Abstract
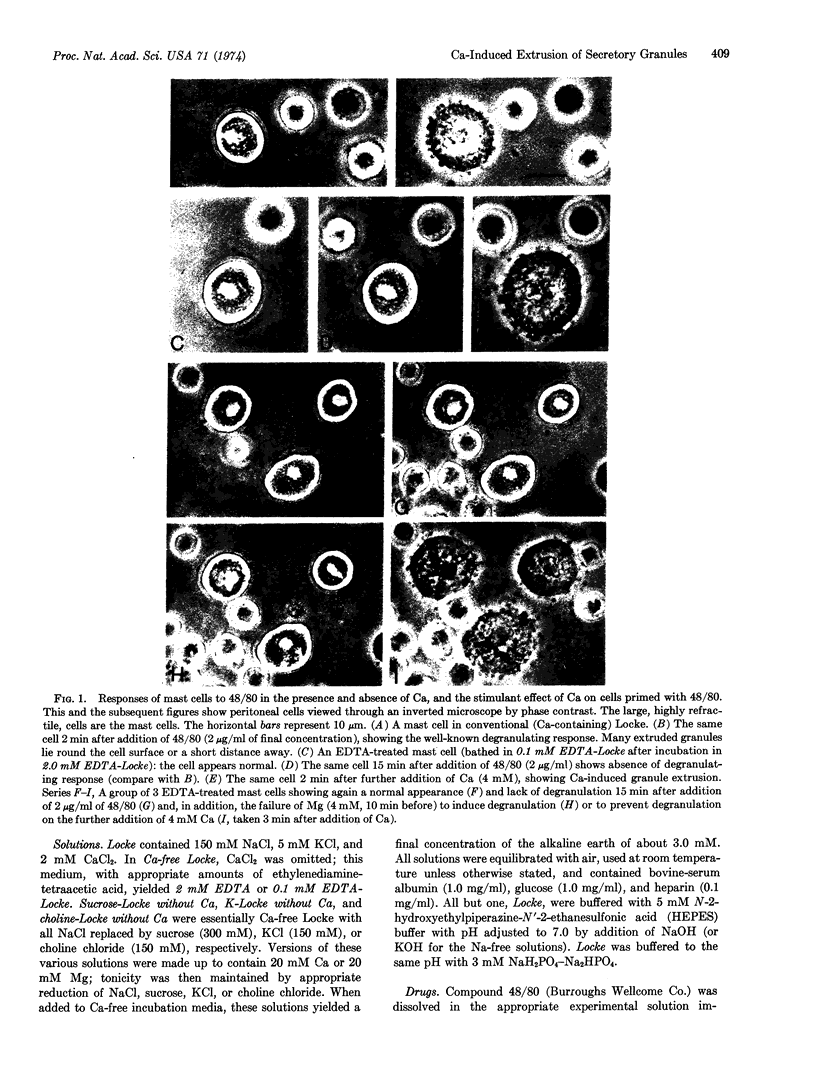
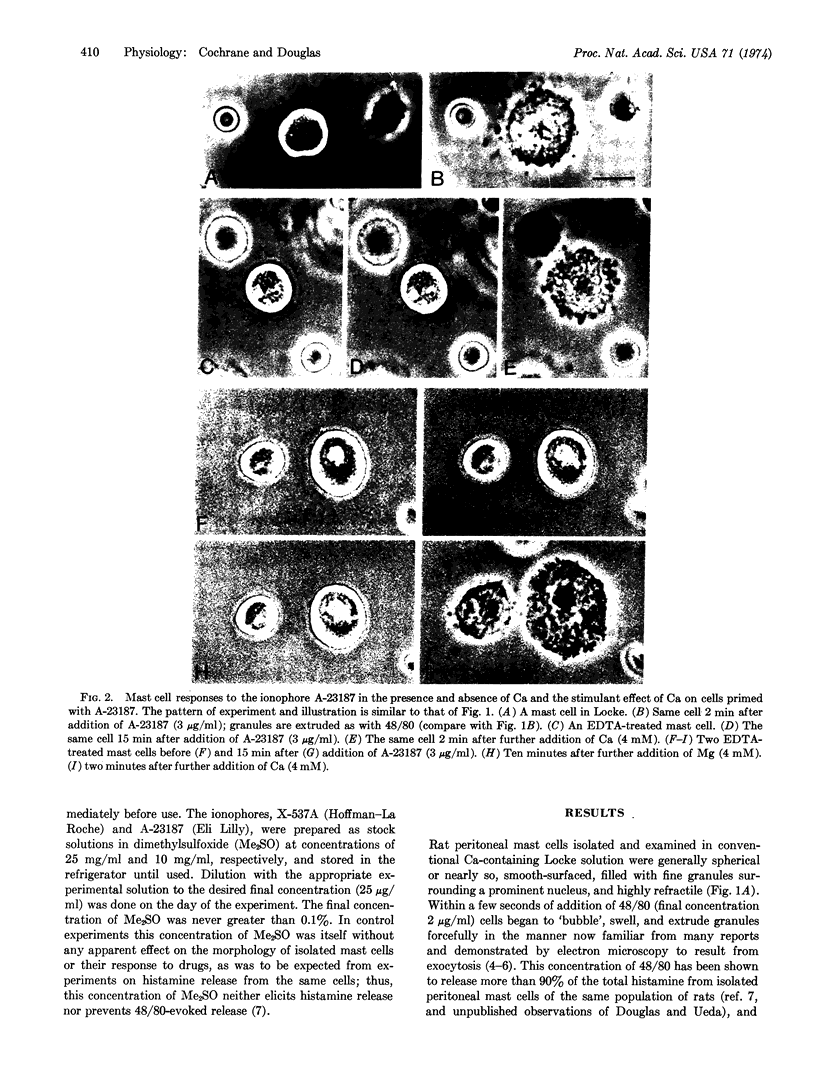
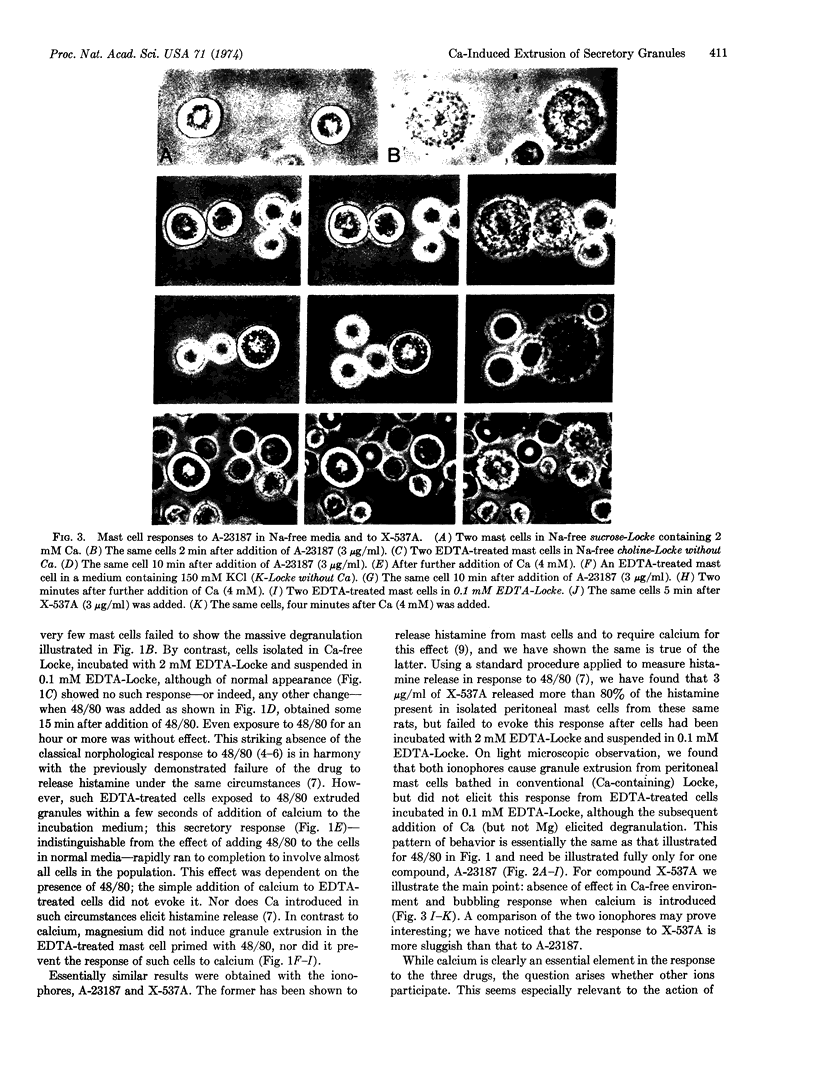
Isolated peritoneal mast cells from rats were observed, by phase contrast microscopy, to extrude secretory granules when exposed to 48/80 or the ionophores A-23187 and X-537A, which are known to facilitate transmembrane fluxes of calcium. These effects were abolished when the cells were treated with EDTA and suspended in a Ca-free environment. Ca-deprived cells exposed to any one of the three drugs promptly extruded granules when calcium, but not magnesium, was added to the incubation medium. Such Ca-evoked or Ca-dependent responses persisted when Na was omitted from the incubation medium and replaced with sucrose, choline, or K. The responses thus seem independent of possible shifts in the alkali metal ions. The results are considered support for the view that Ca influx mediates stimulus-secretion coupling and does so by initiating exocytosis.
Keywords: histamine secretion, monovalent and divalent cations, light microscopy, stimulus-secretion coupling
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- DOUGLAS W. W., RUBIN R. P. The role of calcium in the secretory response of the adrenal medulla to acetylcholine. J Physiol. 1961 Nov;159:40–57. doi: 10.1113/jphysiol.1961.sp006791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamant B., Kruger P. G., Uvans B. Local degranulation of individual rat peritoneal mast cells induced by compound 48-80. Acta Physiol Scand. 1970 May;79(1):1–5. doi: 10.1111/j.1748-1716.1970.tb04696.x. [DOI] [PubMed] [Google Scholar]
- Douglas W. W. Stimulus-secretion coupling: the concept and clues from chromaffin and other cells. Br J Pharmacol. 1968 Nov;34(3):451–474. doi: 10.1111/j.1476-5381.1968.tb08474.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas W. W., Ueda Y. Proceedings: Mast cell secretion (histamine release) induced by 48-80: calcium-dependent exocytosis inhibited strongly by cytochalasin only when glycolysis is rate-limiting. J Physiol. 1973 Oct;234(2):97P–98P. [PubMed] [Google Scholar]
- Horsfield G. I. The effect of compound 48/80 on the rat mast cell. J Pathol Bacteriol. 1965 Oct;90(2):599–605. doi: 10.1002/path.1700900228. [DOI] [PubMed] [Google Scholar]
- Kanno T., Cochrane D. E., Douglas W. W. Exocytosis (secretory granule extrusion) induced by injection of calcium into mast cells. Can J Physiol Pharmacol. 1973 Dec;51(12):1001–1004. doi: 10.1139/y73-153. [DOI] [PubMed] [Google Scholar]
- Lagunoff D. Membrane fusion during mast cell secretion. J Cell Biol. 1973 Apr;57(1):252–259. doi: 10.1083/jcb.57.1.252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pressman B. C. Properties of ionophores with broad range cation selectivity. Fed Proc. 1973 Jun;32(6):1698–1703. [PubMed] [Google Scholar]
- Rubin R. P. The role of calcium in the release of neurotransmitter substances and hormones. Pharmacol Rev. 1970 Sep;22(3):389–428. [PubMed] [Google Scholar]
- Röhlich P., Anderson P., Uvnäs B. Electron microscope observations on compounds 48-80-induced degranulation in rat mast cells. Evidence for sequential exocytosis of storage granules. J Cell Biol. 1971 Nov;51(21):465–483. doi: 10.1083/jcb.51.2.465. [DOI] [PMC free article] [PubMed] [Google Scholar]