Abstract
Purpose
We performed a UGT1A1 genotype-guided study to determine the maximum tolerated dose (MTD) and evaluate the toxicities and pharmacokinetics of the combination of capecitabine (CAP), oxaliplatin (OX), and irinotecan (IRIN).
Experimental Design
Patients were screened for UGT1A1 *28 genotype prior to treatment. The starting dose (mg/m2) was IRIN (150), OX (85) and CAP (400), days 2-15. Doses were escalated or de-escalated within each genotype group (*28/*28, *1/*28 and *1/*1). IRIN pharmacokinetics was performed at the MTD.
Results
50 patients were evaluable for toxicity [11 (*28/*28); 18 (*1/*28); 21 (*1/*1)]. UGT1A1 *28/*28 patients experienced hematologic dose limiting toxicity (DLT), requiring dose-de-escalation. The UGT1A1 *28/*28 recommended phase 2 dose (RP2D) was IRIN (75), OX (85), and CAP (400). In contrast, both UGT1A1 *1/*28 and *1/*1 tolerated higher doses of IRIN and non-hematologic toxicity was dose limiting for UGT1A1 *1/*1. The RP2D was IRIN (150), OX (85), and CAP (400) for UGT1A1*1/*28 and IRIN (150), OX (100), and CAP (1600) for UGT1A1 *1/*1. UGT1A1 *1/*28 and *1/*1 patients treated with IRIN (150) had similar AUCs for the active irinotecan metabolite, SN38 (366 +/− 278 and 350 +/− 159 ng/ml*hr, respectively). UGT1A1 *28/*28 patients (n=3) treated with a lower IRIN dose (100) had non-significantly higher mean SN38 exposures (604 +/− 289 ng/ml*hr, p=0.14). Antitumor activity was observed in all genotype groups.
Conclusions
UGT1A1 genotype affects the dose and pharmacokinetics of the CAPIRINOX regimen and UGT1A1 genotype-guided dosing of CAPIRINOX is ongoing in a phase II study of small bowel cancer (NCT00433550).
Keywords: Irinotecan, Capecitabine, Oxaliplatin, UGT1A1
INTRODUCTION
Irinotecan, oxaliplatin, and the oral 5-FU prodrug, capecitabine, are active agents in the treatment of colorectal cancer. Studies have demonstrated that patients who are exposed to all 3 drugs in the metastatic setting have prolonged survival compared to those exposed to 2 or fewer drugs [1].
The triple-drug combinations of oxaliplatin, irinotecan and 5-FU/LV (e.g. FOLFOXIRI) or the same in which capecitabine is substituted for 5-FU have shown promising antitumor activity in multiple tumor types [2]. We reported results of a phase I dose escalation study combining irinotecan, oxaliplatin, and 5-FU/leucovorin using an every 3 week schedule [3]. However, a subsequent phase II study using this same regimen in metastatic colorectal cancer was halted because of excess toxicity [4]. Other studies combining oxaliplatin with the FOLFIRI (bi-weekly irinotecan) regimen have demonstrated rates of severe toxicity that were nearly double that of the FOLFIRI regimen [5]. These findings suggest that the greater anti-tumor activity achieved with polychemotherapy may be offset by the higher risk of side-effects as long as patients at highest risk for severe toxicity cannot be identified.
A major dose limiting toxicity of irinotecan is myelosuppression, and multiple studies have demonstrated that UGT1A1 genetic variation is associated with higher rates of irinotecan related neutropenia [6,7,8]. SN-38, the active metabolite of irinotecan, undergoes conjugation by the polymorphic hepatic metabolizing enzyme, uridine diphosphate glucuronosyltransferase enzyme 1A1 (UGT1A1), thus inactivating SN-38G [9]. A dinucleotide repeat polymorphism (TA)7TAA in the TATA sequence of the promoter region of UGT1A1 (designated UGT1A1*28), is associated with lower SN-38 glucuronidation rates [10] and higher rates of hematologic toxicity (neutropenia) with irinotecan therapy [11]. In 2005, the Food and Drug Administration and Pfizer changed the package insert for irinotecan to include a patient’s UGT1A1*28 genotype as a risk factor for severe neutropenia.
Since the FDA label change, there have been multiple studies demonstrating that UGT1A1 genotype affects the tolerable dose of irinotecan containing chemotherapy regimens [12,13,14,15,16,17]. However, there are no reports whether UGT1A1 genotype affects the tolerability of the combination of the capecitabine, irinotecan, and oxaliplatin (CAPIRINOX) regimen. In this study, our primary objective was to define the MTD of the CAPIRINOX regimen in different UGT1A1 genotype groups (*1/*1, *1/*28 and *28/*28). Additionally, we wanted to assess the toxicities and examine the effect of UGT1A1 genotype on the pharmacokinetics of irinotecan and its metabolites.
PATIENTS AND METHODS
Eligibility
Patients with histologic or cytologic confirmed measurable or assessable metastatic or locally advanced cancer for which no established life-prolonging therapy was available or patients unresponsive to conventional therapy were eligible for this study. Other eligibility criteria included the following: age ≥ 18 years; Eastern Cooperative Oncology Group performance status ≤ 2; estimated life expectancy of ≥ 12 weeks; completion of chemotherapy, biologic therapy, or immunotherapy ≥ 4 weeks prior to study entry (≥ 6 weeks in patients treated with mitomycin or nitrosoureas) and recovery from any toxic effects of prior treatment; ≤ three prior chemotherapy regimens; completion of radiation therapy ≥ 4 weeks before enrollment; radiation therapy to ≤ 25% of bone marrow; neutrophil count ≥ 1,500/μL; platelet count ≥ 100,000/μL; hemoglobin ≥ 9.0 g/dL; serum creatinine ≤ 1.5 times the upper limit of normal. AST ≤ 3 times upper limit of normal or AST ≤ 5 times upper limit of normal if liver involvement with malignancy; total bilirubin ≤ upper limit of normal in patients with wild-type UGT1A1 genotype, and total bilirubin ≤ 1.5 times upper limit of normal for patients heterozygous or homozygous for UGT1A1*28 genotype; no active or uncontrolled infection; absence of pregnancy or lactation and willingness to use adequate contraception; no untreated CNS metastases; no uncontrolled seizure disorder; no uncontrolled intercurrent illness including, but not limited to symptomatic congestive heart failure (New York Heart Association classification III or IV); no evident peripheral neuropathy ≥ grade 2; absence of any history of allergy to platinum compounds, irinotecan, or to antiemetics or antidiarrheals appropriate for administration in conjunction with chemotherapy as directed by this protocol; and absence of concomitant antiretroviral therapy. Patients at the MTD were required to participate in pharmacokinetic studies. All patients gave written informed consent according to institutional and federal guidelines.
Dose escalation, Study Design and Statistical Analyses
Potentially eligible patients underwent UGT1A1 genotyping and were assigned into one of 3 cohorts; cohort I (*28/*28), cohort II (*1/*28), and cohort III (*1/*1). A standard cohorts of three design [18] was used for each genotype cohort and patients were assigned to a specific dose level and treated if their genotype cohort was open to accrual. All adverse events were graded according to the National Cancer Institute common toxicity criteria (version 3.0). Toxicity was defined as any adverse event that was deemed at least possibly, probably, or definitely related to study treatment.
Given our prior phase I safety data of the FOLFIRINOX regimen, in which the MTD of irinotecan was 175 mg/m2, oxaliplatin (85 mg/m2), and 5-FU (240 mg/m2) [3] and the observation of excessive toxicity of this regimen in the phase II setting [4], cohort I (*28/*28) and cohort II (*1/*28) patients were started at dose level 1 (Table 1). The dose escalation schema (levels 2-5) was designed to rapidly achieve accepted dose levels of both oxaliplatin (120 mg/m2) and capecitabine (1600 mg/m2) [19] followed later by dose escalation of irinotecan. Because *1/*1 patients (compared with *28/*28 and *1/*28) were known to be at lower risk for neutropenia in the setting of the q3 week irinotecan regimen without an increased risk of diarrhea, the starting dose level for UGT1A1 *1/*1 patients was chosen as dose level 2.
Table 1. Dose Escalation Scheme.
| Dose level |
Dose (mg/m2) |
UGT1A1
*28/*28 |
UGT1A1
*1/*28 |
UGT1A1
*1/*1 |
|---|---|---|---|---|
| −2 | Iri 75 Oxal 85 Cap 400 |
6 | ||
| −1 | Iri 100 Oxal 85 Cap 400 |
3 (2) | ||
| 1* | Iri 150 Oxal 85 Cap 400 |
2 (1) | 3 + 9 | |
| 2** | Iri 150 Oxal 85 Cap 800 |
6 (2) | 3 | |
| 3 | Iri 150 Oxal 120 Cap 800 |
3 | ||
| 4 | Iri 150 Oxal 120 Cap 1600 |
3 + 9 (5) | ||
| 5 | Iri 175 Oxal 120 Cap 1600 |
3 (2) |
Table 2: Dose escalation schema for irinotecan (Iri), oxaliplatin (oxal) and capecitabine (cap).
Starting dose level for UGT1A1 *28/*28 and *1/*28 patients
Starting dose level for UGT1A1 *1/*1 patients
( ) Dose limiting toxicity
Dosage and administration
Irinotecan and capecitabine were obtained commercially. Irinotecan was administered as an intravenous infusion over 90 minutes. The Division of Cancer Treatment and Diagnosis of the National Cancer Institute (Bethesda, MD) supplied oxaliplatin. Patients were treated with irinotecan followed by oxaliplatin on day 1, with capecitabine administered orally on days 2-15 as outlined on Table 1.
Dose-Limiting Toxicity (DLT) and maximum tolerated dose (MTD)
The following toxicities were considered dose limiting: grade 4 absolute neutrophil count > 5 days, grade 4 hemoglobin, platelet count less than 25,000/μL, serum creatinine ≥ two times baseline, treatment delay more than 14 days, and sensory neuropathy ≥ grade 3. Grade 3 or 4 non-hematologic toxicity (with the exception of nausea, vomiting, and diarrhea) was also considered dose limiting. Grade 3 or 4 nausea, vomiting, or diarrhea in patients who had received prophylaxis and treatment with an optimal antiemetic or antidiarrheal regimen were considered dose limiting. Inability to complete 10 days of prescribed dose of capecitabine during cycle one was also considered dose limiting. The MTD was defined as one dose level below the dose that induced DLT in at least one-third of patients (at least 2 of a maximum of 6 new patients).
Pretreatment and Follow-Up Studies
A complete patient history, physical examination, complete blood count, serum electrolytes, and chemistries were performed at baseline and before each course of treatment. Urine pregnancy test was performed at baseline for women with childbearing potential. Complete blood counts were performed weekly during the study. Radiologic studies were performed and objective measurement of tumor mass was assessed in accordance with the RECIST criteria [20] within 3 weeks before the start of treatment and after every two cycles.
UGT1A1 genotyping
All patients were genotyped for UGT1A1 *28 prior to registration. Following DNA extraction, UGT1A1*28 genotyping was performed using a modified method of Akaba et al [21]. Briefly, specific DNA sequences were PCR amplified with a fluorescently labeled primer for genetic polymorphisms occurring in the UGT1A1 promoter [*28 TA(n)]. UGT1A1*28 insertions were determined by DNA fragment size analysis using an ABI 3730 (Foster City, CA). All samples were validated using the Coriell DNA sample sets.
Pharmacokinetic Studies
Specimen Collection
Blood samples (7 mL) were drawn via venipuncture or indwelling intravenous cannula into heparin-containing tubes from the arm contralateral to the infusion line at the following times: prior to beginning the infusion; at the end of the infusion, and 1, 2, 4 and 24 hours following the end of the infusion. If a heparin lock was used, 1 mL of whole blood was withdrawn and discarded prior to sample collection. Collection tubes were immediately placed into a slurry of ice water, the plasma was separated by centrifugation (1000-1200 × g for 20 minutes) and transferred into plastic tubes. The plasma specimens were stored at −70°C until assay for irinotecan and SN-38, SN-38G and APC.
Specimen Analysis
Plasma samples were assayed for irinotecan, SN-38 and APC using validated, sensitive and specific isocratic high-performance liquid chromatography (HPLC) methods [22]. In brief, the plasma specimen was mixed with the internal standard camptothecin in acidified acetonitrile to precipitate plasma proteins, and incubated for 15 minutes at 40°C to convert the analytes to their respective lactones. After addition of triethylamine (TEA) buffer (pH 4.2), the sample was centrifuged and the supernatant was transferred to an amber vial for injection (40 μL) onto the HPLC system.
Chromatographic separation was achieved using a Zorbax-C8 column (MacMod) and a mobile phase consisting of 28:72 (v/v) acetonitrile: 0.025 M TEA buffer (pH 4.2). The fluorescence detector was operated at an excitation wavelength of 372 nm; the irinotecan and IS were monitored at an emission wavelength of 425 nm; SN-38 was monitored at 535 nm.
Irinotecan, APC, and unconjugated SN-38 concentrations were determined by direct analysis of plasma. SN-38G concentrations were determined in a separate portion of each plasma sample in which SN-38G was hydrolyzed to SN-38 by incubation with ß-glucuronidase. SN-38 concentrations determined following incubation of plasma with ß-glucuronidase were labeled as “Total SN-38” (i.e., sum of unconjugated SN-38 and conjugated SN-38). Plasma concentrations of SN-38G were estimated as the difference between the Total SN-38 concentration and the SN-38 concentration.
Pharmacokinetic Analysis
Irinotecan, SN-38 and SN-38G plasma concentration data were analyzed by standard non-compartmental methods using the program WINNONLIN. The CL of irinotecan was calculated as dose/AUC0-∞, where dose is the administered dose of irinotecan expressed in free base equivalents.
Statistical Analyses
Descriptive statistics, frequency distributions and graphs were used to summarize the patient characteristics, PK profile, toxicity patterns and tumor response outcomes within each genotype cohort. The distribution of the PK and hematologic parameters at the MTD were compared across the cohorts using a Kruskal-Wallis test. All analyses were performed using SAS version 9.1.3 computer software.
RESULTS
Patients who presented to the Mayo Phase I clinic who were candidates for several different irinotecan phase I studies were initially screened for potential enrollment. A total of 105 patients underwent UGT1A1*28 genotyping. The minor allele (*28) frequency was 0.34 and results were within Hardy-Weinberg equilibrium. Because screened patients may have enrolled onto another study, not all screened patients were enrolled and treated. Of 105 screened, 53 patients initiated therapy, 3 were non-evaluable (2 patients did not complete the first cycle of treatment for reasons other than toxicity and 1 patient was ineligible). The patient characteristics for each cohort are listed in Table 2. All patients were of good performance status (0 or 1), with the exception of one PS 2 patient in the UGT1A1 *28/*28 cohort.
Table 2. Patient Characteristics.
| UGT1A1 *28/*28 (N=11) |
UGT1A1 *1/*28 (N=18) |
UGT1A1 *1/*1 (N=21) |
|
|---|---|---|---|
| Age, median(range) | 50 (25,68) | 54 (34,69) | 55 (27,74) |
| Gender | |||
| Female | 9 (81.8%) | 10 (55.6%) | 13 (61.9%) |
| Male | 2 (18.2%) | 8 (44.4%) | 8 (38.1%) |
| Race | |||
| White | 11 (100%) | 18 (100%) | 20 (95.2%) |
| American Indian or Alaska Native | 0 (0%) | 0 (0%) | 1 (4.8%) |
| Tumor Type | |||
| Breast | 3 (27.3%) | 2 (11.1%) | 7 (33.3%) |
| Ovary | 1 (9.1%) | 4 (22.2%) | 3 (14.3%) |
| Gallbladder | 1 (9.1%) | 1 (5.6%) | 2 (9.5%) |
| Esophagus | 2 (18.2%) | 0 (0%) | 1 (4.8%) |
| Urinary Bladder | 0 (0%) | 2 (11.1%) | 1 (4.8%) |
| Stomach | 0 (0%) | 3 (16.7%) | 0 (0%) |
| Other | 4 (36.4%) | 6 (33.3%) | 7 (33.3%) |
| Prior Treatments | |||
| Chemotherapy | 10 (90.9%) | 15 (83.3%) | 18 (85.7%) |
| Radiation Therapy | 5 (45.5%) | 4 (22.2%) | 10 (47.6%) |
| Surgery | 10 (90.9%) | 14 (77.8%) | 17 (81%) |
MTD Determination
Cohort I (UGT1A1*28/*28)
The first patient treated at dose level 1 did not experience DLT, but did develop cycle 2 febrile neutropenia (grade 3). The second patient treated at dose level 1 was hospitalized with the following DLT: grade 3 neutropenic fever with prolonged grade 4 neutropenia (8 days), as well as the following grade 3 toxicities: nausea, diarrhea, vomiting, dehydration, fatigue and ileus. Dose de-escalation continued following a 50% reduction of the irinotecan dose (dose level −2). DLT was not observed in 3 patients treated at dose level −2 (irinotecan 75 mg/m2). Enrollment continued at dose level −1 (irinotecan: 100 mg/m2) and 2/3 experienced hematologic DLT including grade 3 neutropenia, nausea, vomiting, diarrhea, anorexia, fatigue, dehydration in 1 patient and grade 3 febrile neutropenia in a second patient. Therefore, accrual to dose level −1 was halted, and an additional 3 patients were enrolled at dose level −2 and did not experience DLT. Therefore, dose level −2 was the recommended phase II dose (RP2D) for Cohort I
Cohort II (UGT1A1*1/*28)
Dose-limiting toxicity was not observed at the starting dose level, but 2/6 patients experienced DLT at dose level 2 including one patient with grade 3 diarrhea another with grade 3 diarrhea, anorexia, nausea, vomiting and grade 4 hyponatremia and dehydration. An additional 9 patients were treated at dose level 1, with no other DLT observed. Thus dose level 1 was determined to be the RP2D.
Cohort III (UGT1A1*1/*1)
Dose-escalation began at dose level 2 (Table 2) and proceeded to dose level 5, with no DLT observed at dose levels 2-4. At dose level 5 (irinotecan 175 mg/m2, oxaliplatin 120 mg/m2, and capecitabine 1600 mg/m2), 2 of 3 patients experienced DLT including neutropenia, dehydration, leukopenia, diarrhea, vomiting, nausea and fatigue. Three additional patients were treated at dose level 4 and 2/3 experienced non-hematologic DLT (total 2/6). Because of the absence of dose limiting hematologic toxicity at dose level 4, an amendment to the protocol was approved to enroll 6 additional patients at dose level 4. No hematologic DLT was observed; however, 3/6 experienced non-hematologic DLT (5/12 total; Table 3). Because of these findings, the recommended phase II dose taken forward for UGT1A1 *1/*1 patients enrolled in the subsequent phase II study (NCT00433550) was capecitabine 1600 mg/m2, irinotecan 150 mg/m2, along with a reduced dose of oxaliplatin 100 mg/m2.
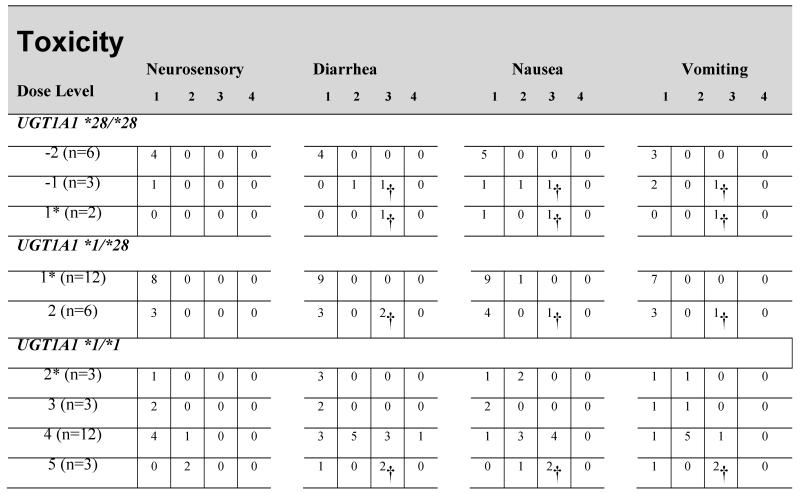
Table 3. Treatment related Nonhematologic toxicity: Cycle 1 only.
|
Starting dose level
Counting one toxicity of each type per patient (maximum grade per patient).
Patients who experienced DLT
Toxicities by genotype
Neutropenia was the predominant toxicity for patients with the UGT1A1 *28/*28 genotype and a significant difference was observed with cycle 1 ANC nadir comparing patients receiving irinotecan at a dose of 100 mg/m2 [0.8 109/L (0.5-1.0)] and 75 mg/m2 [2.4 109/L (1.7-6.3); p=0.02). In contrast, hematologic toxicity was not observed for patients with the UGT1A1 *1/*1 genotype until dose level 5. At dose level 4, non-hematologic toxicities predominated (Table 3).
Pharmacokinetics of Irinotecan and Its Metabolites
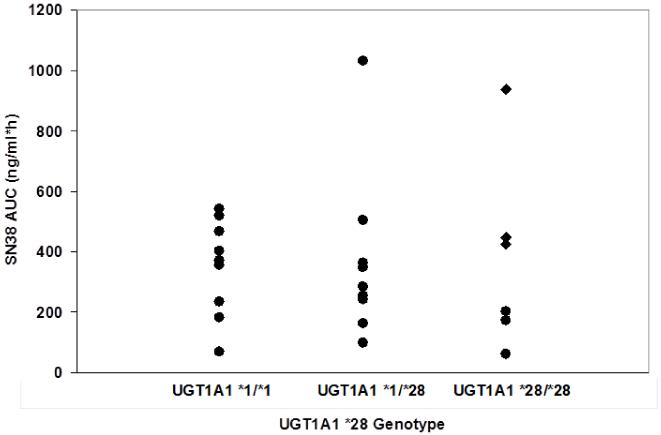
The pharmacokinetics of irinotecan and its metabolites were characterized for UGT1A1 *1/*28 and *1/*1 patients receiving irinotecan 150 mg/m2 (dose level 1 and 4 respectively) and for patients with UGT1A1*28/*28 genotype receiving irinotecan 75 and 100 mg/m2). Both irinotecan and the inactive metabolite, APC, demonstrated Cmax and AUC values that were proportional to the irinotecan dose and not associated with UGT1A1 *28 genotype. In contrast, the pharmacokinetics of SN-38 and SN-38G were associated with UGT1A1*28 genotype (Table 4), with lower SN38G/SN38 AUC ratios for patients with deficient UGT1A1 metabolism [test for trend across genotype (*28/*28 vs *1/*28 vs *1/*1), one-sided p-value=0.04)]. Patients with UGT1A1*1/*28 and UGT1A1*1/*1 genotypes receiving irinotecan 150 mg/m2 had similar mean SN-38 AUC (366 and 350 ng/ml*hr) and Cmax (15.3 and16.1 ng/ml) values. However, patients with the UGT1A1 *28/*28 genotype treated with two-thirds of the irinotecan dose (100 mg/m2) had substantially higher mean SN-38 AUC and Cmax values (604 ng/ml*hr and 20.7 ng/ml, respectively) associated with hematologic DLT in 2 of 3 patients at this dose level. A further dose reduction of irinotecan (75 mg/m2) resulted in significantly lower mean SN38 AUC compared to patients treated at 100 mg/m2 (p=0.05) (Table 4 and Figure 1).
Table 4. Pharmacokinetics of irinotecan and its metabolites by UGT1A1 *28 genotype.
| UGT1A1 Genotype | |||||
|---|---|---|---|---|---|
| *1/*1 | *1/*28 | *28/*28 | |||
|
|
|||||
| Dose (mg/m2) | 150 (N = 9) |
150 (N = 9) |
75 (N = 3) |
100 (N = 3) |
|
| Cmax (ng/ml) | 1469 ± 466 | 1622±280 | 706± 141 | 937± 185 | |
| t1/2 (h) | 11.8 ± 3.9 | 22.4 ± 35.5 | 5.1 ± 1.0 | 10.3 ± 2.7 | |
| CPT-11 | AUC0-48h (ng/ml*h) | 10669 ± 5824 | 10314±2650 | 3734± 1412 | 7523 ± 3008 |
| AUC0-∞ (ng/ml*h) | 10959± 6017 | 11438±3536 | 3713 ± 1421 | 7712±3225 | |
| Cl (L/h/m2) | 20.2 ± 18.5 | 14.6 ± 5.6 | 22.9 ± 10.9 | 14.8 ± 6.8 | |
| Vss (L/m2) | 123 ± 50 | 188±270 | 96 ± 22 | 104 ± 19 | |
|
| |||||
| Cmax (ng/ml) | 228.1 (136.47) | 213 ± 152 | 95 ± 73 | 245 ± 80 | |
| Tmax (h) | 3.6 ± 1.4 | 3.9 ± 1.4 | 2.7 ± 0.1 | 4.1 ± 1.6 | |
| APC | t1/2 (h) | 17.1 ± 16.9 | 119.9 ± 329.1 | 7.0 ± 0.8 | 9.7 ± 2.8 |
| AUC0-48h (ng/ml*h) | 3305± 1583 | 3156±2845 | 1105±588 | 4570±2858 | |
| AUC0-∞ (ng/ml*h) | 3741±1985 | 6377± 8953 | 1111 ± 586 | 4785±3142 | |
|
| |||||
| Cmax (ng/ml) | 16.1 ± 7.9 | 15.3 ± 6.9 | 11.2 ± 2.9 | 20.7 ± 6.7 | |
| Tmax (h) | 2.4 ± 1.3 | 2.2 ± 0.8 | 2.0 ± 0.5 | 2.8 ± 1.0 | |
| SN-38 | t1/2 (h) | 22.0 ± 10.2 | 32.2 ± 16.7 | 11.9 ± 4.0 | 23.0 ± 17.1 |
| AUC0-48h (ng/ml*h) | 291± 146 | 226± 100 | 135 ± 64 | 454 ± 81 | |
| AUC0-∞ (ng/ml*h) | 350± 159 | 366 ± 276 | 146 ± 75 | 604 ± 289 | |
|
| |||||
| Cmax (ng/ml) | 61.4 ± 22.7 | 50.5 ± 30.9 | 33.5 ± 16.6 | 35.5 ± 21.4 | |
| Tmax (h) | 2.4 ± 0.5 | 2.8 ± 0.7 | 2.7 ± 1.0 | 4.1 ± 1.6 | |
| SN-38G | t1/2 (h) | 23.4 ± 15.9 | 25.7 ± 8.4 | 13.6 ± 1.8 | 30.2 ± 33.0 |
| AUC0-48h (ng/ml*h) | 1124 ±543 | 876 ± 543 | 413 ± 84 | 865 ±433 | |
| AUC0-∞ (ng/ml*h) | 1466± 861 | 1136±718 | 446 ± 99 | 1291± 692 | |
Figure 1.
SN38 AUC (ng/ml*hr) according to UGT1A1 *28 genotype. Pharmacokinetic data were obtained for UGT1A1 *1/*1 and *1/*28 patients treated with irinotecan 150 mg/m2 while UGT1A1*28/*28 received either irinotecan 75 mg/m2 (closed circles) or 100 mg/m2 (diamonds).
SN38 AUC and Cycle 1 neutropenia
Cycle 1 hematologic toxicity data were available on 23/24 patients with pharmacokinetic analysis. After adjusting for UGT1A1 genotype, we observed a correlation between log transformed cycle 1 ANC nadir and log transformed SN38 AUC (−0.30, p=0.08)
Antitumor Activity
Anti-tumor activity was observed at all dose levels and in all genotype groups (Table 5). In UGT1A1*28/*28 patients, a partial response was seen in a patient with squamous cell carcinoma of the vulva. In UGT1A1 *1/*28 patients, 2 partial responses were seen, including stomach, and ovary. In UGT1A1*1/*1, one partial response was observed (papillary serous cystadenocarcinoma of the ovary). Notably, the median number of cycles administered overall (Table 5) and at the recommended phase II dose (Table 6) was similar for each UGT1A1 group, despite substantial reductions in dose intensity for each drug based on increasing number of variant UGT1A1 alleles.
Table 5. Tolerability and Efficacy.
| UGT1A1 *28/*28 (N=11) |
UGT1A1 *1/*28 (N=18) |
UGT1A1 *1/*1 (N=21) |
|
|---|---|---|---|
| Number cycles received, median(range) | 3 (1,9) | 4 (1,10) | 4 (1,20) |
| Best Tumor Response | |||
| Partial Response* | 1 (9.1%) | 2 (11.1%) | 1 (4.8%) |
| Stable | 6 (54.5%) | 10 (55.6%) | 10 (47.6%) |
| Disease Progression | 2 (18.2%) | 3 (16.7%) | 7 (33.3%) |
| NA | 2 (18.2%) | 3 (16.7%) | 3 (14.3%) |
UGT1A1 *28/*28 : squamous cell carcinoma of Vulva;
UGT1A1 *1/*28: adenocarcinoma of Stomach, cystadenocarcinoma of Ovary;
UGT1A1 *1/*1: Papillary serous cystadenocarcinoma of Ovary
Table 6.
Cycle and Dose Information by Genotype for each Dose Level*
| Genotype | Dose Level |
N | Median (Range) | |||
|---|---|---|---|---|---|---|
| Treatment Cycles |
OXAL Dose given (mg/m2) |
IRI Dose given (mg/m2) |
CAP Dose given (mg/m2) |
|||
| UGT1A1 (*28/*28) |
−2 | 6 | 3 (1,9) | 158 (146, 170) | 139 (125, 150) | 9100 (4833, 9100) |
| −1 | 3 | 4 (1, 7) | 173 (142, 179) | 161 (142, 203) | 5538 (4000, 8157) | |
| 1 | 2 | 1.5 (1,2) | 153 (151, 154) | 256 (245, 267) | 4750 (2600, 6900) | |
| UGT1A1 (*1/*28) |
1 | 12 | 3.5 (1, 10) |
150 (128, 180) | 264 (225, 320) | 9100 (7700, 13440) |
| 2 | 6 | 4.5 (1, 7) | 161 (139, 205) | 256 (246, 362) | 19786 (16220, 26250) | |
| UGT1A1 (*1/*1) |
2 | 3 | 6 (1, 8) | 153 (126, 180) | 236 (222, 318) | 13094 (13083, 20450) |
| 3 | 3 | 4 (4, 5) | 215 (187, 230) | 258 (173, 288) | 17588 (10838, 21000) | |
| 4 | 12 | 3 (1, 20) | 243 (177, 307) | 261 (177, 383) | 33038 (7600, 95161) | |
| 5 | 3 | 2 (1, 2) | 229 (195, 233) | 334 (317, 340) | 21000 (9850, 39200) | |
Calculated by computing the median dose of the mean total dose for each patient across all cycles for all patients treated at a given dose level.
Discussion
In this phase I trial, we sought to determine whether UGT1A1 genotype affected the MTD of the CAPIRINOX regimen, based on the substantial data linking UGT1A1 genetic variation with severe hematologic toxicity in patients receiving irinotecan combination chemotherapy. In the setting of irinotecan based chemotherapy, there are multiple studies which have evaluated the feasibility of escalating the dose of irinotecan according to UGT1A1 genotype [12,13,14,15,16,17] using multiple different irinotecan containing regimens. Therefore the approach of genotype guided dosing is not unique. However, there are no data using this approach in the setting of the CAPIRINOX regimen, which makes this study unique.
We determined that the MTD of this regimen differed according to UGT1A1*28 genotype, demonstrating that UGT1A1 *1/*28 and *1/*1 patients tolerated twice the dose of irinotecan (150 mg/ m2) compared to patients with the UGT1A1 *28/*28 genotype (irinotecan 75 mg/m2). Based on our observation of severe toxicity in UGT1A1 *28/*28 patients at the starting dose level, our decision to utilize a markedly reduced dose of irinotecan and to use a low dose of capecitabine (compared to our prior published data with the FOLFIRINOX regimen) [3,4] was justified. However, a limitation of this study is that the specific irinotecan MTD within the UGT1A1 *1/*28 and *1/*1 patients is uncertain. Specifically, the dose escalation goal for UGT1A1 *1/*28 and *1/*1 patients was to first achieve capecitabine (1600 mg/m2/day) and oxaliplatin (120 mg/m2) doses most typically used in the two drug capecitabine and oxaliplatin (CAPOX) regimen [19]followed by either irinotecan dose escalation or de-escalation. However, we were unable to achieve these previously published dose levels, due to unexpected differences in tolerability for UGT1A1 *1/*28 (dose level 2) and UGT1A1 *1/*1 (dose level 4) patients, based solely on differences in capecitabine and oxaliplatin dosing (while irinotecan dosing did not change). As expected, the toxicities observed were mainly non-hematologic for these patients, with no differences in SN38 exposure. Therefore, the reason for the differences in tolerability for these two groups and dose levels remains unexplained. An alternative approach for dose escalation for UGT1A1 *1/*28 and *1/*1 group would be to fix the doses of oxaliplatin (120 mg/m2) and capecitabine (1600 mg/m2) while escalating or de-escalating the irinotecan dose.
For UGT1A1 *1/*1 patients, hematologic toxicity was not dose limiting at dose level 4. However, the rate of non-hematologic toxicity exceeded our predefined definition of MTD (33% patients with DLT); therefore the recommended phase II dose taken forward for UGT1A1 *1/*1 patients enrolled in the subsequent phase II study (NCT00433550) reflected a reduced dose of oxaliplatin (capecitabine 1600 mg/m2, irinotecan 150 mg/m2, and oxaliplatin 100 mg/m2).
A secondary objective of our study was to determine the effect of UGT1A1 genotype on the pharmacokinetics of irinotecan and its metabolites. Compared to UGT1A1 *1/*28 and *1/*1 patients who received irinotecan at a dose of 150 mg/m2, UGT1A1 *28/*28 patients receiving a 33% lower irinotecan dose (100 mg/m2) had higher SN-38 exposures which were associated with dose limiting hematologic toxicity in 2 of 3 patients enrolled at this dose level. However, a further dose reduction of irinotecan (75 mg/m2) resulted in significantly lower mean SN-38 AUC compared to patients treated at 100 mg/m2 (p=0.05). While these data should be considered exploratory given the small sample size, they raise the possibility of a steep relationship between SN38 AUC and neutropenia for patients with the UGT1A *28/*28 genotype.
A meta-analysis of irinotecan studies suggested that UGT1A1 deficient patients are at increased risk for hematologic toxicity when irinotecan is administered in higher doses (>150 mg/m2) in an every 2 or 3 week schedule [6]. However, our demonstration that UGT1A1 genotype affects the MTD of irinotecan and oxaliplatin combination chemotherapy suggest that individualized dosing for UGT1A1 deficient patients may not only be critical in the setting of high dose irinotecan (>150 mg/m2), but additionally when other cytotoxic drugs are co-administered with irinotecan. Our findings are consistent with data from a prospective phase III study in which UGT1A1 *28/*28 patients receiving the combination of irinotecan and oxaliplatin (IROX) had significantly higher rates of Grade 4 hematologic toxicity (55%) compared to UGT1A1 *1/*1 (10%) and UGT1A1 *1/*28 (15%), respectively, p=0.002 [23].
We observed anti-tumor activity in multiple different tumor types with this phase I study (Table 5). Partial responses were observed in all genotype groups, including dose level −2 (UGT1A1 *28/*28), dose level 1 (UGT1A1 *1/*28) and dose level 4 (UGT1A1 *1/*1). These findings provide support for the hypothesis that antitumor activity can be maintained despite the delivery of lower doses of irinotecan based chemotherapy for patients with deficient UGT1A1 activity. A phase II study of this regimen is ongoing in advanced small bowel cancer using UGT1A1 genotype specific dosing (NCCTG N0543).
In summary, we demonstrate that UGT1A1 *28 genotype affects the tolerable dose of the CAPIRINOX regimen and the pharmacokinetics of irinotecan containing combination chemotherapy. These findings add to a growing body of literature that suggests that UGT1A1 genotype is an important determinant of irinotecan drug response and provide further evidence to support the use of UGT1A1 genotyping to determine the dose of irinotecan based chemotherapy.
ACKNOWLEDGMENTS
We thank Sacha Nelson and Deb Kaul (Protocol Development Coordinators), Kim Jensen and Janet Lensing (Certified Research Associates), Jill Piens (Research nurse) and the patients who participated in this trial.
Footnotes
CONFLICTS OF INTEREST
The authors have no conflicts of interest to report.
Presented in part at the ASCO annual meeting (2005) and orally at the 2007 GI ASCO meeting.
REFERENCES
- 1.Grothey A, Sargent D, Goldberg RM, Schmoll HJ. Survival of patients with advanced colorectal cancer improves with the availability of fluorouracil-leucovorin, irinotecan, and oxaliplatin in the course of treatment. J Clin Oncol. 2004;22:1209–1214. doi: 10.1200/JCO.2004.11.037. [DOI] [PubMed] [Google Scholar]
- 2.Conroy T, Desseigne F, Ychou M, Bouche O, Guimbaud R, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. The New England journal of medicine. 2011;364:1817–1825. doi: 10.1056/NEJMoa1011923. [DOI] [PubMed] [Google Scholar]
- 3.Goetz MP, Erlichman C, Windebank AJ, Reid JM, Sloan JA, et al. Phase I and pharmacokinetic study of two different schedules of oxaliplatin, irinotecan, Fluorouracil, and leucovorin in patients with solid tumors. J Clin Oncol. 2003;21:3761–3769. doi: 10.1200/JCO.2003.01.238. [DOI] [PubMed] [Google Scholar]
- 4.McWilliams RR, Goetz MP, Morlan BW, Salim M, Rowland KM, et al. Phase II trial of oxaliplatin/irinotecan/5-fluorouracil/leucovorin for metastatic colorectal cancer. Clin Colorectal Cancer. 2007;6:516–521. doi: 10.3816/CCC.2007.n.017. [DOI] [PubMed] [Google Scholar]
- 5.Falcone A, Ricci S, Brunetti I, Pfanner E, Allegrini G, et al. Phase III trial of infusional fluorouracil, leucovorin, oxaliplatin, and irinotecan (FOLFOXIRI) compared with infusional fluorouracil, leucovorin, and irinotecan (FOLFIRI) as first-line treatment for metastatic colorectal cancer: the Gruppo Oncologico Nord Ovest. J Clin Oncol. 2007;25:1670–1676. doi: 10.1200/JCO.2006.09.0928. [DOI] [PubMed] [Google Scholar]
- 6.Hoskins JM, Goldberg RM, Qu P, Ibrahim JG, McLeod HL. UGT1A1*28 genotype and irinotecan-induced neutropenia: dose matters. J Natl Cancer Inst. 2007;99:1290–1295. doi: 10.1093/jnci/djm115. [DOI] [PubMed] [Google Scholar]
- 7.Innocenti F, Undevia SD, Iyer L, Xian Chen P, Das S, et al. Genetic Variants in the UDP-glucuronosyltransferase 1A1 Gene Predict the Risk of Severe Neutropenia of Irinotecan. J Clin Oncol. 2004 doi: 10.1200/JCO.2004.07.173. [DOI] [PubMed] [Google Scholar]
- 8.Toffoli G, Cecchin E, Corona G, Russo A, Buonadonna A, et al. The role of UGT1A1*28 polymorphism in the pharmacodynamics and pharmacokinetics of irinotecan in patients with metastatic colorectal cancer. J Clin Oncol. 2006;24:3061–3068. doi: 10.1200/JCO.2005.05.5400. [DOI] [PubMed] [Google Scholar]
- 9.Iyer L, King CD, Whitington PF, Green MD, Roy SK, et al. Genetic predisposition to the metabolism of irinotecan (CPT-11). Role of uridine diphosphate glucuronosyltransferase isoform 1A1 in the glucuronidation of its active metabolite (SN-38) in human liver microsomes. J Clin Invest. 1998;101:847–854. doi: 10.1172/JCI915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Iyer L, Hall D, Das S, Mortell MA, Ramirez J, et al. Phenotype-genotype correlation of in vitro SN-38 (active metabolite of irinotecan) and bilirubin glucuronidation in human liver tissue with UGT1A1 promoter polymorphism. Clin Pharmacol Ther. 1999;65:576–582. doi: 10.1016/S0009-9236(99)70078-0. [DOI] [PubMed] [Google Scholar]
- 11.Ando Y, Saka H, Ando M, Sawa T, Muro K, et al. Polymorphisms of UDP-glucuronosyltransferase gene and irinotecan toxicity: a pharmacogenetic analysis. Cancer Res. 2000;60:6921–6926. [PubMed] [Google Scholar]
- 12.Freyer G, Duret A, Milano G, Chatelut E, Rebischung C, et al. Pharmacogenetic tailoring of irinotecan-based first-line chemotherapy in metastatic colorectal cancer: results of a pilot study. Anticancer research. 2011;31:359–366. [PubMed] [Google Scholar]
- 13.Kanekiyo S, Hazama S, Kondo H, Nagashima A, Eto R, et al. UDP-glucuronosyltransferase (UGT) 1A1*28 Polymorphism-directed Phase II Study of Irinotecan with 5′-deoxy-5-fluorouridine (5′-DFUR) for Metastatic Colorectal Cancer. Anticancer research. 2013;33:3423–3430. [PubMed] [Google Scholar]
- 14.Kim KP, Kim HS, Sym SJ, Bae KS, Hong YS, et al. A UGT1A1*28 and *6 genotype-directed phase I dose-escalation trial of irinotecan with fixed-dose capecitabine in Korean patients with metastatic colorectal cancer. Cancer chemotherapy and pharmacology. 2013;71:1609–1617. doi: 10.1007/s00280-013-2161-6. [DOI] [PubMed] [Google Scholar]
- 15.Marcuello E, Paez D, Pare L, Salazar J, Sebio A, et al. A genotype-directed phase I-IV dose-finding study of irinotecan in combination with fluorouracil/leucovorin as first-line treatment in advanced colorectal cancer. British journal of cancer. 2011;105:53–57. doi: 10.1038/bjc.2011.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Satoh T, Ura T, Yamada Y, Yamazaki K, Tsujinaka T, et al. Genotype-directed, dose-finding study of irinotecan in cancer patients with UGT1A1*28 and/or UGT1A1*6 polymorphisms. Cancer science. 2011;102:1868–1873. doi: 10.1111/j.1349-7006.2011.02030.x. [DOI] [PubMed] [Google Scholar]
- 17.Takano M, Goto T, Hirata J, Furuya K, Horie K, et al. UGT1A1 genotype-specific phase I and pharmacokinetic study for combination chemotherapy with irinotecan and cisplatin: a Saitama Tumor Board study. European journal of gynaecological oncology. 2013;34:120–123. [PubMed] [Google Scholar]
- 18.Simon R, Freidlin B, Rubinstein L, Arbuck SG, Collins J, et al. Accelerated titration designs for phase I clinical trials in oncology. J Natl Cancer Inst. 1997;89:1138–1147. doi: 10.1093/jnci/89.15.1138. [DOI] [PubMed] [Google Scholar]
- 19.Zeuli M, Di Costanzo E, Sdrobolini A, Gasperoni S, Paoloni FP, et al. Capecitabine and oxaliplatin in advanced colorectal cancer: a dose-finding study. Annals of oncology: official journal of the European Society for Medical Oncology / ESMO. 2001;12:1737–1741. doi: 10.1023/a:1013562914125. [DOI] [PubMed] [Google Scholar]
- 20.Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, et al. European Organization for Research and Treatment of Cancer. National Cancer Institute of the United States. National Cancer Institute of Canada New guidelines to evaluate the response to treatment in solid tumors. J Natl Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 21.Akaba K, Kimura T, Sasaki A, Tanabe S, Ikegami T, et al. Neonatal hyperbilirubinemia and mutation of the bilirubin uridine diphosphate-glucuronosyltransferase gene: a common missense mutation among Japanese, Koreans and Chinese. Biochem Mol Biol Int. 1998;46:21–26. doi: 10.1080/15216549800203512. [DOI] [PubMed] [Google Scholar]
- 22.Santisteban M, Buckner JC, Reid JM, Wu W, Scheithauer BW, et al. Phase II trial of two different irinotecan schedules with pharmacokinetic analysis in patients with recurrent glioma: North Central Cancer Treatment Group results. J Neurooncol. 2009;92:165–175. doi: 10.1007/s11060-008-9749-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McLeod HL, Sargent DJ, Marsh S, Fuchs C, Ramanathan R, et al. Pharmacogenetic analysis of systemic toxicity and response after 5-Fluorouracil (5-FU/CPT-11, 5FU/oxaliplatin (oxal) or CPT-11/oxal therapy for advanced colorectal cancer (CRC): Results from an intergroup trial. Proc Am Soc Clin Oncol. 2003;22:253. [Google Scholar]