Abstract
Background
For patients with hepatic nondigestive endocrine metastases (HNEM), the role of liver resection is not well defined.
Methods
We reviewed outcomes for patients who underwent liver resection for HNEM at 2 centers to identify predictors of survival.
Results
From 1991 to 2010, 51 patients underwent liver resection for HNEM. Primary tumor types were adrenal gland (26), thyroid (11), testicular germ cell (9), and ovarian granulosa cell (5). Twenty-eight patients (55%) had synchronous or early (diagnosed within 12 months after primary tumor resection) liver metastases. At liver resection, 26 patients (51%) had extrahepatic metastases, and 7 (14%) had 2 or more sites of extrahepatic metastases. Thirty-two patients (63%) had major liver resection, and 19 (37%) had an extrahepatic procedure. Ninety-day postoperative morbidity and mortality rates were 27% and 2%, respectively. After median follow-up of 20 months (range: 1–144 months), 5-year overall and recurrence-free survival rates were 58% and 37%, respectively. Survival was not affected by primary tumor type. In multivariate analysis, 2 or more sites of extrahepatic metastases (hazard ratio [HR] = 4.80, 95% confidence interval [CI] = 1.18–19.50, P = .028) and interval of 12 months or less between primary tumor resection and diagnosis of liver metastases (HR = 5.33, 95% CI = 1.11–25.71, P = .037) were associated with worse overall survival after liver resection.
Conclusion
For selected patients, liver resection for HNEM is associated with long-term survival. The number of extrahepatic sites of metastasis and the timing of appearance of liver metastases should be considered in patient selection.
INTRODUCTION
Liver resection is currently the treatment of choice for patients with liver metastases from colorectal cancer; 5-year survival rates as high as 58% have been reported with this approach and establish surgery as a curative option in patients with this form of advanced malignant colorectal disease 1,2. Patients with liver metastases from neuroendocrine cancer also benefit from liver resection; 5-year survival rates of up to 74% and reduction of disease-specific symptoms justify liver resection in this patient cohort 3–6.
In contrast, the benefit of liver resection for patients with liver metastases from nondigestive endocrine cancer (hepatic nondigestive endocrine metastases; HNEM) is controversial since most of these patients present with coexistent extrahepatic metastases 7–9. Previously published data on outcomes after resection of HNEM suggest that surgery may improve survival in selected patients. However, these reports included various types of malignancies and relatively small sample sizes, which limits the ability to draw strong conclusions regarding patient selection 9–12. Given improvements in the efficacy of systemic therapy for endocrine tumors 13,14 and improvements in the safety of liver resection 15, the number of patients with HNEM who are potential candidates for surgery is increasing.
In this study, we evaluated the postoperative and long-term outcomes of patients who underwent liver resection for HNEM. In addition, we analyzed pretreatment factors associated with outcome in order to identify a cohort of patients with HNEM who most benefit from surgical therapy.
PATIENTS AND METHODS
Study inclusion criteria
Following Institutional Review Board approval, clinicopathologic data for 51 patients who underwent liver resection for HNEM (at The University of Texas MD Anderson Cancer Center, Houston, Texas, USA [32 patients] or the Charité – Universitätsmedizin, Berlin, Germany [19 patients]) from April 1991 to April 2010 were reviewed. We included all patients with liver metastases from endocrine primary tumors not located in the gastrointestinal tract or pancreas who were offered surgical treatment at 1 of the 2 centers. Patients with nonmetastatic direct liver invasion from an adrenal tumor were excluded.
Pretreatment assessment
Patients were staged with cross-sectional imaging with liver protocol (computed tomography or magnetic resonance imaging). The therapeutic approach was individually formulated for every patient and planned by a multidisciplinary tumor board, which consisted of hepatobiliary surgeons, medical oncologists, and hepatobiliary radiologists. Liver resection was considered in patients in whom computed tomography volumetry indicated that all liver deposits could be safely resected with a sufficient liver remnant. In patients with an anticipated insufficient liver remnant, preoperative portal vein embolization was used to increase the volume of the future liver remnant 16. Patients with extrahepatic metastases were considered for liver resection if the extrahepatic disease could be resected with curative intent and/or systemic therapy had demonstrated the ability to at least stabilize unresectable extrahepatic disease. In patients with synchronous presentation, the decision whether or not to perform combined resection of the primary tumor and liver metastases was based on the extent of the liver metastases and/or the proximity of the primary tumor to the hepatic operative field. Standard perioperative protocols were used to prepare patients with pheochromocytoma for surgery 17.
Surgical procedure
At operation, the peritoneal cavity was carefully examined to rule out previously unrecognized extrahepatic spread of tumor within the abdomen. Intraoperative liver ultrasonography was systematically performed to confirm and better define the location of the liver metastases and their relationship with portal pedicles and hepatic veins. Liver resection was performed under portal triad clamping using standard hepatic transection techniques. Each resected specimen was subjected to standard pathological analysis.
Postoperative period
Postoperative complications were evaluated according to the Clavien-Dindo classification 18,19. Major complications were defined as those requiring surgical, endoscopic, or radiological intervention (grade III); life-threatening complications requiring intensive-care management (grade IV); and death (grade V). Postoperative mortality was defined as death within 90 days after resection, and postoperative morbidity was defined as any complication within the same time period. The decision to treat patients with adjuvant therapy after liver resection was made on a case-by-case basis by each multidisciplinary tumor board.
Statistical analysis
Quantitative and qualitative variables were expressed as mean ± standard deviation, median (range), and frequency. Chi-squared or Fisher’s exact test and the Mann-Whitney U test were used to compare categorical and continuous variables, as appropriate. Overall survival and recurrence-free survival were calculated from the date of liver resection to the date of last follow-up and date of recurrence, respectively, using the Kaplan-Meier method. Log-rank tests were used to assess significance for univariate analysis.
To identify factors associated with outcome after liver resection in patients with HNEM, we examined correlations between overall and recurrence-free survival and the following clinicopathologic variables: primary tumor type (adrenal versus thyroidal versus gonadal); regional lymph node metastases from the primary tumor (positive versus negative); timing of detection of metastases (synchronous [present at the time of resection of the primary tumor] versus metachronous); interval between resection of the primary tumor and diagnosis of liver metastases (≤ 12 months versus > 12 months); number of HNEM (> 3 versus ≤ 3); size of the largest HNEM (≥ 5 cm versus < 5 cm); extrahepatic metastases (present versus absent); 2 or more sites of extrahepatic metastases (present versus absent); major postoperative complications (present versus absent); preoperative and postoperative systemic therapy for the liver metastases (administered or not); extent of major hepatectomy (major [≥ 3 liver segments] versus minor); histological grade of the primary tumor (low or intermediate versus high); status of the resection margins on microscopic analysis (positive for tumor cells versus negative) for both the primary tumor and the HNEM; associated extrahepatic resection (performed or not); and blood transfusions (required or not). All variables associated with survival with P less than or equal to .1 in univariate proportional hazards models were subsequently entered into a Cox multivariate regression model with backward elimination. In multivariate analysis, P less than .05 was considered statistically significant.
Statistical analysis was performed using the statistical software package SPSS version 17.2 (SPSS, Chicago, IL).
RESULTS
Patient characteristics
Preoperative patient characteristics are summarized in Table 1. The mean patient age for the whole group was 44 ± 14 years. Twenty-five patients were male, and 26 were female.
Table 1.
HNEM patient preoperative characteristics (n = 51)
| Characteristic | |
|---|---|
| Mean age (SD) | 44 years (14) |
| Sex (M/F) | 25/26 |
| Mean body mass index (SD) | 25.5 kg/m2 (5) |
| Primary tumor, no. of patients (%) | |
| Adrenal cortical carcinoma | 23 (45) |
| Pheochromocytoma | 3 (6) |
| Medullary thyroid cancer | 8 (16) |
| Hürthle cell carcinoma of thyroid | 3 (6) |
| Ovarian granulosa tumor | 5 (10) |
| Germ cell testicular tumor | 9 (17) |
| Primary tumor grade, no. of patients (%) | |
| Well differentiated | 19 (37) |
| Intermediate or poorly differentiated | 19 (37) |
| Unknown | 13 (26) |
| Node-positive tumor, no. of patients (%) | 22 (43) |
| Liver metastases | |
| Synchronous, no. of patients (%) | 19 (37) |
| Detected ≤ 12 months after primary tumor resection, no. of patients (%) | 28 (55) |
| Mean number (SD) | 2.5 (2.5) |
| Mean size (SD) | 5.3 cm (4.2) |
| Extrahepatic disease, no. of patients (%) | 26 (51) |
| 1 site | 19 |
| 2 sites | 5 |
| 3 or more sites | 2 |
| Extrahepatic disease sites, no. of patients* | |
| Lymph nodes | 13 |
| Bone | 3 |
| Lung | 11 |
| Peritoneal disease | 7 |
| Preoperative systemic therapy targeting liver metastases, no. of patients (%) | 24 (47) |
Abbreviation: HNEM, Hepatic nondigestive endocrine metastases; SD, Standard deviation.
Some patients had more than 1 extrahepatic site of metastases.
The primary tumor was adrenal cortical carcinoma in 23 patients (45%), pheochromocytoma in 3 patients (6%), medullary thyroid cancer in 8 patients (16%), Hürthle cell carcinoma of the thyroid in 3 patients (6%), ovarian granulosa cell tumor in 5 patients (10%) and germ cell tumor of the testis in 9 patients (17%). Tumor cell invasion of the surgical margins was identified in 9 of the primary tumor resection specimens (18%), and 22 (43%) of the primary malignancies were associated with regional lymph node metastases. Compared to patients with adrenal cortical carcinoma, those with primary disease at other sites were more likely to have regional lymph node metastases (P = .006).
Nineteen patients (37%) presented with synchronous metastases. An additional 9 patients (18%) developed liver metastases within 12 months after resection of the primary tumor. The majority of patients with synchronous or early liver metastases (17/28) had adrenal cortical carcinoma (P = .013). The mean number of liver metastases was 2.5 ± 2.5, and 14 patients (27%) had more than 3 liver metastases. The mean size of the largest metastasis was 5.3 ± 4.2 cm, and 25 patients (49%) had metastases 5 cm or larger removed. At the time of liver resection, extrahepatic metastases were present in 26 patients (51%), and the sites of extrahepatic metastases were lymph nodes (n = 13), lung (n = 11), bone (n = 3), and peritoneal disease (n = 7). Nineteen patients presented with 1 site of extrahepatic metastasis; 5 patients had extrahepatic metastases at 2 sites. Two patients had extrahepatic metastases at more than 2 sites; 1 had a primary tumor originating from the adrenal cortex and the other had a testicular germ cell tumor.
Twenty-four patients (47%) received systemic therapy prior to liver resection. Preoperative systemic therapy differed by tumor type and included tegafur-uracil in 1 patient with ovarian metastases, radioiodine for 5 patients with thyroid metastases, and cisplatin/etoposide for 8 patients with testicular metastases. The 10 patients with adrenal metastases treated with preoperative chemotherapy received a wide range of agents, including mitotane, cisplatin, etoposide, sorafenib and adriamycin. The duration of preoperative therapy was dependent on the radiologic response, ranging from 3 to 12 months. In all patients, the systemic therapy was discontinued at least four weeks before surgery.
Intraoperative and postoperative characteristics of the 51 study patients are summarized in Table 2. A major hepatectomy (≥ 3 contiguous liver segments) was performed in 32 patients (63%), and extended hepatectomy (more than a hemihepatectomy) was required in 8 patients (16%). An associated extrahepatic procedure, such as nephrectomy, adrenalectomy, extended lymph node dissection, or resection of a peritoneal mass, was necessary in 19 patients (37%) in order to achieve complete resection of the visible tumor burden. Radiofrequency ablation was used in only 1 patient (2%), for the treatment of a central liver mass. Resection margins microscopically positive for tumor cells were found in only 7 (14%) of the liver resection specimens. Mean estimated blood loss during hepatectomy was 700 ± 1100 mL, and in 19 patients (37%), perioperative transfusions were required. Seventeen patients (33%) received postoperative systemic therapy.
Table 2.
HNEM patient intraoperative and postoperative characteristics
| Characteristic | No. (%) of patients (n = 51) |
|---|---|
| Major liver resection (≥ 3 contiguous liver segments) | 32 (63) |
| Associated extrahepatic procedure | 19 (37) |
| Nephrectomy and/or adrenalectomy | 9 |
| Extended lymph node dissection | 4 |
| Resection of peritoneal mass | 4 |
| Other | 2 |
| Radiofrequency ablation | 1 (2) |
| Mean estimated blood loss (SD) | 700 mL (1100) |
| Transfusion required | 19 (37) |
| Postoperative mortality | 1 (2) |
| Postoperative morbidity | 14 (27) |
| Major postoperative complication | 7 (14) |
| Surgical margin positive for liver metastases | 7 (14) |
| Postoperative systemic therapy | 17 (33) |
Abbreviation: HNEM, Hepatic nondigestive endocrine metastases; SD, Standard deviation.
Postoperative mortality and morbidity
The postoperative mortality rate was 2% (1 patient died). The postoperative course of the patient who died was complicated by a biliary leak, ascites, pleural effusions, and acute renal failure, which led to the death of the patient 86 days after an extended right hepatectomy for 5 metastases from an adrenal cortical carcinoma.
The postoperative morbidity rate was 27% (14 patients). Seven patients (14%) had major postoperative complications, such as intraabdominal fluid collection necessitating drainage, bleeding necessitating repeat laparotomy, and acute renal failure necessitating hemodialysis.
Survival
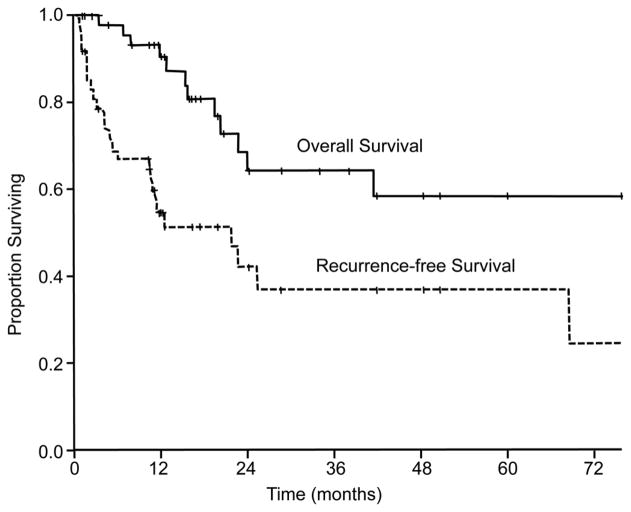
At a median follow-up time of 20 months (range: 1–144 months), 3-year and 5-year overall survival rates were 64% and 58%, respectively. Three-year and 5-year recurrence-free survival rates were 37% and 37%, respectively (Figure 1). Twenty-eight patients (55%) developed a recurrence, and the median time to recurrence after liver resection was 5 months (range: 0.8–81 months). Recurrent lesions were identified in the lung, liver, brain, bones, lymph nodes, peritoneum, and primary tumor site. Lung (9 cases) and liver (8 cases) were the most common sites of tumor recurrence. Three patients had 2 sites of recurrence at the same time. Nine patients underwent a re-resection for the recurrent metastases; 4 of these patients had a repeat hepatectomy.
Figure 1.
Overall and recurrence-free survival in 51 patients who underwent resection of hepatic nondigestive endocrine metastases (HNEM).
Predictors of outcome
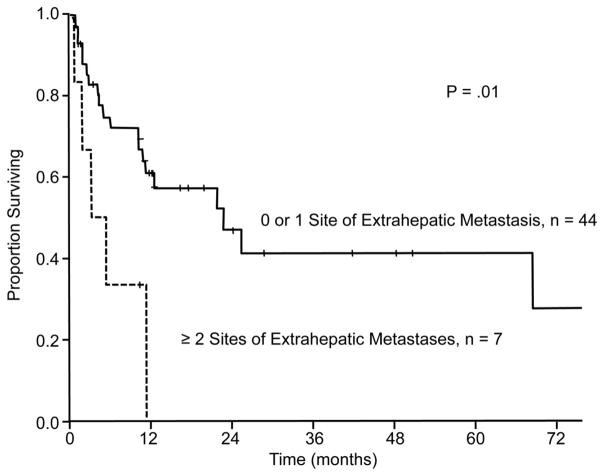
Only 1 factor was predictive of recurrence-free survival: the presence of 2 or more sites of extrahepatic disease was associated with shorter recurrence-free survival in the univariate (P = .01) (Figure 2) and multivariate analyses (hazard ratio [HR] = 3.52, 95% confidence interval [CI] = 1.26–9.84, P = .016) (Table 3).
Figure 2.
Recurrence-free survival according to the number of sites of extrahepatic disease. Patients with 2 or more sites of extrahepatic disease had diminished recurrence-free survival in univariate and multivariate analysis (P = .01 and P = .016, respectively).
Table 3.
Prognostic factors for recurrence-free survival after hepatic resection for HNEM (n=51)
| Predictor of recurrence-free survival | n (%) | 5-year survival (%) | Univariate analysis P value | Multivariate Analysis† | |
|---|---|---|---|---|---|
| P value | HR (95% CI) | ||||
| Primary tumor type | .83 | ||||
| Adrenal | 26 (51) | 36 | |||
| Thyroidal | 11 (22) | 36 | |||
| Gonadal | 14 (27) | 42 | |||
| Regional lymph nodes for primary tumor | .45 | ||||
| Positive | 22 (43) | 51 | |||
| Negative | 29 (57) | 10 | |||
| Timing of detection of HNEM | .20 | ||||
| Synchronous | 19 (37) | 37 | |||
| Metachronous | 32 (63) | 34 | |||
| Interval between primary tumor resection and diagnosis of HNEM | .10 | NS | |||
| ≤ 12 months | 28 (55) | 37 | |||
| > 12 months | 23 (45) | 41 | |||
| Number of liver metastases | .83 | ||||
| > 3 | 14 (27) | 31 | |||
| ≤ 3 | 37 (73) | 42 | |||
| Maximum size of liver metastases | .31 | ||||
| ≥ 5 cm | 25 (49) | 22 | |||
| < 5 cm | 26 (51) | 50 | |||
| Extrahepatic disease | 0.55 | ||||
| Present | 26 (51) | 44 | |||
| Absent | 25 (49) | 31 | |||
| Two or more sites of extrahepatic disease | .01 | .016 | 3.52 (1.26–9.84) | ||
| Present | 7 (14) | 0 | |||
| Absent | 44 (86) | 41 | |||
| Major postoperative complication | .58 | ||||
| Present | 7 (14) | 36 | |||
| Absent | 44 (86) | 38 | |||
| Preoperative systemic therapy for liver metastases | .32 | ||||
| Yes | 24 (47) | 32 | |||
| No | 27 (53) | 43 | |||
| Postoperative systemic therapy for liver metastases | .18 | ||||
| Yes | 17 (33) | 19 | |||
| No | 34 (67) | 48 | |||
| Extent of hepatectomy | .18 | ||||
| Major | 32 (63) | 63 | |||
| Minor | 19 (37) | 22 | |||
| Histological grade of primary tumor | .58 | ||||
| Low or intermediate | 19 (37) | 37 | |||
| High | 19 (37) | 35 | |||
| Surgical margins for primary tumor | .98 | ||||
| Positive | 9 (18) | 19 | |||
| Negative | 42 (82) | 39 | |||
| Surgical margins for liver metastases | .43 | ||||
| Positive | 7 (14) | 48 | |||
| Negative | 44 (86) | 34 | |||
| Associated resection | .14 | ||||
| Yes | 19 (37) | 49 | |||
| No | 32 (63) | 28 | |||
| Need for transfusions | .28 | ||||
| Yes | 19 (37) | 35 | |||
| No | 32 (63) | 38 | |||
Abbreviations: CI, confidence interval; HR, hazard ratio; NS, Not significant; HNEM, Hepatic nondigestive endocrine metastases.
Cox regression multivariate analysis included all variables with P < .1 in univariate analysis.
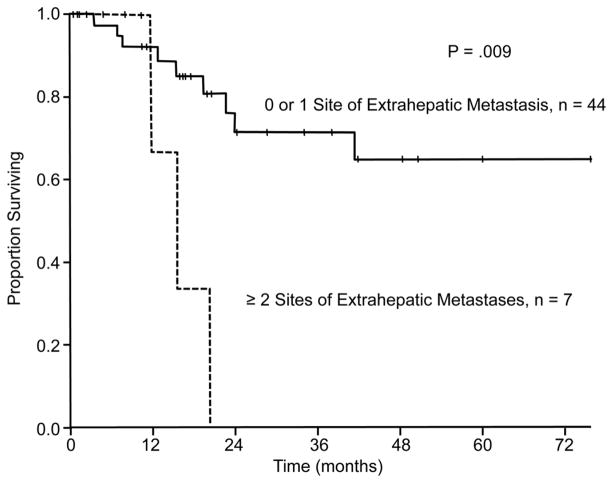
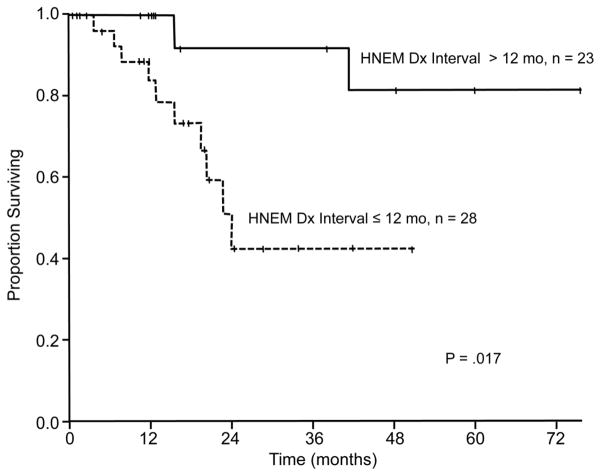
In univariate analysis of overall survival, the presence of 2 or more sites of extrahepatic disease (P = .009) and interval of 12 months or less between resection of the primary tumor and diagnosis of the liver metastases (P = .017) were associated with worse overall survival after resection of liver metastases (Figure 3 and Figure 4, respectively, and Table 4). An additional variable that trended toward significance in univariate analysis was the need for a major hepatectomy (P = .06). Independent associations with overall survival identified in multivariate analysis included the presence of 2 or more sites of extrahepatic disease (HR = 4.80, 95% CI = 1.18–19.50, P = .028) and interval of 12 months or less between resection of the primary tumor and diagnosis of the liver metastases (HR = 5.33, 95% CI = 1.11–25.71, P = .037) (Table 4).
Figure 3.
Overall survival according to the number of sites of extrahepatic disease. Patients with 2 or more sites of extrahepatic disease had diminished overall survival in univariate and multivariate analysis (P = .009 and P = .028, respectively).
Figure 4.
Overall survival according to the interval between resection of the primary tumor and diagnosis (Dx) of HNEM. Patients with an interval of 12 months (mo) or less between primary tumor resection and diagnosis of HNEM had diminished overall survival in univariate and multivariate analysis (P = .017 and P = .037, respectively).
Table 4.
Prognostic factors for overall survival after hepatic resection for HNEM (n=51)
| Predictor of overall survival | n (%) | 5-year survival (%) | Univariate analysis P value | Multivariate Analysis† | |
|---|---|---|---|---|---|
| P value | HR (95% CI) | ||||
| Primary tumor type | .75 | ||||
| Adrenal | 26 (51) | 52 | |||
| Thyroidal | 11 (22) | 67 | |||
| Gonadal | 14 (27) | 66 | |||
| Regional lymph nodes for primary tumor | .85 | ||||
| Positive | 22 (43) | 69 | |||
| Negative | 29 (57) | 49 | |||
| Timing of detection of HNEM | .17 | ||||
| Synchronous | 19 (37) | 57 | |||
| Metachronous | 32 (63) | 62 | |||
| Interval between primary tumor resection and diagnosis of HNEM | .017 | .037 | 5.33 (1.11–25.71) | ||
| ≤ 12 months | 28 (55) | 42 | |||
| > 12 months | 23 (45) | 82 | |||
| Number of liver metastases | .97 | ||||
| > 3 | 14 (27) | 60 | |||
| ≤ 3 | 37 (73) | 56 | |||
| Maximum size of liver metastases | .19 | ||||
| ≥ 5 cm | 25 (49) | 46 | |||
| < 5 cm | 26 (51) | 76 | |||
| Extrahepatic disease | 0.68 | ||||
| Present | 26 (51) | 60 | |||
| Absent | 25 (49) | 58 | |||
| Two or more sites of extrahepatic disease | .009 | .028 | 4.80 (1.18–19.50) | ||
| Present | 7 (14) | 0 | |||
| Absent | 44 (86) | 65 | |||
| Major postoperative complication | .13 | ||||
| Present | 7 (14) | 33 | |||
| Absent | 44 (86) | 62 | |||
| Preoperative systemic therapy for liver metastases | .74 | ||||
| Yes | 24 (47) | 52 | |||
| No | 27 (53) | 61 | |||
| Postoperative systemic therapy for liver metastases | .17 | ||||
| Yes | 17 (33) | 52 | |||
| No | 34 (67) | 63 | |||
| Extent of hepatectomy | .06 | NS | |||
| Major | 32 (63) | 44 | |||
| Minor | 19 (37) | 93 | |||
| Histological grade of primary tumor | .3 | ||||
| Low or intermediate | 19 (37) | 52 | |||
| High | 19 (37) | 50 | |||
| Surgical margins for primary tumor | .75 | ||||
| Positive | 9 (18) | 58 | |||
| Negative | 42 (82) | 59 | |||
| Surgical margins for liver metastases | .21 | ||||
| Positive | 7 (14) | 80 | |||
| Negative | 44 (86) | 53 | |||
| Associated resection | .13 | ||||
| Yes | 19 (37) | 78 | |||
| No | 32 (63) | 43 | |||
| Need for transfusions | .58 | ||||
| Yes | 19 (37) | 65 | |||
| No | 32 (63) | 54 | |||
Abbreviations: CI, confidence interval; HR, hazard ratio; NS, Not significant; HNEM, Hepatic nondigestive endocrine metastases.
Cox regression multivariate analysis included all variables with P less than .1 in univariate analysis.
DISCUSSION
This study examined prognostic factors and outcomes for 51 patients with HNEM treated with liver resection. The low morbidity and mortality rates in this cohort, 27% and 2%, respectively, confirm that this approach is feasible and safe. Results of our survival analysis indicate that liver resection in this cohort was associated with favorable oncologic outcomes, including a 5-year overall survival rate of 58% and a 5-year recurrence-free survival rate of 37%. These long-term outcomes are superior to those in medically treated patients with HNEM, particularly patients with metastases from adrenal cortical carcinoma (5-year overall survival rate = 15%) 20,21.
There are several important differences between patients presenting with liver metastases from colorectal, gastrointestinal neuroendocrine, and nongastrointestinal endocrine primary tumors. Patients with liver metastases from colorectal tumors and gastrointestinal neuroendocrine tumors less commonly present to hepatic surgeons with extrahepatic metastases because 1) liver metastases in such patients occur as the result of tumor spread via gastrointestinal lymphatic channels and/or the portal vein before tumor cells reach the systemic circulation 12,22,23 and 2) the presence of extrahepatic metastases has been reported to be a poor prognostic factor and a relative contraindication to resection in these patients 24. In contrast, HNEM are hematogenous, occurring after malignant cells have already entered the systemic circulation. HNEM, therefore, are associated with extrahepatic metastases in the majority of patients. Despite this mode of tumor spread, the outcomes of our study patients were similar to the favorable outcomes of patients with colorectal liver metastases and neuroendocrine metastases treated with hepatic resection 1,6, validating this therapeutic strategy as a long-term disease-controlling and potentially curative option for HNEM.
Previous studies on liver resection in patients with HNEM have failed to define prognostic factors that could guide patient or therapy selection. Our analysis identified the presence of 2 or more sites of extrahepatic disease and detection of the liver metastases within 12 months after resection of the primary endocrine tumor as patient factors independently associated with worse survival. Each of these factors warrants discussion.
The correlation between extrahepatic disease and diminished survival has previously been reported after resection of liver metastases from noncolorectal primary tumors, including gastrointestinal carcinomas, nonendocrine tumors, and tumors of nonepithelial origin 7,9,25. The presence of a limited burden of extrahepatic disease did not have a significant impact on survival in our patient cohort. However, tumor dissemination to 2 or more extrahepatic locations had a drastic influence on outcome: associated 5-year disease-free and overall survival rates were both 0%. Even though liver metastases could be removed safely in the 7 study patients with 2 or more sites of extrahepatic disease, surgery did not result in prolonged survival. On the basis of these data and the current literature, we consider extensive extrahepatic tumor burden to be a relative contraindication to attempts at curative resection.
The interval between resection of the primary tumor and detection of hepatic metastases also reached significance as a prognostic factor for poor postoperative outcomes. Studies reporting on the surgical approach for noncolorectal liver metastases confirm that early occurrence of liver metastases after resection of the primary tumor signals a more aggressive tumor biology 9,12,26–28. Because of conflicting results in previous analyses, we chose to analyze the time from primary tumor resection to diagnosis of liver metastases using 2 different break points—synchronous versus metachronous and liver metastases diagnosed within 12 months after the primary tumor or later. The difference in outcomes between patients with true synchronous metastases and those with any metachronous presentation was not significant. However, diagnosis of liver metastases within 1 year after resection of the primary tumor was independently predictive of worse overall survival. Because the 5-year survival rate for patients with metastases diagnosed within 1 year after resection of the primary tumor was 42%, we continue to consider liver resection for selected patients with short interval between primary tumor resection and HNEM diagnosis, but only if they are free of extrahepatic disease or have extrahepatic disease in only 1 site.
Various studies investigating the outcome of patients after resection for noncolorectal liver metastases have identified the primary tumor type (location and/or histology) as a significant prognostic factor 7,9–12,26,27. These studies indicated that patients with gastrointestinal primary tumors had in many cases an impaired survival 10,27. Our study is the only report that has focused on the specific group of patients with HNEM. Although each of the histologic tumor types included in this study demonstrates variable response to systemic therapies, interestingly, the primary tumor type was not associated with patient outcome.
For patients with colorectal liver metastases neoadjuvant chemotherapy is an established treatment, likely contributing to improved overall survivals and reduced risk of relapse 29,30. The inhomogeneity in the origin of the liver metastases in the patients in our study prohibits an adequate evaluation of the role of perioperative chemotherapy. Nevertheless, since HNEM are characterized by systemic dissemination, it is reasonable to infer that systemic therapy may contribute to eradication of the circulating tumor cells, preventing tumor recurrence and supporting the curative potential of surgery.
Our study has several limitations. It was a retrospective analysis of selected patients who underwent resection, and the lack of a medical comparison group does not allow a definite therapeutic recommendation for all patients with HNEM. However, the prolonged survival after hepatectomy observed in our study provides evidence that surgery could be effective as part of a multidisciplinary treatment approach. We reviewed the data of patients who underwent resection over a long period of time (20 years) at 2 different centers, and there might be disparities in patient management between the 2 centers and between different time periods. However, characteristics of resected patients, surgical approaches, and outcomes from the two centers were similar. Given the rarity of HNEM, compilation of a cohort large enough to facilitate prognostic factor analysis required the inclusion of patients from a longer time period as well as the cooperation of 2 high-volume hepatobiliary centers. The use of state-of-the-art radiological methods to accurately identify metastatic disease and optimize planning of surgery, the use of advanced methods for parenchymal dissection and control of bleeding during liver resection, and the use of evidence-based postoperative intensive care in both centers are all factors that may have contributed to the excellent short-term and long-term results.
Despite these limitations, we conclude that liver resection is safe and should be regarded as a potentially curative approach in patients with HNEM. Extrahepatic metastases are common in these patients, and although limited extrahepatic disease should not be considered an absolute contraindication to resection, patients with extensive extrahepatic dissemination should be approached cautiously, as this feature is associated with poor survival. Early onset of liver metastases after resection of the primary tumor affects outcome and should be also taken into consideration in evaluation of the resectability of HNEM.
Acknowledgments
Research Support: This research was supported in part by the National Institutes of Health through MD Anderson’s Cancer Center Support Grant CA016672.
The authors thank Stephanie P. Deming and Ruth J. Haynes for their assistance with manuscript preparation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Abdalla EK, Vauthey JN, Ellis LM, et al. Recurrence and outcomes following hepatic resection, radiofrequency ablation, and combined resection/ablation for colorectal liver metastases. Ann Surg. 2004;239:818–25. doi: 10.1097/01.sla.0000128305.90650.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fernandez FG, Drebin JA, Linehan DC, Dehdashti F, Siegel BA, Strasberg SM. Five-year survival after resection of hepatic metastases from colorectal cancer in patients screened by positron emission tomography with F-18 fluorodeoxyglucose (FDG-PET) Ann Surg. 2004;240:438–47. doi: 10.1097/01.sla.0000138076.72547.b1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen H, Hardacre JM, Uzar A, Cameron JL, Choti MA. Isolated liver metastases from neuroendocrine tumors: does resection prolong survival? J Am Coll Surg. 1998;187:88–92. doi: 10.1016/s1072-7515(98)00099-4. [DOI] [PubMed] [Google Scholar]
- 4.Chamberlain RS, Canes D, Brown KT, et al. Hepatic neuroendocrine metastases: does intervention alter outcomes? J Am Coll Surg. 2000;190:432–45. doi: 10.1016/s1072-7515(00)00222-2. [DOI] [PubMed] [Google Scholar]
- 5.Sarmiento JM, Heywood G, Rubin J, Ilstrup DM, Nagorney DM, Que FG. Surgical treatment of neuroendocrine metastases to the liver: A plea for resection to increase survival. J Am Coll of Surg. 2003;197:29–37. doi: 10.1016/S1072-7515(03)00230-8. [DOI] [PubMed] [Google Scholar]
- 6.Mayo SC, de Jong MC, Pawlik TM. Surgical Management and Emerging Therapies to Prolong Survival in Metastatic Neuroendocrine Cancer. Ann Surg Oncol. 2010 doi: 10.1245/s10434-010-1343-2. [DOI] [PubMed] [Google Scholar]
- 7.Yedibela S, Gohl J, Graz V, et al. Changes in indication and results after resection of hepatic metastases from noncolorectal primary tumors: a single-institutional review. Ann Surg Oncol. 2005;12:778–85. doi: 10.1245/ASO.2005.11.018. [DOI] [PubMed] [Google Scholar]
- 8.Carpizo DR, D'Angelica M. Liver resection for metastatic colorectal cancer in the presence of extrahepatic disease. Lancet Oncol. 2009;10:801–9. doi: 10.1016/S1470-2045(09)70081-6. [DOI] [PubMed] [Google Scholar]
- 9.Adam R, Chiche L, Aloia T, et al. Hepatic resection for noncolorectal nonendocrine liver metastases: analysis of 1,452 patients and development of a prognostic model. Ann Surg. 2006;244:524–35. doi: 10.1097/01.sla.0000239036.46827.5f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ercolani G, Grazi GL, Ravaioli M, et al. The role of liver resections for noncolorectal, nonneuroendocrine metastases: experience with 142 observed cases. Ann Surg Oncol. 2005;12:459–66. doi: 10.1245/ASO.2005.06.034. [DOI] [PubMed] [Google Scholar]
- 11.Elias D, Cavalcanti de Albuquerque A, Eggenspieler P, et al. Resection of liver metastases from a noncolorectal primary: indications and results based on 147 monocentric patients. J Am Coll Surg. 1998;187:487–93. doi: 10.1016/s1072-7515(98)00225-7. [DOI] [PubMed] [Google Scholar]
- 12.Weitz J, Blumgart LH, Fong Y, et al. Partial hepatectomy for metastases from noncolorectal, nonneuroendocrine carcinoma. Ann Surg. 2005;241:269–76. doi: 10.1097/01.sla.0000150244.72285.ad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rinke A, Muller HH, Schade-Brittinger C, et al. Placebo-controlled, double-blind, prospective, randomized study on the effect of octreotide LAR in the control of tumor growth in patients with metastatic neuroendocrine midgut tumors: a report from the PROMID Study Group. J Clin Oncol. 2009;27:4656–63. doi: 10.1200/JCO.2009.22.8510. [DOI] [PubMed] [Google Scholar]
- 14.Faiss S, Pape UF, Bohmig M, et al. Prospective, randomized, multicenter trial on the antiproliferative effect of lanreotide, interferon alfa, and their combination for therapy of metastatic neuroendocrine gastroenteropancreatic tumors - The International Lanreotide and Interferon Alfa Study Group. J Clin Oncol. 2003;21:2689–96. doi: 10.1200/JCO.2003.12.142. [DOI] [PubMed] [Google Scholar]
- 15.Vauthey JN, Pawlik TM, Abdalla EK, et al. Is extended hepatectomy for hepatobiliary malignancy justified? Ann Surg. 2004;239:722–30. doi: 10.1097/01.sla.0000124385.83887.d5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ribero D, Abdalla EK, Madoff DC, Donadon M, Loyer EM, Vauthey JN. Portal vein embolization before major hepatectomy and its effects on regeneration, resectability and outcome. Br J Surg. 2007;94:1386–94. doi: 10.1002/bjs.5836. [DOI] [PubMed] [Google Scholar]
- 17.Tauzin-Fin P, Sesay M, Gosse P, Ballanger P. Effects of perioperative alpha1 block on haemodynamic control during laparoscopic surgery for phaeochromocytoma. Br J Anaesth. 2004;92:512–7. doi: 10.1093/bja/aeh083. [DOI] [PubMed] [Google Scholar]
- 18.Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205–13. doi: 10.1097/01.sla.0000133083.54934.ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Clavien PA, Barkun J, de Oliveira ML, et al. The Clavien-Dindo classification of surgical complications: five-year experience. Ann Surg. 2009;250:187–96. doi: 10.1097/SLA.0b013e3181b13ca2. [DOI] [PubMed] [Google Scholar]
- 20.Berruti A, Terzolo M, Sperone P, et al. Etoposide, doxorubicin and cisplatin plus mitotane in the treatment of advanced adrenocortical carcinoma: a large prospective phase II trial. Endocr Relat Cancer. 2005;12:657–66. doi: 10.1677/erc.1.01025. [DOI] [PubMed] [Google Scholar]
- 21.Tacon LJ, Prichard RS, Soon PS, Robinson BG, Clifton-Bligh RJ, Sidhu SB. Current and emerging therapies for advanced adrenocortical carcinoma. Oncologist. 2011;16:36–48. doi: 10.1634/theoncologist.2010-0270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mizuno N, Kato Y, Izumi Y, Irimura T, Sugiyama Y. Importance of hepatic first-pass removal in metastasis of colon carcinoma cells. J Hepatol. 1998;28:865–77. doi: 10.1016/s0168-8278(98)80238-9. [DOI] [PubMed] [Google Scholar]
- 23.Sugarbaker PH. Metastatic inefficiency: the scientific basis for resection of liver metastases from colorectal cancer. J Surg Oncol Suppl. 1993;3:158–60. doi: 10.1002/jso.2930530541. [DOI] [PubMed] [Google Scholar]
- 24.Fong Y, Fortner J, Sun RL, Brennan MF, Blumgart LH. Clinical score for predicting recurrence after hepatic resection for metastatic colorectal cancer: analysis of 1001 consecutive cases. Ann Surg. 1999;230:309–18. doi: 10.1097/00000658-199909000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.O'Rourke TR, Tekkis P, Yeung S, et al. Long-term results of liver resection for non-colorectal, non-neuroendocrine metastases. Ann Surg Oncol. 2008;15:207–18. doi: 10.1245/s10434-007-9649-4. [DOI] [PubMed] [Google Scholar]
- 26.Harrison LE, Brennan MF, Newman E, et al. Hepatic resection for noncolorectal, nonneuroendocrine metastases: a fifteen-year experience with ninety-six patients. Surgery. 1997;121:625–32. doi: 10.1016/s0039-6060(97)90050-7. [DOI] [PubMed] [Google Scholar]
- 27.Earle SA, Perez EA, Gutierrez JC, et al. Hepatectomy enables prolonged survival in select patients with isolated noncolorectal liver metastasis. J Am Coll Surg. 2006;203:436–46. doi: 10.1016/j.jamcollsurg.2006.06.031. [DOI] [PubMed] [Google Scholar]
- 28.Reddy SK, Barbas AS, Marroquin CE, Morse MA, Kuo PC, Clary BM. Resection of noncolorectal nonneuroendocrine liver metastases: a comparative analysis. J Am Coll Surg. 2007;204:372–82. doi: 10.1016/j.jamcollsurg.2006.12.019. [DOI] [PubMed] [Google Scholar]
- 29.Adam R. Developing strategies for liver metastases from colorectal cancer. Semin Oncol. 2007;34:S7–11. doi: 10.1053/j.seminoncol.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 30.Nordlinger B, Sorbye H, Glimelius B, et al. Perioperative chemotherapy with FOLFOX4 and surgery versus surgery alone for resectable liver metastases from colorectal cancer (EORTC Intergroup trial 40983): a randomised controlled trial. Lancet. 2008;371:1007–16. doi: 10.1016/S0140-6736(08)60455-9. [DOI] [PMC free article] [PubMed] [Google Scholar]