Abstract
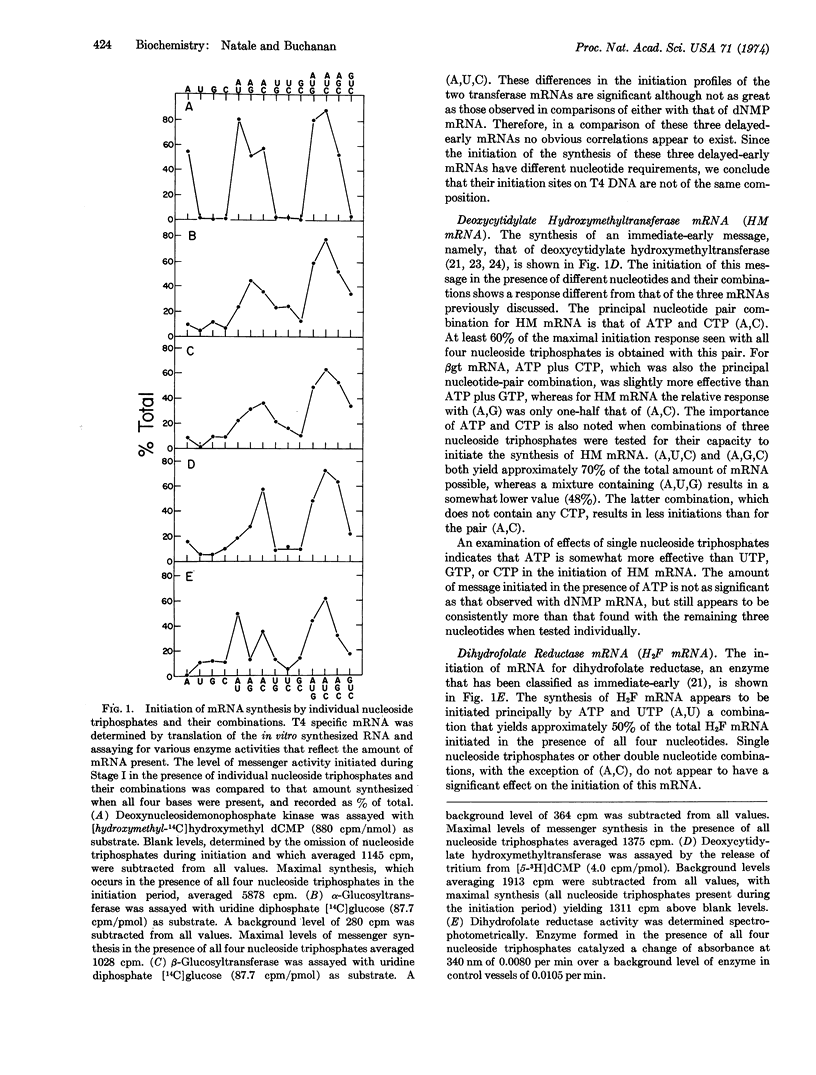
The involvement of the nucleoside triphosphates in the initiation of the synthesis of the messenger ribonucleic acid of five T4 specific enzymes has been studied. Only one of these, the messenger RNA for deoxynucleosidemonophosphate kinase, can be initiated in the presence of one nucleoside triphosphate, namely ATP. All of the remaining four require the presence of at least two nucleoside triphosphates during the initiation period. The combination of ATP and UTP was best for the initiation of messenger RNA for dihydrofolate reductase, ATP and CTP for deoxycytidylate hydroxymethyltransferase and β-glucosyltransferase, and ATP and GTP for α-glucosyltransferase. We have concluded that there is a great variation in the nucleotide composition and sequence of the initiation sites in T4 DNA. No correlation in the requirements of nucleoside triphosphates during the initiation period could be observed among the five systems studied according to their classification as one type or another of “early” T4 messenger RNA.
Keywords: early enzymes, nucleoside triphosphates, initiation signal
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bautz E. K., Bautz F. A., Dunn J. J. E. coli sigma factor: a positive control element in phage T4 development. Nature. 1969 Sep 6;223(5210):1022–1024. doi: 10.1038/2231022a0. [DOI] [PubMed] [Google Scholar]
- Bautz E. K., Bautz F. A. Initiation of RNA synthesis: the function of sigma in the binding of RNA polymerase to promoter sites. Nature. 1970 Jun 27;226(5252):1219–1222. doi: 10.1038/2261219a0. [DOI] [PubMed] [Google Scholar]
- Black L. W., Gold L. M. Pre-replicative development of the bacteriophage T4: RNA and protein synthesis in vivo and in vitro. J Mol Biol. 1971 Sep 14;60(2):365–388. doi: 10.1016/0022-2836(71)90300-7. [DOI] [PubMed] [Google Scholar]
- Blattner F. R., Dahlberg J. E., Boettiger J. K., Fiandt M., Szybalski W. Distance from a promoter mutation to an RNA synthesis startpoint on bacteriophage lambda DNA. Nat New Biol. 1972 Jun 21;237(77):232–236. doi: 10.1038/newbio237232a0. [DOI] [PubMed] [Google Scholar]
- Burgess R. R. A new method for the large scale purification of Escherichia coli deoxyribonucleic acid-dependent ribonucleic acid polymerase. J Biol Chem. 1969 Nov 25;244(22):6160–6167. [PubMed] [Google Scholar]
- DeVries J. K., Zubay G. Characterization of a beta-galactosidase formed between a complementary protein and a peptide synthesized de novo. J Bacteriol. 1969 Mar;97(3):1419–1425. doi: 10.1128/jb.97.3.1419-1425.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downey K. M., Jurmark B. S., So A. G. Determination of nucleotide sequences at promoter regions by the use of dinucleotides. Biochemistry. 1971 Dec 21;10(26):4970–4975. doi: 10.1021/bi00802a021. [DOI] [PubMed] [Google Scholar]
- Grasso R. J., Buchanan J. M. Synthesis of early RNA in bacteriophage T4-infected Escherichia coli B. Nature. 1969 Nov 29;224(5222):882–885. doi: 10.1038/224882a0. [DOI] [PubMed] [Google Scholar]
- Hoffman D. J., Niyogi S. K. RNA initiation with dinucleoside monophosphates during transcription of bacteriophage T4 DNA with RNA polymerase of Escherichia coli. Proc Natl Acad Sci U S A. 1973 Feb;70(2):574–578. doi: 10.1073/pnas.70.2.574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang W. M., Lehman I. R. On the direction of translation of the T4 deoxyribonucleic acid polymerase gene in vivo. J Biol Chem. 1972 Dec 10;247(23):7663–7667. [PubMed] [Google Scholar]
- Jayaraman R. Transcription of bacteriophage T4 DNA by Escherichia coli RNA polymerase in vitro: identification of some immediate-early and delayed-early genes. J Mol Biol. 1972 Sep 28;70(2):253–263. doi: 10.1016/0022-2836(72)90537-2. [DOI] [PubMed] [Google Scholar]
- Maitra U., Nakata Y., Hurwitz J. The role of deoxyribonucleic acid in ribonucleic acid synthesis. XIV. A study of the initiation of ribonucleic acid synthesis. J Biol Chem. 1967 Nov 10;242(21):4908–4918. [PubMed] [Google Scholar]
- Milanesi G., Brody E. N., Grau O., Geiduschek E. P. Transcriptions of the bacteriophage T4 template in vitro: separation of "delayed early" from "immediate early" transcription. Proc Natl Acad Sci U S A. 1970 May;66(1):181–188. doi: 10.1073/pnas.66.1.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller K. The function of the -factor of Escherichia coli RNA polymerase in template site selection. Mol Gen Genet. 1971;111(3):273–296. doi: 10.1007/BF00433112. [DOI] [PubMed] [Google Scholar]
- Natale P. J., Buchanan J. M. DNA-directed synthesis in vitro of T4 phage-specific enzymes. Proc Natl Acad Sci U S A. 1972 Sep;69(9):2513–2517. doi: 10.1073/pnas.69.9.2513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Donnell P. V., Karam J. D. On the direction of reading of bacteriophage T4 gene 43 (deoxyribonucleic acid polymerase). J Virol. 1972 Jun;9(6):990–998. doi: 10.1128/jvi.9.6.990-998.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Farrell P. Z., Gold L. M. Bacteriophage T4 gene expression. Evidence for two classes of prereplicative cistrons. J Biol Chem. 1973 Aug 10;248(15):5502–5511. [PubMed] [Google Scholar]
- O'Farrell P. Z., Gold L. M., Huang W. M. The identification of prereplicative bacteriophage T4 proteins. J Biol Chem. 1973 Aug 10;248(15):5499–5501. [PubMed] [Google Scholar]
- O'Farrell P. Z., Gold L. M. Transcription and translation of prereplicative bacteriophage T4 genes in vitro. J Biol Chem. 1973 Aug 10;248(15):5512–5519. [PubMed] [Google Scholar]
- Peterson R. F., Cohen P. S., Ennis H. L. Properties of phage T4 messenger RNA synthesized in the absence of protein synthesis. Virology. 1972 Apr;48(1):201–206. doi: 10.1016/0042-6822(72)90127-4. [DOI] [PubMed] [Google Scholar]
- Sakiyama S., Buchanan J. M. Control of the synthesis of T4 phage deoxynucleotide kinase messenger ribonucleic acid in vivo. J Biol Chem. 1972 Dec 10;247(23):7806–7814. [PubMed] [Google Scholar]
- Sakiyama S., Buchanan J. M. Relationship between molecular weight of T4 phag-induced deoxynucleotide kinase and the size of its messenger ribonucleic acid. J Biol Chem. 1973 May 10;248(9):3150–3154. [PubMed] [Google Scholar]
- Salser W., Bolle A., Epstein R. Transcription during bacteriophage T4 development: a demonstration that distinct subclasses of the "early" RNA appear at different times and that some are "turned off" at late times. J Mol Biol. 1970 Apr 28;49(2):271–295. doi: 10.1016/0022-2836(70)90246-9. [DOI] [PubMed] [Google Scholar]
- Sauerbier W., Millette R. L., Hackett P. B., Jr The effects of ultraviolet irradiation on the transcription of T4 DNA. Biochim Biophys Acta. 1970;209(2):368–386. doi: 10.1016/0005-2787(70)90735-5. [DOI] [PubMed] [Google Scholar]
- Schäfer R., Zillig W., Zechel K. A model for the initiation of transcription by DNA-dependent RNA polymerase from Escherichia coli. Eur J Biochem. 1973 Mar 1;33(2):207–214. doi: 10.1111/j.1432-1033.1973.tb02671.x. [DOI] [PubMed] [Google Scholar]
- Sugiura M., Okamoto T., Takanami M. RNA polymerase sigma-factor and the selection of initiation site. Nature. 1970 Feb 14;225(5233):598–600. doi: 10.1038/225598a0. [DOI] [PubMed] [Google Scholar]
- Szybalski W., Kubinski H., Sheldrick P. Pyrimidine clusters on the transcribing strand of DNA and their possible role in the initiation of RNA synthesis. Cold Spring Harb Symp Quant Biol. 1966;31:123–127. doi: 10.1101/sqb.1966.031.01.019. [DOI] [PubMed] [Google Scholar]
- Travers A. A. Bacteriophage sigma factor for RNA polymerase. Nature. 1969 Sep 13;223(5211):1107–1110. doi: 10.1038/2231107a0. [DOI] [PubMed] [Google Scholar]
- Travers A. A. Positive control of transcription by a bacteriophage sigma factor. Nature. 1970 Mar 14;225(5237):1009–1012. doi: 10.1038/2251009a0. [DOI] [PubMed] [Google Scholar]
- Trimble R. B., Galivan J., Maley F. The temporal expression of T2r + bacteriophage genes in vivo and in vitro. Proc Natl Acad Sci U S A. 1972 Jul;69(7):1659–1663. doi: 10.1073/pnas.69.7.1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trimble R. B., Maley F. In vitro synthesis of deoxynucleotide kinase, dihydrofolate reductase and deosycytidylate hydroxymethylase from RNA transcripts of T2 phage DNA. Biochem Biophys Res Commun. 1973 Jun 8;52(3):1026–1033. doi: 10.1016/0006-291x(73)91040-1. [DOI] [PubMed] [Google Scholar]


