Abstract
Objective
The real-time tumor-tracking radiotherapy system with fiducial markers has the advantage that it can be used to verify the localization of the markers during radiation delivery in real-time. We conducted a prospective Phase II study of image-guided local-boost radiotherapy for locally advanced bladder cancer using a real-time tumor-tracking radiotherapy system for positioning, and here we report the results regarding the safety and efficacy of the technique.
Methods
Twenty patients with a T2-T4N0M0 urothelial carcinoma of the bladder who were clinically inoperable or refused surgery were enrolled. Transurethral tumor resection and 40 Gy irradiation to the whole bladder was followed by the transurethral endoscopic implantation of gold markers in the bladder wall around the primary tumor. A boost of 25 Gy in 10 fractions was made to the primary tumor while maintaining the displacement from the planned position at less than ±2 mm during radiation delivery using a real-time tumor-tracking radiotherapy system. The toxicity, local control and survival were evaluated.
Results
Among the 20 patients, 14 were treated with concurrent chemoradiotherapy. The median follow-up period was 55.5 months. Urethral and bowel late toxicity (Grade 3) were each observed in one patient. The local-control rate, overall survival and cause-specific survival with the native bladder after 5 years were 64, 61 and 65%.
Conclusions
Image-guided local-boost radiotherapy using a real-time tumor-tracking radiotherapy system can be safely accomplished, and the clinical outcome is encouraging. A larger prospective multi-institutional study is warranted for more precise evaluations of the technological efficacy and patients' quality of life.
Keywords: bladder cancer, image-guided radiation therapy, combined modality therapy
INTRODUCTION
Since the late 1990s, several institutions and cooperative research groups have reported success in the treatment of locally advanced muscle-invasive bladder cancer with the use of combined modality therapy (CMT), including transurethral resection of the bladder tumor (TUR-BT), radiotherapy (RT) and chemotherapy (1–3). However, it is well recognized that the bladder continually changes its volume and position on a daily basis and that treating a bladder by RT traditionally requires at least a 1.5–2 cm isotropic setup margin (4,5). Such a large margin and treatment field may result in late bowel toxicity.
While discussing tolerance doses of the bladder, Marks et al. (6) suggested that it might be useful to classify the bladder complications into ‘global’ injury and ‘focal’ injury. In general, symptoms of global injury include urinary frequency, urgency, decrease in bladder capacity and cystitis. Symptoms of focal injury include bleeding, ulceration, stone formation and fistula. When the entirety of the bladder is irradiated to a modest dose (<40–50 Gy), global injury is infrequent and the development of a bladder injury is most dependent on the maximum bladder dose (focal injury). Total doses of 65–75 Gy delivered to very small portions of the bladder (less than ∼20%) are associated with an ∼5–10% complication rate. When the whole-bladder dose approaches 50–60 Gy, the risk of global bladder dysfunction starts to increase. In this setting, significant bladder complications may be seen even when the maximum bladder dose is low. Based on the findings in their report, a partial bladder boost treatment technique with image guidance was selected to minimize the dose to the whole bladder at our institution.
The real-time tumor-tracking radiotherapy (RTRT) system that we have used clinically since 1999 has the advantage that it enables us to correct the target location and also to observe the location of the target (through the fiducial markers) even during the beam delivery.
To minimize the influence of bladder mobility and subsequent radiation-induced toxicity, we conducted a prospective Phase II study on the safety and efficacy of definitive RT for locally advanced bladder cancer with an image-guided local-boost RT using fiducial markers and an RTRT system for positioning during radiation delivery.
PATIENTS AND METHODS
This study was set up as a single-center Phase II trial following Fleming's single-stage design. The primary endpoint was overall survival and the secondary endpoints were treatment-related toxicity and local control. We set the minimum required 5-year overall survival at 30% and the minimum required level of efficacy at 60% in 5-year overall survival. The required sample size was calculated to be 20 with 80% power and a 2.5% one-sided significance level.
Patient Eligibility
All enrolled patients met each of the following criteria: (i) histologically confirmed urothelial cancer, (ii) T2–T4N0M0 with or without hydronephrosis, (iii) WHO performance status 0–2, (iv) clinically inoperable or refused surgery and (v) provision of written informed consent. Patients with tumors that covered all or most of the bladder wall and patients with small cell carcinoma were excluded. This study was approved by the ethics committee of the Hokkaido University Graduate School of Medicine.
Treatment Protocol
Transurethral tumor resection was followed by 40 Gy irradiation in 16–20 fractions to the whole bladder and the regional lymph node area, or the whole bladder with an anisotropic margin of 1.5–2 cm. To minimize the duration between the implantation of fiducial markers and the end of RT and to avoid the dropout of the markers, implantation was performed after the delivery of 40 Gy. Until January 2002, one or two markers were transurethrally implanted in the bladder wall near the primary tumor bed (i.e. TUR-BT scar); thereafter, four to six markers were implanted around the primary tumor bed. The interruption of RT to implant the marker was scheduled to be <12 days in principle. A localized boost for the primary tumor (25 Gy/10 fractions) was given using the RTRT system. A percutaneous full-thickness tumor biopsy was performed at the time of marker implantation.
Patients with adequate renal function (creatinine clearance ≥45 ml/min) received concurrent chemoradiotherapy (CCRT) with nedaplatin (70 mg/m2 intravenously, Day 1, Day 22 and Day 50); the other patients received radiotherapy alone (RT-alone). The CCRT patients received an initial 40 Gy at 2 Gy/fraction, and the RT-alone patients received an initial 40 Gy at 2.5 Gy/fraction.
Nedaplatin (cis-diammineglycolatoplatinum; Aqupla®, Shionogi & Co., Osaka, Japan) is a second-generation platinum complex with reduced nephrotoxicity and gastrointestinal toxicity (7). It was reported that nedaplatin also has a radiosensitizing effect (8), and that its anticancer efficacy was comparable with that of cisplatin (CDDP) and higher than that of carboplatin (CBDCA) against bladder cancer (9).
For the present patient population, follow-up cystoscopy and urine cytology were performed every 3 months after the completion of the treatment. Local control was defined as freedom from pathologically proven tumor recurrence in the bladder. Toxicity was evaluated using the National Cancer Institute Common Terminology Criteria for Adverse Events (NCI-CTCAE) version 4.0. Five-year local control and survival rates were estimated by the Kaplan–Meier method. All statistical analyses were performed using JMP 9.0.3 (SAS Institute, Cary, NC, USA).
Patient Data Acquisition and Treatment Planning for Local Boost
Until 2004 at our institution, to ensure a constant bladder volume, an intravesical instillation of 100 ml sterile normal saline was performed, followed by computed tomography (CT) scanning of the small pelvis for RT planning with the patient in the supine position on a flat carbon table. The pre-scanning procedure was changed in 2005, because the intravesical instillation was suspected of causing retrograde pyelonephritis in a patient; thereafter, patients were instructed to void 30 min before CT scanning to allow the bladder to fill with urine. Treatment was also administered 30–60 min after the last voiding.
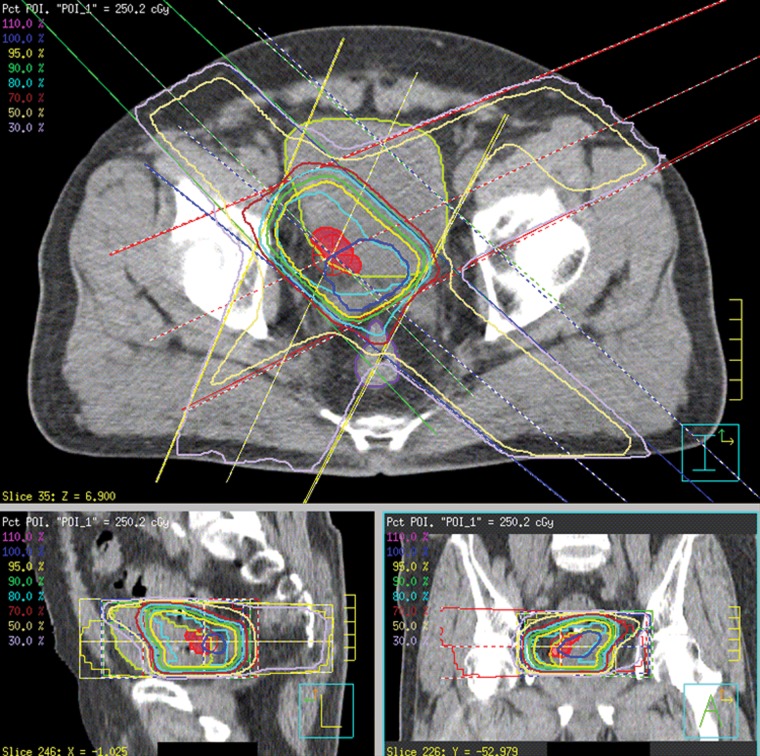
Either a Pinnacle3 (Hitachi Medical, Tokyo) or a XiO (CMS/Elekta, St. Louis, MO, USA) was used as the three-dimensional radiation treatment planning system (3DRTP). The clinical target volume (CTV) was defined as the volume within the contours of the bladder tumor and bladder wall surrounded by fiducial markers. The planning target volume (PTV) was obtained by expanding the CTV by a margin of 5–10 mm in all three dimensions. A 10-mm margin was used for patients in whom only one marker had been implanted. If multiple markers were implanted and we were certain that these markers were outside the tumor based on cystoscopy, a 5-mm margin was applied. The coordinates of the fiducial markers and the CTV were determined on the 3DRTP using CT images. The radiation dose (25 Gy/10 fractions) was prescribed to the isocenter placed at the centroid of the PTV (Fig. 1). At the time we started this study, dose–volume histogram (DVH) was not routinely used and no specific dose constraint has been used for the bladder and organs at risk (OARs) in this study.
Figure 1.
Dose distribution of the boost plan for a patient with right posterolateral bladder tumor. Boost irradiation of 25 Gy after 40 Gy whole-bladder irradiation. Clinical target volume (CTV) (red mesh), planning target volume (PTV) (cyan contour), bladder (yellow-green contour) and rectum (purple contour).
Positioning Procedure Using an RTRT System
The RTRT system consists of a 6 or 10 MV linear accelerator, two diagnostic X-ray fluoroscopic systems in the linear accelerator room, image processing units and an image display unit (originally Mitsubishi; changed to Varian Medical Japan Co., Tokyo), as we have reported previously (10). The actual position of the markers can be visualized on the fluoroscopic image during irradiation. The planned marker position is transferred from 3DRTP and superimposed on the display unit of the RTRT system. For positional registration, one of the markers or the gravity center of the three markers was used as the fiducial point. For patients who were implanted with only one or two markers, one of the markers was used; for patients who were implanted with more than three markers, the gravity center was used.
After the manual setup using skin marks, the coordinates of the fiducial points were measured at the start of treatment and during irradiation. A fluoroscopic image from an RTRT system is digitized with a pixel size of 0.09 × 0.09 mm and image processors compared the digitized image with the template image of a round-shaped metallic marker to detect the location of the marker (11). Details of the calculation of the parallel and rotational setup errors have been reported (12). In short, the position of the patient can be corrected by adjusting the patient's table position by using a remote control bar on the treatment console. When the displacement of the fiducial point exceeds the threshold, the operator can correct the patient's table position using the remote control unit. The threshold used in this study was 2.0 mm in each direction — anterior–posterior, cranial–caudal and left–right — and if the displacement exceeded 2.0 mm in any direction, the table position was corrected accordingly. The table position can be changed in the lateral, vertical and longitudinal directions within 0.1 mm of the specifications. We calculated the rotational setup errors around each axis but intentionally did not correct them in this study.
RESULTS
Patient Characteristics
Between 1999 and 2008, 20 patients with invasive bladder cancer were enrolled, and 14 of these patients were treated by CCRT. The median follow-up period was 55.5 months (range: 9–126 months), and all survivors were followed for more than 2 years. The patient details are listed in Table 1. Six patients had hydronephrosis before treatment. The number of patients for each tumor location when the bladder wall was divided into two portions (left versus right, cranial versus caudal and anterior versus posterior) were 10 versus 10, 4 versus 16 and 6 versus 14, respectively. Ten patients received intravesical instillation of sterile normal saline as a preparation and the remaining 10 patients were instructed to void 30–60 min before CT scanning and treatment.
Table 1.
Characteristics of the 20 bladder cancer patients
| Median age (range) (years) | 77 (58–85) |
| Gender (%) | |
| Male | 15 (75%) |
| Female | 5 (25%) |
| Tumor stage (%) | |
| T2 | 11 (55%) |
| T3 | 7 (35%) |
| T4 | 2 (10%) |
| Pathological grade (%) | |
| 2 | 5 (25%) |
| 3 | 15 (75%) |
| Concurrent chemoradiotherapy (%) | 14 (70%) |
| Median no. of implanted markers (range) | 4 (1–6) |
| No. of patients with less than three markers (%) | 8 (40%) |
Nedaplatin was administered to the 14 CCRT patients, with good compliance; 13 patients completed the planned dose. The median overall treatment time (OTT) was 65 days (range: 51–85 days). All patients completed the planned RT except for one patient who wanted, for religious reasons, to avoid the risk of requiring a blood transfusion in the event of side effects. For this patient, we finished the boost RT at 20 Gy/8 fractions after 40 Gy.
We could not calculate the precise cumulative dose of bladder and other OARs (rectum and intestinal cavity that was defined as the volume of abdominal cavity bordered anteriorly and laterally to the abdominal/pelvic wall and inferiorly to the rectum/bladder including all visible bowel loops) because initial plan for whole bladder (40 Gy) and boost plan (25 Gy) were created based on different treatment planning CT series. Therefore, assuming that the entire bladder and OARs received 40 Gy in the initial plan for whole bladder, we assessed the standard DVH parameters of bladder and OARs of the last nine patients whose data were available for analysis (13–15) although not always the entire OARs were actually received 40 Gy. Median V65 Gy (%) of bladder was 3.5% (range, 0.01–12.7%), median V60 Gy (%) of rectum was 1.3% (range, 0.00–21.4%) and median V45 Gy (cm3) of intestinal cavity was 172.9 cm3 (range, 127.2–419.1 cm3). VxGy denotes the volume receiving at least x Gy.
Treatment-related Toxicities
Two patients experienced acute treatment-related toxicities during treatment; the toxicity was a Grade 3 urinary tract infection in both cases. One of these patients developed retrograde pyelonephritis just after the intravesical instillation of sterile normal saline when the CT scanning was performed; treatment had to be interrupted in this case. Of the 14 patients treated by CCRT, only one patient had to skip chemotherapy due to side effects (Grade 3 thrombocytopenia on Day 50). No other acute toxicity greater than Grade 3 or higher was observed, nor was any significant adverse event thought to be caused by the implantation of the fiducial markers.
With respect to late treatment-related toxicities, five patients developed hemorrhagic cystitis at 2–5 years posttreatment. Of these patients, one required endoscopic hemostasis (Grade 3) and the other patients were improved by conservative treatment (Grade 2). One patient who had a tumor relapse in the bladder and a pelvic lymph node after the initial protocol treatment underwent additional irradiation to the metastatic pelvic lymph node for palliation. She developed a vesicovaginal fistula at 1.5 years after the initial treatment; this was attributed to tumor progression. She also developed an adhesive intestinal obstruction at 1.9 years and required a laparotomy (Grade 3). No other urethral or bowel toxicity of Grade 2 or higher was observed (Table 2). We could not determine the relationship between the tumor location and the gastrointestinal complication in this study; in the patient who had Grade 3 gastrointestinal toxicity (adhesive intestinal obstruction) after irradiation to the metastatic pelvic lymph nodes in addition to the initial protocol study, the tumor was located in the lower posterior part of the bladder.
Table 2.
Number of patients with acute and late complications
| Number of patients |
|||||
|---|---|---|---|---|---|
| Grade (NCI-CTCAE ver.4.0) | 1 | 2 | 3 | 4 | 5 |
| Acute complications | |||||
| Urinary frequency | 7 | 1 | |||
| Diarrhea | 1 | ||||
| Urinary tract infection | 2 | ||||
| Thrombocytopenia | 1 | ||||
| Late complications | |||||
| Cystitis noninfective (hemorrhagic cystitis) | 4 | 1 | |||
| Adhesive intestinal obstruction | 1 | ||||
Tumor Response and Survival
Among the 20 patients, 14 had achieved complete response (CR) at the time of the implantation of fiducial markers, based on re-evaluation with a percutaneous full-thickness bladder biopsy of the primary tumor site. The local-control rate (Fig. 2), overall survival and cause-specific survival (Fig. 3) at 5 years were 64, 61 and 65%, respectively. Eight patients locally relapsed in the bladder; of these, five had relapse in the boost field, two had relapse in the marginal area of CTV (i.e. inside the markers) and one had relapse outside the boost field (extensive superficial relapse). Although the number of patients was less, when dividing patients into two groups according to the preparation method, no significant difference was observed in the rate of local tumor control in this study (50% for the intravesical instillation group and 79% for the patients who voided 30–60 min before treatment, P = 0.22, Wilcoxon test). All patients who had local recurrence were managed with conservative treatment, such as TUR-BT and/or intravesical drug therapy, and no patient received a salvage cystectomy. In other words, the native bladder was preserved for all patients.
Figure 2.
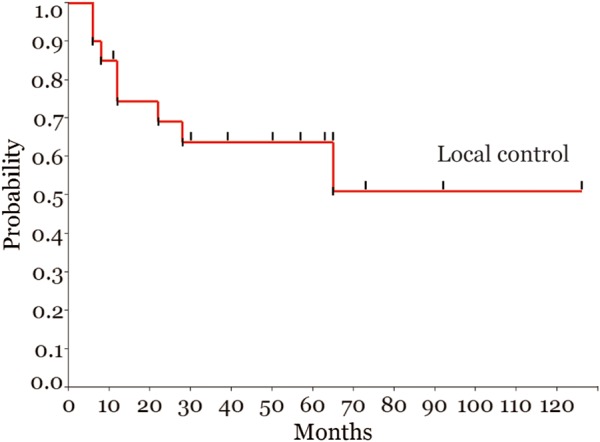
Local-control rate for all patients.
Figure 3.
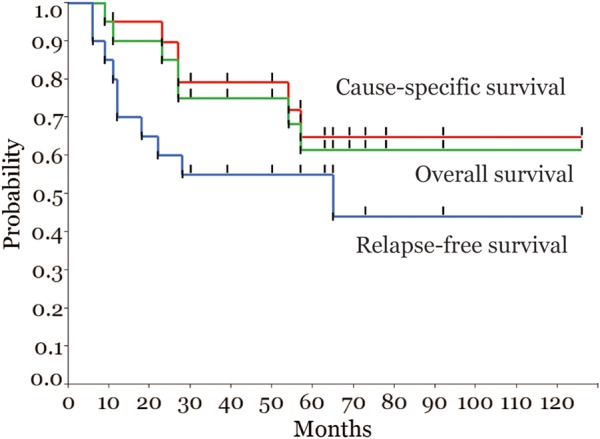
Overall survival, cause-specific survival and relapse-free survival for all patients.
DISCUSSION
Up to the early 1990s, RT for patients with T2-T4N0M0 bladder cancer achieved a 5-year overall survival of ∼30%. One report has suggested that severe and chronic adverse effects affecting the bladder and bowel are not uncommon for this cancer, including a treatment-related death rate of 9.5% (16). Meanwhile, the 5-year overall survival rate after radical cystectomy for these patients was ∼50%.
Since the late 1990s, several groups have reported on bladder preservation therapy through a combined modality approach for muscle-invasive bladder cancer (1–3). In all of these studies, to the best of our knowledge, patients with a CR after induction treatment were selected as candidates for bladder preservation, whereas in all the other patients, cystectomy was performed whenever feasible (i.e. selective bladder preservation). Shipley et al. (17) and Efstathiou et al. (18) reported that, in two series of patients at Massachusetts General Hospital, CMT preserved the native bladder of more than 70% of patients while offering a long-term survival rate similar to that of a contemporary cystectomy series; the details of treatment-related toxicities were not reported. A recent study in Italy on 121 patients reported 5-year overall, local-control and bladder-intact survival rates of 67.7, 56.3 and 51.2%, respectively (19). In this study, 15% (18/121) of patients had Grade 2, 3% (4/121) Grade 3 and 1% (1/121) Grade 4 bladder chronic adverse effects; moreover, 13% (16/121) had Grade 2 and 2% (2/121) had Grade 4 bowel chronic adverse effects. Rodel et al. reviewed the results of bladder-sparing CMT and reported 50–60% 5-year overall survival with 10% (18/186) Grade 2, 3% (5/186) Grade 3 and 2% (3/186) Grade 4 bladder chronic adverse effects; moreover, 5% (20/415) of patients had Grade 2 and 1.5% (6/415) Grade 4 bowel chronic adverse effects (20,21). Efstathiou et al. (22) reported, based on the data of Radiation Therapy Oncology Groups (RTOG) 8903, 9506, 9706 and 9906, that the rate of late Grade 3 or more genitourinary toxicity was 5.7% and that of Grade 3 or more gastrointestinal toxicity was 1.9%. Zietman et al. (23) also reported the results of a urodynamic and quality-of-life study on long-term survivors; the majority of patients retained good bladder function, but one-fifth of them had persistent bowel symptoms such as difficulty with bowel control or abdominal cramping or pain. These studies suggest that recent improvements in the RT technology are reducing bladder and bowel chronic adverse effects but that there is still room for improvement. The large margins and treatment fields used in the past often resulted in chronic adverse effects, but the small margins based on recent static computed tomography in treatment planning may cause geographical error.
The present study was based on a feasibility study conducted between 1998 and 1999 that showed that fiducial markers and two sets of fluoroscopy were useful to reduce setup errors compared with a skin-based setting for bladder and prostate cancer (10). We have also found that a method using multiple gold markers with frequent correction of the table position during RT is useful to reduce the error due to intrafractional organ motion (24). We inserted more than three markers around the tumor bed to be covered in the image-guided boost in principle, since the bladder wall is so flexible. At our institute, as well as at other institutes, a radical cystectomy is generally regarded as the first treatment of choice for muscle-invasive bladder cancer. Patients who were referred for RT were not suited for surgical treatment due to their poor medical condition or they refused radical cystectomy. Therefore, we did not perform radical cystectomy for the patients in this study, even in patients with residual tumor after induction treatment. For these reasons, it was difficult to compare our results with those of selective bladder preservation in both survival and local control. We set the minimum required 5-year overall survival at 30% based on the results of RT without any patient selection. The minimum required level of efficacy was set at 60% in 5-year overall survival because we believed that the efficacy of the image-guided definitive RT combined with maximal TUR-BT and chemotherapy were comparable with radical cystectomy. Moreover, we excluded patients who were unsuited for local-boost RT (i.e. patients with tumors that covered all or most of the bladder wall).
Currently, several types of image-guidance technology using not only implanted fiducial markers but also on-board kilovoltage cone-beam computed tomography (CBCT) and ultrasonography are being used. Offline and online adaptive RT using pre-planned treatment plans and CBCT has received much attention in light of its ability to reduce setup error and the margins required, thereby reducing treatment volumes and the volume of irradiated small bowel in external beam RT for bladder cancer (25–30). As for the motion detected by CBCT, Foroudi et al. (31) reported about the intrafractional bladder motion estimated from pretreatment and posttreatment using CBCT recently. In the article they found that the margins required to cover the intrafractional bladder changes from pretreatment to posttreatment in the superior, inferior, right, left, anterior and posterior were 1.25 cm (range, 1.19–1.50 cm), 0.67 cm (range, 0.58–1.12 cm), 0.74 cm (range, 0.59–0.94 cm), 0.73 cm (range, 0.51–1.00 cm), 1.20 cm (range, 0.85–1.32 cm) and 0.86 cm (range, 0.73–0.99 cm), respectively. They concluded that care is required while using image-guided radiation therapy protocols that reduce CTV to PTV margins based only on daily pretreatment soft tissue position.
The advantage of our method using fiducial markers and the RTRT system is that it enables us to observe the location of the target through the fiducial markers even during the beam delivery. In our series, the incidence of the sessions that the table position adjustments were required was 19.7% for correcting the intrafractional errors. The maximum offset in the superior, inferior, right, left, anterior and posterior direction were 1.47, 0.53, 0.38, 1.33, 0.89 and 0.50 cm, respectively. Based on the Foroudi's findings and ours, we consider that the intrafractional motion of the bladder may not be negligible. The relative positions of the markers in the bladder are not always constant, but the tumor exists in the area surrounded by the markers. Therefore, we believe that the multiple markers implanted around the tumor are still a useful surrogate of the tumor position. In the case that only one or two markers are implanted as in the early phase of this study, a larger margin is considered to be required.
A shortcoming of our method is that we are not seeing the bladder volume or the anatomical relationship between the marker and the tumor during irradiation. Regarding the doses to the bowel, smaller margins could theoretically realize a direct reduction in these doses. Extrapolating from the report of Tuomikoski et al. (27), which concluded that adaptive RT was beneficial to decrease irradiation of the small bowel in bladder cancer treatment, we could apply a smaller margin in boost irradiation than that used in their study without fiducial markers and therefore reduce the doses to the bowel. However, we should investigate more quantitatively the benefit of reducing bowel volume with our technique compared with other methods without fiducial markers and an RTRT system. We suspect that the combination of two sets of fluoroscopy with in-room CT or cone-beam CT is the best solution for bladder cancer at present.
The interruption of RT for marker implantation might deteriorate the effect of RT; however, our method achieved the minimum required level of 5-year overall survival and was comparable with other reports in terms of selective bladder preservation. In general, clinical data on the influence of OTT on the outcome of RT for bladder cancer is scarce and not conclusive. In a recent analysis, Majewski et al. (32) concluded that the OTT did not significantly influence the treatment outcome. Pos et al. (33) also reported that there is no evidence to support short OTTs or large fraction sizes in bladder cancer. Considering these reports, the interruption in this study might have had relatively less influence on the clinical outcome.
Our results showed that the incidence of Grade 3 toxicity was similar to or slightly higher than that in other non-IGRT series, despite the less number of patients. The possible reasons are (i) the overall dose irradiated to the bladder wall adjacent to the tumor could not be reduced and (ii) the patient who experienced Grade 3 gastrointestinal toxicity had received additional irradiation after the protocol treatment for pelvic lymph node recurrence.
The major limitation of our study is the less number of patients enrolled over a long period. Because the current standard treatment for locally advanced muscle-invasive bladder cancer (i.e. radical cystectomy and bladder-sparing CMT) is suboptimal, we were able to treat only patients who were clinically inoperable or refused surgery. In addition, the frequency of bladder cancer varies quite widely by geographic region. Japan has a lower incidence of bladder cancer than those reported in North America, Southern Europe and Western Europe. Therefore, we believe that the less number of patients in this series was not related to the treatment itself. The clinical outcome of the present method was at least comparable with the results of the recent CMT series with respect to local control and survival, despite the inclusion of patients who were considered inoperable and those who had hydronephrosis in this series.
CONCLUSIONS
Although the less number of patients and the long recruitment period limit the value of this study, our data suggest that both the implantation of fiducial markers and image-guided local-boost RT using an RTRT system can be safely accomplished, and the clinical outcome is encouraging. A larger prospective multi-institutional study is warranted for more precise evaluations of the technological efficacy and patients' quality of life.
Funding
This work was supported by the ‘Funding Program for World-Leading Innovative R&D on Science and Technology (FIRST Program),’ initiated by the Council for Science and Technology Policy (CSTP), Japan. This study was also supported by grant-in-aid no. 201020020A of the Ministry of Health, Labour, and Welfare in Japan. Funding to pay the Open Access publication charges for this article was also provided by the ‘FIRST Program’, initiated by the CSTP, Japan.
Conflict of interest statement
None declared.
Acknowledgements
We wish to express our heartfelt appreciation to Dr Toru Harabayashi, Dr Kei Kitamura and Dr Yasuhiro Osaka for their contributions to patient care and data collection.
References
- 1.Kaufman DS, Winter KA, Shipley WU, et al. The initial results in muscle-invading bladder cancer of RTOG 95–06: phase I/II trial of transurethral surgery plus radiation therapy with concurrent cisplatin and 5-fluorouracil followed by selective bladder preservation or cystectomy depending on the initial response. Oncologist. 2000;5:471–6. doi: 10.1634/theoncologist.5-6-471. [DOI] [PubMed] [Google Scholar]
- 2.Hagan M, Winter K, Kaufman D, et al. RTOG 97–06: initial report of a phase I–II trial of selective bladder conservation using TURBT, twice-daily accelerated irradiation sensitized with cisplatin, and adjuvant MCV combination chemotherapy. Int J Radiat Oncol. 2003;57:665–72. doi: 10.1016/s0360-3016(03)00718-1. [DOI] [PubMed] [Google Scholar]
- 3.Kaufman DS, Winter KA, Shipley WU, et al. Phase I–II RTOG study (99–06) of patients with muscle-invasive bladder cancer undergoing transurethral surgery, paclitaxel, cisplatin, and twice-daily radiotherapy followed by selective bladder preservation or radical cystectomy and adjuvant chemotherapy. Urology. 2009;73:833–7. doi: 10.1016/j.urology.2008.09.036. [DOI] [PubMed] [Google Scholar]
- 4.Harris SJ, Buchanan RB. An audit and evaluation of bladder movements during radical radiotherapy. Clin Oncol. 1998;10:262–4. doi: 10.1016/s0936-6555(98)80014-9. [DOI] [PubMed] [Google Scholar]
- 5.Turner SL, Swindell R, Bowl N, et al. Bladder movement during radiation therapy for bladder cancer: implications for treatment planning. Int J Radiat Oncol Biol Phys. 1997;39:355–60. doi: 10.1016/s0360-3016(97)00070-9. [DOI] [PubMed] [Google Scholar]
- 6.Marks L, Carroll P. The response of the urinary bladder, urethra, and ureter to radiation and chemotherapy. Int J Radiat Oncol Biol Phys. 1995;31:1257–80. doi: 10.1016/0360-3016(94)00431-J. [DOI] [PubMed] [Google Scholar]
- 7.Uehara T, Watanabe H, Itoh F, et al. Nephrotoxicity of a novel antineoplastic platinum complex, nedaplatin: a comparative study with cisplatin in rats. Arch Toxicol. 2005;79:451–60. doi: 10.1007/s00204-005-0648-6. [DOI] [PubMed] [Google Scholar]
- 8.Nakamura Y, Hasegawa M, Hayakawa K, et al. Induction of p53-dependent apoptosis in vivo by nedaplatin and ionizing radiation. Oncol Rep. 2000;7:261–5. doi: 10.3892/or.7.2.261. [DOI] [PubMed] [Google Scholar]
- 9.Ota K. Nedaplatin. Gan To Kagaku Ryoho. 1996;23:379–87. [PubMed] [Google Scholar]
- 10.Shimizu S, Shirato H, Kitamura K, et al. Use of an implanted marker and real-time tracking of the marker for the positioning of prostate and bladder cancers. Int J Radiat Oncol Biol Phys. 2000;48:1591–7. doi: 10.1016/s0360-3016(00)00809-9. [DOI] [PubMed] [Google Scholar]
- 11.Shirato H, Shimizu S, Kunieda T, et al. Physical aspects of a real-time tumor-tracking system for gated radiotherapy. Int J Radiat Oncol Biol Phys. 2000;48:1187–95. doi: 10.1016/s0360-3016(00)00748-3. [DOI] [PubMed] [Google Scholar]
- 12.Shirato H, Oita M, Fujita K, et al. Three-dimensional conformal setup (3D-CSU) of patients using the coordinate system provided by three internal fiducial markers and two orthogonal diagnostic X-ray systems in the treatment room. Int J Radiat Oncol Biol Phys. 2004;60:607–12. doi: 10.1016/j.ijrobp.2004.05.042. [DOI] [PubMed] [Google Scholar]
- 13.Viswanathan AN, Yorke ED, Marks LB, et al. Radiation dose–volume effects of the urinary bladder. Int J Radiat Oncol Biol Phys. 2010;76:S116–22. doi: 10.1016/j.ijrobp.2009.02.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Michalski JM, Gay H, Jackson A, et al. Radiation dose–volume effects in radiation-induced rectal injury. Int J Radiat Oncol Biol Phys. 2010;76:S123–9. doi: 10.1016/j.ijrobp.2009.03.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kavanagh BD, Pan CC, Dawson LA, et al. Radiation dose–volume effects in the stomach and small bowel. Int J Radiat Oncol Biol Phys. 2010;76:S101–7. doi: 10.1016/j.ijrobp.2009.05.071. [DOI] [PubMed] [Google Scholar]
- 16.Holmang S, Hedelin H, Borghede G, et al. Long-term followup of a bladder carcinoma cohort: questionable value of radical radiotherapy. J Urol. 1997;157:1642–6. [PubMed] [Google Scholar]
- 17.Shipley W, Zietman A, Kaufman D, et al. Selective bladder preservation by trimodality therapy for patients with muscularis propria-invasive bladder cancer and who are cystectomy candidates—The Massachusetts General Hospital and Radiation Therapy Oncology Group experiences. Semin Radiat Oncol. 2005;15:36–41. doi: 10.1016/j.semradonc.2004.07.008. [DOI] [PubMed] [Google Scholar]
- 18.Efstathiou JA, Spiegel DY, Shipley WU, et al. Long-term outcomes of selective bladder preservation by combined-modality therapy for invasive bladder cancer: the MGH experience. Eur Urol. 2012;61:705–11. doi: 10.1016/j.eururo.2011.11.010. [DOI] [PubMed] [Google Scholar]
- 19.Perdonà S, Autorino R, Damiano R, et al. Bladder-sparing, combined-modality approach for muscle-invasive bladder cancer: a multi-institutional, long-term experience. Cancer. 2008;112:75–83. doi: 10.1002/cncr.23137. [DOI] [PubMed] [Google Scholar]
- 20.Rodel C, Weiss C, Sauer R. Trimodality treatment and selective organ preservation for bladder cancer. J Clin Oncol. 2006;24:5536–44. doi: 10.1200/JCO.2006.07.6729. [DOI] [PubMed] [Google Scholar]
- 21.Rodel C. Combined-modality treatment and selective organ preservation in invasive bladder cancer: long-term results. J Clin Oncol. 2002;20:3061–71. doi: 10.1200/JCO.2002.11.027. [DOI] [PubMed] [Google Scholar]
- 22.Efstathiou JA, Bae K, Shipley WU, et al. Late pelvic toxicity after bladder-sparing therapy in patients with invasive bladder cancer: RTOG 89-03, 95-06, 97-06, 99-06. J Clin Oncol. 2009;27:4055–61. doi: 10.1200/JCO.2008.19.5776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zietman AL, Sacco D, Skowronski U, et al. Organ conservation in invasive bladder cancer by transurethral resection, chemotherapy and radiation: results of a urodynamic and quality of life study on long-term survivors. J Urol. 2003;170:1772–6. doi: 10.1097/01.ju.0000093721.23249.c3. [DOI] [PubMed] [Google Scholar]
- 24.Shimizu S, Osaka Y, Shinohara N, et al. Use of implanted markers and interportal adjustment with real-time tracking radiotherapy system to reduce intrafraction prostate motion. Int J Radiat Oncol Biol Phys. 2011;81:e393–9. doi: 10.1016/j.ijrobp.2011.04.043. [DOI] [PubMed] [Google Scholar]
- 25.Pos F, Remeijer P. Adaptive management of bladder cancer radiotherapy. Semin Radiat Oncol. 2010;20:116–20. doi: 10.1016/j.semradonc.2009.11.005. [DOI] [PubMed] [Google Scholar]
- 26.Lalondrelle S, Huddart R, Warren-Oseni K, et al. Adaptive-predictive organ localization using cone-beam computed tomography for improved accuracy in external beam radiotherapy for bladder cancer. Int J Radiat Oncol Biol Phys. 2011;79:705–12. doi: 10.1016/j.ijrobp.2009.12.003. [DOI] [PubMed] [Google Scholar]
- 27.Tuomikoski L, Collan J, Keyriläinen J, et al. Adaptive radiotherapy in muscle invasive urinary bladder cancer—an effective method to reduce the irradiated bowel volume. Radiother Oncol. 2011;99:61–6. doi: 10.1016/j.radonc.2011.02.011. [DOI] [PubMed] [Google Scholar]
- 28.Murthy V, Master Z, Adurkar P, et al. ‘Plan of the day’ adaptive radiotherapy for bladder cancer using helical tomotherapy. Radiother Oncol. 2011;99:55–60. doi: 10.1016/j.radonc.2011.01.027. [DOI] [PubMed] [Google Scholar]
- 29.Foroudi F, Wong J, Kron T, et al. Online adaptive radiotherapy for muscle-invasive bladder cancer: results of a pilot study. Int J Radiat Oncol. 2011;81:765–71. doi: 10.1016/j.ijrobp.2010.06.061. [DOI] [PubMed] [Google Scholar]
- 30.Button MR, Staffurth JN. Clinical application of image-guided radiotherapy in bladder and prostate cancer. Clin Oncol. 2010;22:698–706. doi: 10.1016/j.clon.2010.06.020. [DOI] [PubMed] [Google Scholar]
- 31.Foroudi F, Pham D, Bressel M, et al. Intrafraction bladder motion in radiation therapy estimated from pretreatment and posttreatment volumetric imaging. Int J Radiat Oncol Biol Phys. 2013;86:77–82. doi: 10.1016/j.ijrobp.2012.11.035. [DOI] [PubMed] [Google Scholar]
- 32.Majewski W, Maciejewski B, Majewski S, et al. Clinical radiobiology of stage T2–T3 bladder cancer. Int J Radiat Oncol Biol Phys. 2004;60:60–70. doi: 10.1016/j.ijrobp.2004.02.056. [DOI] [PubMed] [Google Scholar]
- 33.Pos FJ, Hart G, Schneider C, et al. Radical radiotherapy for invasive bladder cancer: what dose and fractionation schedule to choose? Int J Radiat Oncol Biol Phys. 2006;64:1168–73. doi: 10.1016/j.ijrobp.2005.09.023. [DOI] [PubMed] [Google Scholar]