Abstract
Background
Advances in the surgical management of hepatocellular carcinoma (HCC) have expanded the indications for curative hepatectomy, including more extensive liver resections. The purpose of this study was to examine long-term survival trends for patients treated with major hepatectomy for HCC.
Patients and Methods
Clinicopathologic data for 1,115 patients with HCC who underwent hepatectomy between 1981 and 2008 at five hepatobiliary centers in France, China, and the USA were assessed. In addition to other performance metrics, outcomes were evaluated using resection of ≥4 liver segments as a novel definition of major hepatectomy.
Results
Major hepatectomy was performed in 539 patients. In the major hepatectomy group, median tumor size was 10 cm (range: 1–27 cm) and 22 % of the patients had bilateral lesions. The TNM Stage distribution included 29 % Stage I, 31 % Stage II, 38 % Stage III, and 2 % Stage IV. The postoperative histologic examination indicated that chronic liver disease was present in 35 % of the patients and tumor microvascular invasion was identified in 60 % of the patients. The 90-day postoperative mortality rate was 4 %. After a median follow-up time of 63 months, the 5-year overall survival rate was 40 %. Patients treated with right hepatectomy (n=332) and those requiring extended hepatectomy (n=207) had similar 90-day postoperative mortality rates (4 % and 4 %, respectively, p=0.976) and 5-year overall survival rates (42 % and 36 %, respectively, p=0.523). Postoperative mortality and overall survival rates after major hepatectomy were similar among the participating countries (p>0.1) and improved over time with 5-year survival rates of 30 %, 40 %, and 51 % for the years 1981–1989, 1990–1999, and the most recent era of 2000–2008, respectively (p=0.004). In multivariate analysis, factors that were significantly associated with worse survivals included AFP level >1,000 ng/mL, tumor size >5 cm, presence of major vascular invasion, presence of extrahepatic metastases, positive surgical margins, and earlier time period in which the major hepatectomy was performed.
Conclusions
This multinational, long-term HCC survival analysis indicates that expansion of surgical indications to include major hepatectomy is justified by the significant improvement in outcomes over the past three decades observed in both the East and the West.
Keywords: Hepatocellular carcinoma, Major hepatectomy, Multicenter study, Long-term survival
Introduction
Hepatocellular carcinoma (HCC) is one of the most common malignancies in both Eastern and Western countries and is uniformly fatal without surgical therapy.1,2 For HCC patients with preserved liver function and minimal portal hypertension, hepatic resection is the preferred curative treatment option. Hepatic resection is also the only curative treatment option for patients with large and multifocal tumors, who are less likely to benefit from liver transplantation3 or local ablative therapy.4 In many cases, a complete resection of HCC can only be achieved by a hepatectomy that removes a significant proportion of the liver parenchyma.5 Whether such an extensive resection is possible is determined by the future liver remnant (FLR) function and volume.6 Fortunately, advances in preoperative imaging,7 patient selection, surgical techniques,8-10 and anesthetic monitoring and an improved understanding of liver anatomy11 have increased the safety of liver resection even in patients with chronic liver disease.12-14
Previous studies indicated that hepatectomy for HCC is a safe procedure and can be performed with low postoperative mortality.15-18 Five-year overall survival rates of up to 50 % have been reported at single centers, indicating that liver resection may provide durable long-term oncological results.5,13 As the safety of resection has improved and surgeons have become more aggressive with hepatectomy for HCC,19 the definition of major liver resection has evolved. The use of different definitions for major hepatectomy over time has made the interpretation of outcomes and comparison of studies difficult. To date, major hepatectomy has most commonly been described as resection of either ≥2 liver segments20,21 or ≥3 liver segments.14,22,23 However, resection of ≥4 liver segments, which is achieved by a right or extended (right or left) hepatectomy,11 is becoming increasingly common.24 To our knowledge, no multicenter studies have been published focusing on long-term survival after resection of ≥4 liver segments. Analysis of outcomes after such extensive resection is anticipated to improve clinical decision-making and to facilitate accurate informed consent in patients with advanced HCC.
In this manuscript, we report the results of a multicenter database analysis of the 90-day postoperative and long-term outcomes of patients who underwent major hepatectomy for HCC defined as resection of ≥4 liver segments. Patient outcomes were stratified by multiple factors, including extent of liver resection (right or extended hepatectomy), presence of fibrosis or cirrhosis, country of origin, and time period. In addition, we aimed to identify clinical factors associated with survival in this patient cohort.
Materials and Methods
Patient Inclusion Criteria
Following Institutional Review Board approval, clinicopathological data on 1,115 consecutive patients with HCC who were treated with hepatectomy between April 1981 and January 2008 at five major hepatobiliary centers in three countries were prospectively collected. The centers that participated in the study formed the International Cooperative Study Group on HCC and included The University of Texas MD Anderson Cancer Center (Houston, TX), Mayo Clinic (Rochester, MN), Queen Mary Hospital (Hong Kong, China), Hôpital Beaujon (Clichy, France), and Hôpital Henri Mondor (Créteil, France). Criteria for surgical eligibility included the ability to remove all radiologically evident disease while retaining a sufficient FLR.25
Preoperative Assessment
The preoperative assessment included a medical history, a physical examination, serum laboratory tests, imaging studies, and an anesthesia evaluation. To assess the degree of chronic liver disease, the Child–Pugh score was calculated.26 Ultrasonography of the abdomen and cross-sectional imaging (computed tomography or magnetic resonance imaging) of the abdomen and chest were performed for tumor staging as well as calculation of the FLR volume. Patients with a FLR lower than the minimal safe residual liver volume according to the standards of each center and published data27 underwent percutaneous embolization of the right ± segment IV portal vein branches to induce hypertrophy of the FLR before hepatectomy. At Queen Mary Hospital in Hong Kong, the indocyanine green clearance test28 was additionally used to assess the liver functional reserve. The presence of major vascular invasion, which was defined as gross invasion of the right or left main branches of the portal or hepatic veins,29 was evaluated using the available imaging studies.
Surgical Procedure
For this study, major hepatectomy was defined as resection of ≥4 contiguous liver segments according to Couinaud’s classification.30 This definition included right hepatectomy and extended (right or left) hepatectomy.11 At the beginning of every operation, the peritoneal cavity was inspected to exclude unresectable intraabdominal tumor progression. Intraoperative ultrasonography of the liver was performed to confirm the exact location of the tumors and to assess proximity to the main intrahepatic vessels. Standard transection techniques were used for liver resection under total or selective hepatic vascular exclusion.
Postoperative Evaluation
In all cases, the diagnosis was confirmed by a histological examination of the resection specimen. Tumor cell differentiation was evaluated using the Edmondson–Steiner criteria,31 and nontumoral liver fibrosis or cirrhosis was graded using the scheme established by Ishak et al..32 Pathologic specimens were also examined to identify microvascular invasion, which was defined as gross or microscopic involvement of the lobar or segmental branches of the portal or hepatic veins as well as the presence of tumor emboli within the central hepatic vein, the portal vein, or the large capsular vessels.29
Statistical Analysis
Quantitative and qualitative variables were expressed as median (range) and frequency (percentage), respectively. The clinico-pathological data of patients who underwent resection of ≥4 liver segments was stratified according to the extent of the hepatectomy (right versus extended), the country of origin (France versus USAversus China), and the time period in which major hepatectomy was performed (1981–1989 versus 1990–1999 versus 2000–2008). Comparisons between groups were analyzed with the chi-squared or Fisher’s exact test for categorical variables and the Mann–Whitney U or Kruskal–Wallis H test for continuous variables, as appropriate. The primary outcomes assessed were overall survival and mortality rates after hepatic resection. Postoperative mortality was defined as any death within 90 days after liver resection. Overall survival was calculated from the date of resection to the date of death or last follow-up using the Kaplan–Meier method. Log-rank tests were used to assess significance for univariate analyses.
Among the 1,115 patients who underwent resection of HCC, the effect of major hepatectomy (resection of ≥4 versus <4 liver segments) on overall survival was determined. In patients treated with major hepatectomy, overall survival and postoperative mortality rates were analyzed by extent of resection (right versus extended hepatectomy), country of origin (France versus USA versus China), and time period (1981–1989 versus 1990–1999 versus 2000–2008). Additionally, we examined the differences in outcomes for patients with and without fibrosis or cirrhosis in the subsets of patients who underwent right or extended hepatectomy.
To identify predictors for overall survival after major hepatectomy, the following clinicopathological variables were recorded and analyzed: gender, age, preoperative serum alpha-fetoprotein (AFP) level, Child–Pugh class, size of the largest tumor, bilateral lesions, number of lesions, major vascular invasion, microvascular invasion, fibrosis or cirrhosis, lymph node metastases, extrahepatic metastases, invasion of other adjacent organs by the tumor, status of the resection margins on microscopic analysis, Edmondson–Steiner grade, extent of hepatectomy, American Joint Committee on Cancer TNM stage (7th edition), and time period of treatment. All independent variables that correlated with survival with p<0.05 in the univariate proportional hazards models were subsequently entered into a Cox multivariate regression model with backward elimination.
The same variables were analyzed to identify predictors of 90-day postoperative mortality. All independent variables that correlated with survival with p<0.05 in the univariate proportional hazards models were subsequently entered into a logistic regression multivariate model with backward elimination. P values <0.05 were considered statistically significant in all tests. Statistical analyses were performed using SPSS software, version 17.2 (SPSS Inc., Chicago, IL).
Results
Patient Characteristics
During the study period, 539 of the 1,115 patients with HCC (48 %) underwent major hepatectomy. A right hepatectomy was performed in 332 patients, and an extended hepatectomy was performed in 207 patients.
The clinicopathological data of the patients treated with major hepatectomy are summarized in Table 1. The median age was 56 years (9–87), and 77 % of the patients were male. The median largest tumor diameter was 10 cm (1–27), and multiple lesions were present in 35 % of cases. Among patients treated with major hepatectomy, there were several important differences between subgroups defined by extent of resection (Table 1). Median tumor size was larger in patients who required extended hepatectomy (12 cm2-25) than in patients who underwent right hepatectomy (8 cm1-27) (p< 0.0001). The incidence of bilateral HCC was higher in patients treated with extended hepatectomy (p<0.0001). Chronic liver disease with fibrosis or cirrhosis was less frequent in patients treated with extended hepatectomy (28 %) than in those treated with right hepatectomy (40 %) (p=0.005). The extent of the surgical procedure was associated with the frequency of incomplete resection of HCC: positive surgical margins were identified in 16 % of patients who underwent extended hepatectomy and only 10 % of patients who underwent right hepatectomy (p=0.042).
Table 1.
Clinicopathological data of patients who underwent major hepatectomy for HCC (n = 539) according to extent of resection
| Variable | Major hepatectomy (n=539) |
Right hepatectomy (n=332) |
Extended hepatectomy (n=207) |
P a |
|---|---|---|---|---|
| Male gender (%) | 77 | 81 | 72 | 0.018 |
| Age (years), median (range) | 56 (9–87) | 56 (16–87) | 53 (9–85) | 0.233 |
| AFP (ng/mL), median (range) | 194 (0–1,300,000) | 161 (0–481,000) | 236 (0–1,300,000) | 0.115 |
| Child–Pugh class B (%) | 2 | 2 | 3 | 0.404 |
| Tumor size (cm), median (range) | 10 (1–27) | 8 (1–27) | 12 (2–25) | <0.0001 |
| Tumor size >5 cm (%) | 80 | 74 | 90 | <0.0001 |
| Bilateral lesions (%) | 22 | 5 | 50 | <0.0001 |
| Multiple lesions (%) | 35 | 34 | 38 | 0.296 |
| Major vascular invasion (%) | 12 | 11 | 12 | 0.726 |
| Microvascular invasion (%) | 60 | 57 | 65 | 0.059 |
| Fibrosis/cirrhosis (%) | 35 | 40 | 28 | 0.005 |
| Nodal metastases (%) | 1 | 1 | 2 | 0.305 |
| Extrahepatic metastases (%) | 1 | 1 | 1 | 0.807 |
| Other organ invasion (%) | 9 | 8 | 9 | 0.916 |
| Positive surgical margins (%) | 12 | 10 | 16 | 0.042 |
| Edmondson–Steiner grade (%) | 0.397 | |||
| 1 | 13 | 12 | 14 | |
| 2 | 38 | 36 | 40 | |
| 3 | 42 | 45 | 38 | |
| 4 | 7 | 7 | 8 | |
| 90-Day mortality (%) | 4 | 4 | 4 | 0.976 |
AFP α-fetoprotein
Right hepatectomy versus extended hepatectomy
In this study, 137 patients from France, 111 from the USA, and 291 from China underwent major hepatectomy. There were significant differences between the three countries regarding the extent of tumor and the degree of underlying liver disease. Comparisons of clinicopathological patient data according to country of origin are summarized in Table 2.
Table 2.
Clinicopathological data of patients who underwent major hepatectomy for HCC (n=539) according to country of origin
| Variable | France (n=137) | USA (n=111) | China (n=291) | P a |
|---|---|---|---|---|
| Male gender (%) | 80 | 59 | 84 | <0.0001 |
| Age (years), median (range) | 57 (15–85) | 64 (9–87) | 51 (13–82) | <0.0001 |
| AFP (ng/dL), median (range) | 191 (1–700,000) | 25 (0–249,417) | 354 (2–1,300,000) | <0.0001 |
| Child–Pugh class B (%) | 4 | 0 | 2 | 0.146 |
| Tumor size (cm), median (range) | 10 (1–24) | 10 (2.4–24) | 9.5 (1–27) | 0.050 |
| Tumor size >5 cm (%) | 82 | 86 | 78 | 0.180 |
| Bilateral lesions (%) | 19 | 34 | 19 | 0.004 |
| Multiple lesions (%) | 39 | 33 | 34 | 0.520 |
| Major vascular invasion (%) | 16 | 10 | 10 | 0.145 |
| Microvascular invasion (%) | 68 | 52 | 60 | 0.048 |
| Fibrosis/cirrhosis (%) | 34 | 7 | 46 | <0.0001 |
| Nodal metastases (%) | 2 | 5 | 0 | 0.002 |
| Extrahepatic metastases (%) | 0 | 1 | 2 | 0.196 |
| Other organ invasion (%) | 10 | 4 | 10 | 0.112 |
| Positive surgical margins (%) | 11 | 7 | 14 | 0.297 |
| Edmondson–Steiner grade (%) | <0.0001 | |||
| 1 | 5 | 19 | 14 | |
| 2 | 37 | 45 | 35 | |
| 3 | 46 | 36 | 43 | |
| 4 | 12 | 0 | 8 | |
| Extended hepatectomy (%) | 28 | 43 | 42 | 0.012 |
| 90-Day mortality (%) | 3 | 2 | 5 | 0.237 |
AFP α-fetoprotein
France versus USA versus China
To determine whether the features of patients who underwent major hepatectomy changed over time, we compared the clinicopathological findings from three time periods: 1981–1989, 1990–1999, and 2000–2008. Results of this analysis are summarized in Table 3. Compared to patients treated in the earlier time periods, patients treated in the 2000–2008 era were less likely to have multiple tumors (p=0.002), underlying fibrosis or cirrhosis (p=0.044), or positive surgical margins (p<0.0001). Patients treated in 2000–2008 also had lower preoperative serum AFP levels (p<0.0001).
Table 3.
Clinicopathological data of patients who underwent major hepatectomy for HCC (n=539) according to time period
| Variable | 1981–1989 (n=78) | 1990–1999 (n=340) | 2000–2008 (n=121) | P a |
|---|---|---|---|---|
| Male gender (%) | 77 | 77 | 79 | 0.943 |
| Age (years), median (range) | 57 (17–87) | 52 (13–85) | 59 (9–83) | 0.017 |
| AFP (ng/dL), median (range) | 525 (0–700,000) | 230 (0–1,300,000) | 28 (1–600,000) | <0.0001 |
| Child–Pugh class B (%) | 5 | 2 | 1 | 0.126 |
| Tumor size (cm), median (range) | 11 (1–20) | 10 (1.5–27) | 9 (1.8–24) | 0.119 |
| Tumor size >5 cm (%) | 87 | 80 | 78 | 0.230 |
| Bilateral lesions (%) | 22 | 23 | 19 | 0.631 |
| Multiple lesions (%) | 47 | 37 | 24 | 0.002 |
| Major vascular invasion (%) | 18 | 11 | 10 | 0.154 |
| Microvascular invasion (%) | 64 | 60 | 59 | 0.746 |
| Fibrosis/cirrhosis (%) | 32 | 39 | 26 | 0.044 |
| Nodal metastases (%) | 1 | 2 | 0 | 0.338 |
| Extrahepatic metastases (%) | 1 | 2 | 1 | 0.865 |
| Other organ invasion (%) | 6 | 10 | 6 | 0.278 |
| Positive surgical margins (%) | 27 | 11 | 6 | <0.0001 |
| Edmondson–Steiner grade | 0.157 | |||
| 1 | 14 | 11 | 16 | |
| 2 | 38 | 38 | 36 | |
| 3 | 45 | 44 | 36 | |
| 4 | 3 | 7 | 12 | |
| Extended hepatectomy (%) | 32 | 39 | 40 | 0.620 |
| 90-Day mortality (%) | 12 | 2 | 4 | <0.0001 |
AFP α-fetoprotein
1981–1989 versus 1990–1999 versus 2000–2008
Ninety-Day Postoperative Mortality Rates
The 90-day postoperative mortality rate for all patients undergoing major hepatectomy was 4 %, and it did not differ between patients who underwent right and those who underwent extended hepatectomy (p=0.976; Table 1). However, an analysis according to both extent of resection and underlying liver disease showed that whereas fibrosis or cirrhosis did not negatively affect the 90-day mortality of patients undergoing right hepatectomy (p=0.940), the presence of fibrosis or cirrhosis was associated with worse 90-day mortality rate among patients treated with extended hepatectomy (p=0.002; Table 4).
Table 4.
Five-year survival and 90-day mortality rates following major hepatectomy for HCC according to extent of liver resection and underlying chronic liver disease
| Extent of liver resection | Fibrosis/cirrhosis |
|||
|---|---|---|---|---|
| Absent | Present | p a | ||
| Right hepatectomy (n=332) |
60 % | 40 % | ||
| 5-Year survival rate (%) |
44 | 40 | 0.063 | |
| 90-Day mortality rate (%) |
4 | 4 | 0.940 | |
| Extended hepatectomy (n=207) |
72 % | 28 % | ||
| 5-Year survival rate (%) |
39 | 26 | 0.006 | |
| 90-Day mortality rate (%) |
1 | 11 | 0.002 | |
Fibrosis/cirrhosis absent versus present
Similar 90-day postoperative mortality rates were reported in France (3 %), the USA (2 %), and China (5 %) (p=0.237; Table 2). Interestingly, an improvement in postoperative mortality rates was achieved over time. While the 90-day postoperative mortality rate was 12 % in the 1980s, it was 2 % in the 1990s, and 4 % between 2000 and 2008 (p<0.0001; Table 3).
Long-Term Survival Rates
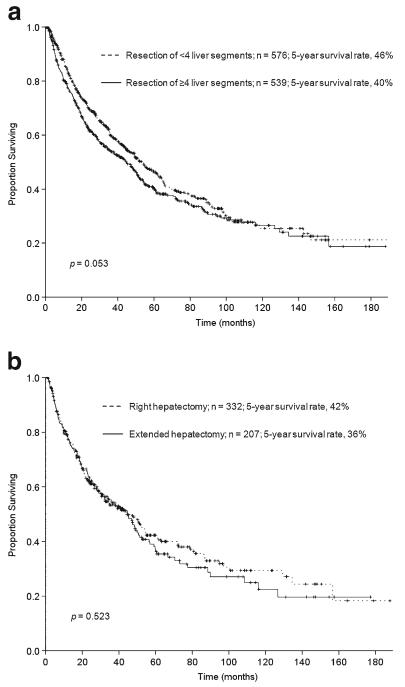
After a median follow-up time of 63 months (1–188), the median survival of the patients who underwent major hepatectomy (n=539) was 45 months. The 3-year, 5-year, and 10-year overall survival rates of these patients were 54 %, 40 %, and, 27 %, respectively. The overall survival rate at 5 years among patients who had major hepatectomy was not significantly different from that of patients who had resection of <4 liver segments (46 %, p=0.053; Fig. 1a). Among patients who underwent major hepatectomy, overall survival did not differ by extent of resection (right versus extended hepatectomy) (p=0.523; Fig. 1b). Likewise, the survival analysis according to both extent of resection and underlying liver disease showed that fibrosis or cirrhosis did not negatively affect the outcome of patients undergoing right hepatectomy (p=0.063). However, even when controlling for 90-day postoperative mortality, the presence of fibrosis or cirrhosis was associated with worse overall survival among patients treated with extended hepatectomy (p=0.006; Table 4).
Fig. 1.
a Overall survival after hepatectomy for HCC (n=1,115) by extent of resection (≥4 liver segments versus <4 liver segments). b Overall survival after major hepatectomy for HCC (n=539) by extent of resection (right versus extended hepatectomy)
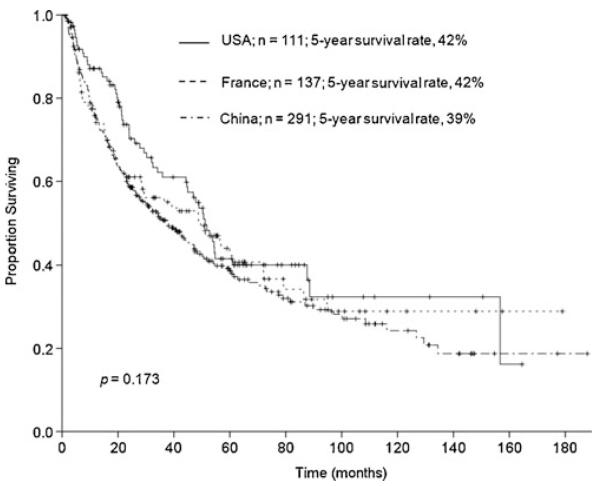
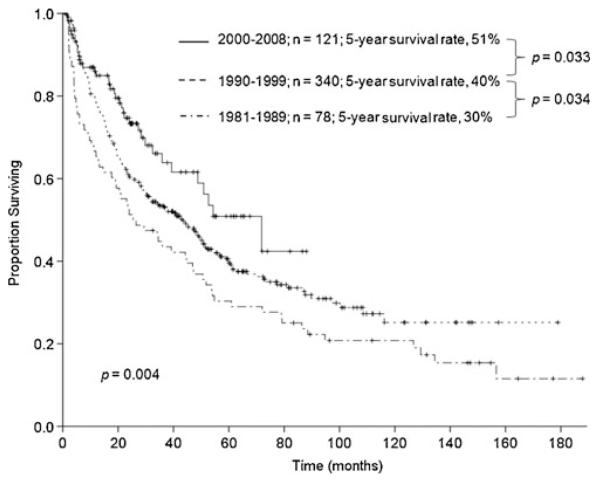
There were no differences in overall survival after major hepatectomy between patients from France, the USA, and China (5-year survival rates were 42 %, 42 %, and 39 %, respectively; p=0.173; Fig. 2). Importantly, overall survival after major hepatectomy increased sequentially over the study period (Fig. 3). The 5-year survival rate was 30 % for 1981–1989, 40 % for 1990–1999, and 51 % for 2000–2008 (p=0.004).
Fig. 2.
Overall survival after major hepatectomy for HCC (n=539) by country (USA versus France versus China)
Fig. 3.
Overall survival after major hepatectomy for HCC (n=539) by time period of treatment (1981–1989 versus 1990–1999 versus 2000–2008)
Predictors of Overall Survival and 90-Day Postoperative Mortality
Results of univariate and multivariate analyses for predictors of overall survival in patients with HCC who underwent major hepatectomy (n=539) are summarized in Table 5. In univariate analysis, 14 of 18 study variables correlated with diminished overall survival after major hepatectomy. In multivariate analysis, AFP level >1,000 ng/mL, tumor size >5 cm, presence of major vascular invasion, presence of extrahepatic metastases, positive surgical margins, and earlier time period in which the major hepatectomy was performed were independently associated with worse survival. The TNM stage was not included in the multivariate analysis in order to avoid the effect of covariance.
Table 5.
Predictors of overall survival after major hepatectomy for HCC (n=539)
| Variable | % of patients | Median survival (months) | Univariate analysis | Multivariate analysisa |
|||
|---|---|---|---|---|---|---|---|
| p | p | HR | 95 % CI | ||||
| Gender | Male | 77 | 40 | 0.021 | NS | ||
| Female | 23 | 67 | |||||
| Age (years) | >60 | 37 | 45 | 0.209 | |||
| ≤60 | 63 | 47 | |||||
| AFP (ng/mL) | >1,000 | 36 | 18 | <0.0001 | <0.0001 | 1.92 | 1.48–2.49 |
| ≤1,000 | 64 | 55 | |||||
| Child–Pugh class | A | 98 | 45 | 0.563 | |||
| B | 2 | 31 | |||||
| Tumor size (cm) | >5 | 80 | 34 | <0.0001 | <0.0001 | 2.30 | 1.51–3.52 |
| ≤5 | 20 | 86 | |||||
| Bilateral lesions | Present | 22 | 30 | 0.005 | NS | ||
| Absent | 78 | 49 | |||||
| Number of lesions | Multiple | 35 | 24 | <0.0001 | NS | ||
| Solitary | 65 | 55 | |||||
| Major vascular invasion | Present | 12 | 12 | <0.0001 | 0.001 | 1.83 | 1.30–2.59 |
| Absent | 88 | 50 | |||||
| Microvascular invasion | Present | 60 | 29 | <0.0001 | NS | ||
| Absent | 40 | 61 | |||||
| Fibrosis/cirrhosis | Present | 35 | 29 | 0.004 | NS | ||
| Absent | 65 | 50 | |||||
| Nodal metastases | Present | 1 | 21 | 0.247 | |||
| Absent | 99 | 45 | |||||
| Extrahepatic metastases | Present | 1 | 11 | <0.0001 | 0.017 | 2.75 | 1.20–6.33 |
| Absent | 99 | 45 | |||||
| Other organ invasion | Present | 9 | 15 | <0.0001 | NS | ||
| Absent | 91 | 49 | |||||
| Surgical margins | Positive | 12 | 16 | <0.0001 | 0.041 | 1.44 | 1.02–2.04 |
| Negative | 88 | 43 | |||||
| Edmondson–Steiner grade | 1 | 13 | 87 | <0.0001 | NS | ||
| 2 | 38 | 47 | |||||
| 3 | 42 | 34 | |||||
| 4 | 7 | 17 | |||||
| Extent of hepatectomy | Extended | 38 | 45 | 0.523 | |||
| Right | 62 | 44 | |||||
| TNM stage (7th edition) | I | 29 | 129 | <0.0001 | NS | ||
| II | 31 | 51 | |||||
| III | 38 | 20 | |||||
| IV | 2 | 15 | |||||
| Time period | 1981–1989 | 15 | 25 | 0.004 | 0.002 | 1.45 | 1.15–1.85 |
| 1990–1999 | 63 | 43 | |||||
| 2000–2008 | 22 | 72 | |||||
CI indicates confidence interval, HR hazard ratio, NS not significant, AFP α-fetoprotein
Cox regression multivariate analysis included all variables with p<0.05 in the univariate analysis
Results of univariate and multivariate analyses for predictors of 90-day postoperative mortality after major hepatectomy (n=539) are summarized in Table 6. In univariate analysis, AFP level >1,000 ng/mL, major vascular invasion, and earlier time period in which the major hepatectomy was performed were associated with higher postoperative mortality rates. In multivariate analysis, only major vascular invasion and earlier time period were independent predictors of 90-day postoperative mortality.
Table 6.
Predictors of 90-day postoperative mortality after major hepatectomy for HCC (n=539)
| Variable | % of patients | 90-Day mortality rate (%) | Univariate analysis | Multivariate analysisa |
|||
|---|---|---|---|---|---|---|---|
| p | p | HR | 95 % CI | ||||
| Gender | Male | 77 | 4 | 0.351 | |||
| Female | 23 | 3 | |||||
| Age (years) | >60 | 37 | 4 | 0.702 | |||
| ≤60 | 63 | 4 | |||||
| AFP (ng/mL) | >1,000 | 36 | 6 | 0.045 | NS | ||
| ≤1,000 | 64 | 3 | |||||
| Child–Pugh class | A | 98 | 4 | 0.422 | |||
| B | 2 | 8 | |||||
| Tumor size (cm) | >5 | 80 | 4 | 0.527 | |||
| ≤5 | 20 | 3 | |||||
| Bilateral lesions | Present | 22 | 3 | 0.379 | |||
| Absent | 78 | 4 | |||||
| Number of lesions | Multiple | 35 | 4 | 0.795 | |||
| Solitary | 65 | 4 | |||||
| Major vascular invasion | Present | 12 | 10 | 0.013 | 0.019 | 3.42 | 1.23–9.53 |
| Absent | 88 | 3 | |||||
| Microvascular invasion | Present | 60 | 5 | 0.284 | |||
| Absent | 40 | 3 | |||||
| Fibrosis/cirrhosis | Present | 35 | 6 | 0.087 | |||
| Absent | 65 | 3 | |||||
| Nodal metastases | Present | 1 | 0 | 0.592 | |||
| Absent | 99 | 4 | |||||
| Extrahepatic metastases | Present | 1 | 14 | 0.153 | |||
| Absent | 99 | 4 | |||||
| Other organ invasion | Present | 9 | 9 | 0.079 | |||
| Absent | 91 | 3 | |||||
| Surgical margins | Positive | 12 | 4 | 0.726 | |||
| Negative | 88 | 5 | |||||
| Edmondson–Steiner grade | 1 | 13 | 0 | 0.054 | |||
| 2 | 38 | 3 | |||||
| 3 | 42 | 5 | |||||
| 4 | 7 | 11 | |||||
| Extent of hepatectomy | Extended | 38 | 4 | 0.976 | |||
| Right | 62 | 4 | |||||
| TNM stage (7th edition) | I | 29 | 2 | 0.385 | |||
| II | 31 | 4 | |||||
| III | 38 | 5 | |||||
| IV | 2 | 7 | |||||
| Time period | 1981–1989 | 15 | 12 | <0.0001 | 0.038 | 2.27 | 1.05–4.93 |
| 1990–1999 | 63 | 2 | |||||
| 2000–2008 | 22 | 4 | |||||
CI indicates confidence interval, HR hazard ratio, NS not significant, AFP α-fetoprotein
Logistic regression multivariate analysis included all variables with p<0.05 in the univariate analysis
Discussion
This multicenter study indicates that major hepatectomy for HCC, defined as resection of ≥4 liver segments, can be performed with a low postoperative mortality rate of 4 % and is associated with a 5-year overall survival rate of 40 %, which is comparable to that achieved by hepatic resection of lesser extent at single centers.2,13,33 The current study showed a steady improvement in survival after major hepatectomy over the past three decades. According to our findings, 10 percentage point increases in the 5-year overall survival rates were achieved between the 1980s and 1990s and between the 1990s and 2000–2008. In addition, among patients undergoing major hepatectomy, long-term survival rates were similar for patients undergoing extended hepatectomy and those treated with right hepatectomy. The reasons for these improvements are likely multifactorial and may include advances in imaging, patient selection, surgical techniques, and patient care. As such, this multicenter analysis of major hepatectomy provides a confirmatory validation of the progress that has been reported in smaller series from single centers.5,6,16,34
Our novel definition of major hepatectomy is in keeping with the fact that the resection of ≥4 liver segments amounts to the removal of more than 50 % of the liver parenchyma.35 We consider the use of this definition appropriate because the postoperative mortality of such major resections is usually related to hepatic insufficiency leading to liver failure, which remains the main cause of mortality after extensive liver resection.36,37 To account for the impact of this important complication, we included in this study all 90-day postoperative deaths in the survival analysis. As such, the improved 5-year survival rate of more than 50 % in the recent era is a comprehensive representation of outcome.
Among the three countries that contributed to our study, postoperative mortality and long-term survival rates were similar. A previous multicenter study that analyzed outcomes after resection for HCC in patients treated in the USA, France, and Japan showed similar results.38 However, in that study, the number of patients who underwent right or extended hepatectomy was small, and definitive conclusions regarding the efficacy of major hepatectomy could not be drawn. In contrast, our study examined outcomes in 332 patients treated with a right hepatectomy and 207 patients treated with an extended hepatectomy and thus represents one of the largest series concentrating on HCC patients with such extensive intrahepatic involvement that major hepatectomy was required.
A better understanding of adequate FLR volume after hepatic resection likely contributed to optimized selection of surgical candidates, particularly in the group of patients with limited hepatic functional reserve. A recent study showed that liver resection can be performed safely if standardized FLR is >20 % in patients with normal liver and >40 % in patients with liver cirrhosis.27,39,40 Portal vein embolization (PVE) has been widely used to induce FLR hypertrophy, facilitating major hepatectomy. Sequential transarterial chemoembolization and PVE have also been investigated as a combined approach to improve posthepatectomy outcomes in patients with HCC.41-44 New techni-ques such as right45 or middle hepatic vein embolization after PVE have been proposed as further means to induce hypertrophy of the FLR before resection; however, further studies are still required to define the role of these new approaches.
Our results indicate that in the entire patient cohort, postoperative and long-term outcomes were similar after right and extended hepatectomy. In patients without underlying liver disease, extended hepatectomy resulted in 90-day postoperative mortality and long-term survival rates similar to those after right hepatectomy, validating the role of extended hepatectomy in the treatment of HCC in patients with normal liver. However, in patients with chronic liver disease, extended resection was associated with an increased postoperative mortality rate of 11 % and a diminished 5-year survival rate of 26 %. Based on these results and those of previous studies,46 right hepatectomy should be considered as a standard surgical therapy for selected patients with advanced HCC and underlying chronic disease. However, these data indicate that extended liver resection should be utilized more selectively in patients with underlying liver disease.
Although extended hepatectomy is associated with worse long-term survival in patients with fibrosis or cirrhosis than in patients without fibrosis or cirrhosis, the association is not absolute. In patients with underlying liver disease, outcomes after extended hepatectomy remain superior to outcomes after nonsurgical treatments (e.g., transarterial chemoembolization).47,48 These findings emphasize the importance of patient selection for surgical therapy. One important tool that may assist in the selection of surgical candidates is the degree of hypertrophy after PVE.49 Particularly in patients with underlying liver disease, substantial hypertrophy of the FLR may predict an uncomplicated postoperative course and prolonged overall survival. This experience confirms reports from previous single-center studies showing that extended hepatectomy for advanced HCC is justifiable in cirrhotic patients with preserved regenerative ability of the liver parenchyma and an adequate liver remnant.5,50
In multivariate analysis, tumor size >5 cm, presence of major vascular invasion, and presence of extrahepatic metastases were identified as independent predictors of worse survival after major hepatectomy; these factors have a critical role in the stratification of HCC patients according to the TNM staging system.29,51-53 The same factors have been associated with survival in patients with HCC eligible for liver transplantation, confirming the significance of these factors in predicting outcome.54 In addition, a preoperative AFP level of >1,000 ng/mL and surgical margins positive for tumor cells reached significance as independent predictors of diminished survival, validating findings from previous studies.5,17,55 Earlier time period in which major hepatectomy was performed predicted both poor overall survival and increased 90-day postoperative mortality in multivariate analysis, confirming the sequential improvement in outcomes following major hepatectomy over the past three decades. Major vascular invasion which may require vascular reconstruction with high risk for increased intraoperative blood loss was also identified as an independent predictor of early postoperative mortality.16
Our study had some limitations. We evaluated the outcome of patients who were selected for surgery at centers in three different countries. Each center may have used different methods to assess resectability and select surgical candidates. In spite of this, the analysis showed that similarly favorable results could be achieved in all cohorts, which confirms the increasing efficacy of major hepatectomy in both the East and the West. Because of the inhomogeneity in the reporting of postoperative complications between centers, this study did not analyze morbidity. However, the objective of this analysis was to focus on long-term survival as a primary endpoint. In this study, 90-day mortality represents a surrogate for morbidity as others have shown an association between postoperative morbidity and mortality.36,37
Conclusion
In conclusion, this multinational experience provides evidence that outcomes after major hepatectomy, defined as resection of ≥4 liver segments, have improved significantly over the past three decades in both the East and the West. Major hepatectomy should be considered an established method of treatment for HCC at hepatobiliary centers and can offer a long-term survival benefit in patients with locally advanced HCC.
Acknowledgments
The authors thank Stephanie Deming and Ruth J. Haynes for their assistance with manuscript preparation.
Funding sources This study was supported, in part, by the National Institutes of Health through MD Anderson’s Cancer Center Support Grant, CA016672.
APPENDIX
Discussant
Dr. Sharon Weber (Madison, WI): The authors are to be congratulated for this outstanding multi-institutional study. The improvements in overall survival and 90-day mortality over time are very encouraging, particularly since this is such a difficult patient population due to the large tumor size and underlying liver dysfunction
Any HPB surgeon who reads this excellent manuscript will likely want to gain a better understanding of the specific changes that occurred over time to account for these fantastic results, particularly in regard to perioperative management and patient selection, in order to better assess how these factors impacted mortality and long-term outcome.
Specific perioperative parameters that should be included in your analyses include EBL, blood transfusions, use of low CVP anesthesia, and use of the Pringle for liver transection, as these factors have been found to have a significant impact on perioperative mortality and, for some of these factors, also on survival.
In addition, can you help us understand how better assessment of liver reserve may have impacted perioperative mortality? How often was PVE or CT volumetry utilized, and were patients excluded from consideration for resection if they were unable to grow their remnant liver following PVE?
Many other series analyzing long-term results following complex resections for cancer have found a detrimental effect of complications on overall survival. I understand why you did not include that data here, but it would be very useful to assess this in order for us to understand the significance of complications relative to the other negative prognostic indicators you have nicely outlined in the paper. This is particularly relevant, since, to some degree, it is more controllable than tumor-specific factors like AFP level, tumor size, and vascular invasion.
Finally, did the improvement in mortality apply to patients with and without cirrhosis? Attempting to better understand the independent predictors of 90-day mortality are so important for this disease; thus, it would be very useful to include a multivariable analysis of predictors of mortality in the final MS.
Again, congratulations on an excellent paper.
Closing Discussant
Dr. Andreas Andreou: On behalf of the authors, I would like to thank Dr. Weber for her positive comments and interesting questions.
In regard to the inquiry about specific factors that have changed over time and may directly account for improved survival, it is difficult to separate the multiple components. Undoubtedly, there have been contributions in the areas of preoperative imaging, patient selection, surgical techniques, and anesthetic monitoring that have increased the safety of liver resection and contributed to forward progress. However, the design of this large multicenter study, which extended over a long period of time, did not allow for analysis of specific perioperative parameters such as EBL, blood transfusions, and use of low CVP anesthesia.
As our study focused on major hepatectomy, one of the most meaningful advances that occurred over time was likely in the area of evaluating the future liver remnant (FLR). With modern cross-sectional imaging, configuration, contour, and volume of the FLR can now be reliably assessed. More recently, the ability to additionally measure the functional capacity of the FLR, through either the indocyanine green clearance test or via hypertrophic response to portal vein embolization, has significantly impacted patient selection and postoperative liver failure rates. Although each center approached FLR evaluation with a unique strategy, the universal development of FLR assessment is certainly a key factor contributing to their shared successes.
In reference to your excellent question regarding the impact of complications on survival, we have reassessed the 90-day outcomes in this study. Although we do not have sufficient detail regarding specific complications to individually report them, the 90-day survival may act as a useful surrogate to differentiate meaningful complications from recoverable complications. The 90-day postoperative mortality rate for all patients undergoing major hepatectomy was 4 %, and it did not differ between patients who underwent right and those who underwent extended hepatectomy (p=0.976; Table 1). However, an analysis according to both extent of resection and underlying liver disease showed that whereas fibrosis or cirrhosis did not negatively affect the 90-day mortality of patients undergoing right hepatectomy (p=0.940), the presence of fibrosis or cirrhosis was associated with worse 90-day mortality rate among patients treated with extended hepatectomy (p= 0.002; Table 4).
Our additional analysis of early outcomes identified major vascular resection and earlier time period for resection as the two independent factors associated with 90-day survival. Interestingly, underlying liver disease in the form of fibrosis or cirrhosis was not a significant factor. Taken together, these data suggest that underlying liver disease has its largest impact when resection requires extended hepatectomy and/or major vascular resection.
Footnotes
This study was presented at the Plenary Session of the 53rd Annual Meeting of The Society for Surgery of the Alimentary Tract, San Diego, CA, May 21, 2012.
Conflict of interest None.
Contributor Information
Andreas Andreou, Department of Surgical Oncology, The University of Texas MD, Anderson Cancer Center, Houston, TX, USA, taaloia@mdanderson.org.
Jean-Nicolas Vauthey, Department of Surgical Oncology, The University of Texas MD, Anderson Cancer Center, Houston, TX, USA, taaloia@mdanderson.org.
Daniel Cherqui, Department of Digestive and Hepatobiliary Surgery and Liver, Transplantation, Hôpital Henri Mondor, Créteil, France.
Giuseppe Zimmitti, Department of Surgical Oncology, The University of Texas MD, Anderson Cancer Center, Houston, TX, USA, taaloia@mdanderson.org.
Dario Ribero, Department of Surgical Oncology, The University of Texas MD, Anderson Cancer Center, Houston, TX, USA, taaloia@mdanderson.org.
Mark J. Truty, Department of Surgical Oncology, The University of Texas MD, Anderson Cancer Center, Houston, TX, USA, taaloia@mdanderson.org
Steven H. Wei, Department of Surgical Oncology, The University of Texas MD, Anderson Cancer Center, Houston, TX, USA, taaloia@mdanderson.org
Steven A. Curley, Department of Surgical Oncology, The University of Texas MD, Anderson Cancer Center, Houston, TX, USA, taaloia@mdanderson.org
Alexis Laurent, Department of Digestive and Hepatobiliary Surgery and Liver, Transplantation, Hôpital Henri Mondor, Créteil, France.
Ronnie T. Poon, Department of Surgery, The University of Hong Kong, Queen Mary Hospital, Hong Kong, China
Jacques Belghiti, Department of Surgery, Hôpital Beaujon, Clichy, France.
David M. Nagorney, Department of Gastroenterologic and General Surgery, Mayo Clinic, Rochester, MN, USA
Thomas A. Aloia, Department of Surgical Oncology, The University of Texas MD, Anderson Cancer Center, Houston, TX, USA, taaloia@mdanderson.org
References
- 1.Ercolani G, Grazi GL, Ravaioli M, et al. Liver resection for hepatocellular carcinoma on cirrhosis: univariate and multivariate analysis of risk factors for intrahepatic recurrence. Ann Surg. 2003;237:536–543. doi: 10.1097/01.SLA.0000059988.22416.F2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Poon RT, Fan ST, Lo CM, et al. Improving survival results after resection of hepatocellular carcinoma: a prospective study of 377 patients over 10 years. Ann Surg. 2001;234:63–70. doi: 10.1097/00000658-200107000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mazzaferro V, Regalia E, Doci R, et al. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med. 1996;334:693–699. doi: 10.1056/NEJM199603143341104. [DOI] [PubMed] [Google Scholar]
- 4.Livraghi T, Meloni F, Di Stasi M, et al. Sustained complete response and complications rates after radiofrequency ablation of very early hepatocellular carcinoma in cirrhosis: Is resection still the treatment of choice? Hepatology. 2008;47:82–89. doi: 10.1002/hep.21933. [DOI] [PubMed] [Google Scholar]
- 5.Poon RT, Fan ST, Lo CM, et al. Extended hepatic resection for hepatocellular carcinoma in patients with cirrhosis: is it justified? Ann Surg. 2002;236:602–611. doi: 10.1097/00000658-200211000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chik BH, Liu CL, Fan ST, et al. Tumor size and operative risks of extended right-sided hepatic resection for hepatocellular carcinoma: implication for preoperative portal vein embolization. Arch Surg. 2007;142:63–69. doi: 10.1001/archsurg.142.1.63. [DOI] [PubMed] [Google Scholar]
- 7.Akai H, Kiryu S, Matsuda I, et al. Detection of hepatocellular carcinoma by Gd-EOB-DTPA-enhanced liver MRI: Comparison with triple phase 64 detector row helical CT. Eur J Radiol. 2010 doi: 10.1016/j.ejrad.2010.07.026. [DOI] [PubMed] [Google Scholar]
- 8.Aloia TA, Zorzi D, Abdalla EK, et al. Two-surgeon technique for hepatic parenchymal transection of the noncirrhotic liver using saline-linked cautery and ultrasonic dissection. Ann Surg. 2005;242:172–177. doi: 10.1097/01.sla.0000171300.62318.f4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Donadon M, Abdalla EK, Vauthey JN. Liver hanging maneuver for large or recurrent right upper quadrant tumors. J Am Coll Surg. 2007;204:329–333. doi: 10.1016/j.jamcollsurg.2006.10.009. [DOI] [PubMed] [Google Scholar]
- 10.Ishizawa T, Mise Y, Aoki T, et al. Surgical technique: new advances for expanding indications and increasing safety in liver resection for HCC: the Eastern perspective. J Hepatobiliary Pancreat Sci. 2010;17:389–393. doi: 10.1007/s00534-009-0231-2. [DOI] [PubMed] [Google Scholar]
- 11.Strasberg SM. Nomenclature of hepatic anatomy and resections: a review of the Brisbane 2000 system. J Hepatobiliary Pancreat Surg. 2005;12:351–355. doi: 10.1007/s00534-005-0999-7. [DOI] [PubMed] [Google Scholar]
- 12.Dahiya D, Wu TJ, Lee CF, et al. Minor versus major hepatic resection for small hepatocellular carcinoma (HCC) in cirrhotic patients: a 20-year experience. Surgery. 2010;147:676–685. doi: 10.1016/j.surg.2009.10.043. [DOI] [PubMed] [Google Scholar]
- 13.Capussotti L, Muratore A, Massucco P, et al. Major liver resections for hepatocellular carcinoma on cirrhosis: early and long-term outcomes. Liver Transpl. 2004;10:S64–68. doi: 10.1002/lt.20035. [DOI] [PubMed] [Google Scholar]
- 14.Ng KK, Vauthey JN, Pawlik TM, et al. Is hepatic resection for large or multinodular hepatocellular carcinoma justified? Results from a multi-institutional database. Ann Surg Oncol. 2005;12:364–373. doi: 10.1245/ASO.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 15.Wei AC, Tung-Ping Poon R, Fan ST, et al. Risk factors for perioperative morbidity and mortality after extended hepatectomy for hepatocellular carcinoma. Br J Surg. 2003;90:33–41. doi: 10.1002/bjs.4018. [DOI] [PubMed] [Google Scholar]
- 16.Melendez J, Ferri E, Zwillman M, et al. Extended hepatic resection: a 6-year retrospective study of risk factors for perioperative mortality. J Am Coll Surg. 2001;192:47–53. doi: 10.1016/s1072-7515(00)00745-6. [DOI] [PubMed] [Google Scholar]
- 17.Zhou L, Rui JA, Wang SB, et al. Outcomes and prognostic factors of cirrhotic patients with hepatocellular carcinoma after radical major hepatectomy. World J Surg. 2007;31:1782–1787. doi: 10.1007/s00268-007-9029-z. [DOI] [PubMed] [Google Scholar]
- 18.Torzilli G, Makuuchi M, Inoue K, et al. No-mortality liver resection for hepatocellular carcinoma in cirrhotic and noncirrhotic patients: is there a way? A prospective analysis of our approach. Arch Surg. 1999;134:984–992. doi: 10.1001/archsurg.134.9.984. [DOI] [PubMed] [Google Scholar]
- 19.Fan ST, Mau Lo C, Poon RT, et al. Continuous improvement of survival outcomes of resection of hepatocellular carcinoma: a 20-year experience. Ann Surg. 2011;253:745–758. doi: 10.1097/SLA.0b013e3182111195. [DOI] [PubMed] [Google Scholar]
- 20.Imamura H, Seyama Y, Kokudo N, et al. One thousand fifty-six hepatectomies without mortality in 8 years. Arch Surg. 2003;138:1198–1206. doi: 10.1001/archsurg.138.11.1198. [DOI] [PubMed] [Google Scholar]
- 21.Miyoshi A, Takahashi T, Otsuka T, et al. Efficacy of major hepatectomy for large hepatocellular carcinoma. Hepatogastroenterology. 2009;56:768–772. [PubMed] [Google Scholar]
- 22.Bismuth H. Surgical Anatomy and Anatomical Surgery of the Liver. World J Surg. 1982;6:3–9. doi: 10.1007/BF01656368. [DOI] [PubMed] [Google Scholar]
- 23.Vauthey JN, Chaoui A, Do KA, et al. Standardized measurement of the future liver remnant prior to extended liver resection: methodology and clinical associations. Surgery. 2000;127:512–519. doi: 10.1067/msy.2000.105294. [DOI] [PubMed] [Google Scholar]
- 24.Kishi Y, Abdalla EK, Chun YS, et al. Three Hundred and One Consecutive Extended Right Hepatectomies: Evaluation of Outcome Based on Systematic Liver Volumetry. Ann Surg. 2009 doi: 10.1097/SLA.0b013e3181b674df. [DOI] [PubMed] [Google Scholar]
- 25.Abdalla EK, Adam R, Bilchik AJ, et al. Improving resectability of hepatic colorectal metastases: Expert consensus statement. Ann Surg Oncol. 2006;13:1271–1280. doi: 10.1245/s10434-006-9045-5. [DOI] [PubMed] [Google Scholar]
- 26.Pugh RN, Murray-Lyon IM, Dawson JL, et al. Transection of the oesophagus for bleeding oesophageal varices. Br J Surg. 1973;60:646–649. doi: 10.1002/bjs.1800600817. [DOI] [PubMed] [Google Scholar]
- 27.Zorzi D, Laurent A, Pawlik TM, et al. Chemotherapy-associated hepatotoxicity and surgery for colorectal liver metastases. Br J Surg. 2007;94:274–286. doi: 10.1002/bjs.5719. [DOI] [PubMed] [Google Scholar]
- 28.Leevy CM, Smith F, Longueville J, et al. Indocyanine green clearance as a test for hepatic function. Evaluation by dichromatic ear densitometry. JAMA. 1967;200:236–240. [PubMed] [Google Scholar]
- 29.Vauthey JN, Lauwers GY, Esnaola NF, et al. Simplified staging for hepatocellular carcinoma. J Clin Oncol. 2002;20:1527–1536. doi: 10.1200/JCO.2002.20.6.1527. [DOI] [PubMed] [Google Scholar]
- 30.Couinaud C. Le foie; études anatomiques et chirurgicales. Masson & Cie; Paris: 1957. [Google Scholar]
- 31.Edmondson HA, Steiner PE. Primary carcinoma of the liver: a study of 100 cases among 48,900 necropsies. Cancer. 1954;7:462–503. doi: 10.1002/1097-0142(195405)7:3<462::aid-cncr2820070308>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 32.Ishak K, Baptista A, Bianchi L, et al. Histological grading and staging of chronic hepatitis. J Hepatol. 1995;22:696–699. doi: 10.1016/0168-8278(95)80226-6. [DOI] [PubMed] [Google Scholar]
- 33.Ishizawa T, Hasegawa K, Aoki T, et al. Neither multiple tumors nor portal hypertension are surgical contraindications for hepatocellular carcinoma. Gastroenterology. 2008;134:1908–1916. doi: 10.1053/j.gastro.2008.02.091. [DOI] [PubMed] [Google Scholar]
- 34.Halazun KJ, Al-Mukhtar A, Aldouri A, et al. Right hepatic trisectionectomy for hepatobiliary diseases. Ann Surg. 2007;246:1065–1074. doi: 10.1097/SLA.0b013e3181492795. [DOI] [PubMed] [Google Scholar]
- 35.Abdalla EK, Denys A, Chevalier P, et al. Total and segmental liver volume variations: implications for liver surgery. Surgery. 2004;135:404–410. doi: 10.1016/j.surg.2003.08.024. [DOI] [PubMed] [Google Scholar]
- 36.Mullen JT, Ribero D, Reddy SK, et al. Hepatic insufficiency and mortality in 1,059 noncirrhotic patients undergoing major hepatectomy. J Am Coll Surg. 2007;204:854–862. doi: 10.1016/j.jamcollsurg.2006.12.032. [DOI] [PubMed] [Google Scholar]
- 37.Kishi Y, Abdalla EK, Chun YS, et al. Three Hundred and One Consecutive Extended Right Hepatectomies: Evaluation of Outcome Based on Systematic Liver Volumetry. Ann Surg. 2009;250:540–548. doi: 10.1097/SLA.0b013e3181b674df. [DOI] [PubMed] [Google Scholar]
- 38.Esnaola NF, Mirza N, Lauwers GY, et al. Comparison of clinicopathologic characteristics and outcomes after resection in patients with hepatocellular carcinoma treated in the United States, France, and Japan. Ann Surg. 2003;238:711–719. doi: 10.1097/01.sla.0000094436.34556.ac. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kubota K, Makuuchi M, Kusaka K, et al. Measurement of liver volume and hepatic functional reserve as a guide to decision-making in resectional surgery for hepatic tumors. Hepatology. 1997;26:1176–1181. doi: 10.1053/jhep.1997.v26.pm0009362359. [DOI] [PubMed] [Google Scholar]
- 40.Azoulay D, Castaing D, Krissat J, et al. Percutaneous portal vein embolization increases the feasibility and safety of major liver resection for hepatocellular carcinoma in injured liver. Ann Surg. 2000;232:665–672. doi: 10.1097/00000658-200011000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Imamura H, Seyama Y, Makuuchi M, et al. Sequential transcatheter arterial chemoembolization and portal vein embolization for hepatocellular carcinoma: the university of Tokyo experience. Semin Intervent Radiol. 2008;25:146–154. doi: 10.1055/s-2008-1076683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yoo H, Kim JH, Ko GY, et al. Sequential Transcatheter Arterial Chemoembolization and Portal Vein Embolization versus Portal Vein Embolization Only before Major Hepatectomy for Patients with Hepatocellular Carcinoma. Ann Surg Oncol. 2011;18:1251–1257. doi: 10.1245/s10434-010-1423-3. [DOI] [PubMed] [Google Scholar]
- 43.Ogata S, Belghiti J, Farges O, et al. Sequential arterial and portal vein embolizations before right hepatectomy in patients with cirrhosis and hepatocellular carcinoma. Br J Surg. 2006;93:1091–1098. doi: 10.1002/bjs.5341. [DOI] [PubMed] [Google Scholar]
- 44.Choti MA, Geschwind JF. Preoperative Sequential TACE and PVE to Increase Resectability in the Cirrhotic Patient With HCC. Gastrointest Cancer Res. 2008;2:47–48. [PMC free article] [PubMed] [Google Scholar]
- 45.Hwang S, Lee SG, Ko GY, et al. Sequential preoperative ipsilateral hepatic vein embolization after portal vein embolization to induce further liver regeneration in patients with hepatobiliary malignancy. Ann Surg. 2009;249:608–616. doi: 10.1097/SLA.0b013e31819ecc5c. [DOI] [PubMed] [Google Scholar]
- 46.Farges O, Malassagne B, Flejou JF, et al. Risk of major liver resection in patients with underlying chronic liver disease: a reap-praisal. Ann Surg. 1999;229:210–215. doi: 10.1097/00000658-199902000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sotiropoulos GC, Druhe N, Sgourakis G, et al. Liver transplantation, liver resection, and transarterial chemoembolization for hepatocellular carcinoma in cirrhosis: which is the best oncological approach? Dig Dis Sci. 2009;54:2264–2273. doi: 10.1007/s10620-008-0604-4. [DOI] [PubMed] [Google Scholar]
- 48.Ruzzenente A, Capra F, Pachera S, et al. Is liver resection justified in advanced hepatocellular carcinoma? Results of an observational study in 464 patients. J Gastrointest Surg. 2009;13:1313–1320. doi: 10.1007/s11605-009-0903-x. [DOI] [PubMed] [Google Scholar]
- 49.Ribero D, Abdalla EK, Madoff DC, et al. Portal vein embolization before major hepatectomy and its effects on regeneration, resectability and outcome. Br J Surg. 2007;94:1386–1394. doi: 10.1002/bjs.5836. [DOI] [PubMed] [Google Scholar]
- 50.Farges O, Belghiti J, Kianmanesh R, et al. Portal vein embolization before right hepatectomy—Prospective clinical trial. Ann Surg. 2003;237:208–217. doi: 10.1097/01.SLA.0000048447.16651.7B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.AJCC Cancer Staging Manual. Seventh Edition Springer; New York: 2010. [Google Scholar]
- 52.Varotti G, Ramacciato G, Ercolani G, et al. Comparison between the fifth and sixth editions of the AJCC/UICC TNM staging systems for hepatocellular carcinoma: multicentric study on 393 cirrhotic resected patients. Eur J Surg Oncol. 2005;31:760–767. doi: 10.1016/j.ejso.2005.04.008. [DOI] [PubMed] [Google Scholar]
- 53.Lei HJ, Chau GY, Lui WY, et al. Prognostic value and clinical relevance of the 6th edition 2002 American Joint Committee on Cancer staging system in patients with resectable hepatocellular carcinoma. J Am Coll Surg. 2006;203:426–435. doi: 10.1016/j.jamcollsurg.2006.06.030. [DOI] [PubMed] [Google Scholar]
- 54.Vauthey JN, Ribero D, Abdalla EK, et al. Outcomes of liver transplantation in 490 patients with hepatocellular carcinoma: validation of a uniform staging after surgical treatment. J Am Coll Surg. 2007;204:1016–1027. doi: 10.1016/j.jamcollsurg.2006.12.043. [DOI] [PubMed] [Google Scholar]
- 55.Pawlik TM, Poon RT, Abdalla EK, et al. Critical appraisal of the clinical and pathologic predictors of survival after resection of large hepatocellular carcinoma. Arch Surg. 2005;140:450–457. doi: 10.1001/archsurg.140.5.450. [DOI] [PubMed] [Google Scholar]