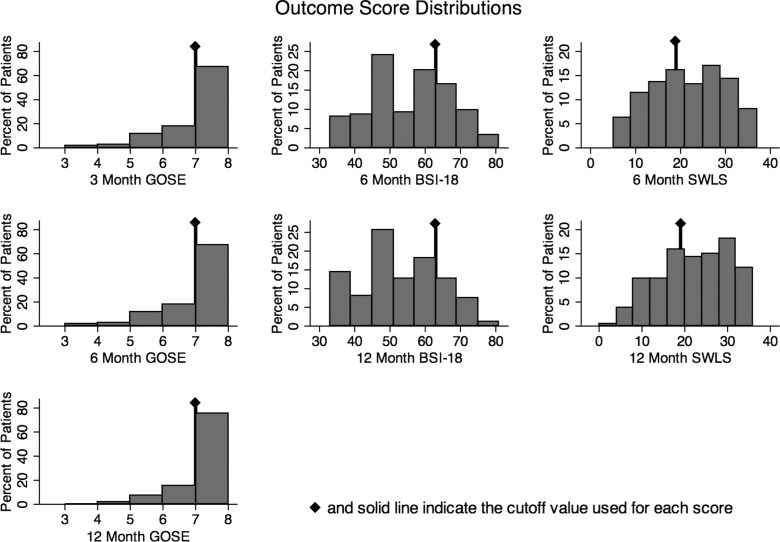
Fig. 2.
Graphs display the percent distribution of patients with each GOSE, BSI-18, or SWLS score, respectively. Solid line indicates the cut-off value used for each score, indicating clinically significant impairment (GOSE ≤6), distress (BSI-18 ≥63), or dissatisfaction (SWLS ≤19). GOSE, Glasgow Outcome Scale-Extended; BSI, Brief Symptom Inventory; SWLS, Satisfaction With Life Scale.
Analysis of the GOSE score using the cut-off value of ≥7 (i.e., good recovery) revealed no significant change in global functional outcome from 3 to 6 months after injury. However, there was a significant improvement from 6 to 12 months (n=185; p=0.002) and overall from 3 to 12 months (n=190; p<0.001), as indicated by a shift from moderate to good recovery on the GOSE scale.
Stratification of patients by CT findings resulted in a significant difference in GOSE score at 3 months, with a greater proportion of CT positive patients with worse scores (p<0.001). There was no significant difference in GOSE scores between CT-positive and CT-negative patients at 6 or 12 months. In both the subgroup with complete follow-up data as well as after removing patients with positive medical history, no significant difference was found between CT-positive and CT-negative patients on GOSE scores at any follow-up time.
Brief Symptom Inventory-18, Satisfaction with Life Scale, and Rivermead Post-Concussion Questionnaire-13
Mean outcome scores for BSI-18, SWLS, and RPQ-13 at 6 and 12 months after mTBI are displayed in Table 3. Analysis of the BSI-18 and the SWLS, as dichotomous scores based on cut-off values, revealed that many patients reported significant levels of distress (BSI-18 ≥63) and dissatisfaction with life (SWLS ≤19) at both 6 and 12 months after injury. However, there were significant improvements in patients over time for BSI-18 and SWLS. At 6 months, 29.6% of patients reported significant distress on the BSI-18 score and 44.5% of patients had a clinically significant dissatisfaction with life per the SWLS score. By 12 months, these scores were marginally, but significantly, reduced to 21.1 and 40.3% for BSI-18 and SWLS, respectively (Fig. 2). The change from 6 to 12 months indicated a significant improvement in patient-reported health-related quality of life on both the BSI-18 (n=159; p=0.01) and the SWLS (n=162; p<0.01).
The change in mean RPQ-13 score from 6 to 12 months indicated a significant reduction in the severity of the symptoms experience (n=165; p<0.001). However, the total RPQ-13 scores at both 6 and 12 months after injury indicated that patients in this mTBI cohort were still experiencing elevated, problematic symptomatology as a direct result of their TBI. The RPQ-3 is the only measure that did not significantly improve between the 6- and 12-month intervals.
Analysis by CT scan findings revealed a significant difference on the BSI-18 score at 6 months between CT-positive and CT-negative patients (53.2±10.8 and 56.8±12, respectively; p=0.013). No significant difference was found between patients with positive and negative CT scans at 12 months for the BSI-18 or for the SWLS at 6 or 12 months. There was a significant difference between CT-positive and CT-negative patients on the RPQ-13 at 6 months (11.9±11.5 and 15.1±12.8, respectively; p=0.035) and the RPQ-3 at 12 months (1.9±2.3 and 2.8±2.9, respectively; p=0.019). Analysis of the subgroup with complete follow-up data revealed no significant difference at 6 or 12 months for the BSI-18, SWLS, or RPQ. After removing those patients with positive medical histories, there was no significant difference between CT-positive and CT-negative patients for any outcome at any follow-up time.
Discussion
The aim of this prospective TRACK-TBI cohort study was to characterize the presence and evolution of PCS symptoms and patient outcomes after mTBI. The results underscore the substantial public health effect of mTBI, because at all follow-up intervals through 1 year after injury, more than three quarters of the sample reported at least one PCS symptom. One third of patients failed to return to full functional status at 3 and 6 months, with 22% of patients having impaired functional status at 1 year (GOSE ≤6). At 1 year after injury, 30% of patients reported dissatisfaction with their overall well-being (SWLS ≤19). These findings appear to be independent of the presence or absence intracranial pathology.
Analysis by PCS symptom domain revealed a trend similar to that of total PCS symptoms. By 1 year, both sleep and emotional symptoms had returned to levels reported at 3 months. However, physical and cognitive symptoms remained elevated at 1 year, with cognitive symptoms showing no sign of improvement overall. The persistence of cognitive symptoms is consistent with the literature, but the lack of improvement by 12 months is not.22,23 A single functional domain was not driving the observed trend in symptomatology from 3 to 6 months, because all four domains followed this pattern. Examining symptoms in the context of CT scan findings, patients with positive CT scans had relatively stable mean PCS symptoms reported across all time points, whereas patients with negative CT scans had significant changes in mean PCS symptoms reported and appeared to drive the overall trend observed. Further, this trend of increasing symptoms from 3 to 6 months remained, looking at only those patients without positive medical histories. Though this was not a significant trend, sample size was markedly reduced in this analysis. Also, those patients without significant medical histories or findings on imaging were still reporting approximately three PCS symptoms, on average, at 12 months. These findings emphasize that recovery from mTBI should be tracked past the conventional 12-month recovery point, regardless of CT findings and perhaps medical history as well.
The GOSE was the only objective outcome measure recorded at the 3-month follow-up, at which time 33% of the patients had failed to regain full functional status. No significant change was noted on the GOSE from 3 to 6 months. Taken in context with the increase in PCS symptoms over the same time period, patients' condition may, in fact, be worsening, and the GOSE is not sensitive to this change in function. The overall GOSE score did improve by 12 months after injury, but nearly one quarter of patients continued to experience impairment at work or with daily activities. Patients with positive CT scans did have worse GOSE scores at 3 months; however, this difference was not appreciated at 6 or 12 months overall or for any other patient subset. Although the GOSE scores at 3 months were consistent with previous reports, as Sigurdardottir and colleagues reported, 30% of mTBI patients with GOSE scores of <7 at 3 months, the nearly one quarter of patients at 1 year experiencing persistent functional impairment has not been fully appreciated in the literature.23
The BSI-18, SWLS, and RPQ-13 measures likewise all indicated persistent changes in function secondary to mTBI in a substantial percentage of the cohort up to 1 year after injury. At 6 months after injury, almost one third of patients had clinically significant levels of distress on the BSI-18 (score, ≥63) and nearly one half of patients experienced clinically significant dissatisfaction with life on the SWLS (score, ≤19). Although there was improvement in the measures by 12 months, 21 and 40% of patients continued to have clinically significant distress or dissatisfaction scores on the BSI and SWLS, respectively. These results are similar to a previous report of BSI-18 indicating 47% of mTBI patients suffering clinically significant distress; however, the follow-up period of the study was anywhere from 6 months to 15 years after injury.17 A mean SWLS score of 21 at 1 year for this study actually indicated increased satisfaction, compared to a previous reported SWLS mean of 17 by Williams and colleagues; however, they included moderate and severe TBI patients in their study.24 On average, at both follow-up times, though the BSI-18 and SWLS scores fell below respective cut-off points of clinical significance, they were still relatively close to their cut-off values. Further, the RPQ-13 scores at both times indicate that patients were experiencing elevated, problematic symptomatology that they ascribed to their injury. Overall outcome trends held regardless of radiographic evidence of intracranial injury, and, in some instances, patients with a negative CT scan had significantly worse outcomes, compared to patients with positive CT scans. Considered together, these findings suggest that even those patients whose outcome scores did not meet a clinically significant level of impairment were nonetheless experiencing an elevated level of distress, dissatisfaction, and/or concussion-related problematic symptomatology at 1 year after mTBI.
Finding no consistent significant difference in reported symptoms or overall outcomes between CT-positive and CT-negative patients was unexpected, because previous reports indicate that patients with CT-positive findings have outcomes closer to moderate TBI patients.9 Though the GOSE scores were worse at 3 months for CT-positive patients, at both 6 and 12 months, GOSE scores were comparable. Further, CT-negative patients had worse 6-month BSI-18 scores as well as significantly increased PCS symptoms reported at both 6 and 12 months. This is despite the fact that CT-positive patients had significantly higher ISS scores. CT-negative patients did, however, have significantly increased rates of neurologic problems, previous TBIs, and drug use, which may contribute to increased symptomatology and worse BSI-18 scores. After examining only those patients with negative medical histories, there was no significant difference between the CT-positive and CT-negative groups on any outcome measure.
Limitations of this study include the inherent weakness of patient self-report outcome scales. This may have introduced increased variability, based on the subjective nature of each patient's experience. Further, PCS symptoms are not necessarily unique to mTBI, which is the source of some controversy, and we lacked a non-head-injury trauma control group with which to compare our findings.25 Whereas Dikmen and colleagues reported increased symptoms in mTBI patients, compared to trauma controls, at 1 year, Ettenhofer and Abeles reported no difference in symptoms between mTBI patients and orthopedic injury controls at 3 months to 6 years postinjury.26,27 Therefore, it must be considered that, to some degree, the symptoms and level of impairment reported may be a result of other health or life issues, unrelated to the mTBI. The use of multiple measures of outcome does help control some of this variability, including the RPQ, which measures change of severity of symptoms since before injury. Another potential issue is loss to follow-up of patients between time points. At 12-month follow-up, only 53.1% of the 375 patients had PCS symptom data recorded. This is, in part, a result of the initial exclusion at 12 months of patients with a GOSE score of 8 at 6 months, but also possibly a result of patients with the best outcomes opting to discontinue follow-up on their own. To help adjust for this, all analysis of change in score across time points only included those patients with data for all applicable times and separate analysis was performed on a subgroup of patients with complete PCS symptom follow-up data for all three follow-up times. Further, each patient presented to an ED and met criteria to receive a CT scan because of their injury.11 This may lead to bias toward worse outcomes, simply because the patients are more severely injured or symptomatic overall at initial evaluation. Last, no analysis of the potential contribution of patient medical history on outcome was performed.
The results of this study indicate that recovery from mTBI is a nonlinear process and the time course to full recovery for some may be protracted. This supports the possibility that, in a subpopulation of patients, full recovery may not ever be achieved.28 After mTBI, symptoms continue to emerge for months subsequent to injury, persisting longer than 1 year, with some symptoms, such as cognitive symptoms, failing to show any trend toward improvement. Even at 1-year follow up, anywhere from 20% to over 30% of patients continue to experience clinically significant sequelae and over 80% remain symptomatic. These outcomes appear independent of complicated or uncomplicated mTBI status. Because of this continued symptomatology, it may be useful to examine the role of unique factors of mTBI, such as the mechanism of injury, more detailed imaging results, or specific patient characteristics, such as past medical and psychosocial history or current medications, all of which may contribute to the course of and recovery from the injury. The findings of this study suggest that there may be subgroups of mTBI patients worth explicating, such that prognostic models of mTBI can be constructed. Developing a standard set of measures, performed at more regular intervals over a longer period of time, would also greatly benefit our understanding of the recovery from mTBI. What is becoming clear though, is that “mild” is indeed a misnomer for this disease, because many patients experience significant and persistent symptoms. For these patients, mTBI is anything but mild.
Contributor Information
Collaborators: the TRACK-TBI investigators including
Acknowledgments
The authors thank the The Doris Duke Charitable Foundation, The National Institute of Mental Health, and Jamie Pardini, PhD. This study is registered under ClinicalTrials.gov (identifier: NCT01565551) and was funded by the National Institutes of Health (grant no.: 1RC2 NS069409).
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Coronado V.G., Xu L., Basavaraju S.V., McGuire L.C., Wald M.M., Faul M.D., Guzman B.R.,and Hemphill J.D. (2011). Surveillance for traumatic brain injury-related deaths—United States, 1997–2007. MMWR Surveill. Summ. 60, 1–32 [PubMed] [Google Scholar]
- 2.Kay T., Harrington D.E., Adams R., Anderson T., Berrol S., Cicerone K., Dahlberg C., Gerber D., Goka R., Harley P., Hilt J., Horn L., Lehmkuhl D., and Malec J. (1993). Definition of mild traumatic brain injury. J. Head Trauma Rehabil. 8, 86–87 [Google Scholar]
- 3.Gouvier W.D., Uddo-Crane M., and Brown L.M. (1988). Base rates of post-concussional symptoms. Arch. Clin. Neuropsychol. 3, 273–278 [PubMed] [Google Scholar]
- 4.Ryan L.M., and Warden D.L. (2003). Post concussion syndrome. Int. Rev. Psychiatry 15, 310–316 [DOI] [PubMed] [Google Scholar]
- 5.Rohling M.L., Binder L.M., Demakis G.J., Larrabee G.J., Ploetz D.M., and Langhinrichsen-Rohling J. (2011). A meta-analysis of neuropsychological outcome after mild traumatic brain injury: re-analyses and reconsiderations of Binder et al. (1997), Frencham et al. (2005), and Pertab et al. (2009). Clin. Neuropsychol. 25, 608–623 [DOI] [PubMed] [Google Scholar]
- 6.Ingebrigtsen T., Waterloo K., Marup-Jensen S., Attner E., and Romner B. (1998). Quantification of post-concussion symptoms 3 months after minor head injury in 100 consecutive patients. J. Neurol. 245, 609–612 [DOI] [PubMed] [Google Scholar]
- 7.Hou R., Moss-Morris R., Peveler R., Mogg K., Bradley B.P., and Belli A. (2012). When a minor head injury results in enduring symptoms: a prospective investigation of risk factors for postconcussional syndrome after mild traumatic brain injury. J. Neurol. Neurosurg. Psychiatry 83, 217–223 [DOI] [PubMed] [Google Scholar]
- 8.Sigurdardottir S., Andelic N., Roe C., Jerstad T., and Schanke A.K. (2009). Post-concussion symptoms after traumatic brain injury at 3 and 12 months post-injury: a prospective study. Brain Inj. 23, 489–497 [DOI] [PubMed] [Google Scholar]
- 9.Kashluba S., Hanks R.A., Casey J.E., and Millis S.R. (2008). Neuropsychologic and functional outcome after complicated mild traumatic brain injury. Arch. Phys. Med. Rehabil. 89, 904–911 [DOI] [PubMed] [Google Scholar]
- 10.Yue J.K., Vass M.J., Lingsma H.F., Cooper S.R., Okonkwo D.O., Veladka A.B., Gordon W.H., Maas A.I., Mukherjee P., Yuh E.L., Poccio A.M., Schnyer D.M., Manley G.T., Casey S.S., Cheong M., Dems-O'Connor K., Hricik A.J., Kinght E.E., Kulubya E.S., Menon D.K., Morabito D.J., Pacheco J.L., Sinha T.K. (2013). Transforming research and clinical knowledge in traumatic brain injury pilot: Multicenter implementation of the common data elements for traumatic brain injury. J. Neurotrauma (Epub ahead of print). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jagoda A.S., Bazarian J.J., Bruns J.J., Jr., Cantrill S.V., Gean A.D., Howard P.K., Ghajar J., Riggio S., Wright D.W., Wears R.L., Bakshy A., Burgess P., Wald M.M., and Whitson R.R.; American College of Emergency Physicians; Centers for Disease Control and Prevention (2008). Clinical policy: neuroimaging and decisionmaking in adult mild traumatic brain injury in the acute setting. Ann. Emerg. Med. 52, 714–748 [DOI] [PubMed] [Google Scholar]
- 12.Shukla D., Devi B.I., and Agrawal A. (2011). Outcome measures for traumatic brain injury. Clin. Neurol. Neurosurg. 113, 435–441 [DOI] [PubMed] [Google Scholar]
- 13.Nichol A.D., Higgins A.M., Gabbe B.J., Murray L.J., Cooper D.J., and Cameron P.A. (2011). Measuring functional and quality of life outcomes following major head injury: common scales and checklists. Injury 42, 281–287 [DOI] [PubMed] [Google Scholar]
- 14.Wilson J.T., Pettigrew L.E., and Teasdale G.M. (1998). Structured interviews for the Glasgow Outcome Scale and the extended Glasgow Outcome Scale: guidelines for their use. J. Neurotrauma 15, 573–585 [DOI] [PubMed] [Google Scholar]
- 15.Pettigrew L.E., Wilson J.T., and Teasdale G.M. (2003). Reliability of ratings on the Glasgow Outcome Scales from in-person and telephone structured interviews. J. Head Trauma Rehabil. 18, 252–258 [DOI] [PubMed] [Google Scholar]
- 16.Wilson J.T., Pettigrew L.E., and Teasdale G.M. (2000). Emotional and cognitive consequences of head injury in relation to the glasgow outcome scale. J. Neurol. Neurosurg. Psychiatry 69, 204–209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Meachen S.J., Hanks R.A., Millis S.R., and Rapport L.J. (2008). The reliability and validity of the brief symptom inventory-18 in persons with traumatic brain injury. Arch. Phys. Med. Rehabil. 89, 958–965 [DOI] [PubMed] [Google Scholar]
- 18.King N.S., Crawford S., Wenden F.J., Moss N.E., and Wade D.T. (1995). The Rivermead Post Concussion Symptoms Questionnaire: a measure of symptoms commonly experienced after head injury and its reliability. J. Neurol. 242, 587–592 [DOI] [PubMed] [Google Scholar]
- 19.Eyres S., Carey A., Gilworth G., Neumann V., and Tennant A. (2005). Construct validity and reliability of the Rivermead Post-Concussion Symptoms Questionnaire. Clin. Rehabil. 19, 878–887 [DOI] [PubMed] [Google Scholar]
- 20.Diener E., Emmons R.A., Larsen R.J., and Griffin S. (1985). The Satisfaction With Life Scale. J. Pers. Assess. 49, 71–75 [DOI] [PubMed] [Google Scholar]
- 21.Pavot W., Diener E., Colvin C.R., and Sandvik E. (1991). Further validation of the Satisfaction with Life Scale: evidence for the cross-method convergence of well-being measures. J. Pers. Assess. 57, 149–161 [DOI] [PubMed] [Google Scholar]
- 22.Pagulayan K.F., Temkin N.R., Machamer J., and Dikmen S.S. (2006). A longitudinal study of health-related quality of life after traumatic brain injury. Arch. Phys. Med. Rehabil. 87, 611–618 [DOI] [PubMed] [Google Scholar]
- 23.Sigurdardottir S., Andelic N., Roe C., and Schanke A.K. (2009). Cognitive recovery and predictors of functional outcome 1 year after traumatic brain injury. J. Int. Neuropsychol. Soc. 15, 740–750 [DOI] [PubMed] [Google Scholar]
- 24.Williams M.W., Rapport L.J., Hanks R.A., Millis S.R., and Greene H.A. (2013). Incremental validity of neuropsychological evaluations to computed tomography in predicting long-term outcomes after traumatic brain injury. Clin. Neuropsychol. 27, 356–375 [DOI] [PubMed] [Google Scholar]
- 25.Donnell A.J., Kim M.S., Silva M.A., and Vanderploeg R.D. (2012). Incidence of postconcussion symptoms in psychiatric diagnostic groups, mild traumatic brain injury, and comorbid conditions. Clin. Neuropsychol. 26, 1092–1101 [DOI] [PubMed] [Google Scholar]
- 26.Dikmen S., Machamer J., Fann J.R., and Temkin N.R. (2010). Rates of symptom reporting following traumatic brain injury. J. Int. Neuropsychol. Soc. 16, 401–411 [DOI] [PubMed] [Google Scholar]
- 27.Ettenhofer M.L., and Abeles N. (2009). The significance of mild traumatic brain injury to cognition and self-reported symptoms in long-term recovery from injury. J. Clin. Exp. Neuropsychol. 31, 363–372 [DOI] [PubMed] [Google Scholar]
- 28.Ruff R.M. (2011). Mild traumatic brain injury and neural recovery: rethinking the debate. NeuroRehabilitation 28, 167–180 [DOI] [PubMed] [Google Scholar]