Abstract
Ovarian cancer mortality ranks highest among all gynecological cancers with growth factor pathways playing an integral role in tumorigenesis, metastatic dissemination and therapeutic resistance. The human epidermal growth factor receptor (HER) and vascular endothelial growth factor receptor (VEGFR) are both overexpressed and/or aberrantly activated in subsets of ovarian tumors. While agents targeting either the HER or VEGF pathways alone have been investigated, the impact of these agents have not led to overall survival benefit in ovarian cancer. We tested the hypothesis that co-targeting HER and VEGFR would maximize anti-tumor efficacy at tolerable doses. To this end, ovarian cancer xenografts grown intraperitoneally in athymic nude mice were tested in response to AC480 (pan HER inhibitor, “HERi”), cediranib (pan VEGFR inhibitor “VEGFRi”), or BMS-690514 (combined HER/VEGFR inhibitor “EVRi”). EVRi was superior to both HERi and VEGFRi in terms of tumor growth, final tumor weight and progression-free survival. Correlative tumor studies employing phosphoproteomic antibody arrays revealed distinct agent-specific alterations, with EVRi inducing the greatest overall effect on growth factor signaling. These data suggest that simultaneous inhibition of HER and VEGFR may benefit select subsets of ovarian cancer tumors. To this end, we derived a novel HER/VEGF signature that correlated with poor overall survival in high-grade, late stage, serous ovarian cancer patient tumors.
Keywords: Targeted therapy, EVRi, ovarian cancer, VEGF, HER, erbB, AC480, BMS-690514, cediranib
INTRODUCTION
Ovarian cancer symptoms are difficult to identify and as a result, patients commonly present with advanced disease at the time of diagnosis (1). While age, performance status, tumor histology, optimal cytoreduction and chemotherapy (e.g. platinum-based regimens) are key prognostic indices and despite improvements in disease management, the overall survival rate for high-grade ovarian cancer patients remains poor (2, 3).
Despite marked interpatient tumor heterogeneity, conserved oncogenic mutations (e.g. TP53, BRCA1/2, etc.) leading to aberrant receptor tyrosine kinase (RTK)-mediated signaling support the use of targeted therapies in ovarian cancer (4-6). The vascular endothelial growth factor (VEGF) pathway functions via RTK-mediated activation and plays a key role in ovarian cancer growth, angiogenesis and dissemination (7). As a result, anti-VEGF agents have garnered attention as potential therapeutic adjuncts for ovarian cancer (8-10). For example, combined use of adjuvant bevacizumab (anti-VEGF) with standard chemotherapy improved overall response rates in patients with advanced ovarian cancer (11). In addition, the palliative utility of bevacizumab has been explored in the recurrent setting (12, 13). For example, addition of bevacizumab to platinum-based chemotherapy significantly improved (PFS) in heavily pretreated and recurrent epithelial ovarian cancer patients (14). However, recent meta-analysis from four independent studies (>4,000 patients) found that combined chemotherapy plus bevacizumab improved progression-free survival (PFS) but not overall survival (8, 15-17). Furthermore, multiple VEGF family members are simultaneously expressed in ovarian tumors and may account for bevacizumab resistance (18). Therefore, alternative anti-VEGF strategies are warranted.
Aberrant epidermal growth factor receptor (HER) activity has been reported in ovarian cancer (19). AC480, a highly selective and potent inhibitor of all erbB/HER receptor tyrosine kinases (HER1, HER2 and HER4), abrogated ovarian tumor cell proliferation (20). Unfortunately, the utility of anti-HER targeting in the patient setting remains unclear, as numerous clinical trials examining the efficacy of anti-HER agents in ovarian cancer have been negative (21). A recent phase II clinical trial evaluating the concomitant use of topotecan with lapatinib, a small molecule pan-HER inhibitor, demonstrated no benefit in chemoresistant ovarian cancer patients (22).
Resistance to anti-VEGF and anti-HER agents in ovarian cancer is significant and new strategies are warranted (23). Early evidence demonstrating EGF-induced VEGF production in tumor cells support crosstalk between the two pathways (24). As a result, numerous preclinical studies have demonstrated benefit via combined use of VEGF and HER agents (25-30). In ovarian cancer, HER ligand overexpression was sufficient to induce VEGF production in ovarian carcinoma cells (31). Therefore, we hypothesized that a dual HER/VEGF targeting strategy would effectively limit ovarian cancer tumor growth as compared to either pathway alone. To this end, the pan HER/VEGFR small molecule inhibitor BMS-690514 was employed (32). In a comprehensive kinase inhibition screen, BMS-690514 demonstrated the highest selectivity and potency towards both HER (HER1, HER2 and HER4) and VEGF (VEGFR1, VEGFR2, VEGFR3) family members. While the anti-tumor activity of BMS-690514 has been established in non-small-cell lung cancer xenografts, its utility in ovarian cancer is unknown (33). Intraperitoneal ovarian cancer xenografts were tested in response to single agent AC480 (pan HER inhibitor, “HERi”), single agent cediranib (pan VEGFR [VEGFR1, VEGFR2 and VEGFR3] inhibitor, here in referred to as “VEGFRi”), or dual targeting BMS-690514 (combined HER/VEGFR inhibitor, here in referred to as “EVRi”). EVRi was superior to both HERi and VEGFRi in terms of tumor growth, final tumor weight and progression-free survival. In addition, we identified a subset of ovarian cancer patients with increased VEGF/HER activity and may benefit from dual targeting.
MATERIALS AND METHODS
Cell lines & reagents
SKOV3.ip1 cells were generously provided by Dr. Ellen Vitetta (passaged for less than 1 month prior to experimentation), cultured in DMEM-F12 (Sigma Chemical Co., St. Louis, MO) supplemented with 10% fetal bovine serum (HyClone, Logan, UT) and 100 U/ml penicillin G and maintained at 37 C in a humidified atmosphere of 5% CO2. SKOV3.ip1 cells were authenticated as per our prior work and stably transduced with luciferase-expressing lentivirus (SKOV3.ip1-LUC) as previously described (34, 35). Cediranib (Recentin, AZD2171) was purchased from Selleckchem (Houston, TX) and Bristol-Myers Squibb (Princeton, NJ) generously provided both AC480 (BMS-599626) and BMS-690514. The chemical structures for cediranib, BMS-599626 and BMS-690514 have previously been reported (20, 32, 36).
Xenografts and in vivo imaging
Female nu/nu athymic nude mice (Harlan Laboratories, Indianapolis, IN) were housed in a pathogen-free environment under controlled conditions of light and humidity receiving food and water ad libitum. All animal studies were conducted according to the Mayo Clinic Institutional Animal Care and Use Committee. Eight- to ten-week-old mice were injected I.P. with SKOV3.ip1-LUC cells (1.0 × 106) for subsequent drug treatment and imaging studies. Specifically, daily treatments were initiated 72-hours post I.P. tumor injection for a total of 15 days and tumor growth was measured non-invasively via whole animal bioluminescence (xenogen) initiated the following day (96-hours post-inoculation). Whole body bioluminescence (photons/sec) was carried out using the IVIS 200 Bioluminescence Imaging System (Perkin Elmer, Waltham, MA). Immmediately prior to image acquisition, anesthetized animals (ketamine/xylazine) were injected I.P. with 100 μl of D-luciferin (150 mg/kg) (Gold Biotechnology, Inc., St. Louis, MO). Dosing was carried via oral gavage according to the predetermined maximally tolerated dose for each of the following cohorts with n≥10 mice per cohort: HERi (180mg/kg), VEGFRi (6mg/kg) and EVRi (50 mg/kg). Animals were sacrificed 35 days post drug initiation and tumors harvested for subsequent correlative analyses.
Human phospho-RTK array
Pan RTK phosphorylation was assessed in xenograft tumors via the Proteome Profiler 96 (R&D Systems, Minneapolis, MN) as previously described (37). Briefly, tumor lysates were subjected to the provided two-site sandwhich ELISA plate and subsequent procedures carried out as per the manufacturer guidelines. Signals were detected using a luminescent image analyzer (LAS-4000; FUJIFILM, Tokyo, Japan) and spot intensities quantified via the Quantity One software package (Bio-Rad, Hercules, CA).
Statistical analysis
Statistical significance between two groups was tested using analysis of variance (ANOVA) with Bonferroni’s post hoc test for multiple-comparison analysis using Prism 5.0 (GraphPad Software, La Jolla, CA). Pearson coefficient correlations were calculated for each treatment sample, hierarchical clustering performed (Cluster 3.0) and visualized (Java TreeView) (38, 39). A strong correlation between a pair of samples is revealed by close proximity on the heatmap. Univariate survival analyses were performed using the Kaplan-Meier method and corresponding log-rank test for intergroup differences. Time to event was employed as a surrogate marker of PFS was calculated as time for whole body bioluminescence of each animal to reach five times the initial whole body bioluminescence. All analyses were conducted using JMP 9.0 software.
RESULTS
EVRi inhibits tumor growth and progression of ovarian cancer xenografts
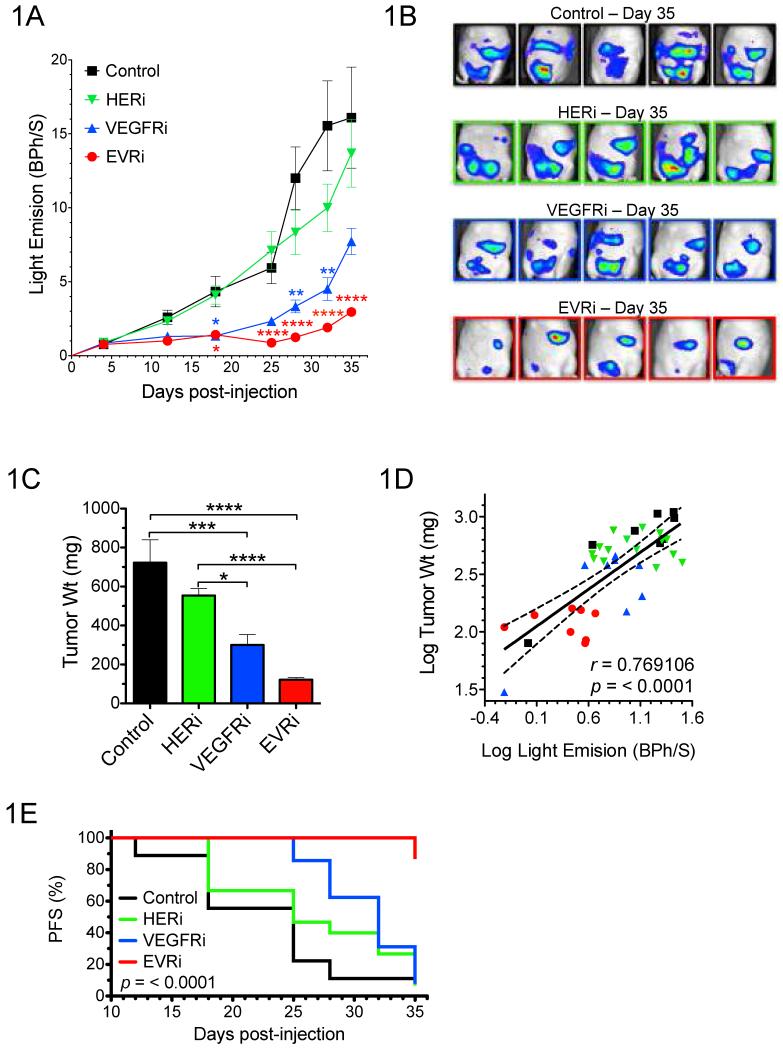
To determine if co-targeted inhibition of these pathways via the single agent BMS-690514 (EVRi) effectively limits ovarian cancer tumor growth, SKOV3.ip1-LUC xenografts were established and drug response determined (Fig. 1). Tumor growth was significantly abrogated in response to both EVRi and VEGFRi (although to a lesser extent) while no significant difference between the HERi and control group was observed (Fig. 1A). Representative bioluminescent images were acquired at the time of study cessation (35 days post tumor inoculation) and included for depiction of the in vivo anti-tumor effect of EVRi compared to HERi and VEGFRi (Fig. 1B). In addition, animals were sacrificed and tumors harvested immediately following Day 35 bioluminescent imaging (Fig. 1C) to correlate bioluminescence with actual tumor weight (Fig. 1D). These data demonstrate that whole body in vivo imaging was an accurate surrogate of ovarian cancer xenograft tumor burden. Importantly, non-specific cytotoxicity was not a factor in any of the treatment groups as bodyweight was maintained throughout the course of the experiment (Supplemental 1).
Figure 1. EVRi inhibits tumor growth and progression of ovarian cancer xenografts.
(A) Tumor growth curves for mice bearing intraperitoneal SKOV3.ip1-LUC xenografts treated with HERi, VEGFRi, EVRi, or saline control. Representative bioluminescent images for each of the treatment groups at 35 days post tumor inoculation. In addition, final tumor weight (C), correlation between bioluminescence and actual tumor weight (D), and progression-free survival (PFS) (E) are depicted. *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001.
Finally, in an effort to translate the potential benefit of EVRi towards tumor suppression, progression-free survival (PFS) was assessed in ovarian cancer xenografts (Fig.1E). For each animal, an event was defined as ≥five-fold increase from baseline bioluminescent tumor signal. In conjunction with both tumor growth and final weight, EVRi treatment resulted in the greatest anti-tumor effect as is demonstrated by significant improvement in PFS versus control (P = <0.0001) (Table 1). No change in PFS was observed in the HERi or VEGFRi versus control.
Table 1.
Hazard ratios (HR) and 95% CIs of HERi, VEGFRi and EVRi vs. Control related to PFS.
| Group | HR (95% CI) | P |
|---|---|---|
| Control | - | - |
| HERi | 0.6339 (0.268 – 1.603) | 0.3210 |
| VEGFRi | 0.4275 (0.174 – 1.109) | 0.0789 |
| EVRi | 0.0558 (0.014 – 0.194) | <0.0001 |
EVRi abrogates oncogenic growth factor signaling
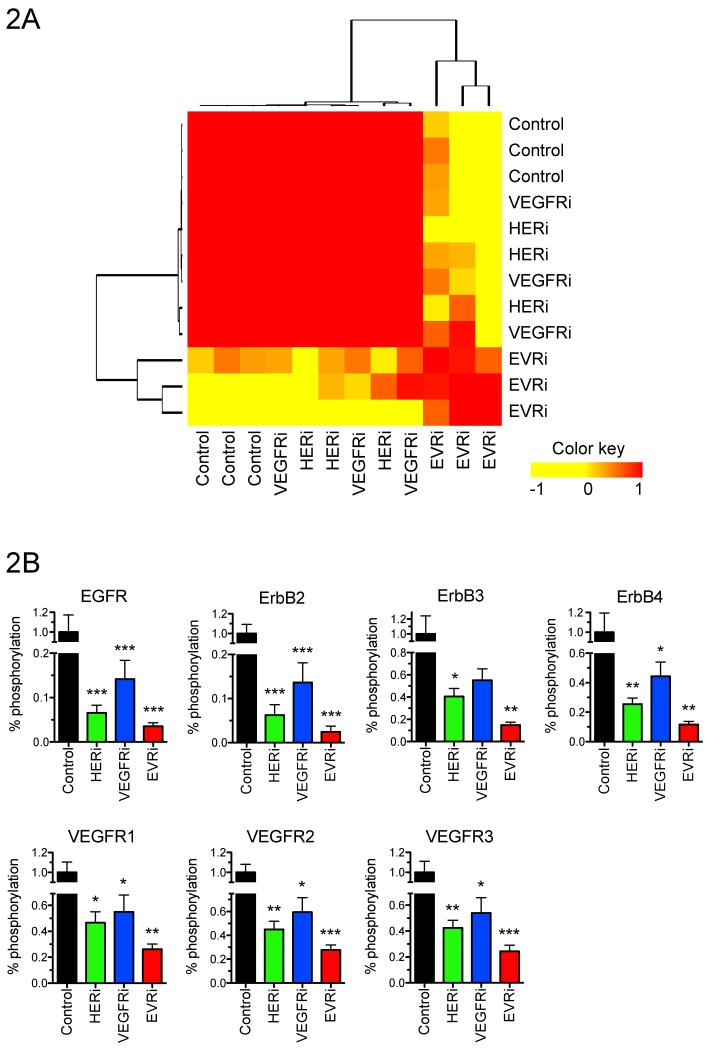
To investigate the mechanism of the anti-tumor effects of EVRi compared with HERi and VEGFRi at the molecular level, phosphoproteomic analysis of multiple growth factor receptor tyrosine kinases (RTKs) known to play a role in cancer was performed via antibody microarray. Briefly, SKOV3.ip1-LUC xenograft tumors were established, randomized to receive a single dose of HERi, VEGFRi or EVRi and tumors harvested 72 hours post-treatment. Tumor protein lysates (n=3/cohort) were prepared and subjected to antibody microarray analysis for quantification of the following cancer-related RTKs: EGFR, ErbB2, ErbB3, ErbB4, HGFR, IGF-1R, INSR, M-CSF, MSPR, PDGFRa, PDGFRb, SCFR, Tie-2, VEGFR1, VEGFR2, and VEGFR3. While strong similarities existed between HERi, VEGFRi and untreated control samples, EVRi treatment samples clustered both according to treatment and independently of HERi, VEGFRi and untreated control samples (Fig. 2A). EVRi resulted in the most significant downregulation of HER and VEGF RTK phosphorylation (Fig. 2B). It is noteworthy to mention that a combined single agent strategy (HERi plus VEGFRi) did not inhibit pan-RTK phosphorylation to that of EVRi (data not shown). These data demonstrate that EVRi inhibits ovarian cancer tumors via pan-RTK suppression and subsequent disruption of functional proteomic dynamics.
Figure 2. EVRi abrogates oncogenic growth factor signaling.
Phosphoproteomic RTK antibody arrays were performed on xenografts harvested at the end of study. (A) Heatmap depicting sample-to-sample correlation by unsupervised hierarchical clustering of Pearson coefficient values. (B) HER (EGFR, ErbB2, ErbB3, ErbB4) and VEGF (VEGFR1, VEGFR2, VEGFR3) receptor phosphorylation. *p<0.05, **p<0.01, ***p<0.001.
A novel HER/VEGF signature correlates with poor overall survival in high-grade serous ovarian cancer patient tumors
One of the greatest challenges towards the clinical advancement of novel therapeutics as a means to improve patient outcome is the ability to accurately identify targetable tumor subsets. In the context of HER and VEGF signaling, both tumor angiogenesis and ErbB2 amplification have been independently examined as poor outcome markers in ovarian cancer (40-42). Moreover, ErbB2 expression directly correlates to EVRi sensitivity (43). It is therefore plausible that combining these two variables (tumor angiogenesis and ErbB2 expression) may provide a predictive surrogate for anti-HER/VEGF agents.
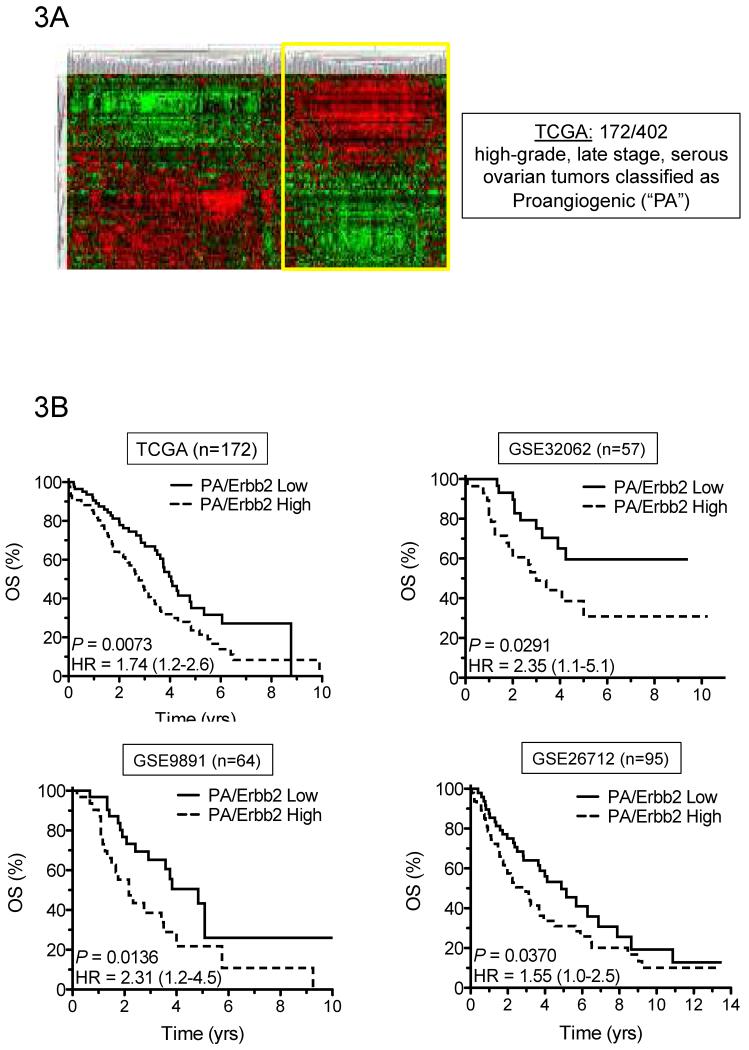
In an effort to first identify VEGF pathway-dependent tumors, a recently defined angiogenic ovarian-specific gene set was applied to high-grade, late stage, serous ovarian tumors from The Cancer Genome Atlas (TCGA) (44). Unsupervised hierarchical clustering stratified patients into two groups, one of which is defined as proangiogenic (“PA”) (Fig. 3A). Amplification of ErbB2 mRNA correlates with increased HER activity and may predict anti-HER targeted therapeutic response (45, 46). As a result, PA tumors were ranked according to ErbB2 expression and split evenly into two cohorts, where the top 50% was defined as “PA/ErbB2 High” and the bottom 50% defined as “PA/ErbB2 Low”. Univariate analysis revealed that overall survival was significantly poorer for the PA/ErbB2 High cohort (P = 0.0073) (Fig. 3B). The prognostic value of this novel HER/VEGF signature was validated independently within three additional public data sets comprised exclusively of high-grade, late state, serous ovarian tumors (GEO accession numbers GSE32062, GSE9891 and GSE26712) (47-49). In combined analyses, the 5- and 10-year estimates of overall survival are presented (Table 2). At 5 and 10 years, patients with PA/ErbB2 High tumors had shorter survival (60%) rates than patients with PA/ErbB2 Low tumors (40%).
Figure 3. A novel HER/VEGF signature correlates with poor overall survival in high-grade serous ovarian cancer patient tumors.
(A) Heatmap depicting unsupervised hierarchical clustering of high-grade, late stage, serous ovarian tumors from the TCGA using a proangiogenic (PA) gene signature. (B) Kaplan Meier analysis of PA tumors stratified by ErbB2 expression into PA/ErbB2 High (dotted line) vs. PA/ErbB2 Low (solid line) and confirmed in four independent cohorts (identified by TCGA or GEO accession number).
Table 2.
Odds ratios of the combined cohorts depicting OS at 5 and 10 years in the PA/ErbB2 High vs. PA/ErbB2 Low groups.
| OS, y | No. of patients |
PA/ErbB2 High (%) |
PA/ErbB2 Low (%) |
Odds Ratio (95% CI) |
P |
|---|---|---|---|---|---|
| <5 | 192 | 60 | 40 | 2.68 (1.68 – 4.28) |
<0.0001 |
| >5 | 123 | 36 | 64 | ||
| <10 | 213 | 60 | 40 | 2.76 (1.80 – 4.25) |
<0.0001 |
| >10 | 153 | 35 | 65 |
DISCUSSION
The complex and dynamic molecular heterogeneity often associated with aggressive ovarian tumors (e.g. high-grade, metastatic, platinum-refractory disease) has challenged the notion of targeted therapeutics as effective tools to improve overall survival. It is likely that de novo and acquired resistance to platinum-based chemotherapy regimens relies on multiple growth factor pathways. Targeted inhibition of oncogenic pathways normally overexpressed/activated (e.g. VEGF pathway) could promote off target compensation by growth factor pathways (e.g. HER pathway). Therefore, an optimal strategy to overcome the limitations of single pathway targeting agents may involve simultaneously inhibition of multiple growth factor pathways. These data suggest that simultaneous inhibition of HER and VEGF pathways via the dual targeting agent EVRi may benefit select subsets of ovarian cancer tumors.
Angiogenesis involves multiple RTK-related pathways and can act as a rate limiting step in tumorigenesis and malignant progression (50). Single agent antiangiogenic (e.g. bevacizumab) resistance and subsequent therapeutic escape is clinically problematic in ovarian cancer and is likely related to the plethora of ligands/cognate receptors involved in ovarian tumor neovascularization (e.g. platelet-derived growth factor (PDGF/R), epidermal growth factor (EGFR/R), placenta growth factor (PlGF/R), KIT, fibroblast growth factor (FGF/R), hepatocyte growth factor (HGF/R)) (51). While EVRi simultaneously targets VEGF and EGFR, it was demonstrated herein to be cross-specific and limit the phosphorylation/activation of additional signaling substrates involved in angiogenesis. It is therefore plausible that the pan-RTK phosphorylation inhibition via EVRi is not due to non-specificity. Instead, we postulate that simultaneous inhibition of two key tumor-dependent growth factor pathways (VEGF and HER) results in global RTK disruption and subsequent tumor growth inhibition. As off target compensation may provide therapeutic escape from chemotherapy and effectively favor the rapid propagation of more aggressive ovarian tumor cells, the ability of EVRi to inhibit multiple RTK pathways in addition to VEGF and HER is favorable. It is important to note that no other commercially available agents targeting both HER and VEGF currently exist. As result, EVRi is uniquely positioned as a novel dual HER/VEGF inhibitor in ovarian cancer.
Similar to previous reports, targeting either VEGF or HER receptor signaling alone demonstrates evidence of crosstalk (Figure 2B) (52). It is likely that blockade of either VEGF or HER receptor pathways confer resistance via up regulation of the unblocked pathway, thus resulting in better tumor control when both pathways are blocked simultaneously. Alternatively, it is conceivable that a portion of the activity of EVRI may stem from undefined off-target effects. Nonetheless, given the anti-tumor activity of EVRI in vivo, clinical investigations with a combined HER/VEGFR inhibitor may be an attractive area of development in the treatment of ovarian cancer.
Supplementary Material
Acknowledgments
This work was supported by the United States National Institutes of Health Grants T32 DK07352 (M.A. Becker) and CA136393, Mayo Clinic SPORE in Ovarian Cancer (S.C. Harrington, J.W. Krempski, K.R. Kalli, and P. Haluska).
Footnotes
Authors’ Disclosure of Potential Conflicts of Interests:
T.W. Wong is an employee of Bristol Meyers Squibb Pharmaceutical Research Institute.
REFERENCES
- 1.Clarke-Pearson DL. Clinical practice. Screening for ovarian cancer. The New England journal of medicine. 2009;361:170–7. doi: 10.1056/NEJMcp0901926. [DOI] [PubMed] [Google Scholar]
- 2.Landrum LM, Java J, Mathews CA, Lanneau GS, Jr., Copeland LJ, Armstrong DK, et al. Prognostic factors for stage III epithelial ovarian cancer treated with intraperitoneal chemotherapy: A Gynecologic Oncology Group study. Gynecologic oncology. 2013 doi: 10.1016/j.ygyno.2013.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McGuire WP, Hoskins WJ, Brady MF, Kucera PR, Partridge EE, Look KY, et al. Cyclophosphamide and cisplatin compared with paclitaxel and cisplatin in patients with stage III and stage IV ovarian cancer. The New England journal of medicine. 1996;334:1–6. doi: 10.1056/NEJM199601043340101. [DOI] [PubMed] [Google Scholar]
- 4.Cancer Genome Atlas Research N Integrated genomic analyses of ovarian carcinoma. Nature. 2011;474:609–15. doi: 10.1038/nature10166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bartholomeusz C, Oishi T, Saso H, Akar U, Liu P, Kondo K, et al. MEK1/2 inhibitor selumetinib (AZD6244) inhibits growth of ovarian clear cell carcinoma in a PEA-15-dependent manner in a mouse xenograft model. Molecular cancer therapeutics. 2012;11:360–9. doi: 10.1158/1535-7163.MCT-11-0400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hirai H, Sootome H, Nakatsuru Y, Miyama K, Taguchi S, Tsujioka K, et al. MK-2206, an allosteric Akt inhibitor, enhances antitumor efficacy by standard chemotherapeutic agents or molecular targeted drugs in vitro and in vivo. Molecular cancer therapeutics. 2010;9:1956–67. doi: 10.1158/1535-7163.MCT-09-1012. [DOI] [PubMed] [Google Scholar]
- 7.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–74. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 8.Heitz F, Harter P, Barinoff J, Beutel B, Kannisto P, Grabowski JP, et al. Bevacizumab in the treatment of ovarian cancer. Advances in therapy. 2012;29:723–35. doi: 10.1007/s12325-012-0041-9. [DOI] [PubMed] [Google Scholar]
- 9.Yu L, Deng L, Li J, Zhang Y, Hu L. The prognostic value of vascular endothelial growth factor in ovarian cancer: a systematic review and meta-analysis. Gynecologic oncology. 2013;128:391–6. doi: 10.1016/j.ygyno.2012.11.002. [DOI] [PubMed] [Google Scholar]
- 10.Stark D, Nankivell M, Pujade-Lauraine E, Kristensen G, Elit L, Stockler M, et al. Standard chemotherapy with or without bevacizumab in advanced ovarian cancer: quality-of-life outcomes from the International Collaboration on Ovarian Neoplasms (ICON7) phase 3 randomised trial. The lancet oncology. 2013;14:236–43. doi: 10.1016/S1470-2045(12)70567-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Micha JP, Goldstein BH, Rettenmaier MA, Genesen M, Graham C, Bader K, et al. A phase II study of outpatient first-line paclitaxel, carboplatin, and bevacizumab for advanced-stage epithelial ovarian, peritoneal, and fallopian tube cancer. Int J Gynecol Cancer. 2007;17:771–6. doi: 10.1111/j.1525-1438.2007.00886.x. [DOI] [PubMed] [Google Scholar]
- 12.Backes FJ, Richardson DL, McCann GA, Smith B, Salani R, Eisenhauer EL, et al. Should bevacizumab be continued after progression on bevacizumab in recurrent ovarian cancer? Int J Gynecol Cancer. 2013;23:833–8. doi: 10.1097/IGC.0b013e318290ea69. [DOI] [PubMed] [Google Scholar]
- 13.Wenham RM, Lapolla J, Lin HY, Apte SM, Lancaster JM, Judson PL, et al. A phase II trial of docetaxel and bevacizumab in recurrent ovarian cancer within 12 months of prior platinum-based chemotherapy. Gynecologic oncology. 2013 doi: 10.1016/j.ygyno.2013.04.049. [DOI] [PubMed] [Google Scholar]
- 14.O’Malley DM, Richardson DL, Rheaume PS, Salani R, Eisenhauer EL, McCann GA, et al. Addition of bevacizumab to weekly paclitaxel significantly improves progression-free survival in heavily pretreated recurrent epithelial ovarian cancer. Gynecologic oncology. 2011;121:269–72. doi: 10.1016/j.ygyno.2011.01.009. [DOI] [PubMed] [Google Scholar]
- 15.Aghajanian C, Blank SV, Goff BA, Judson PL, Teneriello MG, Husain A, et al. OCEANS: a randomized, double-blind, placebo-controlled phase III trial of chemotherapy with or without bevacizumab in patients with platinum-sensitive recurrent epithelial ovarian, primary peritoneal, or fallopian tube cancer. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2012;30:2039–45. doi: 10.1200/JCO.2012.42.0505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Burger RA, Brady MF, Bookman MA, Fleming GF, Monk BJ, Huang H, et al. Incorporation of bevacizumab in the primary treatment of ovarian cancer. The New England journal of medicine. 2011;365:2473–83. doi: 10.1056/NEJMoa1104390. [DOI] [PubMed] [Google Scholar]
- 17.Perren TJ, Swart AM, Pfisterer J, Ledermann JA, Pujade-Lauraine E, Kristensen G, et al. A phase 3 trial of bevacizumab in ovarian cancer. The New England journal of medicine. 2011;365:2484–96. doi: 10.1056/NEJMoa1103799. [DOI] [PubMed] [Google Scholar]
- 18.van der Bilt AR, van der Zee AG, de Vries EG, de Jong S, Timmer-Bosscha H, ten Hoor KA, et al. Multiple VEGF family members are simultaneously expressed in ovarian cancer: a proposed model for bevacizumab resistance. Current pharmaceutical design. 2012;18:3784–92. doi: 10.2174/138161212802002661. [DOI] [PubMed] [Google Scholar]
- 19.Sheng Q, Liu J. The therapeutic potential of targeting the EGFR family in epithelial ovarian cancer. British journal of cancer. 2011;104:1241–5. doi: 10.1038/bjc.2011.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wong TW, Lee FY, Yu C, Luo FR, Oppenheimer S, Zhang H, et al. Preclinical antitumor activity of BMS-599626, a pan-HER kinase inhibitor that inhibits HER1/HER2 homodimer and heterodimer signaling. Clinical cancer research: an official journal of the American Association for Cancer Research. 2006;12:6186–93. doi: 10.1158/1078-0432.CCR-06-0642. [DOI] [PubMed] [Google Scholar]
- 21.Hirte H, Oza A, Swenerton K, Ellard SL, Grimshaw R, Fisher B, et al. A phase II study of erlotinib (OSI-774) given in combination with carboplatin in patients with recurrent epithelial ovarian cancer (NCIC CTG IND.149) Gynecologic oncology. 2010;118:308–12. doi: 10.1016/j.ygyno.2010.05.005. [DOI] [PubMed] [Google Scholar]
- 22.Weroha SJ, Oberg AL, Ziegler KL, Dakhilm SR, Rowland KM, Hartmann LC, et al. Phase II trial of lapatinib and topotecan (LapTop) in patients with platinum-refractory/resistant ovarian and primary peritoneal carcinoma. Gynecologic oncology. 2011;122:116–20. doi: 10.1016/j.ygyno.2011.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vaughan S, Coward JI, Bast RC, Jr., Berchuck A, Berek JS, Brenton JD, et al. Rethinking ovarian cancer: recommendations for improving outcomes. Nature reviews Cancer. 2011;11:719–25. doi: 10.1038/nrc3144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Goldman CK, Kim J, Wong WL, King V, Brock T, Gillespie GY. Epidermal growth factor stimulates vascular endothelial growth factor production by human malignant glioma cells: a model of glioblastoma multiforme pathophysiology. Molecular biology of the cell. 1993;4:121–33. doi: 10.1091/mbc.4.1.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ciardiello F, Bianco R, Damiano V, Fontanini G, Caputo R, Pomatico G, et al. Antiangiogenic and antitumor activity of anti-epidermal growth factor receptor C225 monoclonal antibody in combination with vascular endothelial growth factor antisense oligonucleotide in human GEO colon cancer cells. Clinical cancer research: an official journal of the American Association for Cancer Research. 2000;6:3739–47. [PubMed] [Google Scholar]
- 26.Shaheen RM, Ahmad SA, Liu W, Reinmuth N, Jung YD, Tseng WW, et al. Inhibited growth of colon cancer carcinomatosis by antibodies to vascular endothelial and epidermal growth factor receptors. British journal of cancer. 2001;85:584–9. doi: 10.1054/bjoc.2001.1936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sini P, Wyder L, Schnell C, O’Reilly T, Littlewood A, Brandt R, et al. The antitumor and antiangiogenic activity of vascular endothelial growth factor receptor inhibition is potentiated by ErbB1 blockade. Clinical cancer research: an official journal of the American Association for Cancer Research. 2005;11:4521–32. doi: 10.1158/1078-0432.CCR-04-1954. [DOI] [PubMed] [Google Scholar]
- 28.Yokoi K, Kim SJ, Thaker P, Yazici S, Nam DH, He J, et al. Induction of apoptosis in tumor-associated endothelial cells and therapy of orthotopic human pancreatic carcinoma in nude mice. Neoplasia. 2005;7:696–704. doi: 10.1593/neo.05193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tonra JR, Deevi DS, Corcoran E, Li H, Wang S, Carrick FE, et al. Synergistic antitumor effects of combined epidermal growth factor receptor and vascular endothelial growth factor receptor-2 targeted therapy. Clinical cancer research: an official journal of the American Association for Cancer Research. 2006;12:2197–207. doi: 10.1158/1078-0432.CCR-05-1682. [DOI] [PubMed] [Google Scholar]
- 30.Naumov GN, Nilsson MB, Cascone T, Briggs A, Straume O, Akslen LA, et al. Combined vascular endothelial growth factor receptor and epidermal growth factor receptor (EGFR) blockade inhibits tumor growth in xenograft models of EGFR inhibitor resistance. Clinical cancer research: an official journal of the American Association for Cancer Research. 2009;15:3484–94. doi: 10.1158/1078-0432.CCR-08-2904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hsieh CY, Chen CA, Chou CH, Lai KP, Jeng YM, Kuo ML, et al. Overexpression of Her-2/NEU in epithelial ovarian carcinoma induces vascular endothelial growth factor C by activating NF-kappa B: implications for malignant ascites formation and tumor lymphangiogenesis. Journal of biomedical science. 2004;11:249–59. doi: 10.1007/BF02256568. [DOI] [PubMed] [Google Scholar]
- 32.Wong TW, Lee FY, Emanuel S, Fairchild C, Fargnoli J, Fink B, et al. Antitumor and antiangiogenic activities of BMS-690514, an inhibitor of human EGF and VEGF receptor kinase families. Clinical cancer research: an official journal of the American Association for Cancer Research. 2011;17:4031–41. doi: 10.1158/1078-0432.CCR-10-3417. [DOI] [PubMed] [Google Scholar]
- 33.Loriot Y, Mordant P, Dorvault N, De la motte Rouge T, Bourhis J, Soria JC, et al. BMS-690514, a VEGFR and EGFR tyrosine kinase inhibitor, shows anti-tumoural activity on non-small-cell lung cancer xenografts and induces sequence-dependent synergistic effect with radiation. British journal of cancer. 2010;103:347–53. doi: 10.1038/sj.bjc.6605748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hasegawa K, Pham L, O’Connor MK, Federspiel MJ, Russell SJ, Peng KW. Dual therapy of ovarian cancer using measles viruses expressing carcinoembryonic antigen and sodium iodide symporter. Clinical cancer research: an official journal of the American Association for Cancer Research. 2006;12:1868–75. doi: 10.1158/1078-0432.CCR-05-1803. [DOI] [PubMed] [Google Scholar]
- 35.Haluska P, Carboni JM, TenEyck C, Attar RM, Hou X, Yu C, et al. HER receptor signaling confers resistance to the insulin-like growth factor-I receptor inhibitor, BMS-536924. Mol Cancer Ther. 2008;7:2589–98. doi: 10.1158/1535-7163.MCT-08-0493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brave SR, Ratcliffe K, Wilson Z, James NH, Ashton S, Wainwright A, et al. Assessing the activity of cediranib, a VEGFR-2/3 tyrosine kinase inhibitor, against VEGFR-1 and members of the structurally related PDGFR family. Molecular cancer therapeutics. 2011;10:861–73. doi: 10.1158/1535-7163.MCT-10-0976. [DOI] [PubMed] [Google Scholar]
- 37.Stiehl DP, Bordoli MR, Abreu-Rodriguez I, Wollenick K, Schraml P, Gradin K, et al. Non-canonical HIF-2alpha function drives autonomous breast cancer cell growth via an AREG-EGFR/ErbB4 autocrine loop. Oncogene. 2012;31:2283–97. doi: 10.1038/onc.2011.417. [DOI] [PubMed] [Google Scholar]
- 38.Saldanha AJ. Java Treeview--extensible visualization of microarray data. Bioinformatics. 2004;20:3246–8. doi: 10.1093/bioinformatics/bth349. [DOI] [PubMed] [Google Scholar]
- 39.Eisen MB, Spellman PT, Brown PO, Botstein D. Cluster analysis and display of genome-wide expression patterns. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:14863–8. doi: 10.1073/pnas.95.25.14863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mendiola M, Barriuso J, Redondo A, Marino-Enriquez A, Madero R, Espinosa E, et al. Angiogenesis-related gene expression profile with independent prognostic value in advanced ovarian carcinoma. PloS one. 2008;3:e4051. doi: 10.1371/journal.pone.0004051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gomez-Raposo C, Mendiola M, Barriuso J, Casado E, Hardisson D, Redondo A. Angiogenesis and ovarian cancer. Clinical & translational oncology: official publication of the Federation of Spanish Oncology Societies and of the National Cancer Institute of Mexico. 2009;11:564–71. doi: 10.1007/s12094-009-0406-y. [DOI] [PubMed] [Google Scholar]
- 42.Meden H, Kuhn W. Overexpression of the oncogene c-erbB-2 (HER2/neu) in ovarian cancer: a new prognostic factor. European journal of obstetrics, gynecology, and reproductive biology. 1997;71:173–9. doi: 10.1016/s0301-2115(96)02630-9. [DOI] [PubMed] [Google Scholar]
- 43.Wong TW, Lee FY, Emanuel S, Fairchild C, Fargnoli J, Fink B, et al. Antitumor and antiangiogenic activities of BMS-690514, an inhibitor of human EGF and VEGF receptor kinase families. Clin Cancer Res. 2011;17:4031–41. doi: 10.1158/1078-0432.CCR-10-3417. [DOI] [PubMed] [Google Scholar]
- 44.Bentink S, Haibe-Kains B, Risch T, Fan JB, Hirsch MS, Holton K, et al. Angiogenic mRNA and microRNA gene expression signature predicts a novel subtype of serous ovarian cancer. PloS one. 2012;7:e30269. doi: 10.1371/journal.pone.0030269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.de Graeff P, Crijns AP, Ten Hoor KA, Klip HG, Hollema H, Oien K, et al. The ErbB signalling pathway: protein expression and prognostic value in epithelial ovarian cancer. British journal of cancer. 2008;99:341–9. doi: 10.1038/sj.bjc.6604471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lassus H, Sihto H, Leminen A, Joensuu H, Isola J, Nupponen NN, et al. Gene amplification, mutation, and protein expression of EGFR and mutations of ERBB2 in serous ovarian carcinoma. Journal of molecular medicine. 2006;84:671–81. doi: 10.1007/s00109-006-0054-4. [DOI] [PubMed] [Google Scholar]
- 47.Yoshihara K, Tsunoda T, Shigemizu D, Fujiwara H, Hatae M, Fujiwara H, et al. High-risk ovarian cancer based on 126-gene expression signature is uniquely characterized by downregulation of antigen presentation pathway. Clinical cancer research: an official journal of the American Association for Cancer Research. 2012;18:1374–85. doi: 10.1158/1078-0432.CCR-11-2725. [DOI] [PubMed] [Google Scholar]
- 48.Tothill RW, Tinker AV, George J, Brown R, Fox SB, Lade S, et al. Novel molecular subtypes of serous and endometrioid ovarian cancer linked to clinical outcome. Clinical cancer research: an official journal of the American Association for Cancer Research. 2008;14:5198–208. doi: 10.1158/1078-0432.CCR-08-0196. [DOI] [PubMed] [Google Scholar]
- 49.Bonome T, Levine DA, Shih J, Randonovich M, Pise-Masison CA, Bogomolniy F, et al. A gene signature predicting for survival in suboptimally debulked patients with ovarian cancer. Cancer research. 2008;68:5478–86. doi: 10.1158/0008-5472.CAN-07-6595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 51.Hiss D. Optimizing molecular-targeted therapies in ovarian cancer: the renewed surge of interest in ovarian cancer biomarkers and cell signaling pathways. Journal of oncology. 2012;2012:737981. doi: 10.1155/2012/737981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Weroha SJ, Haluska P. IGF-1 receptor inhibitors in clinical trials--early lessons. J Mammary Gland Biol Neoplasia. 2008;13:471–83. doi: 10.1007/s10911-008-9104-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.