Abstract
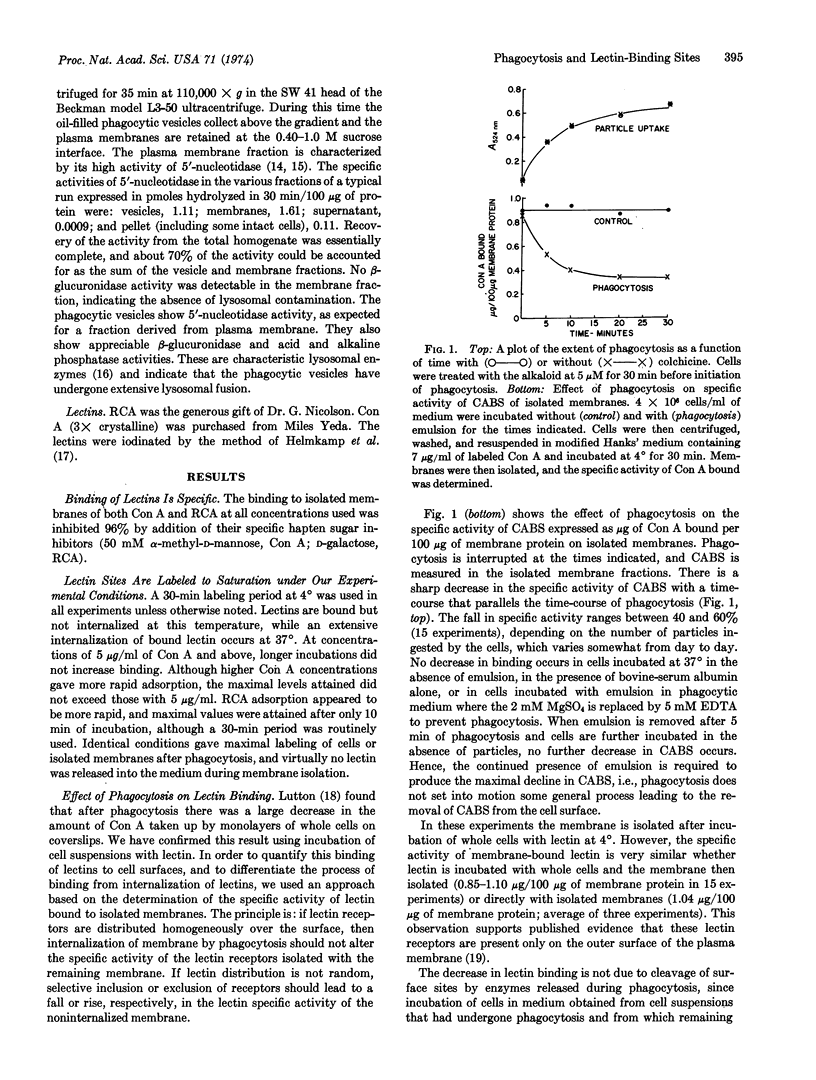
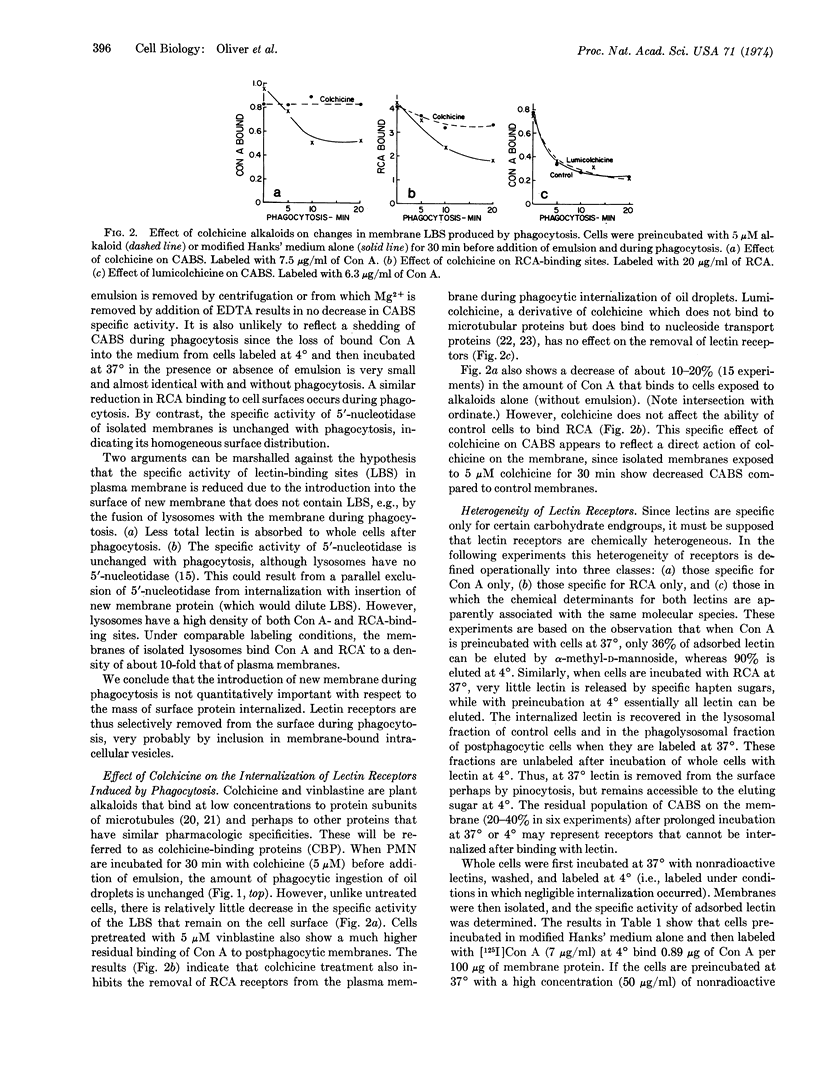
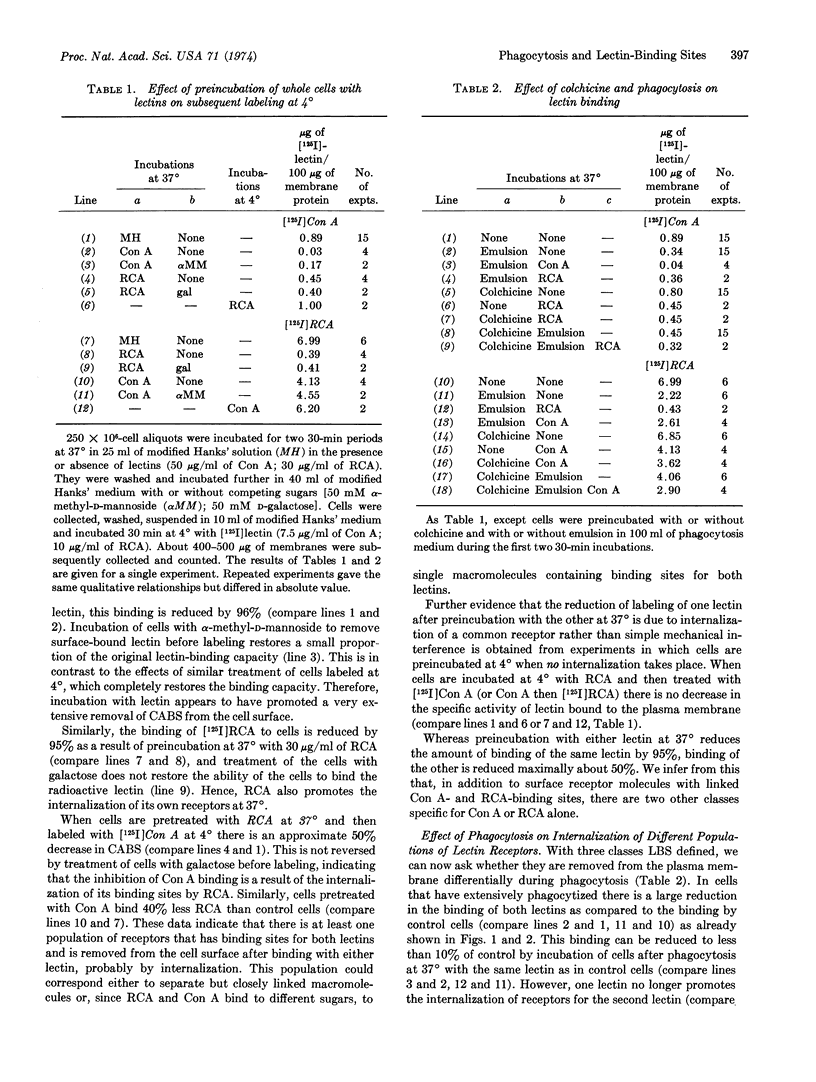
The effect of phagocytosis on lectin binding to plasma membranes of polymorphonuclear leukocytes was examined. The specific activities of binding sites of concanavalin A and Ricinus communis agglutinin (defined as μg of lectin bound per 100 μg of membrane protein) were measured on isolated membranes; they decreased in parallel with phagocytosis. Our data suggest that this removal occurs by concentration of binding sites into internalized membrane. Colchicine and vinblastine, which did not inhibit phagocytosis, prevented the selective removal of lectin-binding sites from the surface. It was also shown that at 37° lectins induce their own internalization. This property was used to define operationally three classes of lectin receptors, one of which is most extensively removed from plasma membrane during phagocytosis. Based on other morphological studies in which it is shown that before phagocytosis the surface distribution of concanavalin-binding sites is random, it is inferred that phagocytosis alters surface topography by inducing the selective movement of binding sites into membrane undergoing internalization and that colchicine-sensitive proteins are essential for this imposed topographical reorganization.
Keywords: polymorphonuclear leukocytes, concanavalin A, Ricinus communis agglutinin
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berlin R. D. Effect of concanavalin A on phagocytosis. Nat New Biol. 1972 Jan 12;235(54):44–45. doi: 10.1038/newbio235044a0. [DOI] [PubMed] [Google Scholar]
- Berlin R. D. Temperature dependence of nucleoside membrane transport of rabbit alveolar macrophages and polymorphonuclear leukocytes. J Biol Chem. 1973 Jul 10;248(13):4724–4730. [PubMed] [Google Scholar]
- Berlin R. D., Ukena T. E. Effect of colchicine and vinblastine on the agglutination of polymorpho-nuclear leucocytes by concanavalin A. Nat New Biol. 1972 Jul 26;238(82):120–122. doi: 10.1038/newbio238120a0. [DOI] [PubMed] [Google Scholar]
- Brittinger G., Hirschhorn R., Douglas S. D., Weissmann G. Studies on lysosomes. XI. Characterization of a hydrolase-rich fraction from human lymphocytes. J Cell Biol. 1968 May;37(2):394–411. doi: 10.1083/jcb.37.2.394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DePierre J. W., Karnovsky M. L. Plasma membranes of mammalian cells: a review of methods for their characterization and isolation. J Cell Biol. 1973 Feb;56(2):275–303. doi: 10.1083/jcb.56.2.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edelman G. M., Yahara I., Wang J. L. Receptor mobility and receptor-cytoplasmic interactions in lymphocytes. Proc Natl Acad Sci U S A. 1973 May;70(5):1442–1446. doi: 10.1073/pnas.70.5.1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gyure W. L. A protein precipitant useful in pteridine and purine analysis. Anal Biochem. 1972 Nov;50(1):309–312. doi: 10.1016/0003-2697(72)90508-8. [DOI] [PubMed] [Google Scholar]
- HELMKAMP R. W., GOODLAND R. L., BALE W. F., SPAR I. L., MUTSCHLER L. E. High specific activity iodination of gamma-globulin with iodine-131 monochloride. Cancer Res. 1960 Nov;20:1495–1500. [PubMed] [Google Scholar]
- KAISER H. K., WOOD W. B., Jr Studies on the pathogenesis of fever. X. The effect of certain enzyme inhibitors on the production and activity of leucocvtic pvrogen. J Exp Med. 1962 Jan 1;115:37–47. doi: 10.1084/jem.115.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lutton J. D. The effect of phagocytosis and spreading on macrophage surface receptors for concanavalin A. J Cell Biol. 1973 Feb;56(2):611–617. doi: 10.1083/jcb.56.2.611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marantz R., Ventilla M., Shelanski M. Vinblastine-induced precipitation of microtubule protein. Science. 1969 Aug 1;165(3892):498–499. doi: 10.1126/science.165.3892.498. [DOI] [PubMed] [Google Scholar]
- Mizel S. B., Wilson L. Nucleoside transport in mammalian cells. Inhibition by colchicine. Biochemistry. 1972 Jul 4;11(14):2573–2578. doi: 10.1021/bi00764a003. [DOI] [PubMed] [Google Scholar]
- Nicolson G. L. Temperature-dependent mobility of concanavalin A sites on tumour cell surfaces. Nat New Biol. 1973 Jun 13;243(128):218–220. doi: 10.1038/newbio243218a0. [DOI] [PubMed] [Google Scholar]
- Rosenblith J. Z., Ukena T. E., Yin H. H., Berlin R. D., Karnovsky M. J. A comparative evaluation of the distribution of concanavalin A-binding sites on the surfaces of normal, virally-transformed, and protease-treated fibroblasts. Proc Natl Acad Sci U S A. 1973 Jun;70(6):1625–1629. doi: 10.1073/pnas.70.6.1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer S. J., Nicolson G. L. The fluid mosaic model of the structure of cell membranes. Science. 1972 Feb 18;175(4023):720–731. doi: 10.1126/science.175.4023.720. [DOI] [PubMed] [Google Scholar]
- Stossel T. P., Pollard T. D., Mason R. J., Vaughan M. Isolation and properties of phagocytic vesicles from polymorphonuclear leukocytes. J Clin Invest. 1971 Aug;50(8):1745–1747. doi: 10.1172/JCI106664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsan M. F., Berlin R. D. Effect of phagocytosis on membrane transport of nonelectrolytes. J Exp Med. 1971 Oct 1;134(4):1016–1035. doi: 10.1084/jem.134.4.1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ukena T. E., Berlin R. D. Effect of colchicine and vinblastine on the topographical separation of membrane functions. J Exp Med. 1972 Jul 1;136(1):1–7. doi: 10.1084/jem.136.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unanue E. R., Perkins W. D., Karnovsky M. J. Ligand-induced movement of lymphocyte membrane macromolecules. I. Analysis by immunofluorescence and ultrastructural radioautography. J Exp Med. 1972 Oct 1;136(4):885–906. doi: 10.1084/jem.136.4.885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisenberg R. C., Borisy G. G., Taylor E. W. The colchicine-binding protein of mammalian brain and its relation to microtubules. Biochemistry. 1968 Dec;7(12):4466–4479. doi: 10.1021/bi00852a043. [DOI] [PubMed] [Google Scholar]
- Yin H. H., Ukena T. E., Berlin R. D. Effect of colchicine, colcemid, and vinblastine on the agglutination, by concanavalin A, of transformed cells. Science. 1972 Nov 24;178(4063):867–868. doi: 10.1126/science.178.4063.867. [DOI] [PubMed] [Google Scholar]
- Zeya H. I., Spitznagel J. K. Isolation of polymorphonuclear leukocyte granules from rabbit bone marrow. Lab Invest. 1971 Mar;24(3):237–245. [PubMed] [Google Scholar]



