Abstract
Individuals with anorexia nervosa (AN) engage in relentless, restrictive eating and often become severely emaciated. Because there are no proven treatments, AN has high rates of relapse, chronicity, and death. Those with AN tend to have childhood temperament and personality traits, such as anxiety, obsessions, and perfectionism, which may reflect neurobiological risk factors for developing AN. Restricted eating may be a means of reducing negative mood caused by skewed interactions between serotonin aversive or inhibitory and dopamine reward systems. Brain imaging studies suggest altered eating is a consequence of dysregulated reward, and/or awareness of homeostatic needs, perhaps related to enhanced executive ability to inhibit incentive motivational drives. Understanding the neurobiology of this disorder is likely to be important for developing more effective treatments.
Keywords: anorexia nervosa, eating disorders, dopamine, serotonin, fMRI, PET brain imaging
Introduction
Anorexia nervosa (AN) is a disorder of unknown etiology that tends to affect young women.1 This illness is characterized by restricted eating, severe emaciation, and distorted body image, as well as high rates of chronicity, morbidity, and mortality.1 It has a narrow range of age of onset (ie. early adolescence), stereotypic presentation of symptoms and course, and tends to be female gender specific. Although often considered to be caused by psychosocial factors, recent studies have shown that genetic heritability accounts for approximately 50–80% of the risk of developing an eating disorder (ED)2 and contributes to neurobiological factors underlying ED.3 A lack of understanding of the pathophysiology of these illnesses has hindered development of effective treatments. How are individuals with AN able to consume a few hundred calories per day and maintain an extremely low weight for many years, when most people struggle to lose a few pounds? It has been controversial as to whether individuals with AN have a primary disturbance of appetite regulation, or whether pathological feeding behavior is secondary to other phenomena, such as an obsessional preoccupation with body image.
Individuals with AN tend to have other puzzling symptoms and behaviors that are poorly understood, such as severe body image distortions, a lack of insight about being ill, as well as depriving themselves of food despite starvation (Box 1). Their disorder is ego-syntonic and they often refuse or resist treatment. How are these unique behaviors encoded in neural processes? Recent studies of obesity suggest that cortico-limbic neural processes, which encode the rewarding, emotional, and cognitive aspects of food ingestion, can drive over-consumption of food, even in the presence of satiety and replete energy stores.4–6 It has been suggested7 that obesity and addictions share overlapping brain circuits and monoamine systems that modulate reward sensitivity, incentive motivation, conditioning (memory/learning), impulse control (behavioral inhibition), stress reactivity, and interoceptive awareness. These studies have created a body of knowledge that could be used to “jump-start” advances towards understanding the neurobiology of AN. Interestingly, AN has contrasting symptoms, such as the under-consumption of food (despite being emaciated) and decreased rates of alcohol and drug abuse8, 9, which suggest differences in these neural processes compared to obesity and addiction. While such understanding is in its infancy, we argue in this review that it is important to know how symptoms and behaviors in AN are encoded in the brain, because this is necessary to improve treatment. Because of space limitations, this review will discuss selected behavioral traits in AN in relation to imaging results: first, evidence that harm avoidance (HA) is related to dopamine (DA) and serotonin (5-HT) function in AN, and second, the use of functional magnetic resonance imaging (fMRI) studies to reveal insights into the neural circuitry of gustatory sensory response, interoception, reward, and executive control.
Box 1: Example of a typical AN case.
E.P. was a woman who developed AN at age 13. Her parents describe her as a compliant but shy child, sensitive to change and criticism. She was anxious and worried about something happening to her parents. She was a perfectionistic child who got all A’s and was obsessively organized in terms of her clothes and desk. Around the age of 13, after starting menarche (she only had one menstrual period) she began to restrict her food intake and complained about feeling fat. At this point she was 5’2” and weighed about 95 lbs. She became a vegetarian, saying that she was disgusted by eating meat, and she started to overexercise, running several miles a day. She had increased concerns about making mistakes and increased worries over perceived consequences.
Over time, she developed more severe restricting behaviors, often refusing to eat with the family, and eating small low calorie meals with unusual food combinations (cabbage and ketchup) in a ritualized manner. While the parents noticed that she was losing weight, they were not terribly concerned about it because she continued to do well in school, and in fact, seemed even more driven towards accomplishment. When they took her to her pediatrician for a routine examination, they found she had lost more than 20 lbs and her blood pressure, pulse, and temperature were abnormally low. The pediatrician made a diagnosis of AN. E.P. was unconcerned and denied being emaciated, holding out her arm to the pediatrician and saying “can’t you see how fat I am.” She was hospitalized because of her medical instability. After many months of inpatient treatment she gained weight, but only with much struggle, as she often stated that gaining weight made her feel fat and nothing was more important to her than being thin. After discharge, she relapsed and was re-hospitalized, starting a cycle of inpatient weight gain and outpatient weight loss over the next 10 years, until her family, exhausted by the struggle gave up forcing her into treatment. Subsequently, she moved out, refused to have anything to do with her family because of their concerns about her weight and she died of malnutrition at the age of 25.
Temperament and personality in AN
While many individuals diet and seek to lose weight in our culture, relatively few develop AN. In fact, the prevalence is less than 1% of women.1 Individuals with AN tend to have certain temperament and personality traits, which often first occur in childhood before the onset of an ED, and may create a vulnerability to develop an ED. In addition to predating the disease, these traits often persist after recovery.10–14 These traits include anxiety, negative emotionality, perfectionism, inflexibility, HA, and obsessive behaviors (particularly with order, exactness, and symmetry). This personality and behavioral profile may constitute an intermediate phenotype between genes and vulnerability to AN. A brief overview of some of these behaviors is given below.
Anxiety
AN is associated with high anxiety that is premorbid to the illness11 and persists after weight restoration. This suggests an underlying anxious trait that is independent of nutritional status.13 Comorbid anxiety disorders occur significantly more frequently in AN individuals than in controls, with lifetime prevalence rates of up to 50%.15 The presence of childhood anxiety disorders predicts more severe ED symptoms, such as lower body mass index (BMI) and more psychopathology15, 16 and elevated anxiety is associated with poor outcome.17
Inhibitory self-control and reward
Individuals with AN have long been noted to be anhedonic and ascetic, able to sustain not only the self-denial of food but also most comforts and pleasures in life.18 Thus, an altered balance between reward and inhibition appears to be a hallmark of AN. For example, subjects with AN have an enhanced ability to delay reward (i.e., show less reduction in the value of a monetary reward over time) compared to healthy volunteers.19 AN subjects also have high punishment sensitivity and low reward reactivity during both the ill and recovered states.20
Set shifting
Adult ill and recovered AN subjects, as well as their unaffected sisters, show impaired set-shifting (i.e., rigid response to changing rules, elevated perseverative responses and switching errors),21–23 suggesting cognitive inflexibility is a trait marker of AN. However, mixed findings for impaired set-shifting in adolescent AN24–26 call into question whether set-shifting difficulties really are a predisposing trait, a result of chronic AN, or related to a particular stage of brain development.
Harm avoidance
A considerable body of literature shows that HA, a multifaceted temperament trait27 that contains elements of anxiety, inhibition, and inflexibility, is elevated in individuals who are ill with AN and persists after recovery12–14, 28, 29. AN adults are also less tolerant of uncertainty, and this is correlated with both HA and depression.30
Perfectionism
Individuals with AN tend to be perfectionistic, with an over-emphasis on self-imposed standards31. Perfectionism is a risk factor for AN; high levels of perfectionism precede the onset of AN32 and have been associated with poor recovery and shorter duration of remission.33, 34 Moreover, levels of perfectionism do not change with a reduction in the ED and comorbid psychiatric symptoms during recovery.34 Perfectionism may exert effects on appetite regulation as it has been shown to mediate the relationship between perceived criticism and restrictive dieting.35
Interoception and alexythmia
Altered interoceptive awareness might be a precipitating and reinforcing factor in AN.36–38 The role of the anterior insula in integrating interoceptive information (e.g., internal physical sensations including taste, pain, hunger) and altered insula activity found in individuals with AN (discussed below) supports the idea that they might suffer from a fundamentally and physiologically altered sense of self.39 Indeed, many of the symptoms of AN,40 such as distorted body image, lack of recognition of the symptoms of malnutrition (e.g., a failure to appropriately respond to hunger) and diminished motivation to change, could be related to disturbed interoceptive awareness. Such disturbances could also be tied to the preponderance of alexithymia (i.e. difficulty identifying emotions) in AN.41, 42 Alexithymia and disrupted interoceptive processing persist even after recovery and are thought to contribute to deficits in emotional processing in AN.43
Appetite regulation in AN
It is possible that many of the aforementioned factors contribute to pathological feeding behavior in AN. For example, there is an anxiety-reducing effect of dietary restraint and reduced daily caloric intake associated with AN,44, 45 whereas food consumption stimulates dysphoric mood.46 Moreover, enhanced inhibition, self-control, and/or an ability to delay reward may help to maintain persistent food restriction. Finally, disturbed interoceptive awareness of satiety or hunger, or even a primary alteration of primary gustatory processes, could play a role in assessing body states and responding to hunger cues.
DA and 5-HT: relationship to harm avoidance
Considerable data3 show that AN individuals have disturbances of DA and 5-HT systems. It has been proposed that 5-HT might play a role in altered satiety, impulse control, and mood, and that DA may be implicated in aberrant rewarding effects of food, motivation, or executive functions (i.e. inhibitory control, salience attribution, and decision-making). Because of space limitations, this review will focus on measures that assess 5-HT and DA levels in the brain, such as cerebrospinal fluid (CSF) measures of metabolites, positron emission tomography (PET), and single photon emission computed tomography (SPECT) brain imaging studies (Tables 1 and 2).
Table 1.
Summary of CNS 5-HT receptor and 5-HT transporter (5-HTT) PET and SPECT studies in AN in the ill and recovered state compared to healthy controlsa
| Receptor/ Transporter |
Imaging Type |
Ill | Recovered | Regions | Direction of changeb |
Refs |
|---|---|---|---|---|---|---|
| 5-HT2A | PET | n/a | AN | mesial temporal (amygdala and hippocampus), pre- and subgenual cingulate, occipital/parietal cortical regions | − | [122] |
| n/a | AN-BN | left subgenual cingulate, left parietal cortex, right occipital cortical regions | − | [123] | ||
| AN, AN-BN | n/a | n/a | = | [124] | ||
| SPECT | AN | n/a | left frontal, bilateral parietal, occipital cortical regions | − | [125] | |
| 5-HT1A | PET | n/a | AN | n/a | = | [126] |
| n/a | AN-BN | cingulate, lateral and mesial temporal, lateral and medial orbital frontal, parietal, and prefrontal cortical regions; dorsal raphe | + | [126] | ||
| AN, AN-BN | n/a | cingulate, lateral and mesial temporal, lateral and medial orbital frontal, parietal, and prefrontal cortical regions; dorsal raphe | + | [124] | ||
| n/a | AN | Right superior temporal, inferior frontal gyrus; parietal operculum, temporoparietal junction; left amygdala, parahippocampal gyrus, left temporal pole | + | [117] | ||
| AN | n/a | Right superior temporal, inferior frontal gyrus; parietal operculum, temporoparietal junction; left amygdala, parahippocampal gyrus, left temporal pole | = | [117] | ||
| 5-HTT | PEt | n/a | AN | n/a | = | [57] |
| n/a | AN-BN | n/a | = | [57] |
Abbreviations: AN, restricting-type AN; AN-BN, bulimic type AN.
+, increased compared to healthy controls; −, decreased compared to healthy controls; =, normal.
Table 2.
Correlations of 5-HT and DA D2/D3 receptor binding and Harm Avoidance (HA) in recovered and ill AN, as measured with PET imaginga.
| Receptor/ Transporter |
Ill | Recovered | Correlation with HAb | Regions | Refs |
|---|---|---|---|---|---|
| 5-HT2A | n/a | AN | no | n/a | [122] |
| n/a | AN-BN | + | Subgenual cingulate, mesial temporal | [123] | |
| AN, AN-BN | + | Supragenual cingulate, frontal, parietal | [124] | ||
| 5-HT1A | n/a | AN | + | Subgenual cingulate, mesial temporal | [126] |
| n/a | AN-BN | no | n/a | [126] | |
| DA D2/D3 | n/a | AN, AN-BN | + | Dorsal caudate, dorsal putamen | [18] |
| n/a | AN, AN-BN, BN | + | Dorsal caudate, dorsal putamen | [47] |
Abbreviations: AN, restricting-type AN; AN-BN, bulimic type AN; BN, bulimia nervosa
+, positive correlation
Alterations in the dopaminergic system
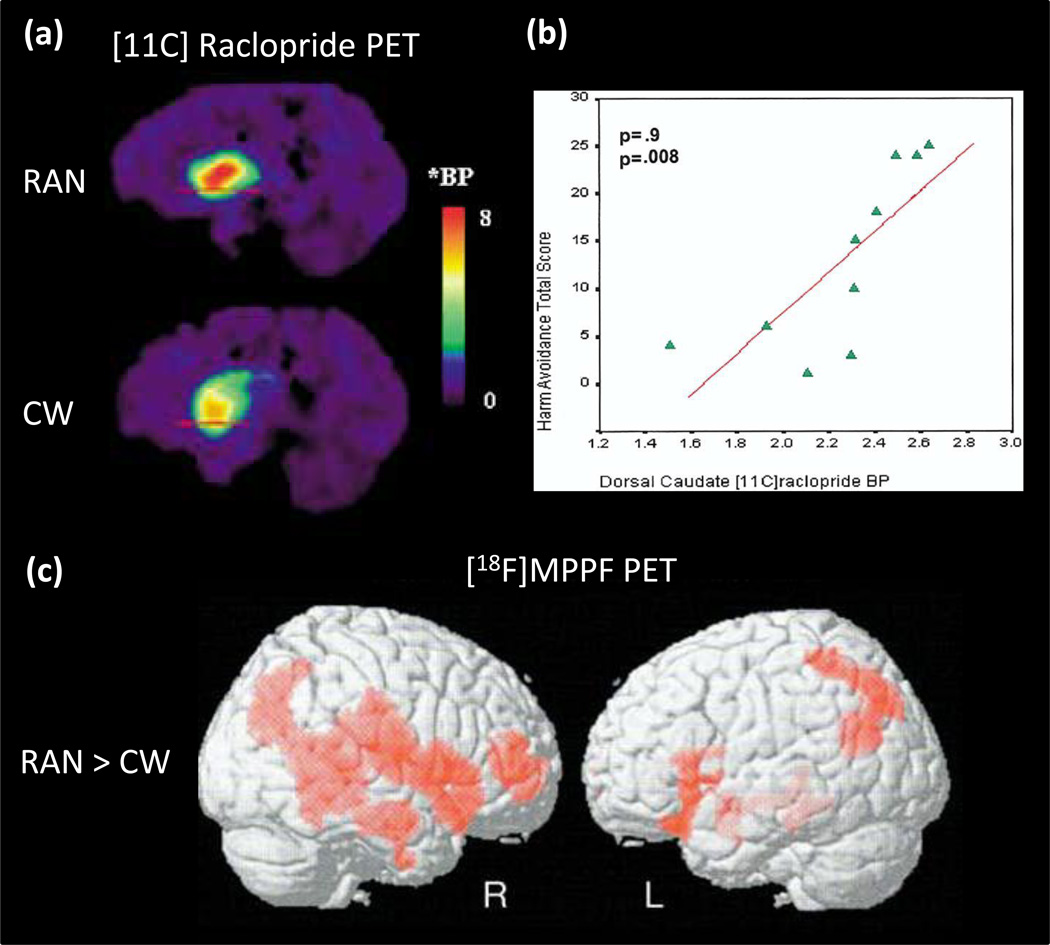
Using the radioligand [11C]raclopride, several PET studies have found that recovered AN subjects have increased binding of DA D2/D3 receptors in the anterior ventral striatum relative to controls18, 47 (Figure 1a). Because PET measures of [11C]raclopride binding are sensitive to endogenous DA concentrations,48 this difference could be due to either a reduction in the intrasynaptic DA concentration or an elevation of the density and/or affinity of the D2/D3 receptors in this region. The former hypothesis is supported by a finding that recovered AN individuals have a reduction of the DA metabolite homovanillic acid (HVA) in the CSF compared to control women.49
Figure 1.
Positron emission tomography (PET) studies show altered DA and 5HT function in AN. (a) Comparison of DA D2/D3 receptor binding in one recovered AN (RAN) and one age-matched control woman (CW)18; Red line indicates level of the right anteroventral striatum;BP, binding potential; *BP = [(region of interest/cerebellum/-1]. (b)Correlation (Spearman [rho] coefficient) of Harm Avoidance Total Score and Dorsal Caudate [11C]raclopride binding potential (BP) in recovered AN subjects18; p value Bonferroni corrected.(c) Statistical parametric mapping analysis the of 4-(2-methoxyphenyl)-1-[2-(N-2-pyridinyl)-p-fluorobenzamido]-ethylpiperazine ([18F]MPPF) binding for the “clinically recovered AN (REC AN)-Control subjects” contrast. Increased 5-HT1A binding (represented by red) is seen in recovered and lean (not shown) AN in widespread regions including the right temporofrontal cortex, amygdala-parahippocampal complex and temporoparietal junction117.
Alterations in the serotonergic system
PET and SPECT studies have assessed 5-HT1A, 5-HT2A receptors and the 5-HT transporter (5-HTT) in AN (for review, see50). Most, but not all studies, show that ill and recovered individuals with AN tend to have increased 5-HT1A binding (Figure 1c) and reduced 5-HT2A binding (Table 1). 5-HT2A and 5-HT1A postsynaptic receptors are highly co-localized (~80%) in the rodent frontal cortex51 and other cortical regions.52 Through interneurons, they mediate direct hyperpolarizing and depolarizing actions of 5-HT on prefrontal neurons that project to cortical and subcortical areas.53, 54 This leads to the interesting speculation that exaggerated 5-HT1A versus diminished 5-HT2A could result in hyperpolarizing effects on prefrontal neurons in AN. Interactions between 5-HT1A and 5-HT2A receptors in the medial prefrontal cortex (mPFC) and related regions have been found to modulate anxiety, attentional functioning55, impulsivity and compulsive perseveration,54 as well as exploration of novel environments.56 Differences in 5-HTT function could contribute to differences in impulse control, and thus explain why individuals develop AN (of the restricting type) versus AN-bulimic type (AN-BN ). While 5-HTT levels in AN and AN-BN individuals are comparable to controls (Table 1), 5-HTT differences have been observed when AN and AN-BN subgroups are compared. 57 These findings may be consistent with a recent study58 showing that the S-allele of the 5-HTTLPR (serotonin-transporter-linked polymorphic region in SLC6A4) genotype increases the risk susceptibility for diagnostic crossover over time in ill subjects.
Relationship between 5-HT, DA, and HA
Many of the PET studies show striking and consistent correlations between the binding potential of both 5-HT1A or 5-HT2A, DA D2/D3 (Figure 1b) and HA in cingulate, mesial temporal and parietal regions (Table 2). While the mechanisms by which 5-HT1A and 5-HT2A receptor function are related to HA remain uncertain, it is still possible to speculate how restricted eating may be one means of reducing anxiety. Recovered individuals were shown to have increased CSF levels of 5-hydroxyindoleacetic acid, the major brain metabolite of 5-HT,59 while those who were ill with AN had reduced concentrations.60 One study61 showed that depletion of tryptophan (the precursor of 5-HT), which results in a reduction of 5-HT activity, decreased anxiety in AN. The 5-HT system consists of 14 or more receptors, and an integration of many other neurochemicals. Whether HA is solely related to 5-HT1A and 5-HT2A receptors or, in all likelihood, involves more complex mechanisms, remains beyond the abilities to determine using current technology.
HA is also positively associated with dorsal caudate DA D2/D3 binding potential in recovered AN/AN-BN18, 47 (Table 2). Consistent with this finding, an fMRI study62 showed baseline trait anxiety to be positively correlated with dorsal caudate blood oxygen level dependent (BOLD) response to positive and negative feedback. These findings are consistent with studies in rats that show D2 receptors within the dorsomedial striatum are associated with response inhibition,63 and that rats characterized as risk-averse have greater D2 mRNA expression within the dorsal striatum.64 This suggests that DA signaling within executive corticostriatal circuitry may reflect a heightened response to adverse consequences and/or increased inhibitory control.65 These data support the provocative possibility that aberrant function of the dorsal caudate creates a vulnerability toward developing anxious, inhibited, shy, inflexible, and risk averse behaviors – traits that are often observed in childhood before the onset of AN.
DA, anxiety, and restricted eating
In order to further explore DA function and behaviors, a recent study used PET [11C]raclopride binding with amphetamine to assess endogenous DA release.66 This study confirmed that DA release in the precommissural dorsal caudate was associated with increased anxiety in recovered subjects, whereas control subjects showed the well-known euphoria associated with anterior ventral striatum DA release. Ingestion of palatable food is associated with striatal endogenous DA release.67, 68 If AN individuals experience endogenous DA release as anxiogenic, rather than hedonic, it may explain their pursuit of starvation, because food refusal may be an effective means of diminishing the anxious feelings associated with the disorder.
Competition between DA and 5-HT
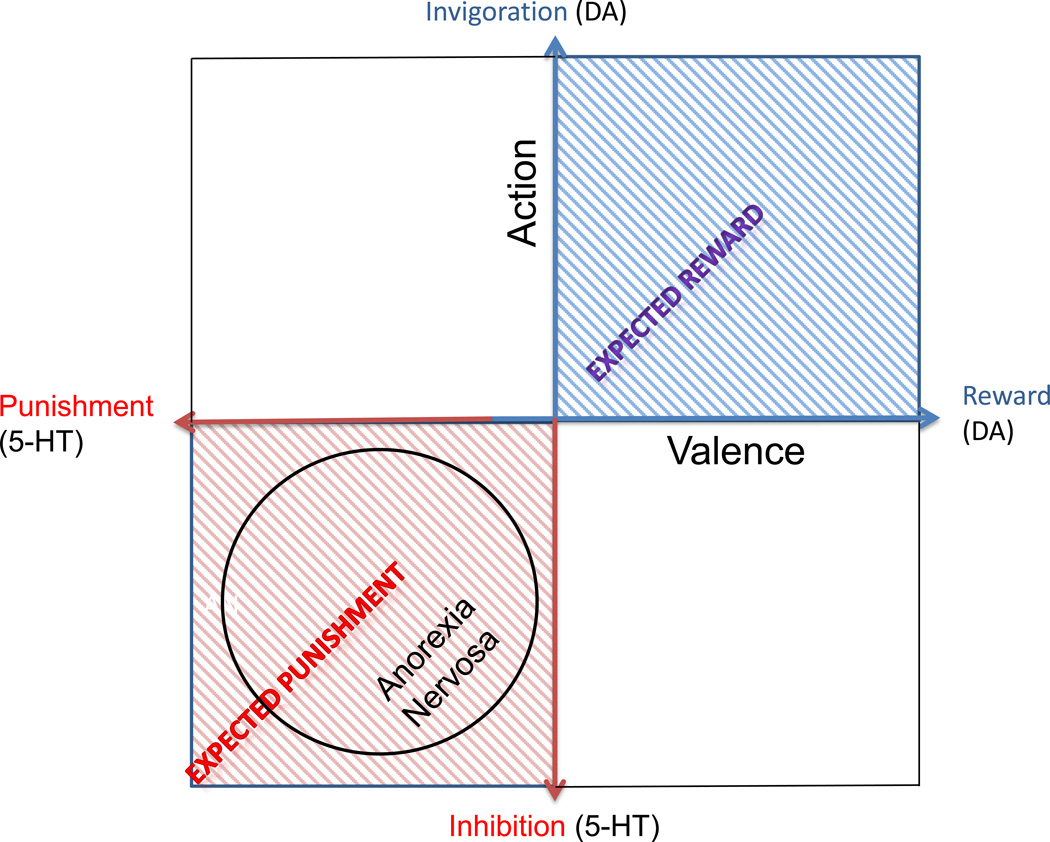
It has been theorized that 5-HT is the crucial substrate of an aversive motivational system that might oppose a DA-related appetitive system.69, 70 That is, 5-HT has a critical role in the adaptation of animals to aversive events71, 72 and may mediate a negative prediction error signal for future threat and punishment.69, 70 While DA has been associated with the expression of an appetitive reward system,73 it is plausible that it works in mutual opponency with a system that signals the prediction of punishment instead of reward.69, 72 From another perspective, studies suggest that 5-HT has a role in action choice by controlling the timescale of delayed rewards through differential effects on ventral and dorsal striatal circuits.74, 75 We hypothesize (Figure 2) that AN individuals have a temperament skewed towards aversive or inhibitory response, rather than reward and motivation. It is important to note that 5-HT and DA neural systems are complex. For example, the 5-HT system has 14 or more receptors and many other components that modulate metabolism, firing rate, neuronal cascades, etc. Still, there is evidence supporting the possibility that interactions between 5-HT and DA contribute to temperament in AN. For instance, a PET study of a mixed group of subjects with recovered ED showed a significant positive correlation between 5-HTT and DA D2/D3 binding for the dorsal caudate, antero-ventral striatum, medial caudate, ventral putamen and dorsal putamen.47 A linear regression analysis showed that the interaction between 5-HTT and DA D2/D3 binding potential in the dorsal putamen significantly predicted HA, while there was a trend towards significance in the dorsal caudate.
Figure 2.
A hypothetical model illustrating the competition/cooperation between DA and 5-HT and the implications for AN. This model is based on previous models118–121 and shows the hypothetical interaction between 5-HT functional activity (aversive or inhibitory) and DA functional activity (reward or motivation).69, 70 Measures of CSF metabolites suggest that recovered AN subjects have increased brain 5-HT59 and decreased brain DA.49 This suggests that individuals with AN may have a temperament that places them in the lower left quadrant of this model, supporting the hypothesis of a skew towards aversive or inhibitory responses, rather than reward and motivation.
A substantial limitation of neurotransmitter studies in humans is that only a few neuromodulatory components can be measured, and there are currently no means to measure or model the functional interactions of the many biochemical mechanisms that make up these complex systems. While an understanding of molecular mechanisms remains far beyond the abilities of technologies available to study in humans, these are still potentially valuable insights. As yet, there are no medications or other treatments that have been proven to reverse core symptoms in AN. These findings may help identify drugs that act on 5-HT and/or DA neural processes that may be best suited to reduce HA behaviors in AN. For example, it has been debatable 76, 77 whether olanzapine, a drug with 5-HT and DA effects, is useful for weight gain in AN, though, such a drug may be effective in diminishing anxiety in AN patients.78
fMRI studies of appetite: Relationship to food taste
fMRI imaging studies of appetitive behaviors in ED have used designs that either administer a taste of some food or present images of food to participants. The neurocircuitry of taste processing in healthy individuals is well understood.5, 7, 79 The anterior insula is the primary gustatory taste cortex and responds to tastes of food (see review3). The anterior insula, as well as the anterior cingulate cortex and orbital frontal cortex, code the sensory-hedonic response to taste and innervate a broad region of the rostral ventral-central striatum, where behavioral repertoires are then computed. This network may play a crucial role in linking sensory-hedonic experiences to the motivational components of reward as well as emotionality, providing conscious awareness of these urges. This review will focus on studies that administer tastants and the interrogation of this circuitry using this method. Though equally valuable, space limitations preclude a discussion of response to food images (see 80, 81).
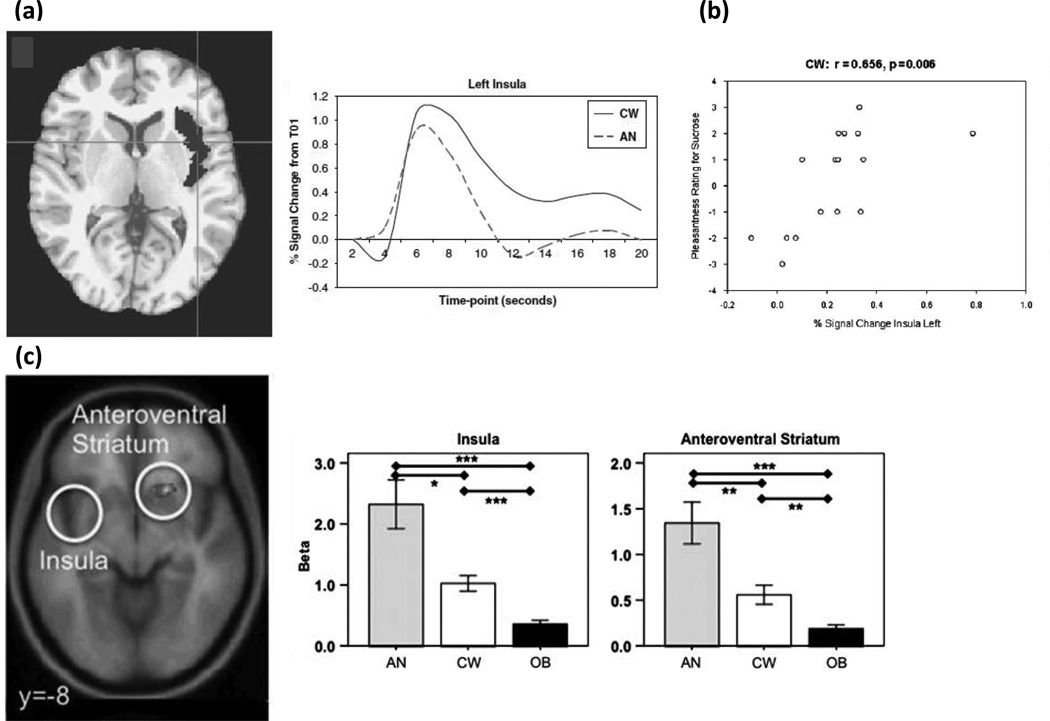
While there are relatively few tastant studies in AN, the literature to date is relatively consistent between the ill and recovered states, supporting the possibility that altered reward and interoceptive processing are trait characteristics of the disorder. In one of the first studies that tested the reward processes behind interoceptive stimuli, tastes of sucrose and water were administered to recovered AN subjects82 (Figure 3a). Compared to control women, recovered AN subjects showed lower neural activation of the ventral and dorsal striatum, as well as the insula (including the primary cortical taste region) to both sucrose and water. Moreover, insular neural activity correlated with pleasantness ratings for sucrose in controls, but not AN subjects (Figure 3b). In a more recent study, hunger and satiety states were compared in ill AN and control subjects when drinking chocolate milk.83 In the fasting state, the insula was significantly activated in response to drinking chocolate milk in controls but not ill AN. These findings suggest minimal insula response in AN to the tastes of food when hungry. In contrast, imaging studies in healthy individuals have consistently shown that food deprivation, when compared to states of satiety, activates the insula and the orbital frontal cortex.84 Considered together, these findings raise the intriguing possibility that AN subjects have an altered set-point, and/or altered sensitivity for sensory-interoceptive-reward processes, when consuming palatable foods.79 Thus, the set-point may mimic a continuous state of satiety in individuals with AN that limits interoceptive and reward processing.
Figure 3.
Altered neurocircuitry of taste consumption and anticipation in AN revealed with fMRI. (a) Decreased taste-related (sucrose and water) BOLD response was found for recovered AN compared to control women (CW) in the left insula region of interest (ROI; highlighted in gray in axial view) and left ventral putamen (not shown). Corresponding time course of the BOLD signal as a mean of 16 AN and 16 CW in the left insula ROI is depicted.82 This suggests in AN the insula may not accurately encode gustatory signals, perhaps as part of a more widespread defect in interoceptive awareness, or perhaps encompassing altered ability to gauge satiety or hunger “set-points.” Insula responses may also be modulated by reward determination (e.g., in anterior ventral striatum circuits, specifically the nucleus accumbens) which may fail to properly recognize, scale, or modulate reward response in AN. (b) In support of altered interoceptive/reward processing, BOLD response is correlated with pleasantness rating for sucrose for control women in the left and right (not shown) insula but not for recovered AN (not shown).82 (c) In response to a taste reward conditioning task that has been associated with activation of dopamine reward circuits, computational model-derived data revealed ill underweight AN had greater brain response during anticipation of taste in the anteroventral striatum, insula, and prefrontal cortex compared to control women and obese (OB) women.86 These results suggest that brain reward circuits are more responsive to food stimuli in AN, but less responsive in obese women. The mechanism for this association is uncertain, but these brain reward response patterns could be biomarkers for the respective weight state.
Previous studies of obese subjects have highlighted the importance of anticipation in the neural response to food stimuli (e.g. 85). Animal studies show that following stimulus conditioning, DA neurons shift firing from the consumption of food to the anticipated consumption of food or to cues associated with food consumption.85, 86 There are a number of studies using tastants in ED in which the design might elicit anticipatory responses. For instance, a recent study (Figure 3c) performed an associative learning task between conditioned visual stimuli and unconditioned sucrose taste stimuli in ill AN, control, and obese participants.86 Reward learning signals in the anterior ventral striatum, insula, and the orbitofrontal cortex (OFC) were greater in the AN subjects and less in the obese participants compared to controls. These findings led to the conclusion that brain reward circuits were more responsive in AN and less responsive in obese participants. In another study, a complex design in which recovered AN and control participants viewed and tasted pleasant/unpleasant stimuli, either alone or paired together was used.87 Recovered AN subjects had increased ventral striatal activity in response to sights and flavors of a pleasant stimuli (i.e. chocolate) and increased insula and posterior dorsal caudate response to flavors and sights of aversive foods as compared to controls. Taken together, these studies suggest that AN subjects may be highly responsive to stimulus cues, perhaps as a mechanism to predict and control the anxiety produced by stimuli that may be associated with subjective unpleasantness. This type of anticipatory sensitivity connected with stimulus avoidance has been previously seen in highly anxious individuals.88
The imbalance between stimulus consumption and anticipation in AN may suggest dysfunctional stimulus integration that could relate to the clinically observed disconnect between reported and actual interoceptive states. Key in this process is the insula. This brain region is thought to code interoceptive prediction error, signaling mismatch between actual and anticipated bodily arousal, which in turn, elicits subjective anxiety and avoidance behavior.89 A study of response to pain confirms a mismatch between anticipation and objective responses in recovered AN individuals.42 In this study, recovered AN compared to control participants showed greater activation within the right anterior insula, dorsolateral prefrontal cortex (DLPFC) and cingulate cortex during pain anticipation, and greater activation within the DLPFC and decreased activation within posterior insula during painful stimulation. Greater anticipatory anterior insula activation correlated positively with alexithymia (i.e. difficulty identifying emotions) in recovered AN subjects. Alexithymia assessment provided additional evidence of an altered ability to accurately perceive or predict bodily signals, which persists even after recovery. Given that food is an inherently emotional stimulus in AN, a mismatch between limbic hyperarousal and executive control may be tied to interoceptive and/or emotional processing deficits in this population.
fMRI studies of reward and executive control
A series of fMRI studies using response to reward or executive control tasks has explored the role of ventral and dorsal corticostriatal systems in AN in comparison to controls to better understand the modulation of reward, emotionality, and behavioral inhibition. Control subjects have been found to have patterns of limbic striatal response that significantly distinguish positive and negative feedback.90 In contrast, another study reported that recovered AN subjects had minimal differences to positive and negative feedback in limbic striatal regions and an exaggerated response in the dorsal caudate and associated executive regions relative to control women.62 Interestingly, and in support of a relationship between anxiety and dorsal caudate DA findings (as described above), dorsal caudate activity was positively associated with baseline trait anxiety for both positive and negative feedback in AN.62 In another study that used a set-shifting fMRI paradigm, ill AN subjects showed predominant activation of frontoparietal networks, indicative of excessive effortful and supervisory cognitive control during task performance, but hypoactivation in the ventral anterior cingulate-striato-thalamic loop involved in motivation-related behavior.91
Other studies using different tasks have found evidence of abnormal function in executive circuitry in AN. For example, using a Go/NoGo task, AN-BN adolescents showed greater activation than control subjects in the anterior cingulate cortex, and greater activation compared to both controls and AN adolescents in the right DLPFC.92 Furthermore, AN participants showed a positive correlation between percent correct on NoGo trials and activation in inferior parietal cortex regions. Another study, using a stop signal task, found that recovered AN subjects had altered task-related activation in the mPFC, a critical node of the inhibitory control network.93
These data support the hypothesis3 that AN individuals have an imbalance within and/or between ventral limbic and dorsal executive circuits. Specifically, a ventral limbic neural circuit [which includes the amygdala, anterior insula, anterior ventral striatum, ventral regions of the anterior cingulate cortex (ACC) and the OFC] is involved in identifying rewarding and emotionally significant stimuli required to generate affective responses to these stimuli94, 95. The anterior ventral striatum processes motivational aspects of stimuli by modulating the influence of limbic inputs on striatal activity96, 97. Decreased anterior ventral striatum responses to cues may reflect a failure to appropriately bind, scale, or discriminate responses to salient stimuli. This supports the possibility that recovered AN subjects may have an impaired ability to identify the emotional significance of stimuli.
A dorsal executive function neural circuit (which includes the dorsal regions of the caudate, DLPFC, parietal cortex, and other regions) is thought to modulate selective attention, planning, and effortful regulation of affective states. The ventral limbic and dorsal executive neural circuits are critically involved in inhibitory decision-making processes, especially involving reward-related behaviors. Together they process the reward value and/or affective valence of environmental stimuli, assess the future consequences of one’s own actions (response selection) and inhibit inappropriate behaviors (response inhibition). Dysfunction within these regions has been proposed to be a key neural mechanism underlying altered behavioral regulation, reward regulation, or cognition found in drug addiction.98, 99 In summary, individuals with AN may have an imbalance in information processing, with impaired ability to identify the emotional significance of a stimulus, but increased traffic in neurocircuits concerned with planning and consequences leading to heightened anxiety. This over-reliance on executive brain circuits involved in linking action to outcome may constitute an attempt at "strategic" (as opposed to hedonic) means of responding to reward stimuli. 100 These studies provide further evidence that exaggerated dorsal executive function is associated with enhanced inhibition and may reflect a possible means of modulating anxiety in AN.
Concluding remarks
Considerable evidence shows that individuals with AN have anxious, inhibited, and inflexible premorbid characteristics. It is possible that these premorbid traits are related to altered monoamine neuronal modulation, or dorsal caudate function, that predates the onset of AN. Several factors might exacerbate these vulnerabilities to cause the onset of AN in adolescence. First, puberty-related female gonadal steroids101 might exacerbate 5-HT and DA system dysregulation. Brain changes associated with puberty might further challenge these processes. For example, increased activity of orbital and DLPFC regions during and after puberty102 might contribute to excessive worry, perfectionism, and strategizing in AN patients. Finally, stress and/or cultural and societal pressures might contribute by increasing anxious and obsessional temperament. Still, relatively little work has been done investigating the psychobiology of this disorder compared to other severe psychiatric disorders, leaving many questions still unanswered (Box 2).
Box 2. Outstanding questions.
Why is it important to understand the neurobiology of disturbed behavior in AN? Several potential hypotheses might explain why AN individuals are perfectionistic. They may have poor prediction certainty, as evidenced by alexithymia. Alternatively, they may have exaggerated responses to negative feedback and a diminished response to reward, so they perceive their world constantly having errors. New treatments could be developed to teach skills to overcome these traits. However, these two hypotheses might require very different psychological interventions. Thus, understanding how perfectionistic behavior is manifested and encoded in neural circuits may be necessary to develop specific and effective treatments.
State and trait in AN: Cause and effect between pathological eating and the impact of malnutrition on neural processes in AN remain a major methodological question. Malnutrition in AN is associated with changes in brain structure (e.g., reduction in gray matter, altered white matter integrity) and profound metabolic, electrolyte, and endocrine disturbances.106–110 Studies in animals suggest that diet and weight can influence DA111, 112 and 5-HT metabolism.61 Strategies to avoid the confounding effects of abnormal nutritional status include: 1) characterizing behavioral traits that occur in childhood, prior to the onset of an ED, and 2) studying recovered anorexics, although it remains conjectural whether abnormal findings reflect traits or scars.
AN subtypes: Restricting-type anorexics (i.e. AN) lose weight purely by restricted dieting. Over time, up to 50% of these individuals will also develop purge and/or binge behaviors (AN-BN) while the rest remain “pure” restrictors.113 Many studies do not differentiate between these subtypes. Currently, the neurobiological factors that differ between the subtypes or are associated with the transitioning between the subtypes are not well understood. One possibility114 is that the subtypes have differential effects on impulse control modulation.
What is the underlying neural circuitry of AN?: Understanding how puzzling AN behavior is encoded in neural circuitry is a major methodological challenge for the ED field. Several fMRI strategies can be used to investigate brain and behavior. One is to provoke AN symptoms (e.g. body image distortions) and determine what regions are abnormal. Alternatively, a focus on tasks designed to interrogate reward processing (e.g., response to positive and negative feedback) can be used to determine differences in a priori identified neural circuitry. Both methods are valuable. Still, symptoms associated with AN (e.g., body image distortion) could be secondary, and serve to make sense of other powerful aberrant drives. We think it is most critical to understand how premorbid temperament traits are encoded in brain circuits, because these may be key traits essential for developing AN.
What about body image distortion? This may be the most puzzling of all AN symptoms, in part because AN individuals feel fat, but tend to have normal perceptions of other people’s bodies. A recent review115 concluded that the perceptive component was related to alterations of the precuneus and the inferior parietal lobe, and the affective component was related to alterations of the prefrontal cortex, the insula and the amygdala. While the mechanism remains obscure, a recent study116 points to impaired integration of visual and proprioceptive information.
AN individuals tend to find that restricting food intake provides a temporary respite from dysphoric mood. People with AN may enter a vicious cycle — which could account for the chronicity of this disorder — because eating exaggerates, and food refusal reduces, an anxious mood. Imaging studies of neural processes are in their infancy and molecular mechanisms remain unexplored. Still, studies that have investigated monoamine function support the hypothesis that AN temperament and personality might be related to exaggerated performance of serotonin systems that mediate a negative prediction error signal for future threat and punishment and diminished effects of DA appetitive reward systems. From another perspective, fMRI studies suggest there are disturbances of higher-order circuits related to interoception, reward, emotionality, and inhibition that regulate approach and avoidance of food. However, cause and consequence remain unclear. For example, it is possible that hyperactive executive circuits directly motivate actions when the ability of the ventral striatal pathways to direct more ‘automatic’ or intuitive motivated responses is impaired. Another possibility is that in AN patients (otherwise adequate) limbic-striatal information processing in the ventral circuit is too strongly inhibited by converging inputs from executive domains. Moreover, diminished reward, combined with increased executive control could contribute to alexithymia through the suppression or misinterpretation of perturbed body signals from the insula.
It has been difficult to modify core traits of ED in treatment settings. New insights into the neurobiology of personality and temperament in AN hold promise in this regard. First, they may inform the development of medications targeting the biology of AN that contributes to certain symptoms, such as anxiety. While this may not be a “cure” for this illness, it may substantially improve the ability to eat and maintain weight. Second, they may guide the development of more effective psychotherapies to elicit behavioral changes that are educated by robust and neurally derived principles of the internal mechanisms of AN.
Acknowledgements
Supported by grants from the National Institute of Mental Health (MH046001, MH042984, MH066122; MH001894 and MH092793), the Price Foundation, and the Davis/Wismer Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclaimer statement: Authors have no conflicts to declare
References
- 1.APA. Diagnostic & Statistical Manual of Mental Disordes: DSM:VI-TR. 4th edition. Washington, DC: 2000. [Google Scholar]
- 2.Bulik C, Sullivan PF, et al. Prevalence, heritability and prospective risk factors for anorexia nervosa. Archives of General Psychiatry. 2006;63:305–312. doi: 10.1001/archpsyc.63.3.305. [DOI] [PubMed] [Google Scholar]
- 3.Kaye W, Fudge J, et al. New insight into symptoms and neurocircuit function of anorexia nervosa. Nat Rev Neurosci. 2009;10:573–584. doi: 10.1038/nrn2682. [DOI] [PubMed] [Google Scholar]
- 4.Berthoud H. Homeostatic and non-homeostatic pathways involved in the control of food intake and energy balance. Obesity. 2006;14(Suppl 5):197S–200S. doi: 10.1038/oby.2006.308. [DOI] [PubMed] [Google Scholar]
- 5.Rolls E. Brain mechanisms underlying flavour and appetite. Philos Trans R Soc Lond B Biol Sci. 2006;361:1123–1136. doi: 10.1098/rstb.2006.1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Small D. Central gustatory processing in humans. Adv Otorhinolaryngol. 2006;63:191–220. doi: 10.1159/000093761. [DOI] [PubMed] [Google Scholar]
- 7.Volkow N, Wang G, et al. Food and drug reward: overlapping circuits in human obesity and addiction. Curr Top Behav Neurosci. 2012;11:1–24. doi: 10.1007/7854_2011_169. [DOI] [PubMed] [Google Scholar]
- 8.Calero-Elvira A, Krug I, et al. Meta-analysis on drugs in people with eating disorders. Eur Eat Disord Rev. 2009;17:243–259. doi: 10.1002/erv.936. [DOI] [PubMed] [Google Scholar]
- 9.Gadalla T, Piran N. Co-occurrence of eating disorders and alcohol use disorders in women: a meta analysis. Arch Womens Ment Health. 2007;10:133–140. doi: 10.1007/s00737-007-0184-x. [DOI] [PubMed] [Google Scholar]
- 10.Anderluh MB, Tchanturia K, et al. Childhood obsessive-compulsive personality traits in adult women with eating disorders: defining a broader eating disorder phenotype. American Journal of Psychiatry. 2003;160:242–247. doi: 10.1176/appi.ajp.160.2.242. [DOI] [PubMed] [Google Scholar]
- 11.Kaye W, Bulik C, et al. Comorbidity of anxiety disorders with anorexia and bulimia nervosa. Am J Psychiatry. 2004;161:2215–2221. doi: 10.1176/appi.ajp.161.12.2215. [DOI] [PubMed] [Google Scholar]
- 12.Cassin S, von Ranson K. Personality and eating disorders: a decade in review. Clinical Psychology Review. 2005;25:895–916. doi: 10.1016/j.cpr.2005.04.012. [DOI] [PubMed] [Google Scholar]
- 13.Wagner A, Barbarich N, et al. Personality traits after recovery from eating disorders: Do subtypes differ? International Journal of Eating Disorders. 2006;39:276–284. doi: 10.1002/eat.20251. [DOI] [PubMed] [Google Scholar]
- 14.Lilenfeld L. Personality and temperament in eating disorders. In: Kaye WH, Adan R, editors. Current Topics in Behavioral Neurosciences. New York: Springer; 2011. [DOI] [PubMed] [Google Scholar]
- 15.Raney T, Thornton L, et al. Influence of overanxious disorder of childhood on the expression of anorexia nervosa. Int J Eat Disord. 2008;41:326–332. doi: 10.1002/eat.20508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dellava J, Thornton LH, RS, et al. Childhood anxiety associated with low BMI in women with anorexia nervosa. Behav Res Ther. 2010;48:60–67. doi: 10.1016/j.brat.2009.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bloss C, Berrettini W, et al. Genetic association of recovery from eating disorders: The role of GABA receptor SNPs. Neuropsychopharm. 2011;36:2222–2232. doi: 10.1038/npp.2011.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Frank G, Bailer UF, et al. Increased dopamine D2/D3 receptor binding after recovery from anorexia nervosa measured by positron emission tomography and [11C]raclopride. Biological Psychiatry. 2005;58:908–912. doi: 10.1016/j.biopsych.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 19.Steinglass J, Albano A, et al. Fear of food as a treatment target: Exposure and response prevention for anorexia nervosa in an open series. Int J Eat Disord. 2012;45:615–621. doi: 10.1002/eat.20936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Harrison A, O'Brien N, et al. Sensitivity to reward and punishment in eating disorders. Psy Res. 2010;177:1–11. doi: 10.1016/j.psychres.2009.06.010. [DOI] [PubMed] [Google Scholar]
- 21.Friederich H, Herzog D. Cognitive-behavioral flexibility in anorexia nervosa. Curr Top Behav Neurosci. 2011;6:111–123. doi: 10.1007/7854_2010_83. [DOI] [PubMed] [Google Scholar]
- 22.Roberts M, Tchanturia K, et al. Exploring the neurocognitive signature of poor set-shifting in anorexia and bulimia nervosa. J Psychiatr Res. 2010;44:964–970. doi: 10.1016/j.jpsychires.2010.03.001. [DOI] [PubMed] [Google Scholar]
- 23.Tchanturia K, Davies H, et al. Altered social hedonic processing in eating disorders. Int J Eat Disord. 2012;45:962–969. doi: 10.1002/eat.22032. [DOI] [PubMed] [Google Scholar]
- 24.McAnarney E, Zarcone J, et al. Restrictive anorexia nervosa and set-shifting in adolescents: a biobehavioral interface. J Adolesc Health. 2011;49:99–101. doi: 10.1016/j.jadohealth.2010.11.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shott M, Filoteo J, et al. Altered implicit category learning in anorexia nervosa. Neuropsychology. 2012;26:191–201. doi: 10.1037/a0026771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fitzpatrick K, Darcy A, et al. Set-shifting among adolescents with anorexia nervosa. Int J Eat Disord. 2012;45:909–912. doi: 10.1002/eat.22027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cloninger C, Przybeck T, et al. The Temperament and Character Inventory (TCI): A guide to its development and use. St. Louis. 1994:19–28. [Google Scholar]
- 28.Fassino S, Amianto F, et al. Temperament and character in eating disorders: ten years of studies. Eat Weight Disord. 2004;9:81–90. doi: 10.1007/BF03325050. [DOI] [PubMed] [Google Scholar]
- 29.Klump K, Strober M, et al. Personality characteristics of women before and after recovery from an eating disorder. Psych Med. 2004;34:1407–1418. doi: 10.1017/s0033291704002442. [DOI] [PubMed] [Google Scholar]
- 30.Frank G, Roblek T, et al. Heightened fear of uncertainty in anorexia and bulimia nervosa. Int J Eat Disord. 2012;45:227–232. doi: 10.1002/eat.20929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lampard A, Byrne S, et al. Avoidance of affect in the eating disorders. Eat Behav. 2011;12:90–93. doi: 10.1016/j.eatbeh.2010.11.004. [DOI] [PubMed] [Google Scholar]
- 32.Halmi K, Bellace D, et al. An examination of early childhood perfectionism across anorexia nervosa subtypes. Int J Eat Disord. 2012;45:800–807. doi: 10.1002/eat.22019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rigaud D, Verges B, et al. Hormonal and psychological factors linked to the increased thermic effect of food in malnourished fasting anorexia nervosa. J Clin Endocrinol Metab. 2007;92:1623–1629. doi: 10.1210/jc.2006-1319. [DOI] [PubMed] [Google Scholar]
- 34.Nilsson K, Sundbom E, et al. A longitudinal study of perfectionism in adolescent onset anorexia nervosa-restricting type. Eur Eat Disord Rev. 2008;16:386–394. doi: 10.1002/erv.850. [DOI] [PubMed] [Google Scholar]
- 35.Sassaroli S, Apparigliato M, et al. Perfectionism as a mediator between perceived criticism and eating disorders. Eat Weight Disord. 2011;16:e37–e44. doi: 10.1007/BF03327519. [DOI] [PubMed] [Google Scholar]
- 36.Bruch H. Perceptual and conceptual disturbances in anorexia nervosa. Psychosom Med. 1962;24:187–194. doi: 10.1097/00006842-196203000-00009. [DOI] [PubMed] [Google Scholar]
- 37.Fassino S, Piero A, et al. Clinical, psychopathological and personality correlates of interoceptive awareness in anorexia nervosa, bulimia nervosa and obesity. Psychopathology. 2004;37:168–174. doi: 10.1159/000079420. [DOI] [PubMed] [Google Scholar]
- 38.Lilenfeld L, Wonderlich S, et al. Eating disorders and personality: a methodological and empirical review. Clinical Psychology Review. 2006;26:299–320. doi: 10.1016/j.cpr.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 39.Pollatos O, Kurz A-L, et al. Reduced perception of bodily signals in anorexia nervosa. Eat Behav. 2008;9:381–388. doi: 10.1016/j.eatbeh.2008.02.001. [DOI] [PubMed] [Google Scholar]
- 40.Nunn K, Frampton I, et al. The fault is not in her parents but in her insula--a neurobiological hypothesis of anorexia nervosa. Eur Eat Disord Rev. 2008;16:355–360. doi: 10.1002/erv.890. [DOI] [PubMed] [Google Scholar]
- 41.Sexton M, Sunday S, et al. The relationship between alexithymia, depression, and axis II psychopathology in eating disorder inpatients. Int J Eat Disord. 1998;23:277–286. doi: 10.1002/(sici)1098-108x(199804)23:3<277::aid-eat5>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 42.Strigo I, Matthews S, et al. Altered insula activation during pain anticipation in individuals recovered from anorexia nervosa: evidence of interocetive dysregulation. Int J Eat Disord. 2013;46:23–33. doi: 10.1002/eat.22045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Heinzel A, Schafer RM, H, et al. Differential modulation of valence and arousal in high-alexithymic and low-alexithymic individuals. Neuroreport. 2010;21:998–1002. doi: 10.1097/WNR.0b013e32833f38e0. [DOI] [PubMed] [Google Scholar]
- 44.Kaye W, Strober M, et al. Neurobiology of eating disorders. In: Martin A, et al., editors. Pediatric Psychopharmacology, Principles & Practice. New York: Oxford University Press; 2003. pp. 224–237. [Google Scholar]
- 45.Steinglass J, Sysko R, et al. Pre-meal anxiety and food intake in anorexia nervosa. Appetite. 2010;55:214–218. doi: 10.1016/j.appet.2010.05.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Frank G, Kaye W. Current status of functional imaging in eating disorders. Int J Eat Disord. 2012;45:723–736. doi: 10.1002/eat.22016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bailer U, Frank G, et al. Interaction between serotonin transporter and dopamine D2/D3 receptor radioligand measures is associated with harm avoidant symptoms in anorexia and bulimia nervosa. Psych Res Neuroimaging. 2012 doi: 10.1016/j.pscychresns.2012.06.010. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Drevets W, Gautier C, et al. Amphetamine-induced dopamine release in human ventral striatum correlates with euphoria. Biological Psychiatry. 2001;49:81–96. doi: 10.1016/s0006-3223(00)01038-6. [DOI] [PubMed] [Google Scholar]
- 49.Kaye WH, Frank GK, et al. Altered dopamine activity after recovery from restricting-type anorexia nervosa. Neuropsychopharmacology. 1999;21:503–506. doi: 10.1016/S0893-133X(99)00053-6. [DOI] [PubMed] [Google Scholar]
- 50.Bailer U, Kaye W. Serotonin: imaging findings in eating disorders. Chapter in Behavioral Neurobiology of Eating Disorder Book Series Volume. Curr Topics Behav Neuroscience. 2011;6:59–79. doi: 10.1007/7854_2010_78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Amargos-Bosch M, Bortolozzi A, et al. Co-expression and in vivo. interaction of serotonin1A and serotonin2A receptors in pyramidal neurons of prefrontal cortex. Cerebral Cortex. 2004;14:281–299. doi: 10.1093/cercor/bhg128. [DOI] [PubMed] [Google Scholar]
- 52.Varnas K, Halldin C, et al. Autoradiographic distribution of serotonin transporters and receptor subtypes in human brain. Human Brain Mapping. 2004;22:246–260. doi: 10.1002/hbm.20035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Santana N, Bortolozzi A, et al. Expression of serotoinin1A and serotonin2A receptor in pyramidal and GABAergic neurons of the rat prefrontal cortex. Cereb Cortex. 2004;14:1100–1109. doi: 10.1093/cercor/bhh070. [DOI] [PubMed] [Google Scholar]
- 54.Carli M, Baviera M, et al. Dissociable contribution of 5-HT1A and 5-HT2A receptors in the medial prefrontal cortex to different aspects of executive control such as impulsivity and compulsive perseveration in rats. Neuropsychopharm. 2006;31:757–767. doi: 10.1038/sj.npp.1300893. [DOI] [PubMed] [Google Scholar]
- 55.Winstanley CA, Chudasama Y, et al. Intra-prefrontal 8-OH-DPAT and M100907 improve visuospatial attention and decrease impulsivity on the five-choice serial reaction time task in rats. Psypchopharm (Berl) 2003;167:304–314. doi: 10.1007/s00213-003-1398-x. [DOI] [PubMed] [Google Scholar]
- 56.Krebs-Thomson K, Geyer MA. Evidence for a functional interaction between 5-HT1A and 5-HT2A receptors in rats. Psychopharmacology (Berl) 1998;140:69–74. doi: 10.1007/s002130050740. [DOI] [PubMed] [Google Scholar]
- 57.Bailer UF, Frank G, et al. Serotonin transporter binding after recovery from eating disorders. Psychopharmacology. 2007;195:315–324. doi: 10.1007/s00213-007-0896-7. [DOI] [PubMed] [Google Scholar]
- 58.Castellini G, Ricca V, et al. Association between serotonin transporter gene polymorphism and eating disorders outcome: a 6-year follow-up study. Am J Med Genet B Neuropsychiatr Genet. 2012;159b:491–500. doi: 10.1002/ajmg.b.32052. [DOI] [PubMed] [Google Scholar]
- 59.Kaye WH, Gwirtsman HE, et al. Altered serotonin activity in anorexia nervosa after long-term weight restoration. Does elevated cerebrospinal fluid 5-hydroxyindoleacetic acid level correlate with rigid and obsessive behavior? Archives of General Psychiatry. 1991;48:556–562. doi: 10.1001/archpsyc.1991.01810300068010. [DOI] [PubMed] [Google Scholar]
- 60.Kaye WH, Ebert MH, et al. Abnormalities in CNS monoamine metabolism in anorexia nervosa. Archives of General Psychiatry. 1984;41:350–355. doi: 10.1001/archpsyc.1984.01790150040007. [DOI] [PubMed] [Google Scholar]
- 61.Kaye WH, Barbarich NC, et al. Anxiolytic effects of acute tryptophan depletion in anorexia nervosa. International Journal of Eating Disorders. 2003;33:257–267. doi: 10.1002/eat.10135. [DOI] [PubMed] [Google Scholar]
- 62.Wagner A, Aizenstein H, et al. Altered reward processing in women recovered from anorexia nervosa. Am J Psychiatry. 2007;164:1842–1849. doi: 10.1176/appi.ajp.2007.07040575. [DOI] [PubMed] [Google Scholar]
- 63.Eagle D, Wong J, et al. Contrasting roles for dopamine D1 and D2 receptor subtypes in the dorsomedial striatum but not the nucleus accumbens core during behavioral inhibition in the stop-signal task in rats. J Neuroscience. 2011;31:7349–7356. doi: 10.1523/JNEUROSCI.6182-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Simon N, Montgomery K, et al. Dopaminergic modulation of risky decision-making. J Neuroscience. 2011;31:17460–17470. doi: 10.1523/JNEUROSCI.3772-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ghahremani D, Lee B, et al. Striatal Dopamine D2/D3 Receptors Mediate Response Inhibition and Related Activity in Frontostriatal Neural Circuitry in Humans. J Neuroscience. 2012;32:7316–7324. doi: 10.1523/JNEUROSCI.4284-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bailer U, Narendran R, et al. Amphetamine induced dopamine release increases anxiety in individuals recovered from anorexia nervosa. Int J Eat Disord. 2012;45:263–271. doi: 10.1002/eat.20937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Avena N, Bocarsly M. Dysregulation of brain reward systems in eating disorders: Neurochemical information from animal models of binge eating, bulimia nervosa, and anorexia nervosa. Neuropharm. 2012;63:87–96. doi: 10.1016/j.neuropharm.2011.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bassareo V, Di Chiara G. Differential responsiveness of dopamine transmission to food-stimuli in nucleus accumbens shell/core compartments. Neuroscience. 1999;89:637–641. doi: 10.1016/s0306-4522(98)00583-1. [DOI] [PubMed] [Google Scholar]
- 69.Daw ND, Kakade S, et al. Opponent interactions between serotonin and dopamine. Neural Networks. 2002;15:603–616. doi: 10.1016/s0893-6080(02)00052-7. [DOI] [PubMed] [Google Scholar]
- 70.Cools R, Roberts A, et al. Serotoninergic regulation of emotional and behavioural control processes. Trends in Cognitive Sciences. 2008;12:31–40. doi: 10.1016/j.tics.2007.10.011. [DOI] [PubMed] [Google Scholar]
- 71.Bari A, Theobald D, et al. Serotonin modulates sensitivity to reward and negative feedback in a probabilistic reversal learnign task in rats. Neuropsychopharmacology. 2010;35:1290–1301. doi: 10.1038/npp.2009.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Deakin J, Graeff F. 5-HT and mechanisms of defense. Journal of Psychopharmacology. 1991;5:305–315. doi: 10.1177/026988119100500414. [DOI] [PubMed] [Google Scholar]
- 73.Schultz W. Dopamine neurons and their role in reward mechanisms. Current Opinions in Neurobiology. 1997;7:191–197. doi: 10.1016/s0959-4388(97)80007-4. [DOI] [PubMed] [Google Scholar]
- 74.Schweighofer N, Tanaka S, et al. Serotonin and the evaluation of future rewards: theory, experiments, and possible neural mechanisms. Annals of the New York Academy of Science. 2007;1104:289–300. doi: 10.1196/annals.1390.011. [DOI] [PubMed] [Google Scholar]
- 75.McClure S, Laibson D, et al. Separate neural systems value immediate and delayed monetary rewards. Science. 2004;306:503–507. doi: 10.1126/science.1100907. [DOI] [PubMed] [Google Scholar]
- 76.Brewerton T. Antipsychotic agents in the treatment of anorexia nervosa: Neuropsychopharmacologic rationale and evidence from controlled trials. Curr Psychiatry Rep. 2012;14:398–405. doi: 10.1007/s11920-012-0287-6. [DOI] [PubMed] [Google Scholar]
- 77.Lebow J, Sim L, et al. The effect of atypical antipsychotic medications in individuals with anorexia nervosa: A systematic review and meta-analysis. Int J Eat Disord. 2012 doi: 10.1002/eat.22059. [DOI] [PubMed] [Google Scholar]
- 78.Malina A, Gaskill J, et al. Olanzapine treatment of anorexia nervosa: a restrospective study. Int J Eat Disord. 2003;33:234–237. doi: 10.1002/eat.10122. [DOI] [PubMed] [Google Scholar]
- 79.Small D. Individual differences in the neurophysiology of reward and the obesity epidemic. Int J Obes. 2009;33(Suppl 2):S44–S48. doi: 10.1038/ijo.2009.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Giel K, Friederich H, et al. Attentional processing of food pictures in individuals with anorexia nervosa--an eye-tracking study. Biol Psych. 2011;69:661–667. doi: 10.1016/j.biopsych.2010.09.047. [DOI] [PubMed] [Google Scholar]
- 81.Brooks S, O'Daly O, et al. Differential neural responses to food images in women with bulimia versus anorexia nervosa. Public Library of Science One. 2011;6:1–8. doi: 10.1371/journal.pone.0022259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wagner A, Aizenstein H, et al. Altered insula response to a taste stimulus in individuals recovered from restricting-type anorexia nervosa. Neuropsychopharmacology. 2008;33:513–523. doi: 10.1038/sj.npp.1301443. [DOI] [PubMed] [Google Scholar]
- 83.Vocks S, Herpertz S, et al. Effects of gustatory stimulation on brain activity during hunger and satiety in females with restricting-type anorexia nervosa: An fMRI study. J Psychiatr Res. 2011;45:395–403. doi: 10.1016/j.jpsychires.2010.07.012. [DOI] [PubMed] [Google Scholar]
- 84.Haase L, Cerf-Ducastel B, et al. Cortical activation in response to pure taste stimuli during the physiological states of hunger and satiety. Neuroimage. 2009;44:1008–1021. doi: 10.1016/j.neuroimage.2008.09.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Stice E, Spoor S, et al. Relation between obesity and blunted striatal response to foods is moderated by TaqIA A1 allele. Science. 2008;322:449–452. doi: 10.1126/science.1161550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Frank G, Reynolds J, et al. Anorexia nervosa and obesity are associated with opposite brain reward response. Neuropsychopharm. 2012;37:2031–2046. doi: 10.1038/npp.2012.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Cowdrey F, Park R, et al. Increased neural processing of rewarding and aversive food stimuli in recovered anorexia nervosa. Biol Psych. 2011;70:736–743. doi: 10.1016/j.biopsych.2011.05.028. [DOI] [PubMed] [Google Scholar]
- 88.Simmons A, Strigo I, et al. Anticipation of aversive visual stimuli is associated with increased insula activation in anxiety-prone subjects. Biol Psychiatry. 2006;60:402–409. doi: 10.1016/j.biopsych.2006.04.038. [DOI] [PubMed] [Google Scholar]
- 89.Paulus M, Stein MB. An insular view of anxiety. Biol Psychiatry. 2006;60:383–387. doi: 10.1016/j.biopsych.2006.03.042. [DOI] [PubMed] [Google Scholar]
- 90.Delgado M, Nystrom L, et al. Tracking the hemodynamic responses to reward and punishment in the striatum. J Neurophysiol. 2000;84:3072–3077. doi: 10.1152/jn.2000.84.6.3072. [DOI] [PubMed] [Google Scholar]
- 91.Zastrow A, Kaiser SS, C, et al. Neural correlates of impaired cognitive-behavioral flexibility in anorexia nervosa. Am J Psychiatry. 2009;166:608–616. doi: 10.1176/appi.ajp.2008.08050775. [DOI] [PubMed] [Google Scholar]
- 92.Lock J, Garrett A, et al. Aberrant brain activation during a response inhibition task in adolescent eating disorder subtypes. Am J Psychiatry. 2011;168:55–64. doi: 10.1176/appi.ajp.2010.10010056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Oberndorfer T, Kaye W, et al. Demand-specific alteration of medial prefrontal cortex response during an ihhibition task in recovered anorexic women. Int J Eat Disord. 2011;44:1–8. doi: 10.1002/eat.20750. [DOI] [PubMed] [Google Scholar]
- 94.Phillips M, Drevets W, et al. Neurobiology of emotion perception II: implications for major psychiatric disorders. Biol Psych. 2003;54:515–528. doi: 10.1016/s0006-3223(03)00171-9. [DOI] [PubMed] [Google Scholar]
- 95.Phillips M, Drevets WR, SL, et al. Neurobiology of emotion perception I: The neural basis of normal emotion perception. Biol Psych. 2003;54:504–514. doi: 10.1016/s0006-3223(03)00168-9. [DOI] [PubMed] [Google Scholar]
- 96.Schultz W. Neural coding of basic reward terms of animal learning theory, game theory, microeconomics and behavioural ecology. Science. 2004;14:139–147. doi: 10.1016/j.conb.2004.03.017. [DOI] [PubMed] [Google Scholar]
- 97.Yin H, Knowlton B. The role of the basal ganglia in habit formation. Nature Neuroscience Rev. 2006;7:464–476. doi: 10.1038/nrn1919. [DOI] [PubMed] [Google Scholar]
- 98.Goldstein R, Volkow ND. Drug addiction and its underlying neurobiological basis: neuroimaging evidence for the involvement of the frontal cortex. Am J Psychiatry. 2002;159:1642–1652. doi: 10.1176/appi.ajp.159.10.1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Feil J, Sheppard D, et al. Addiction, compulsive drug seeking, and the role of frontostriatal mechanisms in regulating inhibitory control. Neurosci Biobehav Rev. 2012;35:248–275. doi: 10.1016/j.neubiorev.2010.03.001. [DOI] [PubMed] [Google Scholar]
- 100.Kober H, Mende-Siedlecki P, et al. Prefrontal-striatal pathway underlies cognitive regulation of craving. Proc Natl Acad Sci USA. 2010;107:14811–14816. doi: 10.1073/pnas.1007779107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Keating C. Sex differences precipitating anorexia nervosa in females: the estrogen paradox and a novel framework for targeting sex-specific neurocircuits and behavior. Curr Top Behav Neurosci. 2011;8:189–207. doi: 10.1007/7854_2010_99. [DOI] [PubMed] [Google Scholar]
- 102.Casey B, Jones R, et al. The storm and stress of adolescence: insights from human imaging and mouse genetics. Dev Psychobiol. 2010;52:225–235. doi: 10.1002/dev.20447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.De Silva A, Salem V, et al. The use of functional MRI to study appetite control in the CNS. Hindawi Publishing Corporation, Experimental Diabetes Research. 2012:1–13. doi: 10.1155/2012/764017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Fudge J, Breitbart M, et al. Insular and gustatory inputs to the caudal ventral striatum in primates. J Comp Neurol. 2005;490:101–118. doi: 10.1002/cne.20660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Kelley A, Bakshi P, et al. Opioid modulation of taste hedonics within ventral striatum. Physiol Behav. 2002;76:365–377. doi: 10.1016/s0031-9384(02)00751-5. [DOI] [PubMed] [Google Scholar]
- 106.Castro-Fornieles J, Bargallo N, et al. Adolescent anorexia nervosa: cross-sectional and follow-up frontal gray matter disturbances detected with proton magnetic resonance spectroscopy. J Psychtr Res. 2007;41:952–958. doi: 10.1016/j.jpsychires.2006.09.013. [DOI] [PubMed] [Google Scholar]
- 107.Friederich H, Walther S, et al. Grey matter abnormalities within cortico-limbic-striatal circuits in acute and weight-restored anorexia nervosa patients. Neuroimage. 2012;59:1106–1113. doi: 10.1016/j.neuroimage.2011.09.042. [DOI] [PubMed] [Google Scholar]
- 108.Van den Eynde F, Suda M, et al. Structural magnetic resonance imaging in eating disorders: a systematic review of voxel-based morphometry studies. Eur Eat Disord Rev. 2012;20:94–105. doi: 10.1002/erv.1163. [DOI] [PubMed] [Google Scholar]
- 109.Mainz V, Schulte-Ruther M, et al. Structural brain abnormalities in adolescent anorexia nervosa before and after weight recovery and associated hormonal changes. Psychosom Med. 2012;74:574–582. doi: 10.1097/PSY.0b013e31824ef10e. [DOI] [PubMed] [Google Scholar]
- 110.Blasel S, Pilatus U, et al. Metabolic gray matter changes of adolescents with anorexia nervosa in combined MR proton and phosphorus spectroscopy. Neuroradiology. 2012;54:753–764. doi: 10.1007/s00234-011-1001-9. [DOI] [PubMed] [Google Scholar]
- 111.Avena N, Borcarsly M, et al. Animal models of sugar and fat bingeing: relationship to food addiction and increased body weight. Methods Mol Biol. 2012;829:351–365. doi: 10.1007/978-1-61779-458-2_23. [DOI] [PubMed] [Google Scholar]
- 112.Johnson P, Kenny P. Dopamine D2 receptors in addiction-like reward dysfunction and compulsive eating in obese rats. Nat Neurosci. 2010;13:635–641. doi: 10.1038/nn.2519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Eddy KT, Keel PK, et al. Longitudinal comparison of anorexia nervosa subtypes. Int J Eat Disord. 2002;31:191–201. doi: 10.1002/eat.10016. [DOI] [PubMed] [Google Scholar]
- 114.Brooks S, Rask-Andersen M, et al. A debate on current eating disorder diagnoses in light of neurobiological findings: is it time for a spectrum model? BMC Psychiatry. 2012;12:76. doi: 10.1186/1471-244X-12-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Gaudio S, Quattrocchi C. Neural basis of a multidimensional model of body image distortion in anorexia nervosa. Neurosci Biobehav Rev. 2012;36:1839–1847. doi: 10.1016/j.neubiorev.2012.05.003. [DOI] [PubMed] [Google Scholar]
- 116.Case L, Wilson R, et al. Diminished size-weight illusion in anorexia nervosa: evidence for visuo-proprioceptive integration deficit. Exp Brain Res. 2012;217:79–87. doi: 10.1007/s00221-011-2974-7. [DOI] [PubMed] [Google Scholar]
- 117.Galusca B, Costes N, et al. Organic background of restrictive-type anorexia nervosa suggested by increased serotonin(1A) receptor binding in right frontotemporal cortex of both lean and recovered patients: [(18)F]MPPF PET scan study. Biol Psychiatry. 2008;64:1009–1013. doi: 10.1016/j.biopsych.2008.06.006. [DOI] [PubMed] [Google Scholar]
- 118.Boureau Y-L, Dayan P. Opponency revisited: Competition and Cooperation Between Dopamine and Serotonin. Neuropsychopharmacology Reviews. 2011;36:74–97. doi: 10.1038/npp.2010.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Larsen R, Diener E. Promises and problems with the circumplex model of emotion. In: Clark M, editor. Review of Personality and Social Psychology: Emotion. Vol. 13. Newbury Park, CA: Sage; 1992. pp. 25–29. [Google Scholar]
- 120.Knutson B, Greer S. Anticipatory affect: neural correlates and consequences for choice. Philos Trans R Soc London B Biol Sci. 2008;363:3771–3786. doi: 10.1098/rstb.2008.0155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Watson D, Wiese D, et al. The two general activation systems of affect: structural findings, evolutionary considerations, and psychobiological evidence. J Pers Soc Psychol. 1999;76:820–838. [Google Scholar]
- 122.Frank GK, Kaye WH, et al. Reduced 5-HT2A receptor binding after recovery from anorexia nervosa. Biological Psychiatry. 2002;52:896–906. doi: 10.1016/s0006-3223(02)01378-1. [DOI] [PubMed] [Google Scholar]
- 123.Bailer UF, Price JC, et al. Altered 5-HT2A receptor binding after recovery from bulimia-type anorexia nervosa: relationships to harm avoidance and drive for thinness. Neuropsychopharmacology. 2004;29:1143–1155. doi: 10.1038/sj.npp.1300430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Bailer UF, Frank G, et al. Exaggerated 5-HT1A but normal 5-HT2A receptor activity in individuals ill with anorexia nervosa. Biological Psychiatry. 2007;61:1090–1099. doi: 10.1016/j.biopsych.2006.07.018. [DOI] [PubMed] [Google Scholar]
- 125.Audenaert K, Van Laere K, et al. Decreased 5-HT2a receptor binding in patients with anorexia nervosa. J Nucl Med. 2003;44:163–169. [PubMed] [Google Scholar]
- 126.Bailer UF, Frank GK, et al. Altered brain serotonin 5-HT1A receptor binding after recovery from anorexia nervosa measured by positron emission tomography and [11C]WAY100635. Archives of General Psychiatry. 2005;62:1032–1041. doi: 10.1001/archpsyc.62.9.1032. [DOI] [PubMed] [Google Scholar]