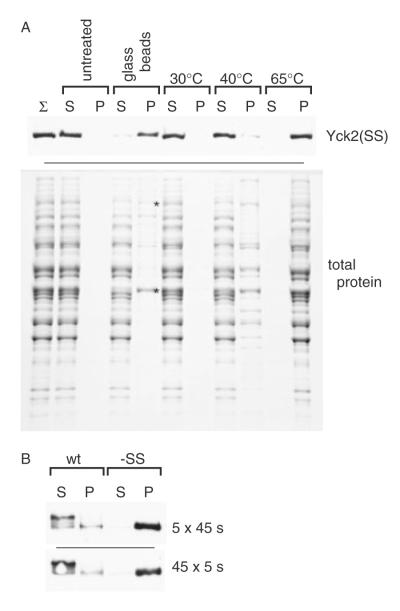
Figure 6.
Tests of the contribution of elevated temperature to glass bead-induced aggregation. (A) Comparison of the effects of elevated temperature to those of glass bead agitation on the aggregation of both Yck2(SS) (upper panel) and bulk yeast proteins (lower panel). A supernatant fraction, prepared by high-speed centrifugation (no Triton X-100 present) of a mortar–pestle lysate from yeast cells expressing GAL1-driven Yck2(SS), was subjected to a variety of treatments, including either a mock glass bead lysis (glass beads) or 10 min incubations at the indicated temperatures. The samples then were subjected to a second high-speed centrifugation yielding S100 and P100 fractions that were analysed either by anti-HA Western blotting [Yck2(SS), upper panel] or by SDS–PAGE and Coomassie staining (total protein, lower panel). Two proteins that show differential fractionation to the pellet from the glass bead-treated sample, identified by LC–MS/MS as both being translational elongation factors, namely the 116 kDa EF-3 (Yef3) and the 50 kDa EF-1A (Tef1/2), are indicated by asterisks (lower panel). (B) Lysates were prepared from yeast cells expressing GAL1-driven Yck2(wt) or Yck2(SS), either by our standard glass bead lysis protocol (i.e. five 45 s vortexings interspersed with 1 min ice-bath rests) or by a revised protocol intended to minimize heating (45 5 s vortexings interspersed with 1 min slushy ice-bath rests). Subsequent lysate processing and fractionation (i.e. Triton X-100 solubilization and centrifugation) was as described for Figure 1A