Abstract
Background
Altered interoception, i.e., processing of stimuli from inside the body, has been considered an important component of drug-taking behavior. However, approaches to examine interoceptive sensitivity in humans have been limited. This study examined the hypothesis that adolescents with substance use disorder show altered interoceptive processing, measured by stimulating mechanoreceptive C-fibers (MR-CF) via soft touch.
Methods
Adolescents with substance use disorders (SUD, n=15) and comparison youth (CON, n=17) underwent functional magnetic resonance imaging (fMRI) during anticipation or reception of a positively valenced “Soft Touch” consisting of MR-CF stimulation to the palm or forearm. Visual analog scales (VAS) indexed subjective interoceptive experience (e.g., pleasantness, intensity).
Results
Across all conditions, SUD displayed attenuated left posterior insula activation compared to CON . Greater left anterior insula and right lentiform nucleus activation was evident during the application of soft touch for SUD but not for CON. Whereas for CON, greater left anterior insula activation was associated with higher pleasantness ratings, pleasantness was linked to less anterior insula activation in SUD. Finally, within SUD, attenuated posterior insula activation was related to more recent cannabis use.
Conclusions
SUD adolescents exhibit blunted somatovisceral processing of pleasant stimulation, heightened sensitivity in regions responsible for processing reward value, and altered relationships between interoceptive processing and subjective experience.
Keywords: alcohol, cannabis, interoception, reward, fMRI
1. INTRODUCTION
Adolescence, a time of increased independence, is often accompanied by experimentation with alcohol and drugs. By 12th grade, 70% of teenagers have tried alcohol and 50% have tried an illicit drug (Johnston et al., 2012). Moreover, 6.9% of adolescents have met criteria for substance abuse or dependence in the past year (Substance Abuse and Mental Health Services Administration, 2012) and early alcohol and drug use is a predictor of dependence on illicit substances in adulthood (Anthony and Petronis, 1995; Bonomo et al., 2004; Grant et al., 2001, 2006; Wells et al., 2004). Examining biological and behavioral markers of problematic substance use in adolescence may elucidate mechanisms involved in the transition to chronic substance dependence in adulthood and aid in prevention and treatment for youth at risk for addiction.
Interoception comprises sensing the physiological condition of the body (Craig, 2002), and representation of the internal state (Craig, 2009) in the context of ongoing activities, and is associated with motivated action to regulate homeostasis (Critchley et al., 2004). The insular cortex helps integrate ascending interoceptive afferents with ongoing behavior (Craig, 2009; Paulus et al., 2009) and based on findings in smokers with insula lesions, interoceptive dysregulation has been proposed as a mechanism for the development and maintenance of addiction (Naqvi and Bechara, 2009, 2010; Paulus, 2007; Paulus et al., 2009; Verdejo-Garcia et al., 2012). The pleasure of intoxication, displeasure of withdrawal, and visceral feelings of craving may depend upon the interaction of interoceptive sensitivity with the current homeostatic state (Naqvi et al., 2009) and subsequent appraisal of bodily feelings could motivate continuing or discontinuing substance use.
As interoception may be dysfunctional in substance dependence (for a recent review see Verdejo-Garcia et al., 2012), examination of the interoceptive system in adolescent substance users is a promising and relatively unexplored area of research. Although no studies have yet investigated interoception in adolescent substance users, several neuroimaging studies have linked early use to the insular cortex. For example, greater lifetime alcohol use in adolescents was associated with lower insula response during a functional magnetic resonance imaging (fMRI) spatial working memory task (Tapert et al., 2004) and adolescent cannabis users have shown decreased cortical thickness and cerebral blood flow in the insula (Jacobus et al., 2012; Lopez-Larson et al., 2011). Further, adolescent cigarette smokers demonstrated attenuated insular activation to pleasurable, appetitive images (Rubinstein et al., 2011), and alcohol-dependent young women showed greater insula response to pictures of alcohol-related words (Tapert et al., 2004), suggesting that the insula may be hypoactive to non-substance related stimuli but hyperactive to substance-related stimuli in young substance users.
Youth at risk for substance use disorders have also shown abnormalities in reward processing, which may relate to interoceptive regulation. For example, adolescent smokers have shown lower striatum activation during reward anticipation that is correlated with smoking frequency (Peters et al., 2011). Studies have also shown that adolescents with a family history of alcoholism demonstrate blunted striatal activation during reward anticipation (Schneider et al., 2012; Yau et al., 2012).
The anatomical and functional differentiation within the insula may play a critical role in understanding the representation of interoception in the brain. The posterior insula, considered the primary somatovisceral cortex, receives afferent projections from the lamina I spinothalamocortical system (Craig, 2002) and contains a topographic representation of small diameter afferent activity, likely reflecting homeostatically relevant body signals (Craig, 2002). The next step in the processing stream is the anterior insula, a region central to the integration of visceral information with conscious awareness and affective states (Craig, 2002; Critchley et al., 2004). The anterior insula has bidirectional connections to the striatum (Chikama et al., 1997), which may constitute a direct link between positive feeling states and reward value associated with pleasant stimuli.
Given the above neuroanatomy, one approach to examine interoceptive processing of a visceral stimulus is via positively valenced stimulation of unmyelinated mechanoreceptive C-fibers (MR-CF) thought to be abundant in hairy (such as the forearm), but not glabrous skin (like the palm; Loken et al., 2009; Olausson et al., 2002, 2010; Vallbo et al., 1999, 1993). Because these C-fibers project to posterior insula (Bjornsdotter et al., 2009; Morrison et al., 2011; Olausson et al., 2002, 2010), MR-CF afferents are excellent candidates for examining how pleasant sensations are processed by the insula and how it may be related to substance use during adolescence, when reward sensitivity is at its lifetime peak (Somerville et al., 2010; Steinberg, 2008).
The present investigation used fMRI to examine insula and striatal activation in adolescents with current alcohol or cannabis use disorder. First, we hypothesized that adolescents with substance use disorders (SUD) would show attenuated insula activation given previous findings of decreased volume and blood flow of the insula in young substance users (Jacobus et al., 2012; Lopez-Larson et al., 2011). Second, we expected this group difference to be present during the interoceptive stimulation itself rather than during the anticipation of the stimulus and for activation to be greatest during forearm stimulation, due to anatomical connections between MR-CF and the insula. Third, we hypothesized that activation in the anterior but not posterior insula would be positively related to subjective ratings of the stimulation, because the anterior insula is important for conscious awareness of visceral states (Craig, 2002). Finally, we hypothesized that SUD would exhibit attenuated striatal response during anticipation of pleasant interoceptive stimulation, in light of findings showing lower striatum activation during reward anticipation in adolescents at risk for SUD (Peters et al., 2011; Schneider et al., 2012; Yau et al., 2012).
2. METHODS
2.1 Subjects
Fifteen adolescents with current SUD and 17 demographically matched healthy control adolescents (CON) with no history of SUD, completed clinical assessments, questionnaires, and an fMRI session. The sample was 84% Caucasian, 13% Asian, and 3% Pacific Islander, and 19% of participants were Latino/a. Adolescents ages 15–17 were recruited from local high schools via fliers distributed on campus or through the mail and potential participants and their parents were screened for eligibility. Written informed consent was obtained from each parent or legal guardian and assent was obtained from each adolescent participant in accordance with the University of California, San Diego Human Research Protections Program.
SUD were defined as having: (1) current endorsement of two or more SUD criteria based on Diagnostic and Statistical Manual of Mental Disorders (5th ed., DSM-5; American Psychiatric Association, 2013) criteria for either alcohol or cannabis and (2) any alcohol or cannabis use within the past three months. All SUD were non-treatment seeking. Inclusion criteria for CON were (1) <5 lifetime binge drinking episodes; (2) <5 lifetime cannabis uses; (3) no other illicit drug use in lifetime. Exclusionary criteria for all groups were: any DSM-IV Axis I psychiatric disorders independent of substance use, current use of psychoactive medication; neurological disorder; head injury with loss of consciousness <2 minutes; learning disability; severe medical disorder; major sensory impairments; complicated or premature birth; prenatal alcohol or drug exposure; MRI contraindications; and left handedness.
2.2 Measures
Lifetime substance use and DSM-5 SUD diagnoses were assessed by experienced interviewers using the Semi-Structured Assessment for Drug Dependence and Alcoholism (SSADDA; Pierucci-Lagha et al., 2005). The Diagnostic Interview Schedule for Children (DISC) Predictive Scales (Lucas et al., 2001; Shaffer et al., 2000) was administered to screen for the presence of any Axis I diagnoses independent of substance use. Participants also completed the Barratt Impulsiveness Scale (BIS-11), a 30 item questionnaire tapping frequency of impulsive behaviors (Patton et al., 1995), the Sensation Seeking Scale (SSS-V), a 40 item forced choice inventory of thrill and adventure seeking, experience seeking, disinhibition, and boredom susceptibility (Zuckerman, 1996) and the Youth Self Report (YSR), a 112 item measure of behavioral and emotional problems experienced in the last 6 months (Achenbach and Rescorla, 2001). Participants completed the SSADDA and self-report measures during the interview session, which was scheduled prior to the fMRI session. Participants were instructed to abstain from substance use at least 72 hours prior to the fMRI session. Recent substance use information was verified at the fMRI session using the Timeline Followback (Sobell and Sobell, 1992), urine toxicology, and breathalyzer screens.
For demographic and questionnaire variables, statistical analysis were conducted in SPSS 19.0 (IBM Corporation, Armonk, NY). Age, years of education, and questionnaire scores were compared using independent 2-sample t-tests and gender, race, and ethnicity were compared using Pearson's chi square tests.
2.3 Soft Touch Task
During the fMRI session, participants performed a continuous performance task (CPT) integrated with interoceptive stimulation. The interoceptive stimulus consisted of MR-CF stimulation (“Soft Touch”) that was administered by trained research assistants using a hand held soft boar bristle brush (OXO International Ltd., NY) at designated intervals during fMRI acquisition. MR-CF stimulation was administered on pre-measured and marked 4cm long regions of skin on the ventral surface of the left forearm, thought to be dense in MR-CF, and on the palm, which is thought to be devoid of these fibers (Loken et al., 2009; Vallbo et al., 1993). Each Soft Touch brush stroke was administered at a velocity of 2cm/sec to maximally excite MR-CF (Vallbo et al., 1999) in a proximal to distal direction with a force equal to the weight of the brush.
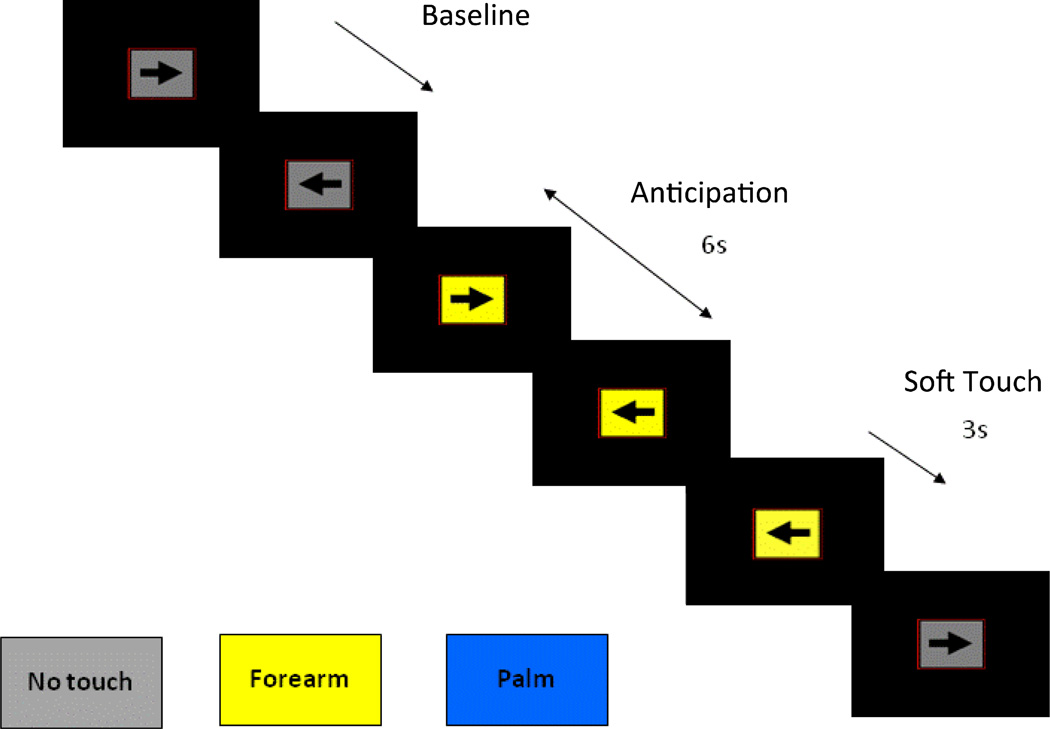
For the CPT, a screen presented a left or right pointing black arrow surrounded by a colored rectangle (see Figure 1). One arrow was present on the screen for each trial and each trial lasted 3 seconds in duration. Subjects were instructed to respond to the orientation of the arrow as quickly and as accurately as possible by pressing a left or right button on a button box. They were also instructed to attend to the colored rectangle background in each trial because it would serve as a cue for MR-CF stimulation. Color cues were used to signify three conditions and the arrows component of the CPT was present in all conditions: (1) a baseline condition (gray) wherein no tactile stimulus was expected or administered (variable duration averaging 8 seconds); (2) an anticipation condition (blue or yellow) lasting 6 seconds (2 trials total) wherein the background color indicated an impending soft touch on the palm (blue) or forearm (yellow); (3) a Soft Touch condition lasting 3 seconds (1 trial total) directly following the anticipation condition where the background color would remain blue or yellow while the corresponding soft touch to the skin was administered. The Soft Touch brush stroke lasted for 2 of the 3 seconds in the trial. Total task duration was 840 seconds.
Figure 1.
Illustration of continuous performance task with integrated interoceptive stimulation.
Response accuracy and reaction time (RT) were obtained during all conditions to examine whether CPT performance varied across group. Participants also completed visual analog scale (VAS) questionnaires prior to the fMRI acquisition during a practice session and directly following their fMRI scan to assess subjective experience of the Soft Touch. VAS instructions indicated that participants should provide a rating from ‘not at all’ to ‘extremely’ (0 to 10) for the Soft Touch on seven dimensions: pleasant, unpleasant, intensity, tickle, warm, cold, and soft.
2.4 fMRI Data Acquisition
The Soft Touch task was presented in an event-related design and was conducted during one fMRI scan sensitive to blood oxygenation level-dependent (BOLD) contrast using a Signa EXCITE (GE Healthcare, USA) 3.0 Tesla scanner (T2*-weighted echo planar imaging (EPI) scans, TR=2000 ms, TE=32 ms, FOV=23 cm, 64×64 matrix, thirty 2.6 mm axial slices with 1.4 mm gap, flip angle=90°, 290 whole-brain acquisitions). For anatomical reference, a high-resolution T1-weighted image (spoiled gradient recalled SPGR, TR=8 ms, TE=3 ms, slices=172, FOV=25 cm approximately 1mm³ voxels) was obtained.
2.5 fMRI Data Analysis
2.5.1 Single subject analysis
fMRI data were preprocessed with the Analysis of Functional NeuroImages (AFNI) software package (Cox, 1996). GE slices were first reconstructed into AFNI BRIK format. A temporal region containing the largest span with the fewest voxel-wise outliers was used as a base for 3d registration. All other time points in dx, dy, dz, and roll, pitch, yaw directions were adjusted to align data to the base image. The functional echoplanar image underwent automatic coregistration to the high-resolution anatomical image and each dataset was inspected to confirm successful alignment. New outliers were generated for the volume-registered dataset based on whether a given time point greatly exceeded the mean number of voxel outliers for the time series. Deconvolution was performed to determine Soft Touch decision phase activations. Three movement regressors (roll, pitch, yaw), a baseline and linear drift regressor, and four condition regressors (trials for anticipation palm, anticipation forearm, soft touch palm, soft touch forearm), were convolved with a modified hemodynamic response function. The baseline condition, wherein participants were neither anticipating nor receiving the soft touch stimulus, served as the baseline for this analysis. A Gaussian Spatial Filter (4mm FWHM) was used to spatially blur data to account for anatomical differences. Voxels were resampled into 4 × 4 × 4 mm space. Automated Talairach transformations were applied to anatomical images and echoplanar images were subsequently transformed into Talairach space. Percent signal change was determined by dividing the signal for each regressor of interest by the baseline regressor.
2.5.2 Group analysis
Whole-brain voxel-wise percent signal change was subjected to linear mixed effects (LME) analysis (Pinheiro et al., 2012) using R (R Core Development Team, 2012), wherein subjects treated as random effects while group (SUD, CON), condition (anticipation, soft touch), and stimulus type (palm, forearm) were treated as fixed effects. Given hypotheses of the present study, the main effect of group, the group by condition interaction, condition by stimulus interaction, and the group by condition by stimulus type interaction were the effects of interest. To guard against identifying false positive activations, a threshold adjustment method based on Monte-Carlo simulations (via AFNI AlphaSim program) was applied. To maintain a cluster significance at p<.02 AlphaSim identified a minimum cluster volume of 512 µL (8 contiguous voxels) for the whole brain analysis. Based on a priori hypotheses regarding interoception, an ROI mask was used to examine bilateral insula and striatum regions of interest at smaller cluster sizes than was permitted in the whole brain analysis. For the ROI mask, AlphaSim identified minimum cluster volumes for bilateral anterior/posterior insula (256 µL; 4 contiguous voxels) and bilateral striatum (192 µL; 3 contiguous voxels) to maintain a cluster significance at p<.02. The associated individual voxel p-value corrected for multiple comparisons was 0.00001788 for both whole brain and ROI clusters.
Average percent signal change was extracted for significant clusters. Given hypotheses regarding group differences in interoceptive processing, simple effects analyses were calculated to examine the nature of the group by condition interaction by comparing SUD to CON within each level of condition using independent samples t-tests. For interactions involving insula or striatum regions, simple effects analyses comparing anticipation vs. soft touch within each group was also performed. Type I error control was maintained at an alpha of 0.025 for regions where two simple effects were calculated and 0.0125 for interoceptive regions where four simple effects were calculated. Equivalent procedures were followed for calculating two simple effects per region in all other 2-way interactions, presented in Supplementary Materials1.
2.5.3 Follow-up analysis: Robust Regressions
Voxel-based robust regression analyses (Huber, 1964) were used to provide a more conservative estimate of associations between brain activation and VAS ratings and substance use variables while minimizing the effect of outliers. First, regressions were performed within SUD and CON separately to examine whether brain activation for group by condition LME results were associated with subjective VAS ratings of two predictors: pleasantness (positive valence) and intensity (arousal). Four regressions were performed for each group, one for reach of the following dependent variables: percent signal change (1) anticipation palm, (2) anticipation forearm, (3) soft touch palm, and (4) soft touch forearm. Second, to examine the relationship between brain activation and substance use, two additional regression models were conducted within SUD using the following predictors: (1) lifetime days of alcohol and cannabis use, and (2) number of days since last cannabis and alcohol use. Regression results are reported for a priori regions of interest (insula, striatum) that demonstrated consistent findings for anticipation palm or soft touch conditions.
2.6. Behavioral Data Analysis
2.6.1. VAS scales
Group differences in two VAS dimensions (Pleasantness and Intensity) for palm and forearm were examined using independent 2-sample t-tests. Because scores did not differ across time, VAS scores were averaged across pre- and post-fMRI administrations.
2.6.2. RT and accuracy
Participants’ responses to the arrows during the Soft Touch task were subjected to repeated measures analysis of variance (ANOVA) tests to examine group differences in two repeated factors: condition (anticipation, soft touch) and stimulus type (forearm, palm).
3. RESULTS
3.1. Subject Characteristics
Groups did not differ significantly in age, gender, race, ethnicity, or years of education. SUD reported greater lifetime substance use and endorsed higher scores on the YSR internalizing and externalizing syndrome scales. While SUD had higher SSS total scores, SUD did not differ from CON on BIS total (see Table 1).
Table 1.
Subject Characteristics
| SUD M (SD) or % |
CON M (SD) or % |
P | |
|---|---|---|---|
| % Female | 33% | 35% | .91 |
| Age | 16.5 (0.6) | 16.8 (0.6) | .99 |
| Education | 10.6 (1.0) | 10.4 (0.5) | .51 |
| Barratt Impulsivity Scale Total | 61.9 (9.4) | 55.1 (8.4) | .21 |
| Sensation Seeking Scale Total | 25.4 (5.9) | 14.3 (3.4) | .001 ** |
| Youth Self Report Internalizing T-score | 54.0 | 44.3 | .012 * |
| Youth Self Report Externalizing T-score | 59.6 | 43.7 | .001 ** |
| Lifetime Alcohol Use (days) | 94.8 (70.3) | 2.4 (5.4) | .001 ** |
| % Used Alcohol in Lifetime | 100% | 29% | .001 ** |
| % Used Alcohol in Past Week | 60% | 5% | .004 ** |
| Lifetime Cannabis Use (days) | 338.9 (286.7) | 0.2 (1.0) | .001 ** |
| % Used Cannabis in Lifetime | 100% | 12% | .001 ** |
| % Used Cannabis in Past Week | 66% | 0% | .001 ** |
| Lifetime Other Drug Use (days) | 43.6 (66.6) | 0.0 (0.0) | .030 * |
| % Used Other Drugs in Lifetime | 73% | 0% | .001 ** |
| % Used Other Drugs in Past Week | 13% | 0% | .001 ** |
| Primary Diagnosis: | |||
| % Alcohol Use Disorder | 27% | 0% | .001 ** |
| % Cannabis Use Disorder | 73% | 0% | .001 ** |
Note: SUD = adolescents with substance use disorders. CON = control subjects.
= groups significantly differ at p <.05
= groups significantly differ at p<.01. Other drug use included MDMA, hallucinogens, methamphetamine, cocaine, opiates, benzodiazepines, prescription stimulants, and inhalants.
3.2. Behavioral Data
3.2.1. VAS scales
No main effects or interactions were found for pleasant and intense VAS ratings of the Soft Touch (ps>.05). Groups endorsed similar ratings for palm pleasant (SUD: M=4.89, SE=.54; CON: M=5.57, SE=.59), forearm pleasant, (SUD: M=4.50, SE=.57; CON: M=4.98, SE=.54), palm intense (SUD: M=1.67, SE=.43; CON: M=1.04, SE=.33), and forearm intense (SUD: M=1.93, SE=.50; CON: M=1.60, SE=.39). Exploratory analysis of other VAS dimensions revealed no significant differences (ps>.05).
3.2.2. RT and accuracy
No main effects or interactions emerged for task RT and accuracy (ps>.05). Groups demonstrated comparable performance during the CPT across all four conditions and were highly accurate (all >99% accuracy).
3.3. fMRI Data
3.3.1. Group main effect
Across condition and stimulus type, SUD exhibited less activation in the bilateral posterior insula than CON (see Table 2).
Table 2.
fMRI Results for the Main Effect of Group (SUD, CON) (n=32).
| Volume (µL) | Region | X | y | z | Main Effect | |
|---|---|---|---|---|---|---|
| Whole Brain | ||||||
| 1216 | Left Posterior Insula | −37 | −24 | 19 | CON>SUD | |
| 704 | Left Cuneus | −14 | −83 | 23 | CON>SUD | |
| 576 | Right Inferior Temporal Gyrus |
−52 | −17 | −18 | CON>SUD | |
| ROI Mask | ||||||
| 896 | Left Posterior Insula | −37 | −24 | 16 | CON>SUD | |
| 320 | Right Posterior Insula |
41 | −14 | 0 | CON>SUD |
SUD = adolescents with substance use disorders. CON = control subjects. All main effects significant at p< .02 corrected for multiple comparisons via AlphaSim. Coordinates reflect center of mass.
3.3.2. Group by condition interaction
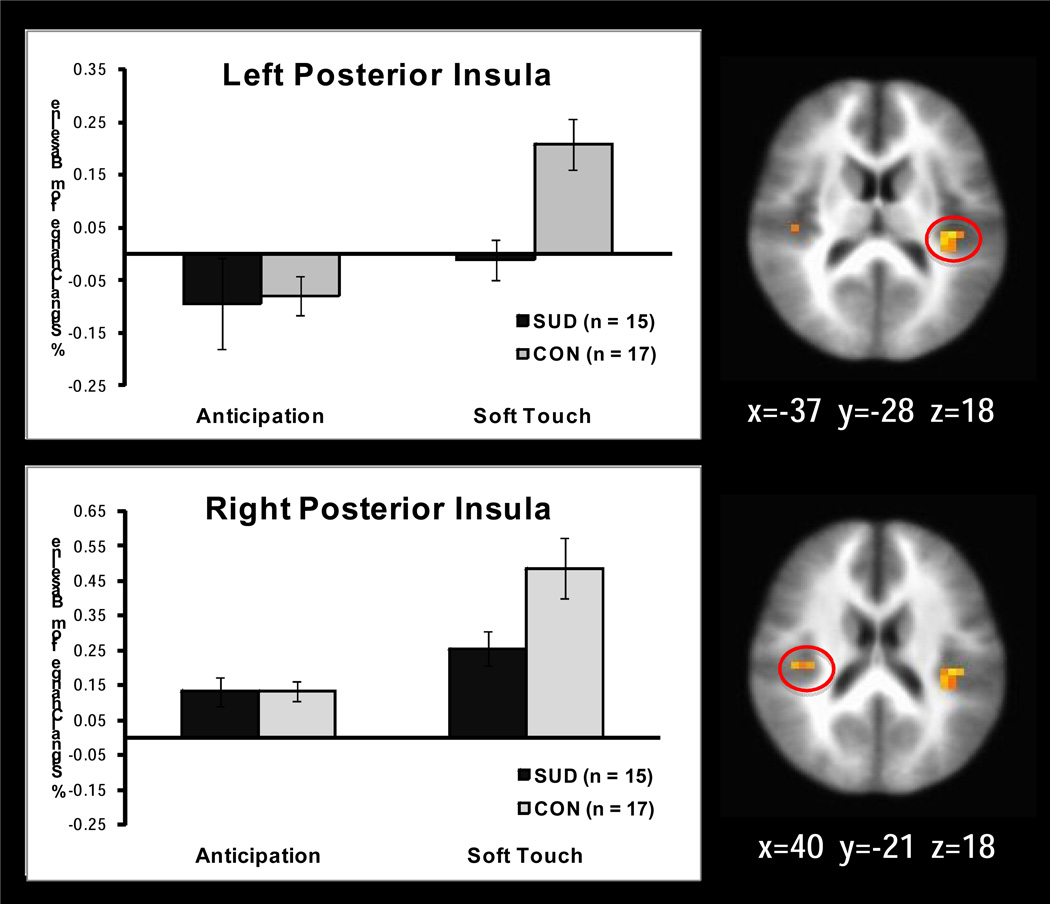
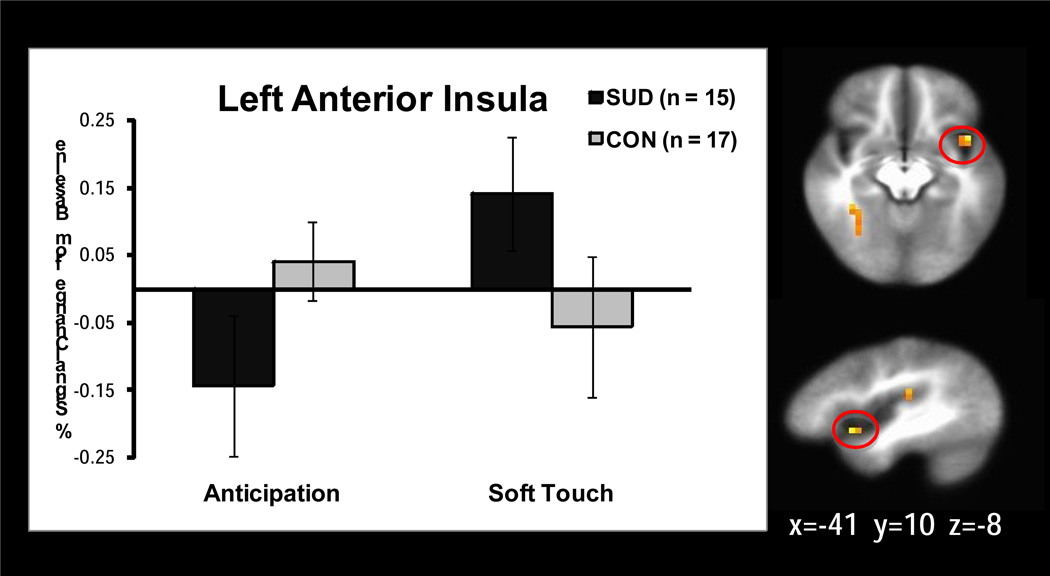
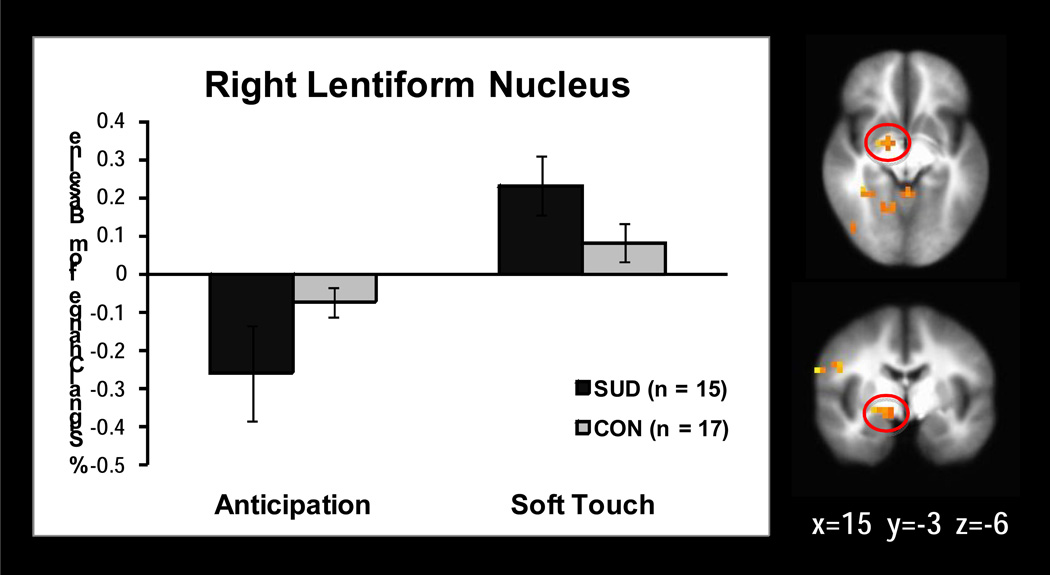
Significant interactions emerged in 18 regions of the whole brain analysis while 3 significant insula regions and no significant striatum regions were found within the ROI mask (see Table 3). Follow up simple effects analysis revealed the following relationships. SUD exhibited less activation than CON in the left (p=.001) and right (p=.02) posterior insula (see Figure 2), right medial frontal gyrus (p=.009), and right middle frontal gyrus (p=.01) during soft touch but not during anticipation. In addition, left anterior insula (see Figure 3) showed significantly greater activation during soft touch than anticipation for SUD but not for CON (p=.03). The right lentiform nucleus (see Figure 4) had greater activation during soft touch than anticipation for both groups, but the difference between soft touch and anticipation was greater for SUD than for CON (ps<.01).
Table 3.
fMRI Results for the Group (SUD, CON) by Condition (Anticipation, Soft Touch) Interaction (n=32).
| Volume (µL) |
Region | x | y | z | Simple Effect of Group | |
|---|---|---|---|---|---|---|
| Whole Brain | ||||||
| 4160 | Left Postcentral Gyrus | −41 | −25 | 50 | CON>SUD during ST | |
| 2240 | Right Precentral Gyrus | 54 | −12 | 28 | CON>SUD during ST | |
| 1600 | Right Culmen | 16 | −54 | −9 | ns | |
| 1088 | Right Inferior Temporal Gyrus | 44 | −72 | 1 | ns | |
| 1088 | Left Posterior Insula | −37 | −27 | 19 | CON>SUD during ST | |
| 1088 | Left Precentral Gyrus | −15 | −33 | 67 | CON>SUD during ST | |
| 832 | Right Middle Frontal Gyrus | 52 | 19 | 26 | CON>SUD during ST | |
| 832 | Left Postcentral Gyrus | −55 | −25 | 16 | CON>SUD during ST | |
| 768 | Right Parahippocampal Gyrus | 31 | −47 | −7 | ns | |
| 768 | Right Lentiform Nucleus | 15 | −3 | −6 | ns | |
| 704 | Right Medial Frontal Gyrus | 1 | −18 | 48 | CON>SUD during ST | |
| 704 | Left Cerebellar Lingual | 0 | −40 | −7 | SUD>CON during ST | |
| 640 | Right Cingulate Gyrus | 3 | −34 | 36 | CON>SUD during ST | |
| 576 | Left Superior Frontal Gyrus | −8 | −21 | 48 | ns | |
| 512 | Right Culmen | 26 | −47 | −19 | ns | |
| 512 | Right Cuneus | 4 | −75 | 22 | CON>SUD during ST | |
| 512 | Right Medial Frontal Gyrus | 15 | 4 | 49 | CON>SUD during ST | |
| 512 | Left Precuneus | −21 | −73 | 50 | CON>SUD during ST | |
| ROI Mask | 896 | Left Posterior Insula | −37 | −28 | 18 | CON>SUD during ST |
| 320 | Right Posterior Insula | 40 | −21 | 18 | CON>SUD during ST | |
| 256 | Left Anterior Insula | −41 | 10 | −8 | ns |
SUD = adolescents with substance use disorders. CON = control subjects. ST = Soft Touch stimulation condition. All interactions significant at p< .02 corrected for multiple comparisons via Alphasim; Simple effects significant at p<.025. ns = no simple main effect of group. Coordinates reflect center of mass.
Figure 2.
Group by condition interaction. Simple effect of group revealed that adolescents with substance use disorder (SUD) exhibited attenuated activation in the left and right posterior insula compared to healthy adolescents (CON) during the experience of soft touch (p<.001 left posterior insula; p=.02 right posterior insula).
Figure 3.
Group by condition interaction. Simple effect of condition revealed that the left anterior insula showed significantly greater activation during soft touch than anticipation for adolescents with substance use disorder (SUD) but not for healthy adolescents (CON; p=.03).
Figure 4.
Group by condition interaction. Simple effect of condition revealed that the right lentiform nucleus showed greater activation during soft touch than anticipation for adolescents with substance use disorder (SUD) and healthy adolescents (CON), but the difference between soft touch and anticipation activation was greater for SUD than for CON (ps<.01).
3.3.3. Condition by stimulus type interaction
Significant interactions emerged in 16 regions of the whole brain analysis while 2 insula regions and 2 caudate regions were found within the ROI mask (see Supplementary Table 42). Simple effects analysis revealed that across groups, palm stimulation produced greater left posterior insula activation than forearm stimulation during soft touch (p=.005), whereas the left (p=.017) and right (p=.021) caudate nuclei and left anterior cingulate cortex (p=.003) showed the opposite pattern.
3.3.4. Group by condition by stimulus type interaction
No group by condition by stimulus type interactions emerged for interoceptive brain regions (see Supplementary Table 53).
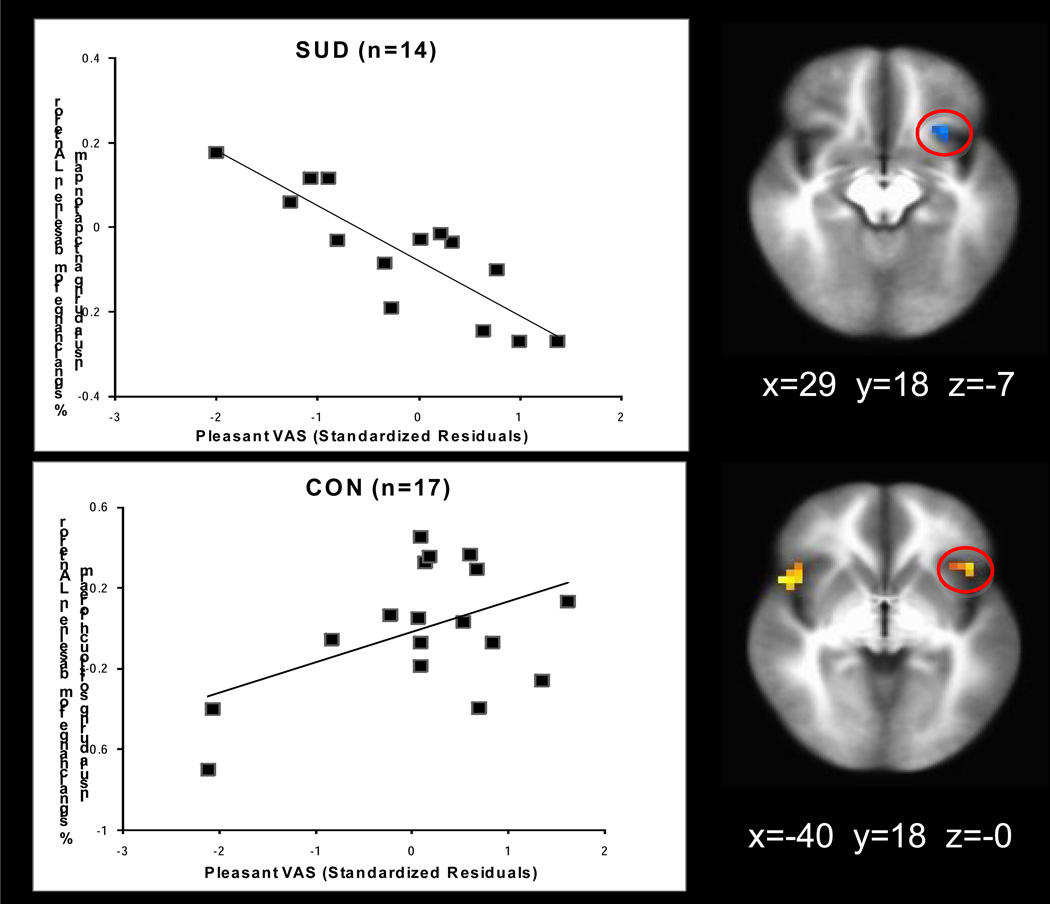
3.3.5. Robust regressions
As shown in Figure 5, for CON greater left anterior insula activation was associated with higher pleasantness ratings during soft touch forearm (r= .48, p=.05). In contrast, SUD with lower left anterior insula activation reported greater pleasantness ratings during anticipation palm (r= −.86, p<.001). SUD with more recent cannabis use also showed lower left posterior insula activation during soft touch palm (r= −.66, p<.01) (see Supplementary Figure 14).
Figure 5.
Adolescents with substance use disorder (SUD) exhibited a negative relationship between left anterior insula activation and pleasantness ratings during anticipation of soft touch (r= −.86, p<.001) while control adolescents (CON) exhibited a positive relationship between left anterior insula activation and visual analog scale pleasantness ratings during the experience of soft touch (r= .48, p=.05).
4. DISCUSSION
To our knowledge, this is the first study to examine the neural basis of interoceptive functioning differences between healthy and substance using adolescents. First, consistent with our predictions, SUD exhibited attenuated activation in bilateral posterior insula during soft touch stimulation. Contrary to our second hypothesis, palm elicited greater insula activation than the forearm. A collection of other interoceptive brain regions such as the anterior cingulate and the caudate did exhibit greater forearm activation, however. Relating to our third prediction, SUD individuals showed greater anterior insula activation during soft touch stimulation relative to anticipation, and subjective pleasantness ratings were associated with the anterior insula. Whereas the insula activation during soft touch appears to process positive valence for healthy adolescents, activation is related to aversiveness in SUD adolescents even though groups were equivalent in their subjective ratings. Finally, while groups did not differ in striatal activation during anticipation as predicted, the increase in lentiform nucleus activation from anticipation to soft touch was greater for SUD. Taken together, these findings suggest that SUD adolescents may have blunted somatovisceral processing of pleasant C-fiber stimulation, heightened sensitivity in regions responsible for processing reward value, and differential processing of conscious feeling states.
One consequence of these discrepancies in interoceptive and reward processing could be a less-well developed system in SUD adolescents for predicting physiological changes in the body. The principle of homeostatic regulation relies in part upon an individual’s ability to accurately process visceral information, incorporate that information with awareness and appraisal, and produce motivational states aimed at maintaining or altering the current body state. Any difference between the expected body state and the experienced body state has been called the body prediction error (Seth et al., 2011) and it has been hypothesized that addiction represents a chronic imbalance in the homeostatic condition of the body (Koob and Le Moal, 2008) caused by an altered body prediction error, leading to maladaptive regulation of the internal state through substance use (Paulus et al., 2009). This investigation supports the notion that SUD youth may have an aberrant body prediction error. However, given evidence linking the anterior insula to emotional experience (Carlson et al., 2011; Zaki et al., 2012), it may also be the case that altered interoceptive processing is directly connected to an emotional, rather than visceral, motivation to use substances. The interoceptive system may be involved in the urge to use substances to cope with, enhance, or alter affective states, in line with the self-medication theory of substance use (Hughes et al., 1986).
There are several limitations to this investigation. First, due to the cross-sectional nature of the study, it is unclear whether observed insular dysfunction reflects pre-existing interoceptive sensitivities or may be a consequence of neurotoxic effects of substances on the developing brain. Future studies should include adolescents who are just beginning to experiment with alcohol and drugs to examine interoceptive differences as a risk factor for substance use initiation. Furthermore, the Soft Touch paradigm did not elicit the hypothesized differences in insula activation expected for palm versus forearm stimulation. This hypothesis was based on the putative distribution of C-fiber afferents in adults and it is possible that these connections differ during adolescent development. Despite this concern, the Soft Touch task produced overall robust activation in the bilateral insula, which appears highly sensitive to this visceral stimulation, permitting a valid examination of interoceptive processing in SUD adolescents. Additionally this study was limited to the examination of stimulation rated as moderately pleasant. It is unknown if adolescents with SUD would demonstrate differential insula activation to extremely pleasant stimuli or unpleasant sensations. Finally, this investigation included a modest sample size, high-functioning participants, and was limited only to those adolescents who endorsed SUD criteria for alcohol or cannabis. Future research should recruit larger clinical samples who endorse SUD criteria for other illicit substances in order to better generalize across divergent groups of adolescents.
To our knowledge, this is the first fMRI examination of interoceptive functioning in adolescents with SUD. This study suggests that youth with clinically significant substance involvement may have aberrant neural activation related to interoceptive and reward processing of a pleasant physiological stimulus, characterized by somatovisceral hypoactivation, altered reward sensitivity, and disconnect between self-report and neural activation. Findings lend support to theories of interoceptive dysregulation in addiction. Because teenage alcohol and drug use is closely linked to future substance dependence, additional research is needed to elucidate the relationship between addiction and the physiological body state in adolescence. Ultimately, interoceptive dysfunction could serve as a potential treatment target for youth at risk for addiction.
Supplementary Material
Acknowledgments
Role of Funding Source
Funding for this study was provided by NIDA Grant 5P20DA027843. This work was also supported by NIAAA Grant T32 AA013525; NIDA and NIAAA had no further role in study design; in the collection, analysis and interpretation of data; in the writing of the report; or in the decision to submit the paper for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Supplementary material can be found by accessing the online version of this paper at http://dx.doi.org and by entering doi:…
Supplementary material can be found by accessing the online version of this paper at http://dx.doi.org and by entering doi:…
Supplementary material can be found by accessing the online version of this paper at http://dx.doi.org and by entering doi:…
Supplementary material can be found by accessing the online version of this paper at http://dx.doi.org and by entering doi:…
Supplementary material can be found by accessing the online version of this paper at http://dx.doi.org and by entering doi:…
Contributors
Drs. Martin Paulus, Susan Tapert and Jennifer Stewart designed the study and wrote the protocol. April May assisted in data collection. Robyn Migliorini and Dr. Jennifer Stewart undertook the statistical and neuroimaging analysis. Robyn Migliorini wrote the first draft of the manuscript. All authors contributed to and have approved the final manuscript.
Conflict of Interest
All authors declare that they have no conflicts of interest.
REFERENCES
- Achenbach TM, Rescorla LA. Manual for the ASEBA School-age Forms and Profiles. Burlington: University of Vermont, Research Center for Children, Youth, and Families; 2001. [Google Scholar]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Washington, DC: 2013. [Google Scholar]
- Anthony JC, Petronis KR. Early-onset drug-use and risk of later drug problems. Drug Alcohol Depend. 1995;40:9–15. doi: 10.1016/0376-8716(95)01194-3. [DOI] [PubMed] [Google Scholar]
- Bjornsdotter M, Loken L, Olausson H, Vallbo A, Wessberg J. Somatotopic organization of gentle touch processing in the posterior insular cortex. J. Neurosci. 2009;29:9314–9320. doi: 10.1523/JNEUROSCI.0400-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonomo YA, Bowes G, Coffey C, Carlin JB, Patton GC. Teenage drinking and the onset of alcohol dependence: a cohort study over seven years. Addiction. 2004;99:1520–1528. doi: 10.1111/j.1360-0443.2004.00846.x. [DOI] [PubMed] [Google Scholar]
- Carlson JM, Greenberg T, Rubin D, Mujica-Parodi LR. Feeling anxious: anticipatory amygdalo-insular response predicts the feeling of anxious anticipation. Soc. Cogn. Affect Neurosci. 2011;6:74–81. doi: 10.1093/scan/nsq017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chikama M, McFarland NR, Amaral DG, Haber SN. Insular cortical projections to functional regions of the striatum correlate with cortical cytoarchitectonic organization in the primate. J. Neurosci. 1997;17:9686–9705. doi: 10.1523/JNEUROSCI.17-24-09686.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput. Biomed. Res. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Craig AD. How do you feel? Interoception: the sense of the physiological condition of the body. Nat. Rev. Neurosci. 2002;3:655–666. doi: 10.1038/nrn894. [DOI] [PubMed] [Google Scholar]
- Craig AD. How do you feel--now? The anterior insula and human awareness. Nat. Rev. Neurosci. 2009;10:59–70. doi: 10.1038/nrn2555. [DOI] [PubMed] [Google Scholar]
- Critchley HD, Wiens S, Rotshtein P, Ohman A, Dolan RJ. Neural systems supporting interoceptive awareness. Nat. Neurosci. 2004;7:189–195. doi: 10.1038/nn1176. [DOI] [PubMed] [Google Scholar]
- Grant BF, Stinson FS, Harford TC. Age at onset of alcohol use and DSM-IV alcohol abuse and dependence: a 12-year follow-up. J. Subst. Abuse. 2001;13:493–504. doi: 10.1016/s0899-3289(01)00096-7. [DOI] [PubMed] [Google Scholar]
- Grant JD, Scherrer JF, Lynskey MT, Lyons MJ, Eisen SA, Tsuang MT, True WR, Bucholz KK. Adolescent alcohol use is a risk factor for adult alcohol and drug dependence: evidence from a twin design. Psychol. Med. 2006;36:109–118. doi: 10.1017/S0033291705006045. [DOI] [PubMed] [Google Scholar]
- Huber PJ. Robust estimation of location parameter. Ann. Math. Stat. 1964;35:73–101. [Google Scholar]
- Hughes JR, Hatsukami DK, Mitchell JE, Dahlgren LA. Prevalence of smoking among psychiatric outpatients. Am. J. Psychiatry. 1986;143:993–997. doi: 10.1176/ajp.143.8.993. [DOI] [PubMed] [Google Scholar]
- Jacobus J, Goldenberg D, Wierenga CE, Tolentino NJ, Liu TT, Tapert SF. Altered cerebral blood flow and neurocognitive correlates in adolescent cannabis users. Psychopharmacology (Berl.) 2012;222:675–684. doi: 10.1007/s00213-012-2674-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston LD, O'Malley PM, Bachman JG, Schulenberg JE. Volume I: Secondary School Students. Ann Arbor: Institute for Social Research, The University of Michigan; 2012. Monitoring the Future National Survey Results on Drug Use; pp. 1975–2011. [Google Scholar]
- Koob GF, Le Moal M. Review. Neurobiological mechanisms for opponent motivational processes in addiction. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 2008;363:3113–3123. doi: 10.1098/rstb.2008.0094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loken LS, Wessberg J, Morrison I, McGlone F, Olausson H. Coding of pleasant touch by unmyelinated afferents in humans. Nat. Neurosci. 2009;12:547–548. doi: 10.1038/nn.2312. [DOI] [PubMed] [Google Scholar]
- Lopez-Larson MP, Bogorodzki P, Rogowska J, McGlade E, King JB, Terry J, Yurgelun-Todd D. Altered prefrontal and insular cortical thickness in adolescent marijuana users. Behav. Brain Res. 2011;220:164–172. doi: 10.1016/j.bbr.2011.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas CP, Zhang H, Fisher PW, Shaffer D, Regier DA, Narrow WE, Bourdon K, Dulcan MK, Canino G, Rubio-Stipec M, Lahey BB, Friman P. The DISC Predictive Scales (DPS): efficiently screening for diagnoses. J. Am. Acad. Child Adolesc. Psychiatry. 2001;40:443–439. doi: 10.1097/00004583-200104000-00013. [DOI] [PubMed] [Google Scholar]
- Morrison I, Loken LS, Minde J, Wessberg J, Perini I, Nennesmo I, Olausson H. Reduced C-afferent fibre density affects perceived pleasantness and empathy for touch. Brain. 2011;134:1116–1126. doi: 10.1093/brain/awr011. [DOI] [PubMed] [Google Scholar]
- Naqvi NH, Bechara A. The hidden island of addiction: the insula. Trends Neurosci. 2009;32:56–67. doi: 10.1016/j.tins.2008.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naqvi NH, Bechara A. The insula and drug addiction: an interoceptive view of pleasure, urges, and decision-making. Brain Struct. Funct. 2010;214:435–450. doi: 10.1007/s00429-010-0268-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olausson H, Lamarre Y, Backlund H, Morin C, Wallin BG, Starck G, Ekholm S, Strigo I, Worsley K, Vallbo AB, Bushnell MC. Unmyelinated tactile afferents signal touch and project to insular cortex. Nat. Neurosci. 2002;5:900–904. doi: 10.1038/nn896. [DOI] [PubMed] [Google Scholar]
- Olausson H, Wessberg J, Morrison I, McGlone F, Vallbo A. The neurophysiology of unmyelinated tactile afferents. Neurosci. Biobehav. Rev. 2010;34:185–191. doi: 10.1016/j.neubiorev.2008.09.011. [DOI] [PubMed] [Google Scholar]
- Patton JH, Stanford MS, Barratt ES. Factor structure of the Barratt Impulsiveness Scale. J. Clin. Psychol. 1995;51:768–774. doi: 10.1002/1097-4679(199511)51:6<768::aid-jclp2270510607>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Paulus MP. Neural basis of reward and craving--a homeostatic point of view. Dialogues Clin. Neurosci. 2007;9:379–387. doi: 10.31887/DCNS.2007.9.4/mpaulus. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulus MP, Tapert SF, Schulteis G. The role of interoception and alliesthesia in addiction. Pharmacol. Biochem. Behav. 2009;94:1–7. doi: 10.1016/j.pbb.2009.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters J, Bromberg U, Schneider S, Brassen S, Menz M, Banaschewski T, Conrod PJ, Flor H, Gallinat J, Garavan H, Heinz A, Itterman B, Lathrop M, Martinot JL, Paus T, Poline JB, Robbins TW, Rietschel M, Smolka M, Strohle A, Struve M, Loth E, Schumann G, Buchel C, IMAGEN Consortium Lower ventral striatal activation during reward anticipation in adolescent smokers. Am. J. Psychiatry. 2011;168:540–549. doi: 10.1176/appi.ajp.2010.10071024. [DOI] [PubMed] [Google Scholar]
- Pierucci-Lagha A, Gelernter J, Feinn R, Cubells JF, Pearson D, Pollastri A, Farrer L, Kranzler HR. Diagnostic reliability of the Semi-structured Assessment for Drug Dependence and Alcoholism (SSADDA) Drug Alcohol Depend. 2005;80:303–312. doi: 10.1016/j.drugalcdep.2005.04.005. [DOI] [PubMed] [Google Scholar]
- Pinheiro JC, Bates DM, DebRoy S, Sarkar D. nlme: Linear and Nonlinear Mixed Effects Models. R package version. 2012;3:1–106. R Core Development Team. [Google Scholar]
- R Core Development Team. R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; 2012. [Google Scholar]
- Rubinstein ML, Luks TL, Dryden WY, Rait MA, Simpson GV. Adolescent smokers show decreased brain responses to pleasurable food images compared with nonsmokers. Nicotine Tob. Res. 2011;13:751–755. doi: 10.1093/ntr/ntr046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider S, Peters J, Bromberg U, Brassen S, Miedl SF, Banaschewski T, Barker GJ, Conrod P, Flor H, Garavan H, Heinz A, Itterman B, Lathrop M, Loth E, Mann K, Martinot JL, Nees F, Paus T, Rietschel M, Robbins TW, Smolka MN, Spanagel R, Strohle A, Struve M, Schumann G, Buchel C. Risk taking and the adolescent reward system: a potential common link to substance abuse. Am. J. Psychiatry. 2012;169:39–46. doi: 10.1176/appi.ajp.2011.11030489. IMAGEN Consortium. [DOI] [PubMed] [Google Scholar]
- Seth AK, Suzuki K, Critchley HD. An interoceptive predictive coding model of conscious presence. Front. Psychol. 2011;2:395. doi: 10.3389/fpsyg.2011.00395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaffer D, Fisher P, Lucas CP, Dulcan MK, Schwab-Stone ME. NIMH Diagnostic Interview Schedule for Children Version IV (NIMH DISC-IV): description, differences from previous versions, and reliability of some common diagnoses. J. Am. Acad. Child Adolesc. Psychiatry. 2000;39:28–38. doi: 10.1097/00004583-200001000-00014. [DOI] [PubMed] [Google Scholar]
- Sobell LC, Sobell MB. Timeline Follow-back: A Technique for Assessing Self-reported Alcohol Consumption. New York: Humana Press; 1992. [Google Scholar]
- Somerville LH, Jones RM, Casey BJ. A time of change: behavioral and neural correlates of adolescent sensitivity to appetitive and aversive environmental cues. Brain Cogn. 2010;72:124–133. doi: 10.1016/j.bandc.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg L. A social neuroscience perspective on adolescent risk-taking. Dev. Rev. 2008;28:78–106. doi: 10.1016/j.dr.2007.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration (SAMHSA) Results from the 2011 National Survey on Drug Use and Health: Summary of National Findings. HHS Publication No. (SMA) 12-4713. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2012. [Google Scholar]
- Tapert SF, Brown GG, Baratta MV, Brown SA. fMRI BOLD response to alcohol stimuli in alcohol dependent young women. Addict. Behav. 2004;29:33–50. doi: 10.1016/j.addbeh.2003.07.003. [DOI] [PubMed] [Google Scholar]
- Tapert SF, Schweinsburg AD, Barlett VC, Brown SA, Frank LR, Brown GG, Meloy MJ. Blood oxygen level dependent response and spatial working memory in adolescents with alcohol use disorders. Alcohol. Clin. Exp. Res. 2004;28:1577–1586. doi: 10.1097/01.alc.0000141812.81234.a6. [DOI] [PubMed] [Google Scholar]
- Vallbo AB, Olausson H, Wessberg J. Unmyelinated afferents constitute a second system coding tactile stimuli of the human hairy skin. J. Neurophysiol. 1999;81:2753–2763. doi: 10.1152/jn.1999.81.6.2753. [DOI] [PubMed] [Google Scholar]
- Vallbo AB, Olausson H, Wessberg J, Norrsell U. A system of unmyelinated afferents for innocuous mechanoreception in the human skin. Brain Res. 1993;628:301–304. doi: 10.1016/0006-8993(93)90968-s. [DOI] [PubMed] [Google Scholar]
- Verdejo-Garcia A, Clark L, Dunn BD. The role of interoception in addiction: a critical review. Neurosci. Biobehav. Rev. 2012;36:1857–1869. doi: 10.1016/j.neubiorev.2012.05.007. [DOI] [PubMed] [Google Scholar]
- Wells JE, Horwood LJ, Fergusson DM. Drinking patterns in mid-adolescence and psychosocial outcomes in late adolescence and early adulthood. Addiction. 2004;99:1529–1541. doi: 10.1111/j.1360-0443.2004.00918.x. [DOI] [PubMed] [Google Scholar]
- Yau WY, Zubieta JK, Weiland BJ, Samudra PG, Zucker RA, Heitzeg MM. Nucleus accumbens response to incentive stimuli anticipation in children of alcoholics: relationships with precursive behavioral risk and lifetime alcohol use. J. Neurosci. 2012;32:2544–2551. doi: 10.1523/JNEUROSCI.1390-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaki J, Davis JI, Ochnser KN. Overlapping activity in anterior insula during interoception and emotional experience. Neuroimage. 2012;62:493–499. doi: 10.1016/j.neuroimage.2012.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuckerman M. Item revisions in the sensation seeking scale form V (SSS-V) Pers. Individ. Dif. 1996;20:515–515. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.