Abstract
Anxiety is one of the early symptoms of opioid withdrawal and contributes to continued drug use and relapse. The acoustic startle response (ASR) is a component of anxiety that has been shown to increase during opioid withdrawal in both humans and animals. We investigated the role of corticotropin-releasing factor (CRF) and norepinephrine (NE), two key mediators of the brain stress system, on acute heroin withdrawal-potentiated ASR. Rats injected with heroin (2 mg/kg s.c.) displayed an increased ASR when tested 4 h after heroin treatment. A similar increase in ASR was found in rats 10–20 h into withdrawal from extended access (12 h) to i.v. heroin self-administration, a model that captures several aspects of heroin addiction in humans. Both the α2 adrenergic receptor agonist clonidine (10 μg/kg s.c.) and CRF1 receptor antagonist N,N-bis(2-methoxyethyl)-3-(4-methoxy-2-methylphenyl)-2,5-dimethyl-pyrazolo[1,5-a] pyrimidin-7-amine (MPZP; 20 mg/kg s.c.) blocked heroin withdrawal-potentiated startle. To investigate the relationship between CRF1 and α2 adrenergic receptors in the potentiation of the ASR, we tested the effect of MPZP on yohimbine (1.25 mg/kg s.c.)-potentiated startle and clonidine on CRF (2 μg i.c.v.)-potentiated startle. Clonidine blocked CRF-potentiated startle, whereas MPZP partially attenuated but did not reverse yohimbine-potentiated startle, suggesting that CRF may drive NE release to potentiate startle. These results suggest that CRF1 and α2 receptors play an important role in the heightened anxiety-like behaviour observed during acute withdrawal from heroin, possibly via CRF inducing the release of NE in stress-related brain regions.
Keywords: Acoustic startle response, acute heroin withdrawal, brain stress system
Introduction
Drug addiction is a chronically relapsing disorder characterized by a compulsion to seek and take drugs and the emergence of a negative emotional state during abstinence (Koob and Le Moal, 2005). A negative affective state is defined as a dysphoric state accompanied by depressive-like and anxiety-like symptoms (Koob and Le Moal, 2008) and is thought to contribute to the compulsivity associated with dependence via the process of negative reinforcement (i.e. an increase in the probability of a response by removal of an aversive state). In human opioid addicts, anxiety is a component of the initial stages of withdrawal and the development of anxiety disorders may be a consequence of ongoing opiate addiction (Fatseas et al., 2010). Studies in laboratory animals have also shown that both spontaneous and antagonist-precipitated opioid withdrawal results in significant signs of anxiety-like behaviour (Harris and Aston-Jones, 1993; Schulteis et al., 1998a, b; Zhang and Schulteis, 2008) and neuroadaptations in anxiety-related brain regions (Edwards et al., 2009). Thus, anxiety appears to be an important factor in the continued use of drugs and relapse to drug seeking and taking. However, there is a gap in our knowledge about the neuropharmacological mechanisms that underlie anxiety-like behaviour during opioid withdrawal.
Opioid addiction has been linked to dysregulation of brain emotional systems that mediate reward and stress (Koob, 2008). Opioids act on specific areas of the midbrain and ventral forebrain to produce acute positive reinforcing effects (Koob and Le Moal, 2005). In the transition to dependence, neuroadaptive changes compromise the brain reward system, involving the loss of reward neurotransmission and recruitment of brain stress systems, such as those mediated by corticotropin-releasing factor (CRF) and norepinephrine (NE) in the extended amygdala (Koob and Le Moal, 2008). Extracellular CRF in the extended amygdala is increased during acute withdrawal from drugs of abuse, and CRF receptor antagonists block excessive drug taking during dependence (Funk et al., 2007; Specio et al., 2008; Greenwell et al., 2009). The anxiogenic effects of CRF have been reported to be mediated by CRF1 receptors, whereas CRF2 receptors have been shown to modulate CRF effects on feeding behaviour with a lesser role in anxiety-like behaviour (Spina et al., 1996; Smith et al., 1998; Risbrough et al., 2003). CRF1 receptor-deficient animals did not show opiate withdrawal-induced place aversions, demonstrating a critical role of CRF1 receptors in the negative affective states of opiate withdrawal (Contarino and Papaleo, 2005). Similarly, NE in the extended amygdala and locus coeruleus increases during acute withdrawal from drugs of abuse. Specifically, hyperactivity of brain NE has been implicated in mechanisms of opiate withdrawal in the extended amygdala, which is also accompanied by increased CRF signalling (Maldonado, 1997; Aston-Jones et al., 1999; Smith and Aston-Jones, 2008). It has been hypothesized that a CRF–NE feed-forward system exists, in which CRF from the central nucleus of the amygdala (CeA)/bed nucleus of stria terminalis (BNST) activates brainstem noradrenergic activity, which then activates forebrain CRF (Koob, 1999). However, whether CRF or NE is the driving force in such a feed-forward system in opioid withdrawal-induced anxiety is not yet known.
The acoustic startle response (ASR) is reliably elevated by anxiogenic-like stimuli in both humans and laboratory animals and is blocked by anxiolytic-like agents (Davis et al., 1993). The α2 receptor antagonist yohimbine is an anxiogenic-like agent that increases startle, and its effect is likely mediated through central descending NE neurons (Kehne and Davis, 1985). Administration of i.v.c. CRF also increased ASR, which was blocked by the benzodiazepine chlordiazepoxide and the CRF antagonist α-helical CRF9–41 (Swerdlow et al., 1986, 1989). Potentiated startle responses have been observed during withdrawal from different drugs of abuse, including ethanol, nicotine, diazepam and morphine (Rassnick et al., 1992; Helton et al., 1993; Rasmussen et al., 1994; Harris and Gewirtz, 2004). Therefore, the increased startle response after the discontinuation of drug administration may reflect the anxiety-like effects of withdrawal (Harris and Gewirtz, 2004).
In the present study, we tested the hypothesis that the brain stress system, specifically the CRF and NE systems, contributes to elevated anxiety-like behaviour during withdrawal from heroin and that CRF is the driving force of the CRF–NE brain stress system. Using the ASR, we characterized anxiety-like behaviour during withdrawal from acute heroin exposure and heroin self-administration. We then tested the effects of CRF1 receptor antagonist MPZP and α2 adrenergic receptor agonist clonidine on heroin-potentiated startle. Finally, we tested the effect of MPZP on yohimbine-potentiated startle and the effect of clonidine on CRF-potentiated startle.
Materials and method
Animals
Male Wistar rats (n=129), weighing 200–400 g, were housed in groups of two to three per cage and maintained on a 12 h light/12 h dark cycle (lights on 08:00 hours) with free access to food and water. The animals were allowed to acclimate to these conditions for at least 7 d in our animal facilities before behavioural testing. The animals were regularly handled for 1 wk prior to surgery and any behavioural testing. All of the procedures adhered to the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee of The Scripps Research Institute.
Acoustic startle test
The animals were tested for acoustic startle in SR-LAB startle chambers for rats (San Diego Instruments, USA), which consisted of a Plexiglas cage (8×12.5×25 cm) within a ventilated, sound-attenuating chamber. Four rats were tested in four chambers simultaneously. Background noise (60 dB) and noise bursts (50 ms duration) were presented by a speaker mounted 24 cm above the cylinder. Piezoelectric accelerometers mounted under the cylinders detected the movements of the animal, which were digitized and recorded by an interface and computer assembly. Startle amplitude was defined as the maximal peak-to-peak voltage that occurred during the first 200 ms after the onset of the startle-eliciting stimulus. Each startle test session consisted of a 5 min stimulus-free acclimation period with 60 dB background noise, followed by presentation of 30 startle-eliciting noise bursts (10 each at 90, 95 and 105 dB in a balanced, random order) with a 30 s fixed inter-stimulus interval. A dynamic calibration system was used to ensure comparable sensitivities across chambers. The house light remained off throughout the test sessions.
Drugs
Heroin [3,6-diacetylmorphine; International Union of Pure and Applied Chemistry: (5α,6α)-7,8-didehydro-4,5-epoxy-17-methylmorphinan-3,6-diol diacetate ester] was provided by the National Institute on Drug Abuse, dissolved in 0.9% sterile saline, and injected s.c. The α2 receptor agonist clonidine hydrochloride was purchased from Sigma-Aldrich, dissolved in 0.9% saline, and injected s.c. in a volume of 1 ml/kg body weight. The α2 receptor antagonist yohimbine hydrochloride was purchased from Sigma-Aldrich, dissolved in sterile water, and injected s.c. in a volume of 1 ml/kg body weight. The CRF1 receptor antagonist N,N-bis(2-methoxyethyl)-3-(4-methoxy-2-methylphenyl)-2,5-dimethyl-pyrazolo[1,5-a] pyrimidin-7-amine (MPZP) was prepared for systemic administration by dissolving in 1 M HCl (10% final volume), diluting with 25% (w/v) hydroxypropyl β-cyclodextrin (HBC; Cargill, USA; 80% final volume), and back-titrating with descending concentrations of NaOH (2, 1, and 0.1 M; 10% final volume), resulting in a final suspension of 10 mg/ml MPZP in 20% HBC (pH 4.5). The animals were s.c. administered MPZP in a volume of 2 ml/kg. For the 0 mg/kg dose of MPZP, the animals were given 2 ml 20% HBC vehicle/kg body weight.
Heroin self-administration
The surgery and self-administration procedures have been reported in detail previously (Vendruscolo et al., 2011). Briefly, the rats were anaesthetized with isoflurane (1.5–2%), and chronic i.v. catheters were implanted in the jugular vein. The rats were allowed to recover for 7 d before behavioural testing.
Operant chambers (Med Associates, USA) were located inside ventilated, sound-attenuated chambers equipped with a 1.1-W miniature light bulb synchronized to a 12 h light/12 h dark cycle (lights on 06:00 hours). The catheter fittings on the rat’s back were connected to polyethylene tubing contained inside a protective metal spring suspended into the operant chamber from a liquid swivel attached to a balance arm. Drug was delivered by activating a syringe pump (Razel Scientific Instruments) with a 2-rotations-per-minute motor that pushed on a 30 ml syringe for 4.5 s to deliver a 0.1 ml infusion. Each operant session was performed using two retractable levers that extended 1 inch into the chamber.
The rats were trained to lever press for heroin (60 μg/kg/infusion) 1 h/d on a fixed-ratio (FR) 1 schedule (i.e. every lever press was reinforced) for 5 d/wk. Drug infusions were paired with a 20 s cue light above the active lever that signalled a time-out period. Presses during the time-out period were recorded, but no drug was delivered. Once stable lever pressing was achieved (i.e. three consecutive self-administration sessions with <10% variation in the total number of reinforcers), the animals were split into two groups that were matched for responding: long-access (LgA; 12 h) and short-access (ShA; 1 h) to heroin self-administration. During the self-administration sessions, the rats are allowed to nosepoke for food (45 mg pellets, Bio-Serve) on an FR3 schedule and water on an FR1 schedule. Rats were tested for ASR 10–20 h into withdrawal from heroin self-administration.
Intracerebroventricular CRF infusion
The rats were implanted with indwelling cannulae directed unilaterally at the lateral ventricle. The rats were anaesthetized with isoflurane (1.5–2%) and secured in a stereotaxic frame (Kopf Instruments, USA), and a 22-gauge stainless steel cannula (Plastics One, USA) was aimed 1 mm above the lateral ventricle and secured to the skull with four stainless steel screws and Silux dental cement. The stereotaxic coordinates were the following: anterior/posterior, −0.6 mm; medial/lateral, ±2.0 mm relative to bregma; dorsal/ ventral, −3.2 mm from skull surface. A 7 mm dummy stylet (Plastics One, USA) filled the cannula and maintained patency. The animals were allowed 1 wk to recover from surgery before testing. Rat/human CRF (2 μg/4 μl) was injected i.c.v. using a Hamilton microsyringe and a 30-gauge stainless steel injector attached to polyethylene 20 tubing. The injector projected 1 mm beyond the end of the cannula.
Pharmacological testing
All of the pretreatment times were derived from previous studies. For pharmacological testing that involved acute heroin withdrawal-potentiated startle, the rats were injected with heroin (2 mg/kg) or saline 4 h prior to ASR testing and then administered a pre-treatment drug at different time-points prior to ASR testing. Clonidine (10 μg/kg) and MPZP (20 mg/kg) were administered 60 min prior to ASR testing. Yohimbine (1.25 mg/kg) was injected 30 min prior to testing. All the pharmacological testing was performed using a Latin-square design, in which the pretreatment groups were switched on the following test day (i.e. 2–3 d after the preceding test), with the exception of the experiment involving i.c.v. CRF, in which all of the rats were tested once.
Statistical analysis
All the data are expressed as means and standard error of the mean (S.E.M.). The data were analysed using one-way analysis of variance (ANOVA), with group (repeated saline, repeated heroin) as the between-subjects factor, or two-way repeated-measures ANOVA, with group (saline vs. heroin, vehicle vs. yohimbine, saline vs. CRF) as the between-subjects factor and treatment (clonidine and MPZP vs. vehicle) and intensity (90, 95 and 105 dB) as the within-subjects factors. Fisher’s least significant difference (LSD) post hoc test was used when appropriate. All of the statistical analyses were performed using Statistica 10. Values of p<0.05 were considered statistically significant.
Results
Withdrawal from acute heroin administration or extended access to heroin self-administration potentiated startle
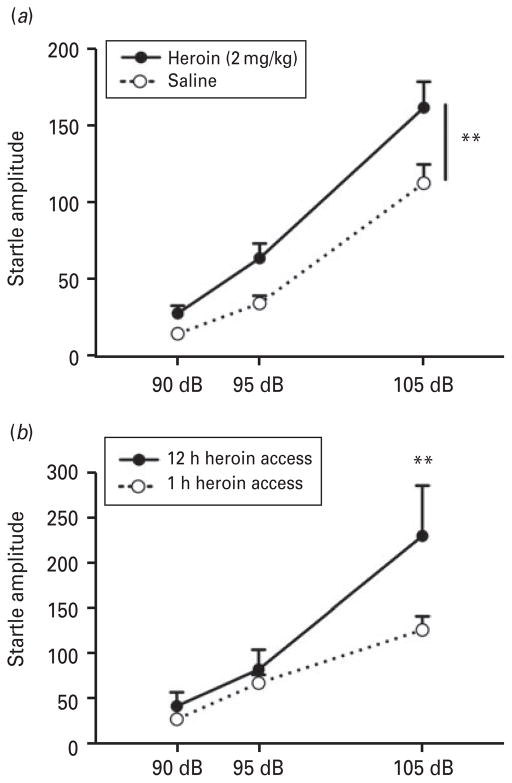
Figure 1 illustrates the ASR in rats during withdrawal from a heroin injection (4 h into withdrawal) or self-administration (10–20 h into withdrawal). For acute heroin injection (Fig. 1a), ANOVA revealed a significant effect of heroin (F1,28 =7.83, p<0.01), with heroin-treated rats showing higher startle amplitudes compared with saline-treated rats. An effect of intensity was also found (F1,56 =133.9, p <0.0001), showing startle amplitudes at 90 dB<95 dB<105 dB. Similarly, Fig. 1b shows that rats with extended access (12 h) to heroin displayed a significant increase in the startle response during withdrawal compared with rats with limited access (1 h) to heroin at 105 dB (treatment× intensity interaction: F2,32 =5.51, p <0.01; LSD post hoc test, p<0.005). An effect of intensity was also present (F2,32 =46.19, p <0.0001). ASR was evaluated after day 1 of self-administration. The average number of lever presses on day 1 for the ShA group was 5.3±0.9, and LgA group was 42.7±9.9.
Fig. 1.
Heroin withdrawal-potentiated startle. (a) The acoustic startle response (ASR) measured during withdrawal from a single injection of heroin (2 mg/kg s.c.). The ASR was measured 4 h after the heroin injection. A significant difference in startle amplitude was found between saline- and heroin-treated rats across all intensities; n=15. ** p<0.01, different from saline. (b) The ASR was measured 10–20 h into withdrawal from i.v. heroin self-administration. Withdrawal-potentiated startle was observed in rats with extended access (12 h) to heroin compared with rats with limited access (1 h) to heroin self-administration; n=7–11. ** p<0.005, different from 1 h self-administration.
MPZP blocked heroin withdrawal-potentiated startle
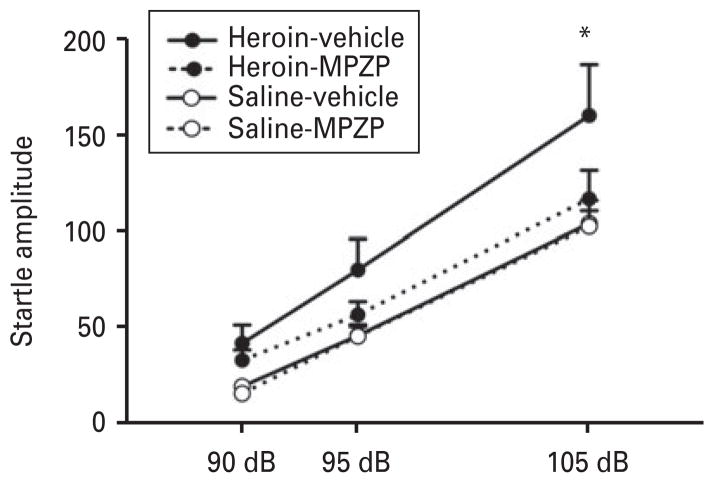
Figure 2 shows the effect of MPZP (20 mg/kg s.c.) on acute heroin withdrawal-potentiated startle. ANOVA revealed a group (heroin)×treatment interaction (F1,23 =4.5, p<0.05). Post hoc comparisons showed that animals treated with heroin+vehicle displayed higher startle amplitudes at 105 dB compared with all of the other groups (p<0.01). MPZP blocked heroin-potentiated startle, in which heroin-MPZP-treated rats displayed significantly lower startle amplitudes compared with heroin-vehicle-treated rats (p<0.01). The ASR was not different between heroin-MPZP-and saline-vehicle-treated rats, and MPZP alone did not alter the ASR. An effect of intensity was found (F1,56 =133.9, p <0.0001), showing startle amplitudes at 90 dB<95 dB<105 dB.
Fig. 2.
MPZP blocked heroin withdrawal-potentiated startle. The CRF1 receptor antagonist MPZP (20 mg/kg s.c.) blocked the heightened startle during withdrawal from heroin. The rats that received heroin-vehicle had significantly higher startle amplitudes compared with the heroin-MPZP, saline-vehicle, and saline-MPZP groups; n=11–14. * p<0.01, different from all groups.
Clonidine blocked heroin withdrawal-potentiated startle
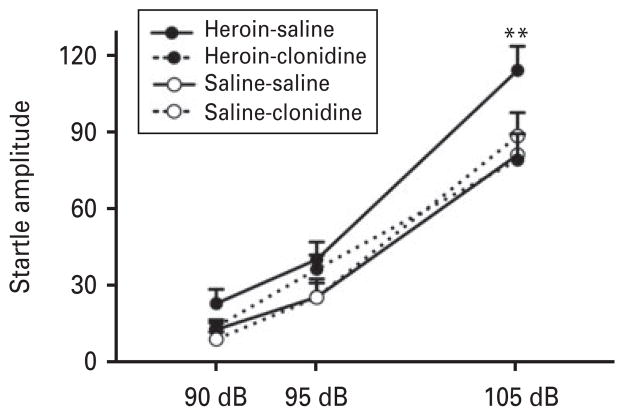
Figure 3 displays the effect of clonidine (10 μg/kg s.c.) on acute heroin withdrawal-potentiated startle. ANOVA revealed a group (heroin)×treatment× intensity interaction (F2,26 =5.6, p <0.01). The post hoc comparisons indicated that heroin-treated rats displayed higher startle amplitudes at 105 dB compared with saline-treated rats (p<0.0005). Importantly, heroin-treated rats that received clonidine displayed significantly lower startle amplitudes at 105 dB than heroin-treated rats that received saline (p<0.0001). At this dose, clonidine alone did not produce an effect on the startle response. An effect of intensity was found (F2,26 =161.4, p <0.0001), showing startle amplitudes at 90 dB<95 dB<105 dB.
Fig. 3.
Clonidine blocked heroin withdrawal-potentiated startle. Heroin-saline treatment produced a significantly higher acoustic startle response at 105 dB compared with rats that received heroin-clonidine, saline-saline, and saline-clonidine; n=7–8. ** p<0.0005, different from all other groups.
MPZP did not block yohimbine-potentiated startle
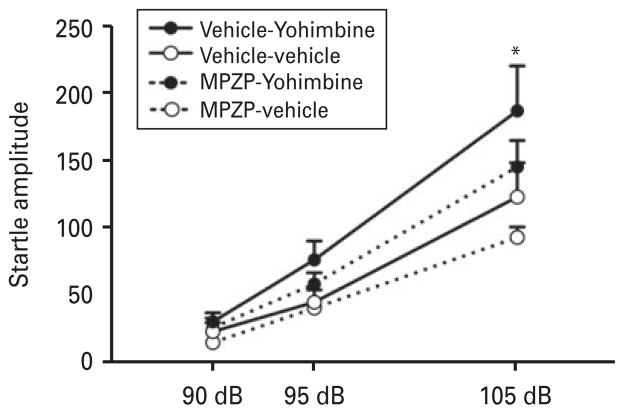
In a preliminary study, we observed a dose-dependent potentiation of startle induced by yohimbine. Table 1 shows the dose-response effect of yohimbine on the ASR. ANOVA revealed a significant yohimbine dose× intensity interaction (F6,42 =9.2, p <0.0001). Subsequent analyses indicated that 1.25 mg/kg yohimbine significantly increased the ASR compared with all the other doses (p<0.0001) at 105 dB. Based on our results and previous findings (Kehne and Davis, 1985), we chose a 1.25 mg/kg dose of yohimbine to determine whether MPZP, a CRF1 receptor antagonist, blocks the startle potentiation induced by yohimbine. Figure 4 shows the effect of systemic MPZP (20 mg/kg s.c.) on yohimbine-potentiated startle. ANOVA revealed a significant group×intensity interaction (F2,40 =3.78, p<0.05). The post hoc comparisons indicated that yohimbine produced a significant increase in startle at 105 dB (p<0.005). MPZP partially attenuated but did not completely block yohimbine-potentiated startle (Yohimbine×MPZP interaction: F1,20 =0.14, p=0.72). MPZP alone (MPZP-vehicle vs. vehicle-vehicle) did not have a significant effect (F1,20 =3.42, p =0.079). An effect of intensity was found (F2,40 =83.38, p <0.0001), showing startle amplitudes at 90 dB<95 dB<105 dB.
Table 1.
Dose–response effect for yohimbine-potentiated startle
| Yohimbine (mg/kg) | 90 dB | 95 dB | 105 dB |
|---|---|---|---|
| 0 | 23.1±3.8 | 61.1±10.5 | 131.6±22.6 |
| 0.3125 | 24.5±4.0 | 54.7±8.6 | 144.6±20.8 |
| 0.625 | 42.7±7.8 | 101.7±20.0*+ | 223.6±39.9*+ |
| 1.25 | 56.1±8.4*+ | 162.7±24.6*+& | 291.9±39.3*+& |
p<0.05, different from 0 mg/kg.
p<0.05, different from 0.3125 mg/kg.
p<0.05, different from 0.625 mg/kg. n=8.
Fig. 4.
MPZP did not block yohimbine-potentiated startle. Yohimbine (1.25 mg/kg s.c.) significantly increased the acoustic startle response compared with vehicle-vehicle, MPZP-yohimbine, and MPZP-vehicle; n=11. * p<0.05, different from vehicle-vehicle.
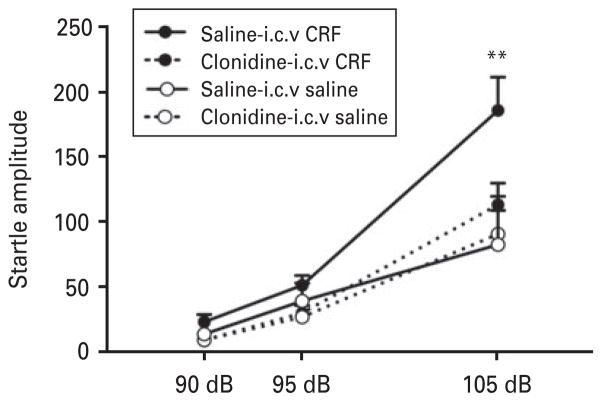
Clonidine blocked CRF-potentiated startle
Figure 5 shows the effect of clonidine (10 μg/kg s.c.) on CRF-potentiated startle. Two-way repeated-measures ANOVA revealed a significant group×treatment× intensity interaction (F2,68 =3.23, p<0.05). The post hoc analysis indicated that CRF significantly potentiated the ASR at 105 dB, and saline+i.c.v. CRF produced significantly higher startle amplitudes compared with clonidine+i.c.v. CRF (p<0.0001), saline +i.c.v. saline (p<0.0001) and clonidine+i.c.v. saline (p<0.0001). These results indicate that clonidine reversed CRF-potentiated startle, with no effect on its own. An effect of intensity was found (F2,68 = 76.53, p<0.0001), showing startle amplitudes at 90 dB <95 dB<105 dB.
Fig. 5.
Clonidine blocked corticotropin-releasing factor (CRF)-potentiated startle. Rats that received i.c.v. CRF exhibited a significant increase in the acoustic startle response compared with rats treated with saline-i.c.v. saline, clonidine-i.c.v. saline, and clonidine-i.c.v. CRF; n=7–12. ** p<0.005, different from clonidine-i.c.v. saline group.
Discussion
We report that the ASR is increased during acute withdrawal from a single injection of heroin. Similarly, rats that underwent withdrawal from 1 d extended access to i.v. heroin self-administration exhibited heightened startle compared with rats which had limited access to heroin self-administration. We found that both MPZP and clonidine were able to block withdrawal-potentiated startle produced by heroin, indicating a functional role for CRF1 and α-adrenergic receptors in mediating this effect. Additionally, we found that clonidine was able to block CRF-potentiated startle, whereas MPZP did not completely block yohimbine-potentiated startle, suggesting that CRF facilitates NE release to potentiate startle.
Previous studies observed a heightened startle response in rodents during withdrawal from ethanol (Rassnick et al., 1992), nicotine (Helton et al., 1993), diazepam (Rasmussen et al., 1994) and morphine (Harris and Gewirtz, 2004). Consistent with these findings, we found that the startle response increased during both acute withdrawal from a single s.c. heroin injection and during withdrawal from extended access (12 h) to heroin self-administration. Rats with limited access (1 h) to heroin did not show an increased ASR. The rats with limited access self-administered approximately 0.3 mg/kg heroin in a 1 h session, whereas rats with extended access self-administered an average of 2 mg/kg heroin in a 12 h session. Interestingly, the same dose (2 mg/kg) of heroin injected in the acute model produced a similar increase in the startle response. The extended access model of heroin self-administration captures several aspects of opioid addiction (Vendruscolo et al., 2011). Thus, the similar results obtained in the startle response during withdrawal from acute heroin injection and extended heroin self-administration support the premise that the acute heroin withdrawal model is a simple and reliable measure to investigate the pharmacological mechanisms that underlie potentiated startle during opioid withdrawal.
We hypothesized that CRF may play a role in the anxiogenic-like effect of heroin withdrawal. Previous studies indicated that the startle response is elevated by i.c.v. CRF administration and anxiolytics, such as chlordiazepoxide, block this effect (Swerdlow et al., 1986). CRF1 receptor antagonists also blocked anxiety-like behaviour measured in the elevated plus maze after i.c.v. CRF administration (Zorrilla et al., 2002). In the self-administration model, CRF1 receptor antagonists reduced the escalation of self-administration of cocaine (Specio et al., 2008), ethanol (Funk et al., 2007) and heroin (Greenwell et al., 2009), as well as the hyperalgesia observed in ethanol- and heroin-dependent animals (Edwards et al., 2012). CRF1 receptor antagonists have been shown to reduce anxiety-like behaviour in rats with high basal anxiety but not in rats with low basal anxiety-like behaviour (Keck et al., 2001). These studies suggest that extrahypothalamic CRF plays an important role in anxiety-like behaviour that is prominent during drug withdrawal. We found that MPZP, a brain-penetrant CRF1 receptor antagonist, blocked withdrawal-potentiated startle, indicating that CRF plays a functional role in anxiety-like behaviour during heroin withdrawal.
Given the CRF×NE systems interaction, we hypothesized that NE also plays a role in anxiety-like behaviour during heroin withdrawal. Clonidine has been shown to produce a rapid and prolonged reduction of opiate withdrawal symptoms in humans (Gossop, 1988). In animals, clonidine has been shown to attenuate conditioned place aversion to opiate withdrawal (Schulteis et al., 1998a, b) and block morphine withdrawal-potentiated startle (Harris and Gewirtz, 2004). Consistent with these results, we found that clonidine blocked heroin withdrawal-potentiated startle. Clonidine is an agonist at presynaptic α2 receptors and decreases both NE release and sympathetic outflow. Our data suggest that CRF and NE modulate the potentiation of startle during heroin withdrawal, with the involvement of CRF1 and α2 receptors.
To further explore the mechanistic relationship between CRF and NE in potentiated startle responses, we tested the effects of MPZP on yohimbine-potentiated startle and clonidine on CRF-potentiated startle. We hypothesized that CRF is the driving force in NE release, leading to an enhanced ASR. Consistent with this hypothesis, the results showed that clonidine was able to block CRF-potentiated startle, whereas systemic MPZP administration only partially attenuated yohimbine-potentiated startle. Neither clonidine nor MPZP significantly altered ASR on its own. MPZP produced a slight decrease of ASR that was not statistically significant. The MPZP dose (20 mg/kg) used in the present study has been shown to effectively decrease alcohol intake, cocaine self-administration, and anxiety-like behaviour in dependent rats (Specio et al., 2008; Richardson et al., 2008) and reduce heroin withdrawal-induced mechanical hypersensitivity (Edwards et al., 2012). Although these findings strongly suggest that 20 mg/kg MPZP produces sufficient receptor occupancy to produce pharmacological effects, we cannot completely rule out a possible interaction of CRF1 receptors on yohimbine-potentiated startle. These results provide evidence that the CRF and NE systems interact to modulate anxiogenic-like effects expressed through the startle response and that CRF potentially drives NE release in the brain stress system to produce this behavioural effect. These results fit with the feed-forward CRF–NE–CRF stress system linking the fore-brain (specifically the CeA, BNST and paraventricular nucleus of the hypothalamus) and brainstem (locus coeruleus), whereby CRF can activate brainstem NE that in turn activates forebrain CRF (Koob, 1999). Evidence suggests that the balance between CRF and opioids may be important in limiting the noradrenergic response to stressors, and disruption in this balance, such as chronic opioid use or stress, alters the balance in favour of CRF activation (Valentino and Van Bockstaele, 2001). Therefore, during opioid withdrawal, CRF may activate the CRF–NE feed-forward brain stress system, leading to a heightened noradrenergic response and increased ASR.
The neurocircuitry involved in the CRF–NE stress system modulation of heroin withdrawal-potentiated startle is a subject for future investigations. Our hypothesis is that the extended amygdala, specifically the CeA and BNST, comprises a key area that mediates anxiety-like behaviour during withdrawal. CRF and NE are abundant in the CeA/BNST, and acute withdrawal from all major drugs of abuse increases CRF/NE release in these regions (Koob, 2008). CRF injected directly into the BNST enhanced the startle response, and lesions of the BNST blocked CRF-potentiated startle (Lee and Davis, 1997). Additionally, infusion of the CRF antagonist α-helical CRF9–41 into the BNST blocked CRF-potentiated startle (Lee and Davis, 1997). These results suggest that the BNST may be a primary receptor site for the excitatory effects of i.c.v. CRF on the ASR. Given these findings, it would be interesting to determine whether manipulations of the CRF and NE systems in the BNST and CeA alter heroin withdrawal-potentiated startle. Furthermore, ASR has been shown to be potentiated by naloxone for at least 80 d into withdrawal (protracted abstinence) after a single morphine exposure (Rothwell et al., 2012), thus, investigating the effects of MPZP and clonidine during protracted withdrawal would be an interesting future study.
In conclusion, we demonstrated that the ASR is potentiated during withdrawal from a single heroin administration and from extended access to heroin self-administration. Both clonidine, by decreasing NE release, and MPZP, by antagonizing CRF1 receptors, blocked heroin withdrawal-potentiated startle. Altogether, these results suggest that CRF may drive the activation of the feed-forward CRF–NE–CRF brain stress system to produce anxiety-like behaviour during opioid withdrawal. Pharmacological manipulation of the CRF and NE systems may be useful in the alleviation of anxiety during withdrawal and may ultimately help in the treatment of opioid addiction.
Acknowledgments
This is publication number 23005 from The Scripps Research Institute. Research was financially supported by National Institutes of Health grants DA004043 from the National Institute on Drug Abuse and AA008459 from the National Institute on Alcohol Abuse and Alcoholism and the Pearson Center for Alcoholism and Addiction Research. Training for P.E.P. was provided by National Institute on Drug Abuse grant T32DA73157. G.S. was supported by a Research Career Scientist Award from the Biomedical Laboratory Research and Development Program, Veterans Health Administration. The authors thank Dr Jean Rivier from the Clayton Foundation Laboratories for Peptide Biology, The Salk Institute, for his generous gift of CRF and Dr Kim Janda, Department of Chemistry, The Scripps Research Institute, for his generous gift of MPZP. The authors thank Michael Arends for proofreading the manuscript.
Footnotes
Statement of Interest
None.
References
- Aston-Jones G, Delfs JM, Druhan J, Zhu Y. The bed nucleus of the stria terminalis. A target site for noradrenergic actions in opiate withdrawal. Ann N Y Acad Sci. 1999;877:486–498. doi: 10.1111/j.1749-6632.1999.tb09284.x. [DOI] [PubMed] [Google Scholar]
- Contarino A, Papaleo F. The corticotropin-releasing factor receptor-1 pathway mediates the negative affective states of opiate withdrawal. Proc Natl Acad Sci USA. 2005;102:18649–18654. doi: 10.1073/pnas.0506999102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis M, Falls WA, Campeau S, Kim M. Fear-potentiated startle: a neural and pharmacological analysis. Behav Brain Res. 1993;58:175–198. doi: 10.1016/0166-4328(93)90102-v. [DOI] [PubMed] [Google Scholar]
- Edwards S, Graham DL, Whisler KN, Self DW. Phosphorylation of GluR1, ERK, and CREB during spontaneous withdrawal from chronic heroin self-administration. Synapse. 2009;63:224–235. doi: 10.1002/syn.20601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards S, Vendruscolo LF, Schlosburg JE, Misra KK, Wee S, Park PE, Schulteis G, Koob GF. Development of mechanical hypersensitivity in rats during heroin and ethanol dependence: alleviation by CRF(1) receptor antagonism. Neuropharmacology. 2012;62:1142–1151. doi: 10.1016/j.neuropharm.2011.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fatseas M, Denis C, Lavie E, Auriacombe M. Relationship between anxiety disorders and opiate dependence–a systematic review of the literature: implications for diagnosis and treatment. J Subst Abuse Treat. 2010;38:220–230. doi: 10.1016/j.jsat.2009.12.003. [DOI] [PubMed] [Google Scholar]
- Funk CK, Zorrilla EP, Lee MJ, Rice KC, Koob GF. Corticotropin-releasing factor 1 antagonists selectively reduce ethanol self-administration in ethanol-dependent rats. Biol Psychiatry. 2007;61:78–86. doi: 10.1016/j.biopsych.2006.03.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gossop M. Clonidine and the treatment of the opiate withdrawal syndrome. Drug Alcohol Depend. 1988;21:253–259. doi: 10.1016/0376-8716(88)90078-6. [DOI] [PubMed] [Google Scholar]
- Greenwell TN, Funk CK, Cottone P, Richardson HN, Chen SA, Rice KC, Zorrilla EP, Koob GF. Corticotropin-releasing factor-1 receptor antagonists decrease heroin self-administration in long-but not short-access rats. Addict Biol. 2009;14:130–143. doi: 10.1111/j.1369-1600.2008.00142.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris AC, Gewirtz JC. Elevated startle during withdrawal from acute morphine: a model of opiate withdrawal and anxiety. Psychopharmacology (Berl) 2004;171:140–147. doi: 10.1007/s00213-003-1573-0. [DOI] [PubMed] [Google Scholar]
- Harris GC, Aston-Jones G. Beta-adrenergic antagonists attenuate withdrawal anxiety in cocaine- and morphine-dependent rats. Psychopharmacology (Berl) 1993;113:131–136. doi: 10.1007/BF02244345. [DOI] [PubMed] [Google Scholar]
- Helton DR, Modlin DL, Tizzano JP, Rasmussen K. Nicotine withdrawal: a behavioral assessment using schedule controlled responding, locomotor activity, and sensorimotor reactivity. Psychopharmacology (Berl) 1993;113:205–210. doi: 10.1007/BF02245698. [DOI] [PubMed] [Google Scholar]
- Keck ME, Welt T, Wigger A, Renner U, Engelmann M, Holsboer F, Landgraf R. The anxiolytic effect of the CRH(1) receptor antagonist R121919 depends on innate emotionality in rats. Eur J Neurosci. 2001;13:373–380. doi: 10.1046/j.0953-816x.2000.01383.x. [DOI] [PubMed] [Google Scholar]
- Kehne JH, Davis M. Central noradrenergic involvement in yohimbine excitation of acoustic startle: effects of DSP4 and 6-OHDA. Brain Res. 1985;330:31–41. doi: 10.1016/0006-8993(85)90005-8. [DOI] [PubMed] [Google Scholar]
- Koob GF. Corticotropin-releasing factor, norepinephrine, and stress. Biol Psychiatry. 1999;46:1167–1180. doi: 10.1016/s0006-3223(99)00164-x. [DOI] [PubMed] [Google Scholar]
- Koob GF. A role for brain stress systems in addiction. Neuron. 2008;59:11–34. doi: 10.1016/j.neuron.2008.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Le Moal M. Plasticity of reward neurocircuitry and the ‘dark side’ of drug addiction. Nat Neurosci. 2005;8:1442–1444. doi: 10.1038/nn1105-1442. [DOI] [PubMed] [Google Scholar]
- Koob GF, Le Moal M. Addiction and the brain antireward system. Annu Rev Psychol. 2008;59:29–53. doi: 10.1146/annurev.psych.59.103006.093548. [DOI] [PubMed] [Google Scholar]
- Lee Y, Davis M. Role of the hippocampus, the bed nucleus of the stria terminalis, and the amygdala in the excitatory effect of corticotropin-releasing hormone on the acoustic startle reflex. J Neurosci. 1997;17:6434–6446. doi: 10.1523/JNEUROSCI.17-16-06434.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maldonado R. Participation of noradrenergic pathways in the expression of opiate withdrawal: biochemical and pharmacological evidence. Neurosci Biobehav Rev. 1997;21:91–104. doi: 10.1016/0149-7634(95)00061-5. [DOI] [PubMed] [Google Scholar]
- Rasmussen K, Helton DR, Berger JE, Scearce E. The CCK-B antagonist LY288513 blocks diazepam-withdrawal-induced increases in auditory startle response. Ann N Y Acad Sci. 1994;713:374–376. doi: 10.1111/j.1749-6632.1994.tb44097.x. [DOI] [PubMed] [Google Scholar]
- Rassnick S, Koob GF, Geyer MA. Responding to acoustic startle during chronic ethanol intoxication and withdrawal. Psychopharmacology (Berl) 1992;106:351–358. doi: 10.1007/BF02245417. [DOI] [PubMed] [Google Scholar]
- Richardson HN, Zhao Y, Fekete EM, Funk CK, Wirsching P, Janda KD, Zorrilla EP, Koob GF. MPZP: a novel small molecule corticotropin-releasing factor type 1 receptor (CRF1) antagonist. Pharmacol Biochem Behav. 2008;88:497–510. doi: 10.1016/j.pbb.2007.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risbrough VB, Hauger RL, Pelleymounter MA, Geyer MA. Role of corticotropin releasing factor (CRF) receptors 1 and 2 in CRF-potentiated acoustic startle in mice. Psychopharmacology (Berl) 2003;170:178–187. doi: 10.1007/s00213-003-1535-6. [DOI] [PubMed] [Google Scholar]
- Rothwell PE, Thomas MJ, Gewirtz JC. Protracted manifestations of acute dependence after a single morphine exposure. Psychopharmacology (Berl) 2012;219:991–998. doi: 10.1007/s00213-011-2425-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulteis G, Yackey M, Risbrough V, Koob GF. Anxiogenic-like effects of spontaneous and naloxone-precipitated opiate withdrawal in the elevated plus-maze. Pharmacol Biochem Behav. 1998a;60:727–731. doi: 10.1016/s0091-3057(98)00034-3. [DOI] [PubMed] [Google Scholar]
- Schulteis G, Stinus L, Risbrough VB, Koob GF. Clonidine blocks acquisition but not expression of conditioned opiate withdrawal in rats. Neuropsychopharmacology. 1998b;19:406–416. doi: 10.1016/S0893-133X(98)00036-0. [DOI] [PubMed] [Google Scholar]
- Smith GW, Aubry JM, Dellu F, Contarino A, Bilezikjian LM, Gold LH, Chen R, Marchuk Y, Hauser C, Bentley CA, Sawchenko PE, Koob GF, Vale W, Lee KF. Corticotropin releasing factor receptor 1-deficient mice display decreased anxiety, impaired stress response, and aberrant neuroendocrine development. Neuron. 1998;20:1093–1102. doi: 10.1016/s0896-6273(00)80491-2. [DOI] [PubMed] [Google Scholar]
- Smith RJ, Aston-Jones G. Noradrenergic transmission in the extended amygdala: role in increased drug-seeking and relapse during protracted drug abstinence. Brain Struct Funct. 2008;213:43–61. doi: 10.1007/s00429-008-0191-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Specio SE, Wee S, O’Dell LE, Boutrel B, Zorrilla EP, Koob GF. CRF(1) receptor antagonists attenuate escalated cocaine self-administration in rats. Psychopharmacology (Berl) 2008;196:473–482. doi: 10.1007/s00213-007-0983-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spina M, Merlo-Pich E, Chan RK, Basso AM, Rivier J, Vale W, Koob GF. Appetite-suppressing effects of urocortin, a CRF-related neuropeptide. Science. 1996;273:1561–1564. doi: 10.1126/science.273.5281.1561. [DOI] [PubMed] [Google Scholar]
- Swerdlow NR, Geyer MA, Vale WW, Koob GF. Corticotropin-releasing factor potentiates acoustic startle in rats: blockade by chlordiazepoxide. Psychopharmacology (Berl) 1986;88:147–152. doi: 10.1007/BF00652231. [DOI] [PubMed] [Google Scholar]
- Swerdlow NR, Britton KT, Koob GF. Potentiation of acoustic startle by corticotropin-releasing factor (CRF) and by fear are both reversed by alpha-helical CRF (9-41) Neuropsychopharmacology. 1989;2:285–292. doi: 10.1016/0893-133x(89)90033-x. [DOI] [PubMed] [Google Scholar]
- Valentino RJ, Van Bockstaele E. Opposing regulation of the locus coeruleus by corticotropin-releasing factor and opioids. Potential for reciprocal interactions between stress and opioid sensitivity. Psychopharmacology (Berl) 2001;158:331–342. doi: 10.1007/s002130000673. [DOI] [PubMed] [Google Scholar]
- Vendruscolo LF, Schlosburg JE, Misra KK, Chen SA, Greenwell TN, Koob GF. Escalation patterns of varying periods of heroin access. Pharmacol Biochem Behav. 2011;98:570–574. doi: 10.1016/j.pbb.2011.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Schulteis G. Withdrawal from acute morphine dependence is accompanied by increased anxiety-like behavior in the elevated plus maze. Pharmacol Biochem Behav. 2008;89:392–403. doi: 10.1016/j.pbb.2008.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zorrilla EP, Valdez GR, Nozulak J, Koob GF, Markou A. Effects of antalarmin, a CRF type 1 receptor antagonist, on anxiety-like behavior and motor activation in the rat. Brain Res. 2002;952:188–199. doi: 10.1016/s0006-8993(02)03189-x. [DOI] [PubMed] [Google Scholar]